Multi-Media Geochemical Exploration in the Critical Zone: A Case Study over the Prairie and Wolf Zn–Pb Deposits, Capricorn Orogen, Western Australia
Abstract
:1. Introduction
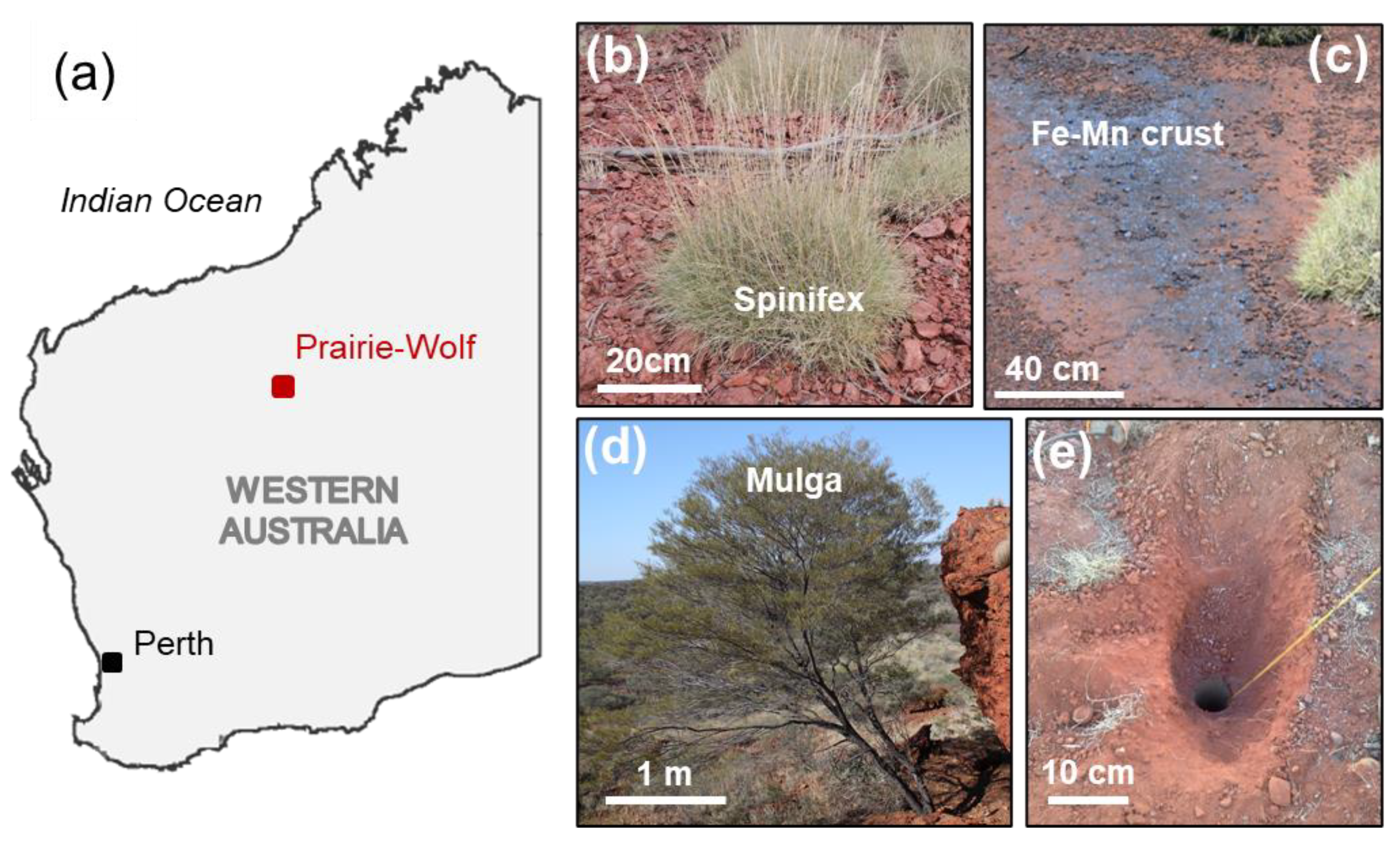
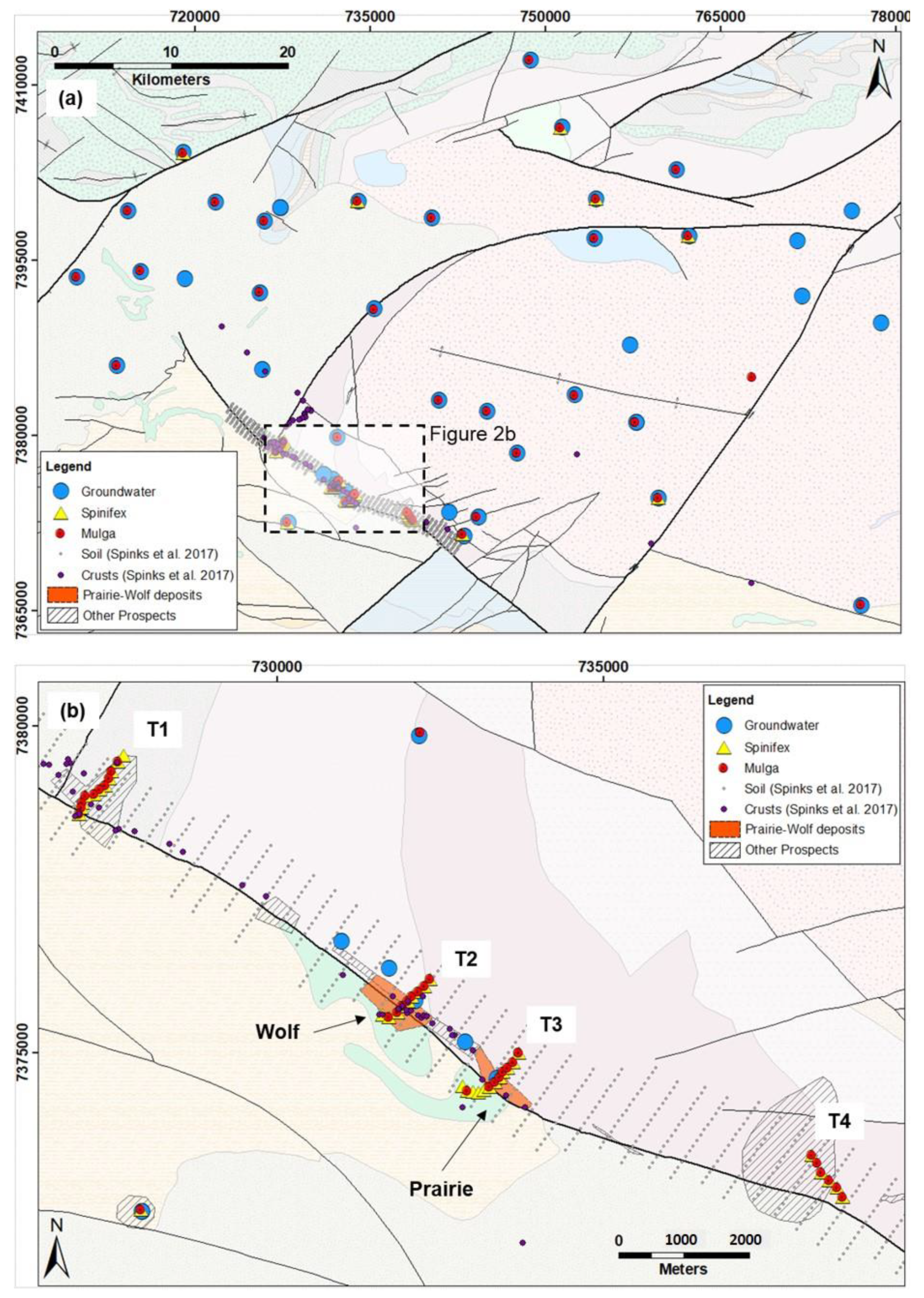
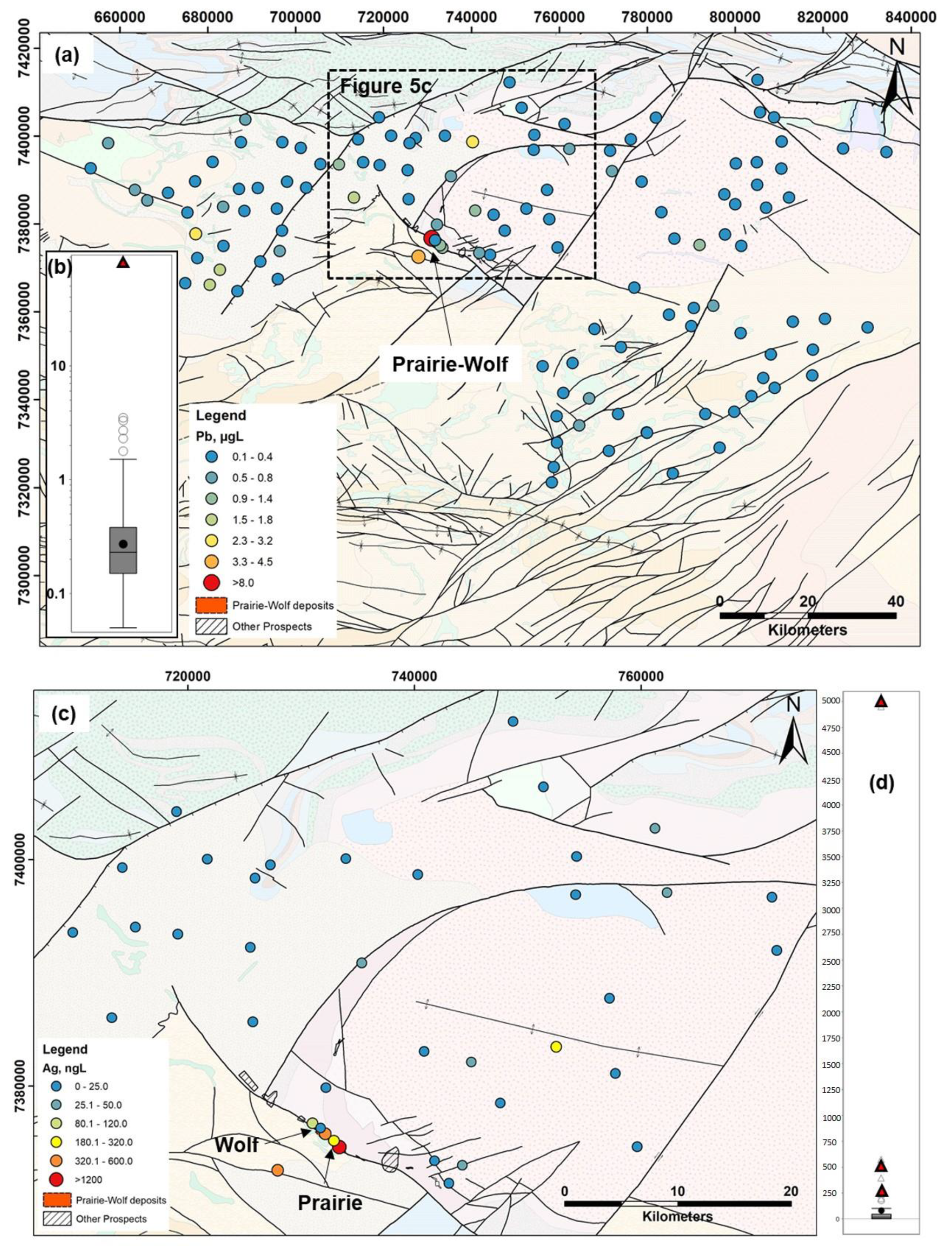
Study Area
