Abstract
There is a growing interest in electronic wastes (e-wastes) recycling for metal recovery because the fast depletion of worldwide reserves for primary resources is gradually becoming a matter of concern. E-wastes contain metals with a concentration higher than that present in the primary ores, which renders them as an apt resource for metal recovery. Owing to such aspects, research is progressing well to address several issues related to e-waste recycling for metal recovery through both chemical and biological routes. Base metals, for example, Cu, Ni, Zn, Al, etc., can be easily leached out through the typical chemical (with higher kinetics) and microbial (with eco-friendly benefits) routes under ambient temperature conditions in contrast to other metals. This feature makes them the most suitable candidates to be targeted primarily for metal leaching from these waste streams. Hence, the current piece of review aims at providing updated information pertinent to e-waste recycling through chemical and microbial treatment methods. Individual process routes are compared and reviewed with focus on non-ferrous metal leaching (with particular emphasis on base metals dissolution) from some selected e-waste streams. Future outlooks are discussed on the suitability of these two important extractive metallurgical routes for e-waste recycling at a scale-up level along with concluding remarks.
1. Introduction
The concomitant increment in metal demand and paucity of primary resources is an emerging challenge for valuable metal production [1,2]. In this regard, urban mining aimed towards green recycling and recovery of metals from electronic waste (e-waste) has been gaining momentum [3,4]. Additionally, the metal recycling approaches are also receiving crucial attention from the United Nations Environment Program (UNEP), since the worldwide demand for metals has been mounting consistently [5,6]. The electrical and electronic equipment (EEE) such as cell phones, smartphones, tablets, laptops, flat panel TVs, video recorders, refrigerators, digital cameras, etc., have turned out to be key requisites for the modern world [7].
E-waste or the waste electrical and electronic equipment (WEEE) consists of several parts including sub-assemblies and consumables that remain a part of the product at the time of disposal [8]. It is developing into a global concern because of its huge volume along with the health impacts pertinent to its inappropriate management and reuse. At the same time, it is also a rich source of valuable materials, which can be extracted from it judiciously for several applications [9,10,11]. In this regard, sustainable recycling processes would assist in increasing the metal production, while at the same time, addressing the environmental and health problems associated with hazardous wastes [5].
According to reports, WEEE is considered as one of the major sources of waste globally and has a high annual growth rate [12,13,14]. The projected worldwide e-waste generation amounted to 53.6 million ton (Mt) in 2019, which increased from 41.8 Mt in 2014 and 44.7 Mt in 2016. It is expected to grow further to 74.7 Mt by 2030 following a yearly growth rate of nearly 2 Mt [8,15,16]. Therefore, proper recycling of this waste for recovery of critical metals would be beneficial considering both from an economic and eco-friendly viewpoint [7,17,18]. According to the European WEEE Directives 2002/96/EC and 2012/19/EU, the e-waste is categorized into distinct varieties, which comprise of big and small domestic appliances, information technology and telecom equipment, monitors and control units, electrical and electronic non-industrial tools, relaxation and sports equipment, lighting devices, consumer equipment, medical non-infected devices and automatic dispensers [19].
The recycling of metals from e-wastes in a resourceful and environmentally feasible manner would assist in meeting the inadequacy of several valuable metals that have applications in technological development [20,21]. In this aspect, hydrometallurgical recycling techniques, e.g., the leaching of metals using chemicals and/or biological (re)agents have gained rapid momentum over the years [22,23]. Since, the concentration of base (non-ferrous) metals, in general, is higher in e-wastes (even higher than that present in the ores); hence, this piece of review emphasizes primarily on their dissolution aspects from some selected e-waste materials/streams. Precious metals (e.g., Au and Ag), critical and/or rare metals (e.g., Li, In, Ga), also considered as non-ferrous metals [24,25], are seen to be extracted along with the base metals. In this review, very limited focus is given on the dissolution aspects of such non-ferrous metals; however, they are discussed in order to reflect the overall research attempts made with respect to the selected e-wastes. The metal dissolution aspects are reviewed in context to providing comparative insights into both the chemical and microbial leaching routes in practice. The article discusses some of the recent attempts in the area where a majority of the literature covered are within the time frame from 2015–2020, also including articles that are published in the current year 2021. To our knowledge, very limited review articles on solar panels and by-products of e-wastes are available. In this piece of review, along with some highlighted e-wastes (e.g., PCBs, batteries, LCD/LED panels), aspects related to the bio and chemical leaching of spent solar panels and certain by-products of e-wastes (e.g., flue dust, slags etc.) have been discussed.
With respect to the collection of literature, search engines such as Google Scholar, Web of Science and Scopus databases were primarily searched. Several key words such as “Electronic waste”, “E-waste”, “Base metal leaching from e-waste”, “Non-ferrous metals”, “Non-ferrous metals in E-waste”, “Chemical leaching of E-waste”, “Bioleaching of E-waste”, “Metal leaching from spent solar panels”, “by-products from e-wastes”, “PCBs”, “LCD panels”, “Alkaline batteries”, “Lithium ion batteries”, “Acidophilic bioleaching of E-waste”, “Fungal bioleaching”, “Heterotrophic bioleaching”, “Authotrophic bioleaching”, “E-waste circular economy”, etc., were used.
2. Hydro-Metallurgical Applications in Metal Recovery
2.1. Chemical Leaching
As part of extractive metallurgy, hydrometallurgy is a well-established process which is applied successfully for metal leaching from both primary and secondary resources [22,26]. Nowadays, several researches are being undertaken on chemical leaching of critical metals including REE [26,27,28,29], base metals [30,31,32,33] and precious metals [34,35,36] from e-waste materials. The process is mainly divided into three steps (leaching, solid liquid separation/purification and metal recovery). The first step consists of deriving the metal value in aqueous phase. In the second stage, the solid part is separated from leach liquor, subsequently followed by pregnant leach solution purification. The last step consists of extracting metal from the purified solution [37].
Different groups of lixiviants such as inorganic acid [30,31,33,38], organic acid [27,36] and alkaline reagents [36,39,40,41], are used for metal leaching from e-waste. Recently, several reports were made with respect to REE and precious metals [26,37,42,43,44]. Table 1 shows a list of chemical leaching approaches that were carried out for various e-waste samples along with their experimental conditions and metal recovery. It is to be noted that the table presents base and some other non-ferrous metals, e.g., Au, Ag, Li, and In, to reflect the overall study. The following sections discuss metal leaching aspects from some selected e-waste streams with primary focus on base metals.
2.1.1. Metal Leaching from Waste Printed Circuit Boards (WPCBs)
Printed circuit boards contain a variety of base metals such as Cu, Fe, Ni, Al, Sn, Pb, Zn and precious metals including Au, Pd, and Ag. The metal composition in PCBs can vary based on the grades (high or low quality); nevertheless, Cu is the element found in a higher concentration ranging from 16 to 38.8% [45]. As per a study carried out by [46], the presence of 24.178% Cu was confirmed by the characterization of PCBs from a mobile phone. Considering the Cu content in the current primary resources (which is approximately 0.62%), the above mentioned values hold a primary importance in extractive metallurgy [47].

Table 1.
List of some chemical leaching studies for metal recovery from selected e-waste streams along with their operation conditions (note: Au, Ag, Li, In, etc., have been presented, wherever applicable, to reflect the overall study along with base metal dissolution).
Table 1.
List of some chemical leaching studies for metal recovery from selected e-waste streams along with their operation conditions (note: Au, Ag, Li, In, etc., have been presented, wherever applicable, to reflect the overall study along with base metal dissolution).
| E-Waste | Chemical Concentration | Pulp Density | Temp | Stirring Rate | Leach Time | Metal Recovery | References |
|---|---|---|---|---|---|---|---|
| PCBs | 0.5 mol/dm3 HCl and 0.074 mol/dm3 FeCl3 | 1/10 S/L (w/v) | Room temp. | 600 rpm | 24 h | 96% Cu, 81% Sb | [31] |
| 4 g CS(NH2)2 + 2.6 g Fe2(SO4)3 + 3.6 N H2SO4 | 1/100 S/L (w/v) | 20 °C | 150 rpm | 7 h | 100% Cu, 100% Au, 100% Ag | [48] | |
| 1.17 M NaBr + 0.77 M Br2 + 2M HCl | 50 g/L | 23.5 °C | 400 rpm | 10 h | 95.21% Ni, 97.88% Cu, 92.50% Zn, 97.61% Pb, 96.79% Sn, 96.52% Ag, 95.59% Au | [32] | |
| 100 mM Fe2(SO4)3 | 10 g/L | 20 °C | 300 rpm | 4 h | 98% Cu | [44] | |
| 20 g/L Fe2(SO4)3 | 1% | 25 °C | 200 rpm | 48 h | 84.3% Cu, 98.4% Ni, 100% Zn, 100% Al | [49] | |
| 1 mol/L glycine + 10% H2O2 | 1/100 S/L ratio | 30 °C | 400 rpm | 8 h | 94.08% Cu | [36] | |
| 0.074 mol/L FeCl3 + 0.5 mol/L HCl | 1/10 S/L ratio | Room temp. | 600 rpm | 24 h | 96% Cu, 81% Sb | [31] | |
| 3.6 mol/L H2SO4 + 6% v/v H2O2 | 75 g/L | 20 °C | - | 186 min | 96% Cu | [33] | |
| 0.5 M glycine | 2% | 23 °C | 100 rpm | 72 h | 96.5% Cu, 92.5% Zn, 46.8% Pb | [50] | |
| PCBs Sludge | 0.84 M H2SO4 | L/S ratio of 100:1 | 60 °C | 200 rpm | 80 min | 96% Cu | [51] |
| 0.2 M H2SO4 | 4% | 25 °C | 250 rpm | 1 h | 95% Cu | [52] | |
| PCB dust | 2 M NH4OH + 17.5 M H2O2 | 1% | 40 °C | 400 rpm | 3 h | 92% Cu, 50% Zn | [39] |
| LCD | 2 M H2SO4 | 0.1 kg/L | 80 °C | - | 10 min | 85–90% In | [53] |
| 6 M HCl | 1.9 to 33.3 L/kg | - | - | 2 h | 968.5 mg/kg In | [54] | |
| 1 M citrate + 0.2 M N2H4 | 20 g/L | 25 °C | 450 rpm | 16.6 h | 98.9% In | [27] | |
| 5 M HCl | 500 g/L | 75 °C | 400 rpm | 2 h | 10.24 × 10−3 g/L Sn, | [55] | |
| 76.16 × 10−3 g/L In | |||||||
| 1 mol/L H2SO4 | 1/8 S/L ratio | 70 °C | 320 rpm | 1 h | 97.07% Sn, 9.25% In | [56] | |
| 0.4 N H2SO4 | 50% | 70 °C | 250 rpm | 30 min | 99.5% In | [57] | |
| 0.5 M H2SO4 | 0.1 g/mL | 90 °C | 360 rpm | 2 h | 98% In | [58] | |
| 3M H2SO4 | 6/1 L/S ratio | 85 °C | 600 rpm | 10 min | 76.1% Sn, 86.3% In | [59] | |
| LIBs | 2.75 mol/L H3PO4 | 6 mL/g L/S ratio | 40 °C | 450 rpm | 10 min | 96.3% Mn, 99.1% Li | [60] |
| 1.5 mol/L malonic acid + 0.5% H2O2 | 20 g/LS/L ratio | 70 °C | 300 rpm | 20 min | 98.27% Ni, 98.6% Co, 98.54% Mn, 95.74% Li | [61] | |
| Alkaline batteries | 2 mol/L H2SO4 | 5 mL/g L/S ratio | 60 °C | - | 2 h | 98% Ni, 90% Mn, 97% Co | [38] |
| H2SO4 | 10/1 L/S ratio | 60 °C | 300 rpm | 2 h | 99.2% Zn, 37.6% Mn | [62] | |
| 1.2 M glycine | 10% | 25 °C | 210 rpm | 150 min | 86% Cd | [63] | |
| 5M HNO3 | 1/35 S/L ratio | 70 °C | - | 180 min | 96.5% Cd | [64] | |
| 3M NaOH | L/S ratio = 50 | 70 °C | - | 3 h | 99.9% Al | [65] | |
| 6M H2SO4 | 1/10 S/L ratio | 93.2 °C | - | 148 min | 95.2% In | [66] |
Hydrometallurgy has been successfully applied for the leaching of various metals. A recent study indicated that using ferric chloride (0.074 mol dm−3 FeCl3) in the presence of hydrochloric acid (0.5 mol dm−3 HCl) led to the successful leaching of antimony (Sb) as a by-product during Cu leaching from PCBs [31]. The leaching step was followed by an intermediate precipitation process for Sb recovery (81% pure Sb) and ended with the electro-winning process for Cu recovery (96% pure copper) (Figure 1a). FeCl3 and HCl are suitable and efficient reagents for antimony leaching [67]. Alongside these mixtures, HCl and hydrogen peroxide (H2O2) were used together for Cu leaching from PCBs. The overall reaction for copper dissolution in the mixture (HCl + H2O2) is given below [68],
Cu + H2O2 + 2HCl → CuCl2 + 2H2O
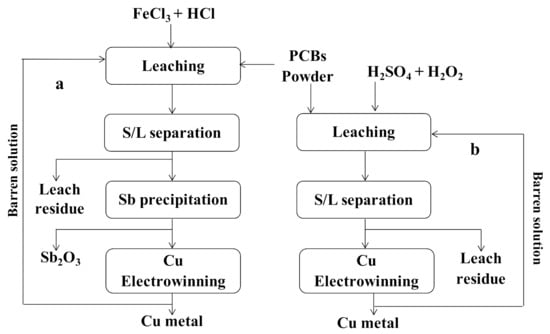
Figure 1.