2. Methods and Materials
2.1. Plant Samples
2.2. Groundwater
2.3. Soil, Ore and Ferro-Manganese Crust Samples
2.4. Data Processing
3. Results
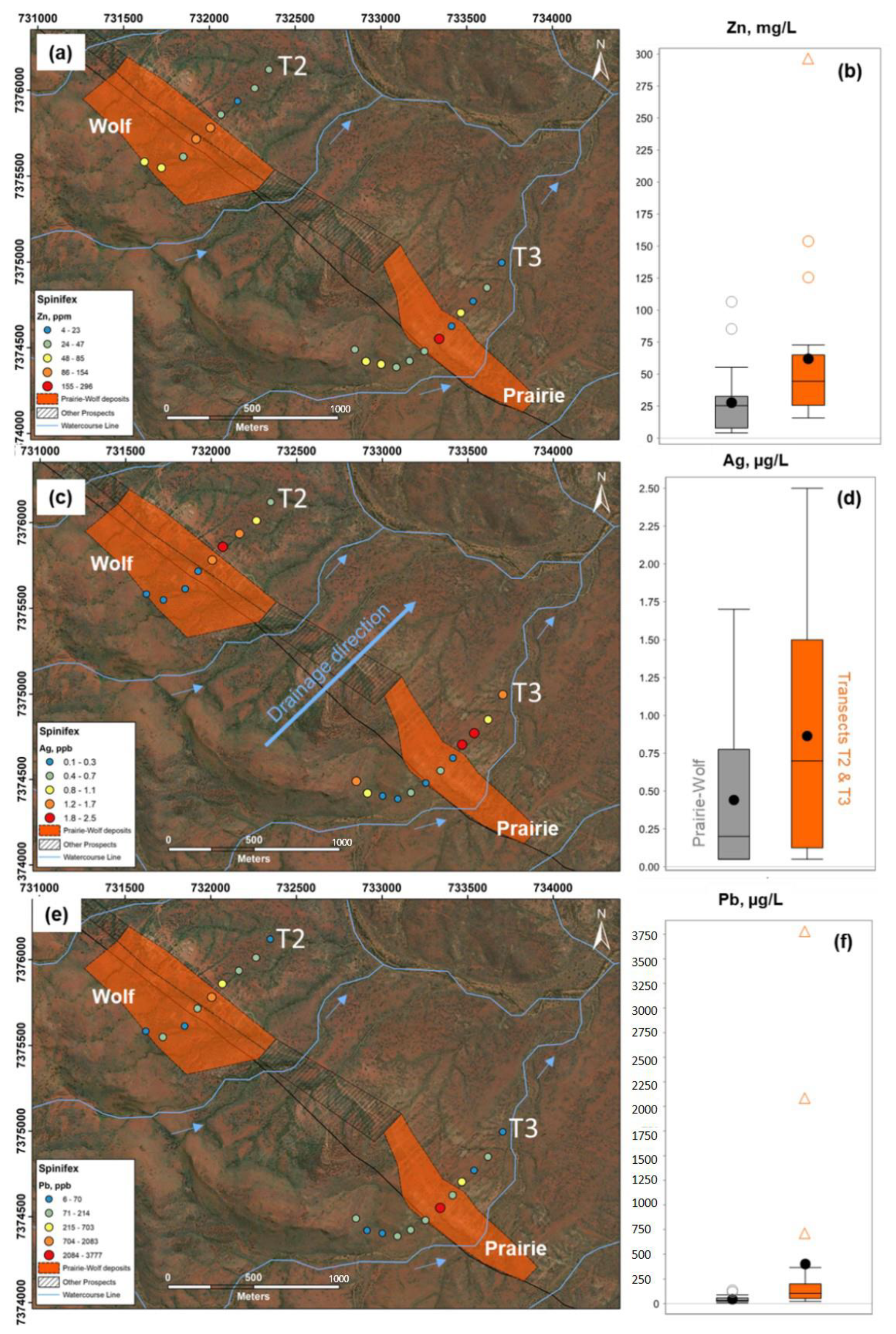
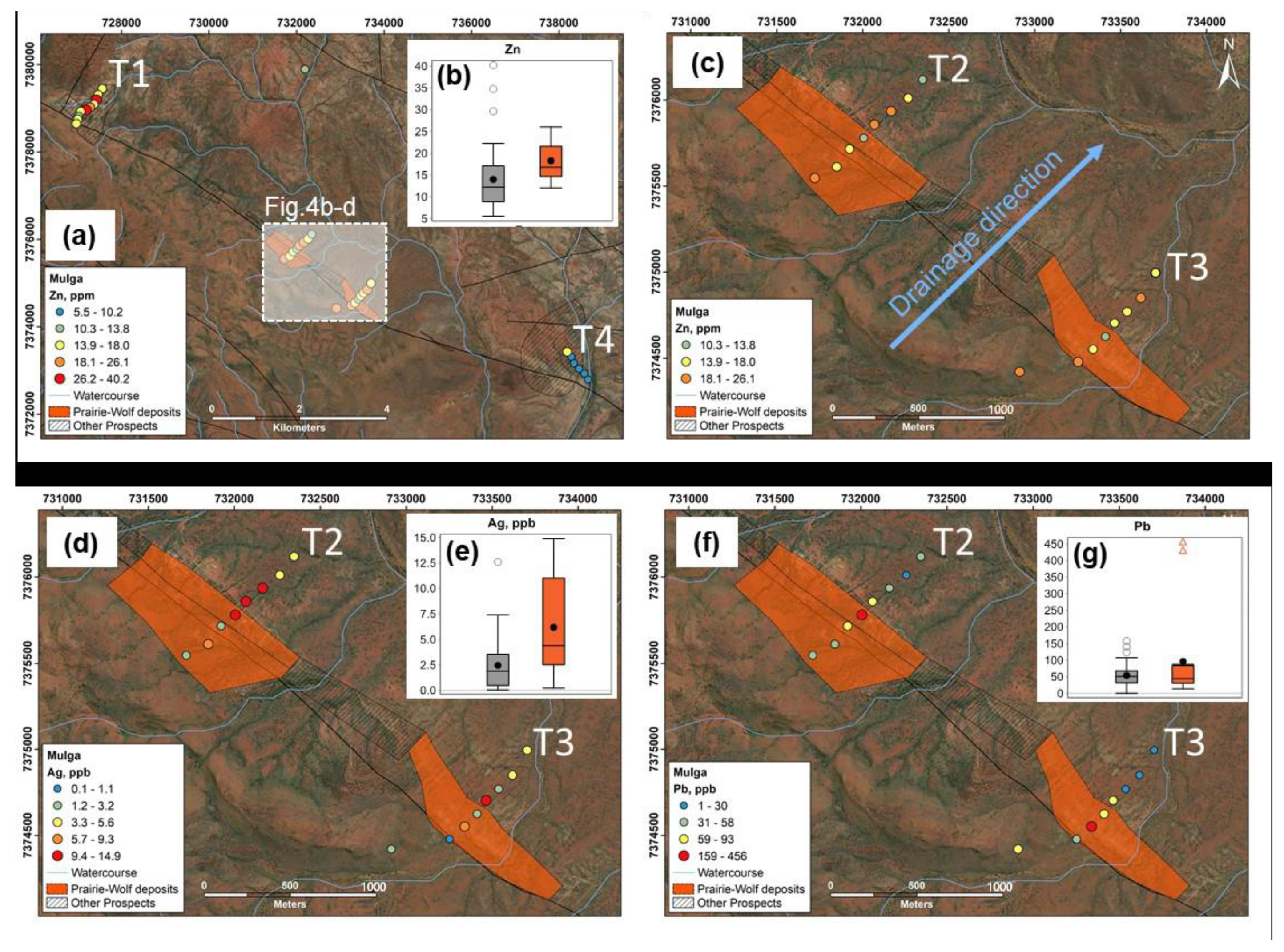
3.1. Plant Samples
3.2. Groundwater Samples
3.3. Soil and Ferro-Manganese Crust Samples
4. Discussion
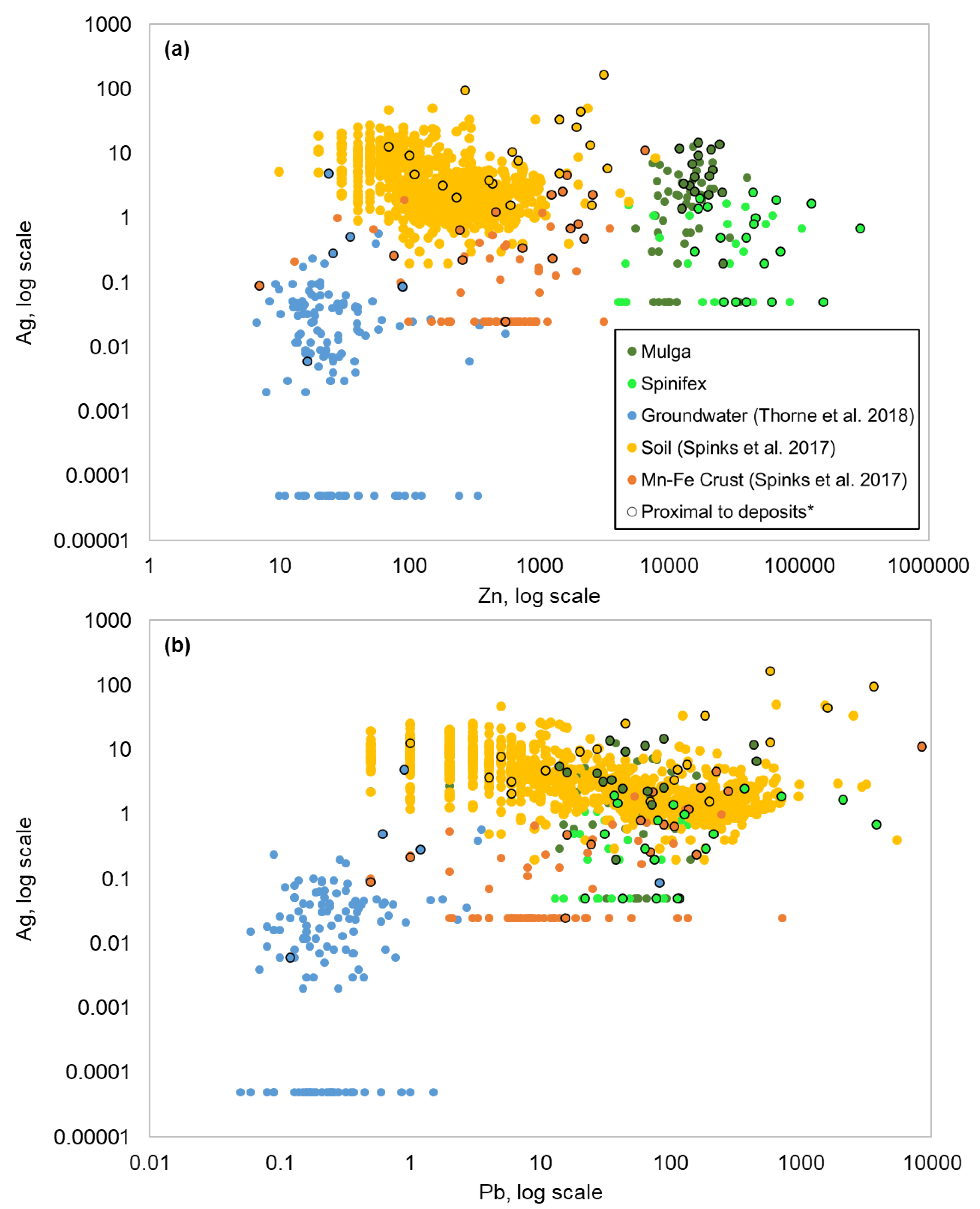
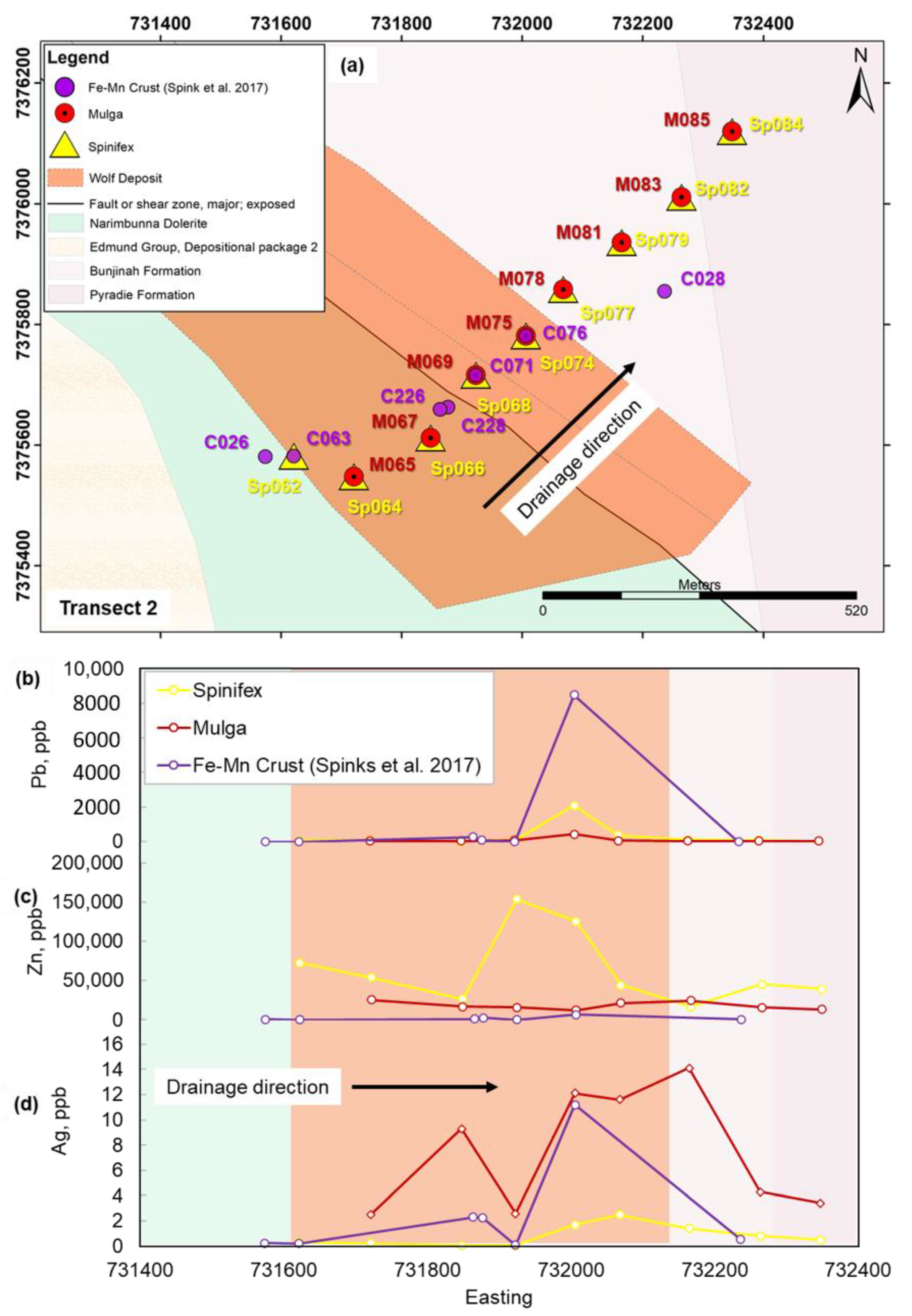
4.1. Controls on Geochemical Dispersion of Elements from Mineralization to Different Near-Surface Sample Media at Surface
4.2. Comparison of Metal Signatures in Near-Surface Sample Media
4.3. Implications for Exploration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butt, C.R.M.; Robertson, I.D.M.; Scott, K.M.; Cornelius, M. Regolith Expression of Australian Ore Systems; CRC LEME: Perth, Australia, 2005; 431p. [Google Scholar]
- Anand, R.R.; Butt, C. A guide for mineral exploration through the regolith in the Yilgarn Craton, Western Australia. Aust. J. Earth Sci. 2010, 57, 1015–1114. [Google Scholar] [CrossRef]
- Brantley, S.L.; White, T.S.; White, A.F.; Sparks, D.; Richter, D.; Pregitzer, K.; Derry, L.; Chorover, J.; Chadwick, O.; April, R.; et al. Frontiers in Exploration of the Critical Zone; National Science Foundation (NSF): Newark, DE, USA, 2006; 30p.