Cu metal recovery from waste PCBs. The flow sheet is drawn following information collected from (a) [31], (b) redrawn following [33].
Recently, a similar investigation was conducted by [33], which focused mainly on copper leaching and was followed by electro-winning process (Figure 1b). In contrast to the earlier study, mentioned in the above paragraph, here the authors used sulfuric acid (H2SO4) in the presence of H2O2 as an oxidant. The optimum concentrations were: H2SO4 (3.6 mol L−1) and H2O2 (6% v/v), temperature (20 °C), pulp density (75 g L−1), the leaching time was 186 min, and the leaching efficiency of Cu reached 96%. The electrowinning process allowed the recovery of over 92% of Cu from leach solution having a purity of 99.9%.
Alkaline leaching agents have been used for metal leaching from PCBs. A recovery of 92% of Cu and 50% of Zn from PCBs was observed by using ammonia (NH4OH), along with hydrogen peroxide (H2O2) [39]. These extraction values were obtained under the following conditions: 2 M NH4OH, 17.5 M H2O2, at 40 °C and 400 rpm agitation.
In addition to strongly acidic and alkaline leaching agents, some researchers have used chemicals that can behave like acid or alkali to leach metals from PCBs. An amino acid such as glycine has been used for extracting Cu from PCBs under both acidic and alkaline leaching environments [36,40]. This reagent can act as an acidic or basic agent because it includes a carboxyl group (COOH) which is considered as an acidic group and also an amino group (NH2) which is a basic group [69]. Based on these properties, a novel method was proposed for Cu leaching from PCBs with a green amino acid (glycine) in the presence of H2O2 [36]. The Cu leaching efficiency reached 94.08% in 8 h at 30 °C with 1 mol/L of glycine concentration, 10% H2O2, 1:100 solid–liquid ratio and 400 rpm of stirring speed. In yet another study, glycine was used in an alkaline environment as a sustainable approach for Cu recovery from PCBs [40]. The authors proposed a two-step alkaline leaching process by simply using glycine for base metal leaching, and a mixture of glycine and sodium cyanide (NaCN) for precious metal leaching in the first and second stages, respectively. The recoveries of base metals were 80.9%, 99.1%, 85.6%, 72.5% and 6.5% for Zn, Al, Pb, Cu and Ni, respectively. A similar study indicated that it is possible to recover about 96.5% Cu from PCBs by using alkaline glycine [50].
2.1.2. Metal Leaching from Liquid Crystal Display (LCD) Panels
Hydrometallurgical processes have been used as an important tool in several researches that were recently carried out for metal leaching from liquid crystal display (LCD) panels. The majority of these studies focus on the recovery of indium from LCD [27,57,58,59]. It is important to note that indium is basically and primarily recovered as a by-product of zinc through the processing of base metal concentrates [70]. The U.S. Customs and Border Protection Agency has included indium in the list of its commercial base metals [71]. Indium (In) is primarily used as indium tin oxide for the making of transparent conductive coatings for LCDs. The composition of LCD panels varies according to the variety, as well as the manufacturer and majorly consists of silicon, aluminium and calcium, which compose the glass of LCD panels. A range of other metals such as Zn, Cu and Fe can also be present in LCD. However, indium is considered as the most desired metal since it occurs in a higher amount in LCD waste. The studies conducted on the leaching of In from LCD panels in the last decade have been reviewed and can be found elsewhere [43,44,72].
Nevertheless, during the last three years, some studies have focused toward the improvement of In leaching from LCD. Several reports have indicated sulfuric acid as a suitable inorganic acid lixiviant due to its high selectivity and dissolution of indium oxide (In2O3) and tin oxide (SnO2) from LCD [53,56,57,58] according to the following reactions [59].
In2O3 + 3H2SO4 → 2In3+ + 3SO42− + 3H2O
SnO2 + 2H2SO4 → Sn4+ + 2SO42− + 2H2O
In one of the studies, [57] used the Box–Behnken-type experimental design methodology to optimize and evaluate the effect of acid concentration (H2SO4), leaching time and temperature in In leaching from LCD panels. From their investigation, it was concluded that H2SO4 and temperature were the main parameters that influenced the metal solubilisation. Under optimum conditions (0.4 N H2SO4; 30 min; 70 °C and 50% of S/L ratio), about 99.5% of In was leached out. In a report made by [58], the leaching of In was carried out by using 0.5 M of H2SO4 at a solid/liquid ratio of 0.1 g/mL, 360 rpm and 90 °C for 2 h. Under these conditions, the leaching efficiency of In was about 98%.
Inorganic acid such as citrate can also be used for efficient In leaching in the presence of an oxidizing agent. However, since LCD panels contain Fe and Sn in addition to In, this reagent can lose its selectivity by co-dissolution of all of these metals [27]. In order to improve the efficiency and selectivity of In leaching, prior to citrate leaching, pre-treatment of LCD sample was done so as to remove the excess of Sn and Fe [27]. As a result, this allowed the recovery of approximately 99% In in 16.6 h by using 1M citrate and 0.2 M N2H4 as an oxidant at pH 5 and solid/liquid ratio of 20 g/L. However, compared to the earlier studies [57,58], it was observed that the leaching time was considerably higher and needed to be improved.
Of late, several researches were diverted towards the leaching of more than a single metal from LCD panels. As per a report made by [56] using 1 mol/L H2SO4 at 70 °C for 1 h, 99.25% of In could be recovered and 97.07% of Sn got precipitated in the solid form by the adoption of a single leaching step. The precipitation of Sn in the leaching process was due to the hydrolysis and precipitation of Sn4+ at pH < 1, according to the reaction shown below. The pregnant leach solution from the previous step was subjected to sequential electrodeposition for Cu and In extraction in the first and second steps, respectively.
Sn4+(aq) + (2 + x)H2O → SnO2·xH2O(s) + 4H+(aq)
Leaching time can be reduced by using a mixture of H2SO4 and H2O2 as an oxidant. Recently, 3 M of H2SO4 with H2O2 was used to recover 86.3% of In and 76.1% Sn within a time period of 10 min at 85 °C [59]. H2O2 was used to enhance In and Sn dissolution during the leaching process. It was observed that In and Sn recovery increased with increasing concentration of H2O2, while the HCl concentration was kept constant. The mechanisms involved in HCl leaching of In2O3 and SnO2 from LCD are presented in the following chemical reactions below [55].
In2O3 + 6HCl → 2InCl3 + 3H2O
InCl3 + 6H2O ⇌ [In(H2O)6]3+ + 3Cl−
SnO2 + 4HCl → SnCl4 + 2H2O
HCl is considered as a corrosive lixiviant [73]. This reagent can be replaced by weak acids for co-leaching of metals from LCD panels. A study carried out by [27] showed that citrate is capable of co-dissolving Fe, Sn and In by forming metal complexes. The results of the study indicated that Fe-citrate was thermodynamically more stable in the leaching environment followed by Sn-citrate due to the high consumption of citrate by Fe and Sn, whereas the In-citrate complex was less stable.
2.1.3. Metal Leaching from Spent Batteries and Solar Cells
Several hydrometallurgical processes are used for leaching of different metals from spent battery based on the type of product and composition [44]. In these processes inorganic acids such as hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3) and phosphoric acid (H3PO4) or organic acids like citric acid, oxalic acid, and tartaric acid were investigated for metal leaching from spent batteries [41]. Additionally, alkaline lixiviants such as ammonium hydroxide (NH4OH), ammonium oxalate ((NH4)2C2O4), ammonium bicarbonate (NH4HCO3) and ammonium carbonate ((NH4)2CO3) were also used for metal leaching from spent batteries [60].
Hydrometallurgical approaches have long been applied for metal extraction from alkaline batteries. In one of the studies, the extraction of base metal recovery from spent zinc-carbon and alkaline battery mixtures was investigated [62]. The work consisted of a stepwise procedure, where the first step included phosphorous removal by neutral leaching and it was subsequently followed by selective sulfuric acid leaching, purification and metal recovery processes. In this approach, 99.2% of Zn and 37.6% Mn were leached at 60 °C, pH 2 and liquid/solid ratio of 10. The whole flow sheet of the work is shown in Figure 2. A similar investigation was conducted for Zn and Mn leaching and recovery from spent alkaline batteries using sulfuric acid [74]. In yet another study, an alkaline glycine leaching was considered for Cd recovery from spent Ni-Cd battery. The findings of this study indicated that 86% of Cd could be selectively leached using 1.2 M of glycine at pH 9.6 and 25 °C for a time period of 150 min [63]. Moreover, HNO3 was used to carry out Cd leaching from Ni-Cd battery. It was observed that 96.5% of Cd could be leached out in 180 min using 5 M HNO3 at 70 °C [64].
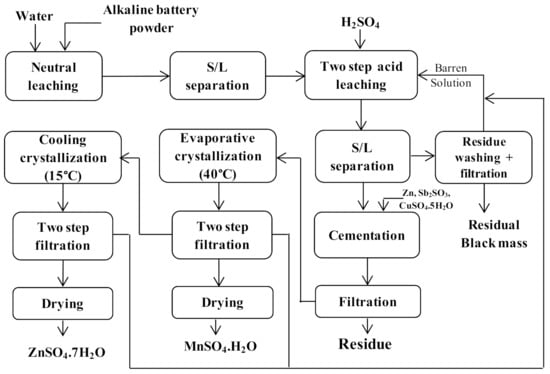
Figure 2.
Chemical leaching flow sheet of Zn and Mn extraction from alkaline battery (redrawn and adapted with modifications from [62].
With advances in studies and certain limitations for alkaline batteries, studies on Li-ion batteries (LIBs) have gained momentum. In case of LIBs, HCl, H2SO4 and HNO3 are commonly used for cobalt leaching. Reactions shown below represent Co dissolution from lithium-cobalt oxide (LiCoO2) by using HCl and H2SO4, respectively [41].
2LiCoO2 + 8HCl → 2CoCl2 + Cl2 + 2LiCl + 4H2O
2LiCoO2 + 3H2SO4 + H2O2 → 2CoSO4 + O2 + Li2SO4+ 4H2O
In a study carried out by [60], the effect of the concentration of H3PO4, temperature, leaching time and liquid/solid ratio on the selective leaching of Li and Mn from a carbothermic reduced spent cathode of LIBs was studied. Using 2.75 mol/L H3PO4 at 40 °C, the leaching efficiencies of Li and Mn were 99.1% and 96.3%, respectively, within 10 min. The reactions involved in such process are illustrated below:
2H3PO4(aq) + MnO(s) ⇌ Mn2+(aq) + 2H2PO4−(aq) + H2O
2H3PO4(aq) + Li2CO3(s) ⇌ 2Li + (aq) + 2H2PO4−(aq) + CO2(g) + H2O
A similar procedure was investigated by [38] for the leaching of Li, Mn, Co and Ni from spent electrical vehicle (EV) power batteries. The study involved the roasting of the material by sulfation, subsequently followed stepwise leaching experiments with water and H2SO4, respectively. In the first stage, the roasted product was leached using water at 30 °C, 4 mL/g of L/S ratio for 2 h, which resulted in Li extraction of 90%. In the second stage, the residue from the first step was leached with 2 mol/L H2SO4 at 60 °C, L/S ratio of 5 mL/g for 2 h. During this last step 98% Ni, 97% Co and 90% Mn was leached from the residue.