- Guo, L.; Lin, H. Critical Zone Research and Observatories: Current Status and Future Perspectives. Vadose Zone J. 2016, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Brooks, R.R.; Dunn, C.E.; Hall, G.E.M. Biological Systems in Mineral Exploration and Processing; Ellis Horwood Limited: Hertfordshire, UK, 1995. [Google Scholar]
- Lintern, M.; Butt, C.; Scott, K. Gold in vegetation and soil—Three case studies from the goldfields of southern Western Australia. J. Geochem. Explor. 1997, 58, 1–14. [Google Scholar] [CrossRef]
- Cohen, D.R.; Silva-Santisteban, C.M.; Rutherford Garnett, D.L.; Waldron, H.M. Comparison of Vegetation and Stream Sediment Geochemical Patterns in Northeastern New South Wales. J. Geochem. Explor. 1999, 66, 469–489. [Google Scholar] [CrossRef]
- Hulme, K.A.; Hill, S.M. River red Gums as a Biogeochemical Sampling Medium in Mineral Exploration and Environmental Chemistry Programs in the Curnamona Craton and Adjacent Regions in NSW and SA. Adv. Regolith 2003, 2003, 205–210. [Google Scholar]
- Dunn, C.E. Biogeochemistry in Mineral Exploration; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Reid, N.; Hill, S.M.; Lewis, D.M. Spinifex biogeochemical expressions of buried gold mineralisation: The great mineral exploration penetrator of transported regolith. Appl. Geochem. 2008, 123, 76–84. [Google Scholar] [CrossRef]
- Reid, N.; Hill, S.M.; Lewis, D.M. Biogeochemical expression of buried Au-mineralisation in semi-arid northern Australia: Penetration of transported cover at the Titania Gold Prospect, Tanami Desert Australia. Geochem. Explor. Environ. Anal. 2009, 9, 1–8. [Google Scholar] [CrossRef]
- Lintern, M.; Anand, R. Using gum trees for Au exploration in the Eastern Goldfields (Western Australia). In Proceedings of the 25th International Applied Geochemistry Symposium 2011, Rovaniemi, Finland, 22–26 August 2011; p. 83. [Google Scholar]
- Collerson, K.D. Application of spinifex biogeochemistry to identify mineralisation targets in obscured basement terranes beneath the Simpson Desert in southwestern Queensland. In Queensland Minerals and Energy Review Series; Natural Resources and Mines: Queensland, Australia, 2014. [Google Scholar]
- Arhin, E.; Boadi, S.; Esoah, M.C. Identifying pathfinder elements from termite mound samples for gold exploration in regolith complex terrain of the Lawra belt, NW Ghana. J. Afr. Earth Sci. 2015, 109, 143–153. [Google Scholar] [CrossRef]
- Gonzalez-Alvarez, I.; Stewart, A.; Anand, R.; Sinclair, P.; Salama, W.; Laird, J.; Ibrahimi, T.; Pinchand, T. Termitaria Geochemistry for Uranium Exploration in Arnhem Land, Northern Territory, Australia. In Proceedings of the SEG 2015: World-Class Ore Deposits: Discovery to Recovery, Hobart, Australia, 27–30 September 2015. [Google Scholar]
- Sinclair, P. Termitaria sampling in uranium exploration: Refining an old technique. In Proceedings of the AGES 2017, NT Geological Survey, Alice Springs, Australia, 28–29 March 2017; pp. 74–75. [Google Scholar]
- Arhin, E.; Captain-Esoah, M.; Berdie, B.S. Economic Importance of Termites and Termitaria in Mineral Exploration. In Termites and Sustainable Management; Springer: Cham, Switzerland, 2018; pp. 241–258. [Google Scholar] [CrossRef]
- Leslie, K.; Van Geffen, P.W.; Macfarlane, B.; Oates, C.J.; Kyser, T.K.; Fowle, D.A. Biogeochemical indicators of buried mineralization under cover, Talbot VMS Cu–Zn prospect, Manitoba. Appl. Geochem. 2013, 37, 190–202. [Google Scholar] [CrossRef]
- Bohu, T.; Anand, R.; Noble, R.; Lintern, M.; Kaksonen, A.H.; Mei, Y.; Cheng, K.Y.; Deng, X.; Veder, J.-P.; Bunce, M.; et al. Evidence for fungi and gold redox interaction under Earth surface conditions. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gray, D.J.; Noble, R.R.P.; Reid, N. Hydrogeochemical Mapping of the Northeast Yilgarn Craton. CSIRO Exploration and Mining Report No. P2009/1612//MERIWA project M402. Minerals and Energy Research Institute of Western Australia Report No. 280. 2009, 73p. Available online: http://hdl.handle.net/102.100.100/115382?index=1 (accessed on 22 October 2021).
- Gray, D.J.; Reid, N.; Noble, R.R.P. Improved Hydrogeochemical Exploration in the northwest Yilgarn—Adding Value to Underexplored Areas. CSIRO Report EP 143875. 2014, 86p. Available online: https://publications.csiro.au/rpr/pub?pid=csiro:EP143875 (accessed on 8 August 2014).
- Spinks, S.C.; Uvarova, U. Fractionation of Zn isotopes in terrestrial ferromanganese crusts and implications for tracing isotopically-heterogeneous metal sources. Chem. Geol. 2019, 529, 119314. [Google Scholar] [CrossRef]
- Spinks, S.C.; Uvarova, Y.; Thorne, R.; Anand, R.; Reid, N.; White, A.; Ley-Cooper, Y.; Bardwell, N.; Gray, D.; Meadows, H.; et al. Detection of zinc deposits using terrestrial ferromanganese crusts. Ore Geol. Rev. 2017, 80, 484–503. [Google Scholar] [CrossRef]
- Thorne, R.L.; Reid, N.; Gray, D.J.; Ballsun-Stanton, B.; Bardwell, N.; Klump, J.; Davis, A.; Ross, S.; Sobotkova, A. Hydrogeochemistry of the Capricon Orogen. EP 182906 CSIRO, 2018, p. 106. Available online: https://publications.csiro.au/rpr/pub?list=ASE&pid=csiro:EP161243&expert=false&sb=RECENT&n=3&rpp=25&page=54&tr=2079&dr=all&csiro.affiliation%7Ccsiro.projectBusinessUnit=50013148 (accessed on 26 June 2016).
- Loftleidir, H. Essential Trace Elements for Plants, Animals and Humans; Agricultural University of Iceland: Reykjavík, Iceland, 2005. [Google Scholar]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef]
- Alekseenko, V.A. Geochemical Methods for Mineral Deposits Prospecting; Logos: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Bech, J.; Duran, P.; Roca, N.; Poma, W.; Sánchez, I.; Roca-Pérez, L.; Boluda, R.; Barceló, J.; Poschenrieder, C. Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. spontaneous species from mine spoils in Peru and their potential use in phytoremediation. J. Geochem. Explor. 2012, 123, 109–113. [Google Scholar] [CrossRef]
- Alekseenko, V.A.; Shvydkaya, N.V.; Alekseenko, A.V.; Machevariani, M.M.; Bech, J.; Pashkevich, M.A.; Puzanov, A.V.; Nastavkin, A.V.; Roca, N. Element Accumulation Patterns of Native Plant Species under the Natural Geochemical Stress. Plants 2020, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.E. The biogeochemical expression of deeply buried uranium mineralisation in Saskatchewan, Canada. J. Geochem. Explor. 1981, 15, 437–452. [Google Scholar] [CrossRef]
- Malyuga, D.P. Biogeochemical Methods of Prospecting; V.I.Vernadskii Institute of Geochemistry and Analytical Chemistry: Moscow, Russia, 1963. [Google Scholar]
- Kovalevsky, A.L. Biogeochemical Exploration for Mineral Deposits; VNU Science Press: Utrecht, The Netherlands, 1987. [Google Scholar]
- Cole, M.M.; Provan, D.M.J.; Tooms, J.S. Geobotany, biogeochemistry and geochemistry in mineral exploration in the Bulman-Waimuna Springs area, Northern Territory, Australia. Trans. Inst. Min. Metall. Sect. 1968, 77, 81–104. [Google Scholar]
- Anand, R.; Cornelius, M.; Phang, C. Use of vegetation and soil in mineral exploration in areas of transported overburden, Yilgarn Craton, Western Australia: A contribution towards understanding metal transportation processes. Geochem. Explor. Environ. Anal. 2007, 7, 267–288. [Google Scholar] [CrossRef]
- Collerson, K.D.; Hutton, L.; Wason, R. Grassroots Exploration under Cover: Spinifex Geochemistry Leads to Discovery of a New Australian Metallogenic Province. 2015. Available online: https://www.ausimmbulletin.com/feature/grassroots-exploration-under-cover/ (accessed on 1 August 2021).
- Marshall, A.E.; Lintern, M.J. Biogeochemical investigation in the Murchison and Telfer regions of arid Western Australia. In Proceedings of the 17th International Geochemical Exploration Symposium, Applied Biogeochemistry in Mineral Exploration and Environmental Studies, Townsville, Australia, 13–14 May 1995. [Google Scholar]
- Gray, D.J.; Noble, R.R.P. Nickel hydrogeochemistry of the northeastern Yilgarn Craton, Western Australia. In CRC LEME Open File Report; CRC LEME: Perth, Australia, 2006; 133p. [Google Scholar]
- Kirste, D.; de Caritat, P.; Dann, R. The application of the stable isotopes of sulfur and oxygen in groundwater sulfate to mineral exploration in the Broken Hill region of Australia. J. Geochem. Explor. 2003, 78, 81–84. [Google Scholar] [CrossRef]
- Kirste, D.M.; de Caritat, P.; McPhail, D.C. Hydrogeochemistry and transport of weathering/oxidation products of buried mineralisation. Geochim. Cosmochim. Acta 2004, 68, A180. [Google Scholar]
- Pirlo, M.C.; Giblin, A.M. Application of groundwater–mineral equilibrium calculationsto geochemical exploration for sediment-hosted uranium:observations from the Frome Embayment,South Australia. Geochem. Explor. Environ. Anal. 2004, 4, 113–127. [Google Scholar] [CrossRef]
- De Caritat, P.; Kirste, D.; Carr, G.; McCulloch, M. Ground water in the Broken Hill region, Australia; recognising interaction with bedrock and mineralisation using S., Sr and Pb isotopes. Appl. Geochem. 2005, 20, 767–787. [Google Scholar] [CrossRef]
- Gray, D.J.; Noble, R.R.; Reid, N.; Sutton, G.J.; Pirlo, M.C. Regional scale hydrogeochemical mapping of the northern Yilgarn Craton, Western Australia: A new technology for exploration in arid Australia. Geochem. Explor. Environ. Anal. 2016, 16, 100–115. [Google Scholar] [CrossRef]
- White, A.J.R.; Pearce, M.A.; Meadows, H.R. Distinguishing regional-and local-scale metasomatic systems at the Prairie Downs Zn-Pb deposit. Lithos 2016, 262, 247–265. [Google Scholar] [CrossRef]
- Reid, N.; Thorne, R.; Folkes, C.; Gilmore, P.J.; Pinchand, T. Hydrogeochemistry of the Cobar Region. CSIRO, Australia. CSIRO EP: 207556, 2020, p. 112, Geological Survey of New South Wales Record GS2021/0054. MinEx Record 2020/21. Available online: https://www.researchgate.net/publication/350295603_Hydrogeochemistry_of_the_Cobar_region_Geological_Survey_of_New_South_Wales_Record_GS20210054_MinEx_record_202021 (accessed on 31 March 2021).
- Thorne, A.M.; Seymour, D.B. Geology of the Ashburton Basin, Western Australia; Geological Survey of Western Australia: Bulletin, CT, USA, 1991; Volume 139, 162p.