Lately, solar cells are becoming a widespread and important source of green energy. They contain several valuable metals such as cadmium (Cd), chromium (Cr), lead (Pb), silver (Ag), selenium (Se) and tellurium (Te), copper (Cu), manganese (Mn), zinc (Zn) [75,76], titanium (Ti) and antimony (Sb) [77] based on the type of product. The production of waste solar cells is slow in comparison to other e-wastes due to their long lifetime (25–30 years) [76]; however, it is expected to increase in the near future [65]. Nevertheless, some hydrometallurgical studies are being conducted for their recycling aimed at metals extraction. Lixiviants, such as HNO3 [65,78,79,80,81] and organic acid [82], are used for Ag leaching, while NaOH [64,76] and KOH [79] are investigated for Al leaching from solar cells.
In the study carried out by [79], a two-step chemical leaching process was applied after a pre-treatment process in order to leach out metals from photovoltaic modules. The first leaching step consisted of Ag dissolution using 3 M HNO3, followed by leaching with 3 M NaOH for Al and Ti removal. These two processes allowed obtaining a final product of Si (99.98%). The same approach was applied recently by [65]. According to the study, 4 M HNO3 was used at 80 °C for 4 h hours in the first step; whereas, in the second step, the same concentration of NaOH was used at 70 °C for 3 h. Leaching efficiencies for each step were 99.7% of Ag and 99.9% of Al, respectively. A typical metal leaching procedure for solar panels is illustrated in Figure 3.
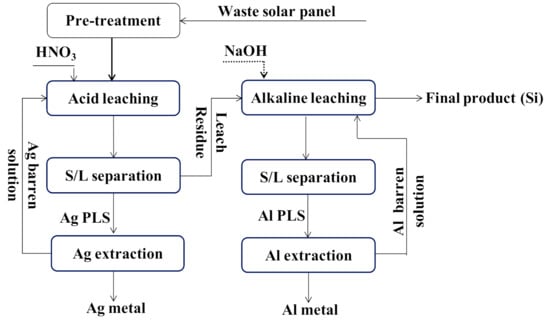
Figure 3.
A typical flow sheet for metal leaching from solar panels (Drawn following information from [65,79].
2.1.4. Metal Leaching from By-Product of E-Waste Sources
Electronic and electric equipment contain diverse elements that can be treated together or separately. During the pre-treatment or pyro-metallurgical processing of e-waste, some by-products such as flue dust [30] and sludge [51,52] may be generated. These by-products are mostly rich in valuable metals that need to be reprocessed. Flue dust from this source contains valuable metals such as Cu, Fe and Al in a ratio of 6%, 27% and 4%, respectively. Cu is generally found in the form of Cu metal, CuO and Cu2O and Fe occurs as Fe3O4 [30].
As per a report made by [30], Cu and Fe could be leached out from the fine product (flue dust) produced by e-waste processing plant. During this investigation, two leaching steps (Figure 4) were proposed under fixed parameters of 20 g/L pulp density and 200 rpm stirring speed. The first step consisted of selective leaching of Fe with 1 M H2SO4 at 60 °C within 4 h; whereas, in the second step, 1 M HNO3 was used for Cu leaching using the same temperature and leaching time. The metal recoveries were 90% of Fe and 98% of Cu for the first and second leaching stages, respectively. The proposed stepwise procedure seemed to be suitable, since under these conditions Fe was efficiently leached out as compared to Cu, which remained almost insoluble under low concentration of 1 M H2SO4. This could be due to the almost stable Gibbs free energy of metallic Fe (−78.364 kJ/mol) compared to metallic Cu (26.283 kJ/mol) according to the following reactions, respectively.
Fe + H2SO4(aq) → FeSO4(aq) + H2(g)
Cu + 2H2SO4(aq) → CuSO4(aq) + 2H2O(l) + SO2(g)
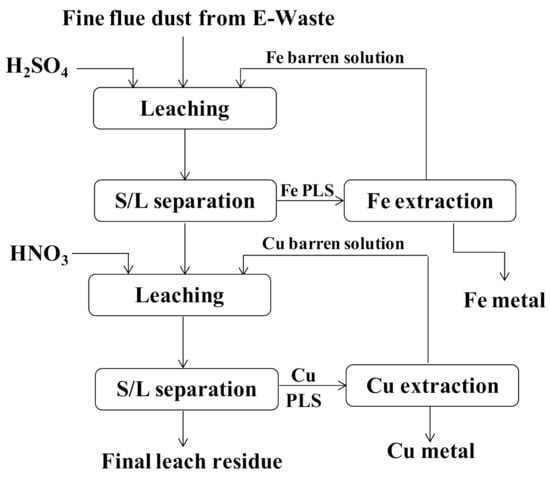
Figure 4.
Metal leaching from e-waste flue dust (redrawn with modifications following [30]).
Apart from flue dust, sludge generated by the PCBs manufacturing plant is also a source of metals, that is mainly composed of Cu(OH)2 and Fe(OH)3 [52]. The hydroxide form is generated due to the treatment of the wastewater by simple alkali precipitation [50]. Strong acids such as HCl, HNO3 and H2SO4, have been tested in order to selectively leach out Cu from this secondary resource. Sulfuric acid was found to be the most suitable lixiviant in comparison to the rest [51,52,83]. The reaction involved in the dissolution of Cu(OH)2 in the presence of sulfuric acid is given below [51,52].
Cu(OH)2 + H2SO4 → CuSO4 + 2H2O
As per reports [51], it is possible to leach out more than 96% Cu from the sludge of PCBs using 0.84 M sulfuric acid, L/S ratio of 100:1, 200 rpm, at a temperature of 60 °C within 80 min. In the study carried out by [52], Cu was selectively leached out from waste sludge generated from PCBs manufacturing process. Tests conducted with three different acids (HCl, HNO3 and H2SO4) revealed that sulfuric acid showed the best Cu leaching efficiency. Under optimum conditions, (0.2 M H2SO4, 4% pulp density, 25 °C, 250 rpm, 60 min) the Cu leaching reached 95%. The study showed that a high acid concentration of 0.5 M led to the complete co-dissolution of Cu and Fe.
2.2. Bioleaching
The fast depletion of natural resources has motivated researchers and industrialists to exploit e-wastes for the recovery of metals in an eco-friendly and energy feasible manner. The biological method is similar to the chemical leaching route, except for it utilizes the reagents generated by microbes for metal extraction [84]. In addition, it is also considered to be an economically feasible and eco-friendly approach with higher efficacy, safety and easier management [85,86]. Several diverse microbial groups are involved in the leaching process including bacteria, fungi and yeast. These are specialized microbes and can be naturally isolated from mine sites or acid mine drainage samples [87].
The acidophiles including sulphur oxidizing bacteria and iron-oxidizing bacteria have found extensive application for bioleaching [88]. Apart from that, several organic and inorganic acids secreted by microorganisms were used for metal recovery from different low-grade sources. For example, microorganisms belonging to the genus Bacillus and Pseudomonas have the ability to extract metals from non-sulphidic sources by using organic carbon as a source of energy and carbon. The metabolic by-products produced by the microorganisms in the form oxalic acid, formic acid, citric acid and other bioreagents assist in the metal extraction from e-waste [89].
2.2.1. Bioleaching Mechanisms for Valuable Metals Recovery
The bioleaching of metals from e-waste mostly involves three mechanisms, namely: acidolysis, redoxolysis and complexolysis. Acidolysis involves the protonation of oxygen atoms that are present on the surface of metallic compound. Several biogenic organic (citric, succinic, formic, gluconic, oxalic formic and pyruvic acids) and inorganic acids (H2SO4) generated by heterotrophs are capable of carrying out acidolysis [90,91]. Redoxolysis involves oxidation-reduction reactions for metal solubiliation and the energy transfer essential for the growth of the microbe is derived through electron transfer. The complexolysis mechanism is mostly used by fungi and cyanogenic bacteria, in which cyanide is generated in the late stationary phase through the decarboxylation of glycine [85]. A mechanism showing the microbial mode of action for e-waste is shown in Figure 5.
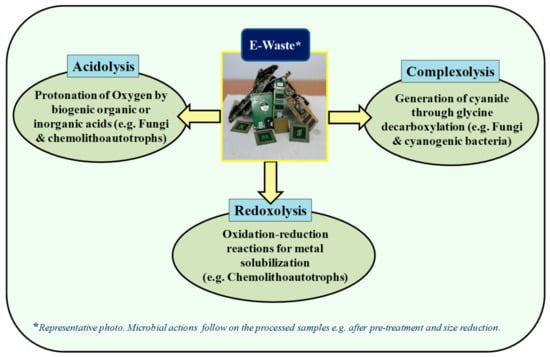
Figure 5.
Microbial mechanisms involved in leaching of metals from e-waste.
The bioleaching process for recovering metals from e-waste is mostly carried out with fungi and bacteria and it is dependent on the culture media composition, system pH and particle size of the waste material. Several microorganisms including Aspergillus niger (A. niger), Aspergillus nominus (A. nominus), Acidithiobacillus thiooxidans (At. thiooxidans) are reported for the recovery of base metals and precious metals such as Cu, Au, etc. [92]. Table 2, Table 3 and Table 4 lists such microbial studies on some selected e-waste streams. It is to be kindly noted that the tables present base and some other non-ferrous metals (wherever applicable) in order to reflect the overall study.
Bacterial Mechanisms
According to studies carried out by some investigators on the bioleaching mechanism of bacteria using Acidithiobacillus ferrooxidans (At. ferrooxidans) and At. thiooxidans, it is observed that no contact needs to be established between the bacteria and e-waste in order to ensure or initiate the bioleaching process [93]. In this process, the oxidation reaction (conversion of Fe2+ to Fe3+) takes place by inorganic acid and enzymes. The reaction rate is enhanced by the production of H+ ion during the oxidation process [94]. Nevertheless, direct bioleaching of metals from e-waste utilizing At. ferrooxidans and At. thiooxidans is also reported [92,95]. The efficacy of the bioleaching process relies on the Fe2+ oxidation rate, concentration of Fe2+ ion and pH of the medium [92]. However, the direct mechanism of bioleaching is yet to be understood completely. The maintenance of high redox potential in the leaching medium due to bacterial action is presumed to facilitate the indirect leaching. Since e-waste is devoid of any energy source, therefore, iron and sulfur are generally added to the medium in the form of ferrous sulphate and elemental sulphur.
The bioleaching process generally consists of the following steps: (a) oxido-reductive reactions, (b) the secretion of organic and inorganic acids and (c) the release or excretion of microbial metabolic products, complexing agents and chelators [89]. The redox reactions take place in the exopolysaccahride layer of the bacteria and the exopolymeric substances such as proteins, amino acid, lipids, etc., secreted by the bacteria play a vital role in the leaching process [89,96]. The microbe-substrate interactions hold prime importance in determining the efficiency of bioleaching. The reactions facilitating bioleaching of metals (combined acidolysis–redoxolysis) are shown in reactions below:
Microbial: S° + 1.5O2 + H2O → 2H+ + SO42−
4Fe2+ + O2 + 2H+ → 4Fe3+ + 2OH−
Chemical: Cu° (e.g., in e-waste) + 2Fe3+ (biogenic) → Cu2+ + 2Fe2+
Cu° (e.g., in e-waste) + H2SO4 (biogenic) + 0.5O2 → Cu2+ + SO42−

Table 2.
A list of bioleaching studies on some selected e-wastes using mesophilic microorganisms their metal recovery aspects. (Note: Metals like Li, In etc. have been included, wherever applicable, to reflect the overall study along with base metal dissolution).
Table 2.
A list of bioleaching studies on some selected e-wastes using mesophilic microorganisms their metal recovery aspects. (Note: Metals like Li, In etc. have been included, wherever applicable, to reflect the overall study along with base metal dissolution).
| E-Waste | Microorganisms | Growing Conditions | Optimum Bioleaching Conditions | Metal Recovery | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Con. | pH | Temp. | Stirring Rate | Cell Con. | pH | Pulp Density | Temp. | Stirring Rate | Time | ||||
| PCBs | Acidithiobacillus ferrooxidans | 10% | NA | 30 °C | 170 rpm | 5% | 3 | 20 g/L | 30 °C | 170 rpm | 20 days | 100% Cu and Ni | [97] |
| Acidithiobacillus ferrooxidans | 10% | NA | 30 °C | 170 rpm | 5% | 1 | 8.5 g/L | 30 °C | 170 rpm | 17 days | 100% of Cu and Ni | [98] | |
| Acidithiobacillus ferrooxidans | NA | 1.75 | 35 °C | 150 rpm | 10% | 1.75 | 10 g/L | 30 °C | 150 rpm | 6 days | 94% Cu | [99] | |
| Acidithiobacillus ferrivorans and Acidithiobacillus thiooxidans | 5% (v/v) | 2.5 | 30 °C | 150 rpm | 1.2 ± 0.4 × 108 CFU/mL | 1.0–1.6 | 10 g/L | 23 ± 2 °C | 150 rpm | 7 days | 98.4% Cu | [100] | |
| Acidithiobacillus ferrooxidans | 5% (v/v) | 1.5 | 30 °C | 180 rpm | 5% | 1.5 | 18 g/L | 30 °C | 180 rpm | 64 h | 94.1% Cu | [101] | |
| Acidithiobacillus ferrooxidans | 10% | 2 | 30 °C | 165 rpm | 10% | 2.25 | 2 g/L | 30 °C | 160 rpm | 78 h | 92.57% Cu | [82] | |
| Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans | NA | 1.5–2.0 | 30 °C | 150 rpm | NA | 1.5 | 10 g/L | 30 °C | 150 rpm | 7 days | 95% Cu | [102] | |
| Acidiphilium acidophilum | NA | 3.5 | 30 °C | 150 rpm | NA | 2.5 | 1 g/L | 30 °C | 170 rpm | 60 days | 79% Cu, 29% Zn, 10% Pb, 39% Ni | [89] | |
| Acidithiobacillus ferrooxidans | 1 × 109 cells/mL | 2.5 | 30 °C | 170 rpm | NA | 2.5 | 7.5 g/L | 30 °C | 170 rpm | 18 days | 94% Cu, 92% Zn, 64% Pb, 81% Ni | [103] | |
| Bacteria consortium dominated by Leptospirillum ferriphilum | 10% | 1.7–1.9 | 30 °C | 150 rpm | NA | 1.8 | 10 g/L | 30 ± 2 °C | 150 rpm | 2–4 days | >99% Cu, 29% Zn, 58% Ni | [104] | |
| Leptospirillum feriphillum | 10% | 2.0 | 30 ± 2 °C | 150 rpm | 10% | 2 | 10 g/L | 30 ± 2 °C | 150 rpm | <4 days | >95% Cu, Zn, Ni | [105] | |
| Acidithiobacillus ferrooxidans | 10% | NA | 30 °C | 130 rpm | 10% | 2 | 15 g/L | 30 °C | 130 rpm | 11–14 days | 99% Cu (11d), 98% Ni (14d) | [106] | |
| Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans | 10% | 1.8 | 30 °C | 150 rpm | 10% | 1.8 | 10% | 30 °C | 150 rpm | 8 days | 98.1% Cu, 55.9% Al, 66.9% Zn, 79.5% Ni | [107] | |
| Acidithiobacillus ferrooxidans | 10% | 1.2 | 35 °C | 250 rpm | NA | 0.6–1.2 | 1% | 25 °C | 200 rpm | 2 days | 86.17% Cu, 100% Al, 100% Ni, 100% Zn | [49] | |
| Leptospirillum ferriphilum and Sulfobacillus benefaciens | 10% v/v | 1.3 | 35 °C | NA | NA | 1.5 | 1% (w/v) | 36 °C | 600 rpm | 2 days | 96% Cu, 73% Ni, 85% Zn, 93% Co | [108] | |
| LCD | Acidothiobacillus ferrooxidans and Acidothiobacillus thiooxidans | NA | NA | 30 °C | NA | 10% | 1.9 | 2.5% (w/v) | 30 °C | NA | 14 days | 90.2% Sn | [109] |
| Acidithiobacillus thiooxidans | 10% | 2 | 30 °C | 170 rpm | 10% | 2.6 | 1.6% (w/v) | 30 °C | 170 rpm | 15 days | 100% In, 10% Sr | [110] | |
| Zn-Mn Batteries | Acidithiobacillus ferrooxidans | 5% | 2 | 30 °C | 140 rpm | NA | 2 | 10 g/L | 30 °C | 140 rpm | 21 days | 99% Zn, 53% Mn | [111] |
| LIBs | Acidothiobacillus thiooxidans | 10% v/v | 4.5 | 30 °C | 200 rpm | NA | 2.4 | 0.25% | 30 °C | 200 rpm | 40 days | 22.6% Co, 66% Li | [112] |
| Acidithiobacillus ferrooxidans, | 10% | 2 | 30 °C | 160 rpm | 10% | 1.93 | 100 g/L | 30 °C | 160 rpm | 3 days | 90% Ni, 92% Mn, 82% Co, 89% Li | [113] | |

Table 3.
A list of bioleaching studies using moderately thermophilic microorganisms and their metal recovery aspects from selected e-wastes. (Note: Li is included, wherever applicable, to reflect the overall study along with base metal dissolution).
Table 3.
A list of bioleaching studies using moderately thermophilic microorganisms and their metal recovery aspects from selected e-wastes. (Note: Li is included, wherever applicable, to reflect the overall study along with base metal dissolution).
| E-Waste | Microorganisms | Growing Conditions | Optimum Bioleaching Conditions | Metal Recovery | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoc. | pH | Temp. | Stirring Rate | Cell Con. | pH | Pulp Density | Temp. | Stirring Rate | Time | ||||
| E-Scrap | Sulfobacillus thermosulfdooxidans and Thermoplasma acidophilum | 10% | NA | 45 °C | 180 rpm | 10% w/v | 2 | 10% w/v | 45 °C | 180 rpm | 12 days | 90% Cu, 80% Al, 82% Ni, 85% Zn | [114] |
| PCBs | Sulfobacillus thermosulfdooxidans | NA | 1.75 | 50 °C | 150 rpm | 10% | 1.75 | 10 g/L | 50 °C | 150 rpm | 6 days | 99% Cu | [99] |
| Leptospirillum ferriphilum and Sulfobacillusthermosulfdooxidans | 10% | 0.9 | 42 °C | 200 rpm | 10% | 0.9 | 100 g/L | 32 °C | 180 rpm | 9 days | 93.4% Cu | [115] | |
| LIBs | Leptospirillum ferriphilum sp. and Sulfobacillus thermosulfidooxidans spp. | 10% | 1.2 | 42 °C | 180 rpm | 10% | 1.2 | 15 g/L | 42 °C | 180 rpm | 3 days | 100.0% Li, 99.3% Co, | [116] |
| Leptospirillum ferriphilum and Sulfobacillus thermosulfidooxidans | 10% | 1.25 | 42 °C | 180 r/min | NA | 1.25 | 5 g/L | 42 °C | 180 r/min | 1.5 days | 98.1% Li, 96.3% Co | [117] | |

Table 4.
A list of bioleaching studies on some selected e-wastes using heterotrophic microorganisms and their metal recovery aspects. (Note: Au, Ag, Li, In, etc., are included, wherever applicable, to reflect the overall study along with base metal dissolution).
Table 4.
A list of bioleaching studies on some selected e-wastes using heterotrophic microorganisms and their metal recovery aspects. (Note: Au, Ag, Li, In, etc., are included, wherever applicable, to reflect the overall study along with base metal dissolution).
| E-Waste | Microorganism | Growth Media | Energy Source | Optimum Bioleaching Conditions | Metal Recovery | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Pulp Density | Temp | Stirring Rate | Time | ||||||
| PCBs | Chromobacterium violaceum | LB medium | 0.5 g glycine | 7.2 | 1% w/v | 30 °C | 150 rpm | 7 days | 79% Cu, 46% Zn, 9% Fe, 69% Au, 7% Ag | [118] |
| Chromobacterium violaceum and Pseudomonas aeruginosa | LB medium | 0.5 g glycine | 7.2. | 1% w/v | 30 °C | 150 rpm | 7 days | 83% Cu, 49% Zn, 13% Fe, 73% Au, 8% Ag | ||
| Bacillus subtilis and Bacillus cereus | NA | NA | 6–8 | 10 g/ 150 mL | 37 °C | 120 rpm | 25 days | 48% Zn, 93% Cd | [119] | |
| Bacillus megaterium | Nutrient broth medium | 0.5 g/L glycine | 10 | 2 g/L | 30 °C | 170 rpm | 10 days | 13.26% Cu, 36.81% Au | [120] | |
| Aspergillus niger | PDA | 50 g/L glucose | 4.4 | NA | 28 °C | 280 rpm | 14 days | 29% Cu, 87% Au | [121] | |
| Aspergillus niger | PDA | 100 g L−1 sucrose | NA | 0.5–20.00 g L−1 | Ambient temp | 120 rpm | 21 days | 100% Zn, 80.39% Ni, 85.88% Cu | [122] | |
| Aspergillus niger | PDA | 20 g Dextrose | 5.0 | 2 g L−1 | 17–24 °C | NA | 35 days | 2.8% Cu, 0.53% Au | [123] | |
| Streptomyces albidofavus | ISP 2 broth medium | NA | NA | 1.5% | 28 °C | 120 rpm | 5 days | 66% Al, 74% Ca, 68% Cu, 65% Cd, 42% Fe, 81% Ni, 82% Zn, 46% Pb | [124] | |
| LIBs | Aspergillus niger MM1 | Sucrose medium | 100 g/L sucrose | 6. | 0.25% | 30 °C | 200 rpm | 40 days | 82% Co, 100% Li | [112] |
| Ni-Cd Batteries | Aspergillus niger | RB medium | NA | NA | 0.3 g/80 mL | 28 °C | 150 rpm | 21 days | 81.41% Ni, 92.31% Cd | [125] |
| LCD | Aspergillus niger | PDA | 100 g/L Sucrose | 4.0 | 1% (w/v) | 70 °C | 125 rpm | 90 min | 100% In | [126] |
| AMOLED Displays | Bacillus foraminis | TSA and TSB | 15% glycerol | 7.7 | 7% | 40 °C | 160 rpm | 12 days | 56.8% Mo, 41.4% Cu, 100% Ag | [127] |
| Solar Cells | Penicillium chrysogenum | Sucrose medium | 100 g/L sucrose | NA | 1% (w/v) | 20 °C | 200 rpm | 3 days | 100% Te, 98% Al | [128] |
Fungal Mechanism
The precious metal recovery from e-waste is generally instigated by biogenic cyanide or organic acids produced by fungi. The organic acids (natural chelating agents) generally act as complexing agents and mostly include oxalic acid, tartaric acid and citric acid. Reports indicate the use of A. niger for the recovery of Au and Ag from e-waste, where organic acids such as gluconic acid and citric acid are produced by the microbe for carrying out the leaching process. The reaction as a result of the microbial (A. niger) action is shown below:
C6H12O6 + 1.5O2 → C6H8O7 + 2H2O
The leaching operations can take place under both aerobic and anaerobic conditions and may be influenced by temperature, solid-liquid ratio and pH of the system [92].