- Nelson, D.R. Compilation of SHRIMP U-Pb Zircon Geochronology Data, 1994; Western Australia Geological Survey: Perth, Australia, 1995; p. 244.
- Thorne, A.M.; Trendall, A.F. Geology of the Fortescue Group, Pilbara Craton; Western Australia; Geological Survey of Western Australia: Bulletin, CT, USA, 2001; Volume 144, 249p.
- White, A.J.R.; Legras, M.; Smith, R.E.; Nadoll, P. Deformation-driven, regional-scale metasomatism in the Hamersley Basin, Western Australia. J. Metamorph. Geol. 2014, 32, 417–433. [Google Scholar] [CrossRef]
- White, A.J.R.; Smith, R.E.; Nadoll, P.; Legras, M. Regional-scale Metasomatism in the Fortescue Group Volcanics, Hamersley Basin, Western Australia: Implications for Hydrothermal Ore Systems. J. Pet. 2014, 55, 977–1009. [Google Scholar] [CrossRef] [Green Version]
- Atlas of Living Australia Databases. Available online: http://www.ala.org.au/ (accessed on 4 January 2013).
- AusGrass2. 2021. Available online: http://ausgrass2.myspecies.info/content/triodia-basedowii (accessed on 22 October 2021).
- Miller, J.T.; Andrew, R.A.; Maslin, B.R. Towards an understanding of variation in the Mulga complex (Acacia aneura and relatives). Conserv. Sci. West. Aust. 2002, 4, 19–35. [Google Scholar]
- Moore, P. A Guide to Plants of Inland Australia; Reed New Holland: Sydney, Australia, 2005. [Google Scholar]
- McWilliam, J.; Mison, K. Significance of the C4 Pathway in Triodia irritans (Spinifex), a Grass Adapted to Arid Environments. Funct. Plant Biol. 1974, 1, 171–175. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Wong, V.N.L.; Wilson, S.; Gore, O. Critical metals in the critical zone: Controls, resources and future prospectivity of regolith-hosted rare earth elements. Aust. J. Earth Sci. 2017, 64, 1045–1054. [Google Scholar] [CrossRef]
- Brugger, J. Encyclopedia of Earth Sciences Series; White, W.M., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Mondillo, N.; Wilkinson, J.J.; Boni, M.; Weiss, D.J.; Mathur, R. A global assessment of Zn isotope fractionation in secondary Zn minerals from sulfide and non-sulfide ore deposits and model for fractionation control. Chem. Geol. 2018, 500, 182–193. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Caine, J.S.; Evans, J.P.; Forster, C.B. Fault zone architecture and permeability structure. Geology 1996, 24, 1025–1028. [Google Scholar] [CrossRef]
- Anand, R.R.; Paine, M. Regolith geology of the Yilgarn Craton, Western Australia: Implications for exploration. Aust. J. Earth Sci. 2002, 49, 3–163. [Google Scholar] [CrossRef]
- Dawson, T.E.; Pate, J.S. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 1996, 107, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Orellana, F.; Verma, P.; Loheide, S.; Daly, E. Monitoring and modeling water-vegetation interactions in groundwater-dependent ecosystems. Rev. Geophys. 2012, 50, RG3003. [Google Scholar] [CrossRef]
- Rasouli-Sadaghiani, M.H.; Sadeghzadeh, B.; Sepehr, E.; Rengel, Z. Root exudation and zinc uptake by barley genotypes differing in Zn efficiency. J. Plant Nutr. 2011, 34, 1120–1132. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.E., Jr.; Henrich, V.E.; Casey, W.H.; Clark, D.L.; Eggleston, C.; Felmy, A.; Goodman, D.W.; Gratzel, M.; Maciel, G.; McCarthy, M.I.; et al. Metal oxide surfaces and their interaction with aqueous solutions and microbial organisms. Chem. Rev. 1999, 99, 77–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.E., Jr.; Parks, G.A. Sorption of trace elements on mineral surfaces: Modern perspectives from spectroscopic studies, and comments on sorption in the marine environment. Int. Geol. Rev. 2001, 43, 963–1073. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Geoffroy, N. Natural speciation of Ni, Zn, Ba, and as in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction. Geochim. Cosmochim. Acta 2007, 71, 95–128. [Google Scholar] [CrossRef]
- Fan, T.; Wang, Y.; Li, C.; He, J.; Gao, J.; Zhou, D.; Friedman, S.; Sparks, D. Effect of Organic Matter on Sorption of Zn on Soil: Elucidation by Wien Effect Measurements and EXAFS Spectroscopy. Environ. Sci. Technol. 2016, 50, 2931–2937. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henne, A.; Reid, N.; Thorne, R.L.; Spinks, S.C.; Pinchand, T.; White, A. Multi-Media Geochemical Exploration in the Critical Zone: A Case Study over the Prairie and Wolf Zn–Pb Deposits, Capricorn Orogen, Western Australia. Minerals 2021, 11, 1174. https://doi.org/10.3390/min11111174
Henne A, Reid N, Thorne RL, Spinks SC, Pinchand T, White A. Multi-Media Geochemical Exploration in the Critical Zone: A Case Study over the Prairie and Wolf Zn–Pb Deposits, Capricorn Orogen, Western Australia. Minerals. 2021; 11(11):1174. https://doi.org/10.3390/min11111174
Chicago/Turabian StyleHenne, Anicia, Nathan Reid, Robert L. Thorne, Samuel C. Spinks, Tenten Pinchand, and Alistair White. 2021. "Multi-Media Geochemical Exploration in the Critical Zone: A Case Study over the Prairie and Wolf Zn–Pb Deposits, Capricorn Orogen, Western Australia" Minerals 11, no. 11: 1174. https://doi.org/10.3390/min11111174