2.2.2. Bioleaching of Metals from Waste Printed Circuit Boards (PCBs)
Bacterial assisted leaching of scrap TV circuit boards was carried out for the recovery of copper using a mixed culture of mesophilic bacteria (comprising of At. ferrooxidans, L. ferrooxidans, At. thiooxidans) [129]. The bioleaching process was seen to be dependent on ferrous iron oxidation efficiency of the bacteria and the initial availability of soluble iron in the medium. Lab-scale shake flask experiments were conducted using At. ferrooxidans for copper extraction from printed circuit boards [130]. Recently, mechanical activation was proposed by [131] in order to enhance the bioleaching efficacy of At. ferrooxidans for metal extraction from waste printed circuit boards (WPCBs). The leaching rates of Cu, Ni and Zn could be enhanced by using mechanical activation, which was approximately 20% higher in comparison to the un-activated WPCBs. Additionally, in one of the studies, a direct DC electric field was applied for enhancing bioleaching and copper recovery from e-waste in a bioelectric reactor. The DC electric field enhanced the bacterial growth and activity, simultaneously facilitating the ferrous iron oxidation and resulting efficient leaching of copper from printed circuit boards (PCBs) [132]. Apart from At. ferrooxidans, a pure culture of the acidophilic bacteria, Acidiphilium acidophilum (A. acidophilum), was also used for the bioleaching of specific metals such as Cu, Zn, Pb and Ni from e-waste [103]. In addition, heterotrophic species such as Acinetobacter sp. CrB2 was used for the bioleaching of copper from e-waste. The copper bioleaching occurred as a result of the combined action of extracellular enzymes and metabolites produced by the bacteria and increasing the number of cycles of operations increased the bioleaching efficiency [133]. Of late, some indigenous cyanogenic bacterial strains isolated from e-waste landfills have been also found to be potential microorganisms for copper extraction from e-waste under optimized conditions [134]. Apart from that, lately a Bacillus sp. isolated from Hymeniacidon heliophila sponge cells has shown the potential to leach copper from e-waste, where copper was produced in the form of copper nanoparticles. The peptides released by bacteria were responsible for leaching of copper, absorption of copper ion and their incorporation into cells for nanoparticle formation [135].
Besides bacterial strains, several fungal strains have been reported for metal extraction from e-waste. Fungi are capable of secreting large number of organic acids, amino acids and other metabolites which assist in metal dissolution. The fungal metabolites act by displacing metal ions with hydrogen ions or by developing soluble metal complexes and chelates, subsequently leading to metal dissolution [136]. Recently, Penicillium simplicissimum (P. simplicissimum) was investigated for copper and nickel extraction from PCBs of mobile phones. Four different carbon sources such as sucrose, cheese whey, sugar, and sugar cane molasses were used for the study and it was observed that non-conventional carbon sources improved the bioleaching efficacy [137]. Apart from that, mixed fungal cultures were used for metal extraction from e-waste [138]. The gradual adaptation of microorganisms to heavy metals helps in the development of heavy metals tolerant microbes, which can be utilized for industrial scale applications [138,139]. Examples of such fungal strains include Asergillus foetius (A. foetius) and Penicillium funiculosum (P. funiculosum) [140,141]. More experimental works on WPCB bioleaching can be found in Table 2, Table 3 and Table 4, respectively.
2.2.3. Bioleaching of Metals from Spent Batteries
A variety of batteries such as lithium-ion batteries (LIBs) and alkaline batteries including nickel-cadmium, nickel-hydrogen [142], Zn-Mn batteries [111], and zinc-carbon batteries [62] exist in the market based on the models.
In case of alkaline batteries, it has been be found that autotrophic acidophiles have been applied for the leaching of base metals. For example, At. ferrooxidans has been used for metal recovery from Ni-Cd batteries [143,144,145]. Alicyclobacillus sp. and Sulfobacillus sp. [146], At. thiooxidans [147,148,149], L. ferriphilum [148] and At. ferrooxidans [111,113] have been used for metal recovery from spent alkaline Zn-Mn batteries. In a study conducted by [111], the culture supernatant of At. ferrooxidans was used for Mn and Zn dissolution from waste alkaline button-cell batteries. At an initial pH of 2, temperature of 30 °C and 10 g/L pulp density, 99% of Mn and 53% of Zn were extracted in 21 days. A similar investigation was conducted by [113] with the same strains of bacteria for metal dissolution from spent Ni, Mn, Co (NMC) batteries. The findings of this study showed that an improved leaching time (3 days) and pulp density (100 g/L), 90%, 92%, 82%, and 89% dissolution for f Ni, Mn, Co and Li, respectively, could be achieved.
Moreover, heterotrophic fungus has been also applied for metals extraction from this type of batteries. Kim et al. [139] showed that most of the Aspergillus species are able to dissolve metal from spent Zn-Mn and Ni-Cd batteries. This assertion was confirmed by [125] through a comparative study on bioleaching of nickel and cadmium recovery from spent Ni-Cd batteries by using A. niger. In this work, different culture media such as potato dextrose broth (PDB), malt extract broth (MEB) and Richards’s broth (RB) were investigated. It appeared that the application of A. niger in RB medium in a two-step bioleaching process, resulted in efficient dissolution of both base metals (Ni—81.41% and Cd—92.31% in 21 days).
Apart from the alkaline batteries, the LIBs are now widely prevalent and studied. Several bioleaching studies have been undertaken on metal extraction from spent lithium-ion batteries (LIBs) by using autotrophic acidophiles [112,150] and heterotrophic microorganisms [112,139,151,152]. A comparative bioleaching study was investigated by using spent medium of A. niger (MM1 and SG1) (heterotroph) and At. thiooxidans 80,191 (autotrophic acidophile) for Co and Li leaching from spent LIBs [112]. It was observed that fungal bioleaching (spent medium of A. niger MM1) was more effective for metal dissolution from LIBs compared to bacterial leaching. The findings of the research indicated Co and Li dissolution of 82%100%, respectively.
The process is illustrated in Figure 6. Moreover, in order to increase the efficiency of bioleaching, the optimization of bioleaching parameters for metal extraction from LIBs was conducted by [152] using A. niger. Additionally, heterotrophic bacterial such as Gluconobacter oxydans (G. oxydans) [153] and another strains of Aspergillus like A. nomius JAMK1 [151] were proposed in the optimization of bioleaching parameters for metal recovery from LIBs.
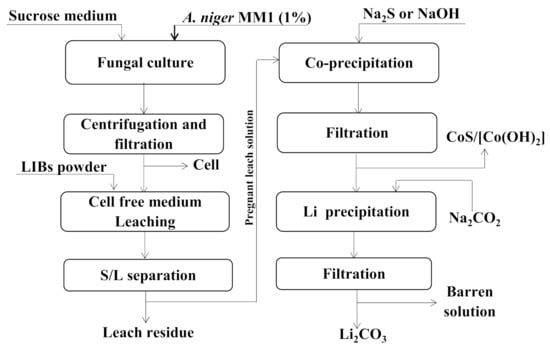
Figure 6.
Bioleaching of LIBs with A. niger (drawn based on information collected from [112]).
On the other hand, some studies have suggested efficient metal extraction from spent LIBs at higher pulp densities and shorter leaching time by using autotrophic meso-acidophilic bacteria such as At. ferrooxidans. A study carried out by [150] indicated that using this bacterium for bioleaching of LIBs at 40 g/L of solid/liquid ratio resulted in 100% of Li, 88% of Co, and 20% of Mn dissolution within 12 days. A consortium of meso-acidophile and moderate thermo-acidophile (L. ferriphilum and S. thermosulfidooxidans) was used to study the effects of higher pulp density on the leaching of waste LIBs. At 5 g/L of pulp density and 42 °C, 98.1% Li and 96.3% Co were leached in 1.5 days [117].
2.2.4. Bioleaching of Metals from LCD/LED Panels
Most of the studies carried out on the bioleaching of liquid crystal displays (LCDs) are focused on the recovery of indium. A brief description on indium application in LCD is presented in Section 2.1.2. Adapted acidophilic strains such as At. ferrooxidans and At. thioxidans were used for the extraction of indium and tin from LCD panels, where a maximum leaching of 55.6% and ˃90% was obtained for indium and tin respectively [109]. Apart from acidophilic strains, heterotrophic strains such as A. niger are used for the extraction of In from waste LCD panels through the optimization of the fermentation method. Through this method, In bioleaching efficiency increased from 12.3% to 100%. The carboxyl groups from organic acids and proteins were identified as the crucial factors for the release of H+ ions required for leaching of indium [126].
Aside from LCDs, LEDs (light emitting diodes) are receiving considerable interest as a secondary source for valuable metals. In addition, they pose a threat to the environment as a source of pollutant. Therefore, of late, studies have been carried out in this regard for the recovery of metals from LEDs. In one of the approaches, a novel stepwise indirect bioleaching technique was studied using adapted cells of At. ferrooxidans. The rate of bioleaching was improved by the stepwise addition of biogenic ferric and was maintained at 4–5 g/L. The results of the study revealed that the direct bioleaching approach, which involves bacterial attachment to the sample resulted in lower yields of metals. In contrast, the indirect bioleaching approach using biogenic ferric resulted in higher metal recovery from LEDs, where at a pulp density of 20 g/L, nearly 83% copper, 97% nickel and 84% gallium could be recovered. Metals such as nickel and copper have a bacteriostatic effect on the acidophiles. This is seen to interfere with the enzyme catalysed oxidation of Fe and reproduction. Consequently, the lower yields with direct bioleaching are attributed to this effect [154]. It is also observed that adapted strains of At. ferrooxidans provide better resistance to the high concentration of metals present in LEDs. In a study carried out by [155], it was observed that the heavy metals tolerance of At. ferrooxidans reduced with increasing pulp density from 5 to 20 g/L. Nevertheless, the adapted cells had higher Fe3+ level, cell number, ORP and lower pH than the non-adapted cells, which resulted in better a leaching efficiency of metals, where 84%, 96%, and 60%, copper, nickel and gallium, respectively, could be extracted.
2.2.5. Bioleaching of Spent Solar Panels and Some By-Products of E-Waste Resources
The application of bioleaching on metal recovery from solar panels is very limited. Nevertheless, recently [128] have investigated the potential metal leaching abilities of four different microorganisms from solar panels which contain B, Mg, V, Ni, Zn, Sr, Cr, and Te. In the study, spent media of two autotrophic (acidophilic) bacteria (At. thiooxidans, At. ferrooxidans) and two heterotrophic fungi (P. chrysogenum and P. simplicissimum) wereused. It was observed that the spent medium of At. ferrooxidans was effective for Mg, Mn, Co, and Zn leaching. At. thiooxidans was capable of B and Zn dissolution, and P. simplicissimum was able to leach only Mg, whereas the spent medium of P. chrysogenum was able to dissolve almost all the aforementioned metals. Based on this comparative investigation, the authors concluded that P. chrysogenum spent medium was the most effective for multiple metal leaching from waste solar cells. Under conditions of 30 °C, 150 rpm and 1% (w/v) pulp density, the leaching efficiencies of B, Mg, V, Ni, Zn, and Sr was 100% and Te was 93%.
Bioleaching has been also applied for metal recovery from the by-product of e-waste. Yan et al. [156] investigated bioleaching of Cu and Ni from electroplating sludge by using At. ferrooxidans and At. thiooxidans. Additionally, [157] have developed two successive bioleaching steps for base metals, precious metals and REE extraction from the dusts generated by e-waste shredding process. In the first step, At. thiooxidans was used for base metals leaching (almost 100%) at pH 3.5 during 8 days, whereas, cyanide producing P. putida WSC361 was applied for gold dissolution (48%) within 3 h.
3. Integrated/Hybrid Approaches
Recent years have been witnessing the utilization of some hybrid approaches for enhancing the metals recovery from e-wastes. The rapid diminution in resources and the significant ecological footprints have compelled investigators to comb for green approaches for metal recovery. The hybrid methods provide a stepwise combination of different approaches in order to deliver an enhanced and resourceful system for metal recycling [158]. In a report made by [159], several ligand–microbe combinations (an example is shown in Figure 7) were proposed and described as hybrid techniques for the efficient extraction of the desired metals. In one of the investigations carried out by [103], the bioleaching of some specific metals such as Cu, Zn, Pb and Ni from high grade PCBs was studied using pure culture of At. ferrooxidans. The study was carried out in the presence and absence of lemon juice, which contains 0.2 M citric acid as an active constituent and natural tetradentate as a chelating agent, that provide a hybrid environment for enhanced metal recovery. Results of the study indicated enhanced metal solubilization under hybrid conditions, as a result of the chelating effect of citric acid and a maximum leaching of 94% Cu, 92% Zn, 64% Pb and 81% Ni was observed after 18 days, using a size range of 0.075–1 mm and pulp density of 7.5 g/L.
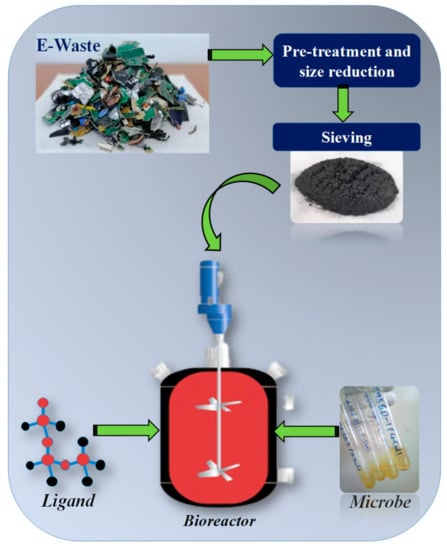
Figure 7.
Pictorial representation of a ligand mediated integrated bioleaching approach.
Yet another study carried out by [158] demonstrated the use of a novel two-step bio-recovery approach, subsequently followed by electrochemical treatment for the recovery of copper from waste PCBs. In the study, an isolated strain, USCT-R010 was used for copper leaching under optimized conditions and the leach liquor was then subjected to a purification step using biosorption technique, where dead biomass of Aspergillus oryzae and Baker’s yeast were used as biosorbents under optimized conditions. Following desorption and electrowinning, 92.7% copper was recovered from the eluate and the characterization studies revealed that the recovered copper had 95.2% purity. This study indicated the utilization of cost-effective biomaterial towards metal purification and recycling while, at the same time, providing an efficient and ecofriendly approach for metal recovery. In addition, the hybrid technique is often less time consuming than the individual approach involving only bioleaching and allows the extraction of valuable metals from low grade ores, secondary wastes, etc.
Likewise, several researchers have reported that ferric sulphate is applicable for metal leaching from PCBs [49,160,161]. A study carried out by [161] revealed that 100 mM Fe2(SO4)3 is able to leach more than 98% of Cu from PCBs at 20 °C, 300 rpm and 10 g·L−1 of pulp density within 4 h. A similar study was conducted by [49]. In their investigation, the authors made a comparative study between chemical and biogenic ferric leaching. The purpose was to understand the function of these two sources of Fe(III) towards the leaching of Cu, Ni, Al and Zn from PCBs. Their findings confirmed that there was no major difference in the leaching efficiency of these metals with the use of either biogenic or chemical ferric sulfate. Under optimum conditions, the chemical leaching attained 84.3% of Cu extraction, 98.4% of Ni extraction and 100% extraction for both Zn and Al.
4. Challenges, Future Prospects and Conclusions
During the last few decades, various studies have been carried out on the management and recycling of electronic and electrical wastes. As discussed in the introduction section, this is due to the fact that mineral resources are becoming increasingly scarce and also due to the environmental problems associated with these wastes. Circular economy, involving the principle of the three Rs (reduce, reuse and recycle), is currently the focus for waste recycling and holds prime significance in the current scenario [17,162]. In this aspect, recently (April 2021), a voluntary certification scheme for waste electrical and electronic equipment (WEEE) and batteries treatment was proposed through the EU Horizon 2020 CEWASTE project [163].
Theoretically, the concept of the circular economy remains the ideal solution for the integrated management (collection and recycling) of electronic waste. However, its applications in certain aspects of extractive metallurgy present an enormous challenge with respect to the implementation of an economically profitable and eco-friendly technology [164,165,166,167]. The foremost challenges that are to be taken into consideration are the long-term availability of electronic waste as raw material for a metallurgical plant, their diversity (PCBs, batteries, LCDs, solar panels, etc.) and their metal content, including the metals of interest. From the technical aspect, the diversity of electronic wastes and their content play an important role in the choice of technology, i.e., hydrometallurgy and bio-hydrometallurgy in the current case. As it can be understood from all the above sections, the contributions of chemical and biological routes are quite effective; however, each process has its own merits and demerits. A list of such aspects is given in Table 5.

Table 5.
The Merits and Demerits of Chemical and biological methods.
For both of these processes, it can be seen that many studies were undertaken for PCBs compared to the rest of e-waste streams. For example, there is limited information available on the metal extraction from solar panels and by-product of e-waste. Consequently, such waste streams need to be deeply investigated within the scope of future works. With respect to chemical leaching (hydrometallurgy), the use of green lixiviants should be practiced more or low-cost, environmentally friendly methods can be developed that can facilitate/promote multi-metal leaching. Though bioleaching (bio-hydrometallurgy) provides more eco-friendly benefits and is considered economic in comparison to chemical methods, still the search for more robust microbes that can enable efficient metal dissolution from specific or wide varieties of e-wastes should be prioritized. In addition, bioinformatic platforms can also contribute to receiving more useful information related to key microbial species involved in bioleaching [168,169,170]. According to a study, a bioleaching bacterial protein finder system was proposed that can predict putative proteins and make an assumption regarding any microbe that is capable of iron and sulphur oxidation (a key aspect in bioleaching operations) [171]. More of such bioinformatic attempts should be made to identify potential bioleaching microbes and then integrate them with the applied aspects of bioleaching. This would allow further validation of the bioinformatic analysis through wet-lab experimental findings. Modifications or upgrading the process engineering aspects can also be an appropriate way to improve performance in both the systems/routes. Moreover, integrated approaches (chemical and bioleaching) can be implemented and tested to monitor the overall process efficiency for treatment of any specific e-waste and subsequently studied on a pilot scale, with the aim of finding industrial applications. Nevertheless, such attempts should be validated through techno-economic feasibility analysis [172] and life cycle assessment (LCA) [173] of the processes, considering the environmental and social impacts.
Author Contributions
Conceptualization, S.M. and S.P.; Methodology, S.M., S.D. and I.A.; Validation, S.P. and A.A.; Resources, A.A.; Writing—original draft preparation, S.M., S.P., S.D. and I.A.; Supervision, S.P. and A.A.; Project administration, S.P. and AA.; Funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This article was funded by European Commission under ERAMIN2-2019 project (BaCLEM) and Türkiye Bilimsel ve Teknolojik Araştirma Kurumu (TUBITAK, Turkey), grant No. 120N273.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panda, S.; Pradhan, N.; Mohapatra, U.; Panda, S.K.; Rath, S.S.; Rao, D.S.; Nayak, B.D.; Sukla, L.B.; Mishra, B.K. Bioleaching of copper from pre and post thermally activated low grade chalcopyrite contained ball mill spillage. Front. Environ. Sci. Eng. 2013, 7, 281–293. [Google Scholar] [CrossRef]
- Sukla, L.B.; Pradhan, N.; Panda, S.; Mishra, B.K. Environmental Microbial Biotechnology. In Soil Biology; Sukla, L.B., Pradhan, N., Sandeep Panda, B.K.M., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-19017-4. [Google Scholar]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E.Y.; Panda, S. A novel approach based on solvent displacement crystallisation for iron removal and copper recovery from solutions of semi-pilot scale bioleaching of WPCBs. J. Clean. Prod. 2021, 294, 126346. [Google Scholar] [CrossRef]
- Zeng, X.; Mathews, J.A.; Li, J. Urban Mining of E-Waste is Becoming More Cost-Effective Than Virgin Mining. Environ. Sci. Technol. 2018, 52, 4835–4841. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, F.; Lindberg, D.; Hamuyuni, J.; Taskinen, P.; Hupa, L. Improving urban mining practices for optimal recovery of resources from e-waste. Miner. Eng. 2017, 111, 209–221. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A. Securing supplies of technology critical metals: Resource recycling and waste management. Waste Manag. 2021, 123, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Ahirwar, R.; Tripathi, A.K. E-waste management: A review of recycling process, environmental and occupational health hazards, and potential solutions. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100409. [Google Scholar] [CrossRef]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential; International Telecommunication Union, United Nations Institute for Training and Research, United Nations University: Bonn, Germany; Geneva, Switzerland; International Solid Waste Association: Rotterdam, The Netherlands, 2020; ISBN 9789280891140. [Google Scholar]
- Charles, R.G.; Douglas, P.; Dowling, M.; Liversage, G.; Davies, M.L. Towards increased recovery of Critical Raw Materials from WEEE—Evaluation of CRMs at a component level and pre-processing methods for interface optimization with recovery processes. Resour. Conserv. Recycl. 2020, 104923. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Lenny Koh, S.C.; Rosa, P. Recycling of WEEEs: An economic assessment of present and future e-waste streams. Renew. Sustain. Energy Rev. 2015, 51, 263–272. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Chong, M.N.; Herath, G.; Moyo, T.; Petersen, J. E-waste in the international context—A review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor—2017: Quantities, Flows, and Resources; United Nations University (UNU): Bonn, Germany; Geneva, Switzerland; Vienna, Austria, 2017. [Google Scholar]
- Baldé, C.P.; Wang, F.; Kuehr, R.; Huisman, J. The Global E-Waste Monitor—2014: Quantities, Flows and Resources; United Nations University (UNU): Tokyo, Japan; Bonn, Germany, 2015. [Google Scholar]
- Akcil, A.; Sun, Z.; Panda, S. COVID-19 disruptions to tech-metals supply are a wake-up call. Nature 2020, 587, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Wang, Z.; Cao, H.; Son, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; El-Sheltawy, C.T.; Abdo, D.M. Green Processes for Electronic Waste Recycling: A Review. J. Sustain. Metall. 2018, 4, 295–311. [Google Scholar] [CrossRef]
- Zang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Tansel, B. From electronic consumer products to e-wastes: Global outlook, waste quantities, recycling challenges. Environ. Int. 2017, 98, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Ibrahim, Y.A.; Meshram, P.; Panda, S.; Abhilash. Hydrometallurgical recycling strategies for recovery of rare earth elements from consumer electronic scraps: A review. J. Chem. Technol. Biotechnol. 2021, 96, 1785–1797. [Google Scholar] [CrossRef]
- Panda, S.; Costa, R.B.; Shah, S.S.; Mishra, S.; Bevilaqua, D.; Akcil, A. Biotechnological trends and market impact on the recovery of rare earth elements from bauxite residue (red mud)—A review. Resour. Conserv. Recycl. 2021, 171, 105645. [Google Scholar] [CrossRef]
- OHSA Guidance for the Identification and Control of Safety and Health Hazards in Metal Scrap Recycling. Available online: https://www.osha.gov/sites/default/files/publications/OSHA3348-metal-scrap-recycling.pdf (accessed on 1 October 2021).
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P. Life cycle analysis of copper-gold-lead-silver-zinc beneficiation process. Sci. Total Environ. 2019, 659, 41–52. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Kopacek, B.; Vegliò, F. Yttrium recovery from primary and secondary sources: A review of main hydrometallurgical processes. Waste Manag. 2014, 34, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- López-Yáñez, A.; Alonso, A.; Vengoechea-Pimienta, A.; Ramírez-Muñoz, J. Indium and tin recovery from waste LCD panels using citrate as a complexing agent. Waste Manag. 2019, 96, 181–189. [Google Scholar] [CrossRef]
- Pavón, S.; Lorenz, T.; Fortuny, A.; Sastre, A.M.; Bertau, M. Rare earth elements recovery from secondary wastes by solid-state chlorination and selective organic leaching. Waste Manag. 2021, 122, 55–63. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, Q.; Li, W.; Dong, Q.; Sun, W. One-step separation and recovery of rare earth and iron from NdFeB slurry via phosphoric acid leaching. J. Rare Earths 2021. [Google Scholar] [CrossRef]
- Lee, H.; Mishra, B. Selective recovery and separation of copper and iron from fine materials of electronic waste processing. Miner. Eng. 2018, 123, 1–7. [Google Scholar] [CrossRef]
- Barragan, J.A.; Ponce De León, C.; Alemán Castro, J.R.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Anderson, C. Hydrometallurgical Treatment of Waste Printed Circuit Boards: Bromine Leaching. Metals 2020, 10, 462. [Google Scholar] [CrossRef]
- Rajahalme, J.; Perämäki, S.; Budhathoki, R.; Väisänen, A. Effective Recovery Process of Copper from Waste Printed Circuit Boards Utilizing Recycling of Leachate. JOM 2021, 73, 980–987. [Google Scholar] [CrossRef]
- Petter, P.M.H.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef]
- Xiu, F.R.; Qi, Y.; Zhang, F.S. Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment. Waste Manag. 2015, 41, 134–141. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; Sun, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glycine. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Elbashier, E.; Mussa, A.; Hafiz, M.A.; Hawari, A.H. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy 2021, 204, 105706. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, P.; Chang, D.; Jie, Y.; Yang, S.; Wu, G.; Chen, H.; Zhu, J.; Hu, F.; Wilson, B.P.; et al. Selective extraction of valuable metals from spent EV power batteries using sulfation roasting and two stage leaching process. Sep. Purif. Technol. 2021, 258, 118078. [Google Scholar] [CrossRef]
- Oluokun, O.O.; Otunniyi, I.O. Kinetic analysis of Cu and Zn dissolution from printed circuit board physical processing dust under oxidative ammonia leaching. Hydrometallurgy 2020, 193, 105320. [Google Scholar] [CrossRef]
- Oraby, E.A.; Li, H.; Eksteen, J.J. An Alkaline Glycine-Based Leach Process of Base and Precious Metals from Powdered Waste Printed Circuit Boards. Waste Biomass Valorization 2020, 11, 3897–3909. [Google Scholar] [CrossRef]
- Zhou, L.-F.; Yang, D.; Du, T.; Gong, H.; Luo, W.-B. The Current Process for the Recycling of Spent Lithium Ion Batteries. Front. Chem. 2020, 8, 578044. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward Sustainability for Recovery of Critical Metals from Electronic Waste: The Hydrochemistry Processes. ACS Sustain. Chem. Eng. 2017, 5, 21–40. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Gorewoda, T.; Eschen, M.; Charasińska, J.; Knapik, M.; Kozłowicz, S.; Anyszkiewicz, J.; Jadwiński, M.; Potempa, M.; Gawliczek, M.; Chmielarz, A.; et al. Determination of Metals’ Content in Components Mounted on Printed Circuit Boards from End-of-Life Mobile Phones. Recycling 2020, 5, 20. [Google Scholar] [CrossRef]
- Terena, L.M.; Almeida Neto, A.F.; Gimenes, M.L.; Vieira, M.G.A. Characterisation of Printed Circuit Boards of Mobile Phones Discarded in Brazil. Chem. Eng. Trans. 2017, 56, 1945–1950. [Google Scholar] [CrossRef]
- Calvo, G.; Mudd, G.; Valero, A.; Valero, A. Decreasing Ore Grades in Global Metallic Mining: A Theoretical Issue or a Global Reality? Resources 2016, 5, 36. [Google Scholar] [CrossRef]
- Lee, C.H.; Tang, L.W.; Popuri, S.R. A study on the recycling of scrap integrated circuits by leaching. Waste Manag. Res. 2011, 29, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Nikoloski, A.N.; Moheimani, N.; Valix, M.; Sahajwalla, V.; Kaksonen, A.H. Potential of metals leaching from printed circuit boards with biological and chemical lixiviants. Hydrometallurgy 2020, 196, 105433. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of copper and the co-leaching behaviour of other metals from waste printed circuit boards using alkaline glycine solutions. Resour. Conserv. Recycl. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Thawornchaisit, U.; Juthaisong, K.; Parsongjeen, K.; Phoengchan, P. Optimizing acid leaching of copper from the wastewater treatment sludge of a printed circuit board industry using factorial experimental design. J. Mater. Cycles Waste Manag. 2019, 21. [Google Scholar] [CrossRef]
- Trinh, H.B.; Kim, S.; Lee, J. Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge. Metals 2020, 10, 293. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Fonti, V.; Ubaldini, S.; De Michelis, I.; Kopacek, B.; Vegliò, F.; Beolchini, F. Cross-current leaching of indium from end-of-life LCD panels. Waste Manag. 2015, 42, 180–187. [Google Scholar] [CrossRef]
- Dodbiba, G.; Nagai, H.; Wang, L.P.; Okaya, K.; Fujita, T. Leaching of indium from obsolete liquid crystal displays: Comparing grinding with electrical disintegration in context of LCA. Waste Manag. 2012, 32, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Mishra, C.; Hong, H.S.; Cho, S.S. Beneficiation and recovery of indium from liquid-crystal-display glass by hydrometallurgy. Waste Manag. 2016, 57, 207–214. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, L.; Xu, Z. Indium recovery from In-Sn-Cu-Al mixed system of waste liquid crystal display panels via acid leaching and two-step electrodeposition. J. Hazard. Mater. 2020, 381, 120973. [Google Scholar] [CrossRef] [PubMed]
- Houssaine Moutiy, E.; Tran, L.H.; Mueller, K.K.; Coudert, L.; Blais, J.F. Optimized indium solubilization from LCD panels using H2SO4 leaching. Waste Manag. 2020, 114, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Wang, S.Y.; Lo, S.L. Indium recovery from spent liquid crystal displays by using hydrometallurgical methods and microwave pyrolysis. Chemosphere 2021, 280, 130905. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ning, S.; Fujita, T.; Wei, Y.; Zhang, S.; Lu, S. Leaching of indium and tin from waste LCD by a time-efficient method assisted planetary high energy ball milling. Waste Manag. 2021, 120, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Hu, J.; Zhang, T.; Xu, S. Stepwise Recovery of Valuable Metals from Spent Lithium Ion Batteries by Controllable Reduction and Selective Leaching and Precipitation. ACS Sustain. Chem. Eng. 2020, 8, 15496–15506. [Google Scholar] [CrossRef]
- Fan, E.; Yang, J.; Huang, Y.; Lin, J.; Arshad, F.; Wu, F.; Li, L.; Chen, R. Leaching Mechanisms of Recycling Valuable Metals from Spent Lithium-Ion Batteries by a Malonic Acid-Based Leaching System. ACS Appl. Energy Mater. 2020, 3, 8532–8542. [Google Scholar] [CrossRef]
- Andak, B.; Özduǧan, E.; Türdü, S.; Bulutcu, A.N. Recovery of zinc and manganese from spent zinc-carbon and alkaline battery mixtures via selective leaching and crystallization processes. J. Environ. Chem. Eng. 2019, 7, 103372. [Google Scholar] [CrossRef]
- Oghabi, H.; Haghshenas, D.F.; Firoozi, S. Selective separation of Cd from spent Ni-Cd battery using glycine as an eco-friendly leachant and its recovery as CdS nanoparticles. Sep. Purif. Technol. 2020, 242, 116832. [Google Scholar] [CrossRef]
- Saleh, M.M.; Bamsaoud, S.F.; Barfed, H.M. Optimization of nitric acid properties for chemical recycling of cadmium from spent Ni-Cd batteries. J. Phys. Conf. Ser. 2021, 1900, 12018. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chen, Y.-J.; Yueh, K.-C.; Cheng, C.-P.; Chang, T.-C. Recovery of valuable metal from Photovoltaic solar cells through extraction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 720, 12007. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Wang, L.; Chen, T.; Zhao, L.; Hu, Y.; Xu, C. Optimization of indium recovery from waste crystalline silicon heterojunction solar cells by acid leaching. Sol. Energy Mater. Sol. Cells 2021, 230, 111218. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Ma, B.; Jie, X.; Xing, P. Extracting antimony from high arsenic and gold-containing stibnite ore using slurry electrolysis. Hydrometallurgy 2019, 186, 284–291. [Google Scholar] [CrossRef]
- Tran, C.D.; Salhofer, S.P. Processes in informal end-processing of e-waste generated from personal computers in Vietnam. J. Mater. Cycles Waste Manag. 2018, 20, 1154–1178. [Google Scholar] [CrossRef]
- Borsook, H.; MacFadyen, D.A. The Effect Of Isoelectric Amino Acids On The Ph+ Of A Phosphate Buffer Solution: A Contribittion In Support Of The “Zwitter Ion” Hypothesis. J. Gen. Physiol. 1930, 13, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Alfantazi, A.M.; Moskalyk, R.R. Processing of indium: A review. Miner. Eng. 2003, 16, 687–694. [Google Scholar] [CrossRef]
- U.S. Customs and Border Protection (CBP). Household Articles of Base Metal. Available online: https://www.cbp.gov/sites/default/files/documents/icp079_3.pdf (accessed on 1 October 2021).
- Akcil, A.; Agcasulu, I.; Swain, B. Valorization of waste LCD and recovery of critical raw material for circular economy: A review. Resour. Conserv. Recycl. 2019, 149, 622–637. [Google Scholar] [CrossRef]
- Domingos, L.F.T.; Azevedo, A.G.S.; Lombardi, C.T.; Strecker, K. Corrosion resistance of fly ash-based geopolymer in hydrochloric and sulfuric acid solutions. Cerâmica 2020, 66, 394–403. [Google Scholar] [CrossRef]
- Tran, L.-H.; Tanong, K.; Jabir, A.D.; Mercier, G.; Blais, J.-F. Hydrometallurgical Process and Economic Evaluation for Recovery of Zinc and Manganese from Spent Alkaline Batteries. Metals 2020, 10, 1175. [Google Scholar] [CrossRef]
- Nain, P.; Kumar, A. Metal dissolution from end-of-life solar photovoltaics in real landfill leachate versus synthetic solutions: One-year study. Waste Manag. 2020, 114, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.B.; Vanapalli, K.R.; Barnwal, V.K.; Dubey, B.; Bhattacharya, J. Evaluation of heavy metal leaching under simulated disposal conditions and formulation of strategies for handling solar panel waste. Sci. Total Environ. 2021, 780, 146645. [Google Scholar] [CrossRef]
- Parize, R.; Katerski, A.; Gromyko, I.; Rapenne, L.; Roussel, H.; Kärber, E.; Appert, E.; Krunks, M.; Consonni, V. ZnO/TiO2/Sb2S3 Core-Shell Nanowire Heterostructure for Extremely Thin Absorber Solar Cells. J. Phys. Chem. C 2017, 121. [Google Scholar] [CrossRef]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H.; Bernardes, A.M. Recycling WEEE: Extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Manag. 2016, 57, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.K.; Kim, H.S.; Tran, T.; Hong, S.K.; Kim, M.J. Recovering valuable metals from recycled photovoltaic modules. J. Air Waste Manag. Assoc. 2014, 64, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, J.; Park, N. A method to recycle silicon wafer from end-of-life photovoltaic module and solar panels by using recycled silicon wafers. Sol. Energy Mater. Sol. Cells 2017, 162, 1–6. [Google Scholar] [CrossRef]
- Wongnaree, N.; Kritsarikun, W.; Ma-ud, N.; Kansomket, C.; Udomphol, T.; Khumkoa, S. Recovery of Silver from Solar Panel Waste: An Experimental Study. Mater. Sci. Forum 2020, 1009, 137–142. [Google Scholar] [CrossRef]
- Yang, E.H.; Lee, J.K.; Lee, J.S.; Ahn, Y.S.; Kang, G.H.; Cho, C.H. Environmentally friendly recovery of Ag from end-of-life c-Si solar cell using organic acid and its electrochemical purification. Hydrometallurgy 2017, 167, 129–133. [Google Scholar] [CrossRef]
- Hu, S.H.; Tsai, M.S.; Yen, F.S.; Onlin, T. Recovery of copper-contaminated sludge in a two-stage leaching process. Environ. Prog. 2006, 25, 72–78. [Google Scholar] [CrossRef]
- Panda, S.; Rout, P.C.; Sarangi, C.K.; Mishra, S.; Pradhan, N.; Mohapatra, U.; Subbaiah, T.; Sukla, L.B.; Mishra, B.K. Recovery of copper from a surface altered chalcopyrite contained ball mill spillage through bio-hydrometallurgical route. Korean J. Chem. Eng. 2014, 31, 452–460. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, S.; Akcil, A. Bioremediation of acidic mine effluents and the role of sulfidogenic biosystems: A mini-review. Euro-Mediterr. J. Environ. Integr. 2016, 1, 8. [Google Scholar] [CrossRef]
- Panda, S. Magnetic separation of ferrous fractions linked to improved bioleaching of metals from waste-to-energy incinerator bottom ash (IBA): A green approach. Environ. Sci. Pollut. Res. 2020, 27, 9475–9489. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Feasibility of Bioleaching of Selected Metals from Electronic Waste by Acidiphilium acidophilum. Waste Biomass Valorization 2017, 9, 871–877. [Google Scholar] [CrossRef]
- Glombitza, F.; Reichel, S. Metal-containing residues from industry and in the environment: Geobiotechnological urban mining. Adv. Biochem. Eng. Biotechnol. 2014, 141, 49–107. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Lee, J. Biometallurgical Recovery of Metals from Waste Electrical and Electronic Equipment: A Review. ChemBioEng Rev. 2014, 1, 148–169. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste—A review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dong, B.; Zhuang, X.; Zhao, J.; Zhang, C.; Wang, J.; Shih, K. Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode. Chem. Eng. J. 2017, 324, 122–129. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Mishra, S.; Erust, C. A novel bioreactor system for simultaneous mutli-metal leaching from industrial pyrite ash: Effect of agitation and sulphur dosage. J. Hazard. Mater. 2018, 342, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.G.; Schippers, A. (Bio)chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Simultaneous recovery of Ni and Cu from computer-printed circuit boards using bioleaching: Statistical evaluation and optimization. Bioresour. Technol. 2014, 174, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Rodrigues, M.L.M.; Leão, V.A.; Gomes, O.; Lambert, F.; Bastin, D.; Gaydardzhiev, S. Copper extraction from coarsely ground printed circuit boards using moderate thermophilic bacteria in a rotating-drum reactor. Waste Manag. 2015, 41, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef]
- Liang, G.; Li, P.; Liu, W.; Wang, B. Enhanced bioleaching efficiency of copper from waste printed circuit boards (PCBs) by dissolved oxygen-shifted strategy in Acidithiobacillus ferrooxidans. J. Mater. Cycles Waste Manag. 2016, 18, 742–751. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Zhou, X.; Yan, W.; Ding, R.; Chen, B.; Wang, C.T.; Zhao, F. Enhanced bioleaching efficiency of copper from printed circuit boards without iron loss. Hydrometallurgy 2018, 180, 65–71. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Khatri, B.R.; Sodha, A.B.; Shah, M.B.; Tipre, D.R.; Dave, S.R. Chemical and microbial leaching of base metals from obsolete cell-phone printed circuit boards. Sustain. Environ. Res. 2018, 28, 333–339. [Google Scholar] [CrossRef]
- Sodha, A.B.; Qureshi, S.A.; Khatri, B.R.; Tipre, D.R.; Dave, S.R. Enhancement in Iron Oxidation and Multi-metal Extraction from Waste Television Printed Circuit Boards by Iron Oxidizing Leptospirillum feriphillum Isolated from Coal Sample. Waste Biomass Valorization 2019, 10, 671–680. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S. Bioleaching of Basic Metals from Electronic Waste PCBs. J. Min. Mech. Eng. 2020, 1, 51–58. [Google Scholar]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. J. Chem. Technol. Biotechnol. 2020, 95, 2272–2285. [Google Scholar] [CrossRef]
- Hubau, A.; Minier, M.; Chagnes, A.; Joulian, C.; Silvente, C.; Guezennec, A.-G. Recovery of metals in a double-stage continuous bioreactor for acidic bioleaching of printed circuit boards (PCBs). Sep. Purif. Technol. 2020, 238, 116481. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A.; Gajda, B.; Saternus, M. Bioleaching of indium and tin from used LCD panels. Physicochem. Probl. Miner. Process. 2018, 54, 639–645. [Google Scholar] [CrossRef]
- Jowkar, M.J.; Bahaloo-Horeh, N.; Mousavi, S.M.; Pourhossein, F. Bioleaching of indium from discarded liquid crystal displays. J. Clean. Prod. 2018, 180, 417–429. [Google Scholar] [CrossRef]
- Sadeghabad, M.S.; Bahaloo-Horeh, N.; Mousavi, S.M. Using bacterial culture supernatant for extraction of manganese and zinc from waste alkaline button-cell batteries. Hydrometallurgy 2019, 188, 81–91. [Google Scholar] [CrossRef]
- Biswal, B.K.; Jadhav, U.U.; Madhaiyan, M.; Ji, L.; Yang, E.H.; Cao, B. Biological Leaching and Chemical Precipitation Methods for Recovery of Co and Li from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 12343–12352. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Srinivasan, M.; Cao, B. Bioleaching as an Eco-Friendly Approach for Metal Recovery from Spent NMC-Based Lithium-Ion Batteries at a High Pulp Density. ACS Sustain. Chem. Eng. 2021, 9, 3060–3069. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.C.; Chi, R.A. Bioleaching of metals from electronic scrap and its potential for commercial exploitation. Hydrometallurgy 2013, 131–132, 138–143. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Li, X.; Qiu, Y.; Zhu, M.; Tan, W. Mechanism underlying the bioleaching process of LiCoO2 by sulfur-oxidizing and iron-oxidizing bacteria. J. Biosci. Bioeng. 2019, 128, 344–354. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wu, W.; Zhang, X.; Gu, T.; Zhu, M.; Tan, W. Oxidative Stress Induced by Metal Ions in Bioleaching of LiCoO2 by an Acidophilic Microbial Consortium. Front. Microbiol. 2020, 10, 3058. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag. Res. 2012, 30, 1151–1159. [Google Scholar] [CrossRef]
- Karwowska, E.; Andrzejewska-Morzuch, D.; Łebkowska, M.; Tabernacka, A.; Wojtkowska, M.; Telepko, A.; Konarzewska, A. Bioleaching of metals from printed circuit boards supported with surfactant-producing bacteria. J. Hazard. Mater. 2014, 264, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mousavi, S.M. Enhancement of simultaneous gold and copper extraction from computer printed circuit boards using Bacillus megaterium. Bioresour. Technol. 2015, 175, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Arias, J.E.; Argumedo-Delira, R.; Alarcón, A.; Mendoza-López, M.R.; García-Barradas, O.; Cruz-Sánchez, J.S.; Ferrera-Cerrato, R.; Jiménez-Fernández, M. Bioleaching of gold, copper and nickel from waste cellular phone PCBs and computer goldfinger motherboards by two Aspergillus niger strains. Braz. J. Microbiol. 2015, 46, 707–713. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial Bioleaching of Ag, Au and Cu from Printed Circuit Boards of Mobile Phones. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef]
- Kaliyaraj, D.; Rajendran, M.; Angamuthu, V.; Antony, A.R.; Kaari, M.; Thangavel, S.; Venugopal, G.; Joseph, J.; Manikkam, R. Bioleaching of heavy metals from printed circuit board (PCB) by Streptomyces albidoflavus TN10 isolated from insect nest. Bioresour. Bioprocess. 2019, 6, 47. [Google Scholar] [CrossRef]
- Netpae, T.; Suckley, S. Comparison of three culture media for one-step and two-step bioleaching of nickel and cadmium from spent Ni-Cd batteries by Aspergillus niger. Adv. Environ. Technol. 2020, 6, 167–172. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Mao, F.; Wu, P.; Dang, Z. Bioleaching of indium from waste LCD panels by Aspergillus niger: Method optimization and mechanism analysis. Sci. Total Environ. 2021, 790, 148151. [Google Scholar] [CrossRef] [PubMed]
- Golzar-Ahmadi, M.; Mousavi, S.M. Extraction of valuable metals from discarded AMOLED displays in smartphones using Bacillus foraminis as an alkali-tolerant strain. Waste Manag. 2021, 131, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Chakankar, M.; Su, C.H.; Hocheng, H. Leaching of metals from end-of-life solar cells. Environ. Sci. Pollut. Res. 2019, 26, 29524–29531. [Google Scholar] [CrossRef] [PubMed]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Bioleaching of copper from low grade scrap TV circuit boards using mesophilic bacteria. Hydrometallurgy 2013, 138, 65–70. [Google Scholar] [CrossRef]
- Annamalai, M.; Gurumurthy, K. Enhanced bioleaching of copper from circuit boards of computer waste by Acidithiobacillus ferrooxidans. Environ. Chem. Lett. 2019, 17, 1873–1879. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Lu, L.; Zhuang, X.; Zhao, J.; Yuan, W.; Zhang, C.; Wang, J. Improved bioleaching efficiency of metals from waste printed circuit boards by mechanical activation. Waste Manag. 2019, 98, 21–28. [Google Scholar] [CrossRef]
- Wei, X.; Dongfang, L.; Huang, W.; Huang, W.; Lei, Z. Simultaneously enhanced Cu bioleaching from E-wastes and recovered Cu ions by direct current electric field in a bioelectrical reactor. Bioresour. Technol. 2020, 298, 122566. [Google Scholar] [CrossRef]
- Jagannath, A.; Vidya Shetty, K.; Saidutta, M.B. Bioleaching of copper from electronic waste using Acinetobacter sp. Cr B2 in a pulsed plate column operated in batch and sequential batch mode. J. Environ. Chem. Eng. 2017, 5, 1599–1607. [Google Scholar] [CrossRef]
- Arab, B.; Hassanpour, F.; Arshadi, M.; Yaghmaei, S.; Hamedi, J. Optimized bioleaching of copper by indigenous cyanogenic bacteria isolated from the landfill of e-waste. J. Environ. Manag. 2020, 261, 110124. [Google Scholar] [CrossRef] [PubMed]
- Rozas, E.E.; Mendes, M.A.; Nascimento, C.A.O.; Espinosa, D.C.R.; Oliveira, R.; Oliveira, G.; Custodio, M.R. Bioleaching of electronic waste using bacteria isolated from the marine sponge Hymeniacidon heliophila (Porifera). J. Hazard. Mater. 2017, 329, 120–130. [Google Scholar] [CrossRef]
- Rezza, I.; Salinas, E.; Elorza, M.V.; de Tosetti, M.I.S.; Donati, E. Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms. Process Biochem. 2001, 36, 495–500. [Google Scholar] [CrossRef]
- Arshadi, M.; Nili, S.; Yaghmaei, S. Ni and Cu recovery by bioleaching from the printed circuit boards of mobile phones in non-conventional medium. J. Environ. Manag. 2019, 250, 109502. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Kim, M.-J.; Seo, J.-Y.; Choi, Y.-S.; Kim, G.-H. Bioleaching of spent Zn-Mn or Ni-Cd batteries by Aspergillus species. Waste Manag. 2016, 51, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Valix, M.; Loon, L. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Valix, M.; Tang, J.Y.; Malik, R. Heavy metal tolerance of fungi. Miner. Eng. 2001, 14, 499–505. [Google Scholar] [CrossRef]
- Wang, S.; Tian, Y.; Zhang, X.; Yang, B.; Wang, F.; Xu, B.; Liang, D.; Wang, L. A Review of Processes and Technologies for the Recycling of Spent Lithium-ion Batteries. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 022025. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Yang, D.; Zhu, N. Bioleaching of spent Ni-Cd batteries and phylogenetic analysis of an acidophilic strain in acidified sludge. Front. Environ. Sci. Eng. China 2007, 1, 459–465. [Google Scholar] [CrossRef]
- Velgosová, O.; Kaduková, J.; Marcınčáková, R. Study of Ni And Cd Bioleaching from Spent Ni-Cd Batteries. Nov. Biotechnol. Chim. 2012, 11, 117–123. [Google Scholar] [CrossRef][Green Version]
- Velgosová, O.; Kaduková, J.; Marcinčáková, R.; Mrážiková, A.; Fröhlich, L. The Role of Main Leaching Agents Responsible for Ni Bioleaching from spent Ni-Cd Batteries. Sep. Sci. Technol. 2014, 49, 438–444. [Google Scholar] [CrossRef]
- Xin, B.; Jiang, W.; Li, X.; Zhang, K.; Liu, C.; Wang, R.; Wang, Y. Analysis of reasons for decline of bioleaching efficiency of spent Zn-Mn batteries at high pulp densities and exploration measure for improving performance. Bioresour. Technol. 2012, 112, 186–192. [Google Scholar] [CrossRef]
- Falco, L.; Martínez, A.; Di Nanno, M.P.; Thomas, H.; Curutchet, G. Study of a pilot plant for the recovery of metals from spent alkaline and zinc-carbon batteries with biological sulphuric acid and polythionate production. Lat. Am. Appl. Res. 2014, 44, 123–129. [Google Scholar] [CrossRef]
- Niu, Z.; Huang, Q.; Wang, J.; Yang, Y.; Xin, B.; Chen, S. Metallic ions catalysis for improving bioleaching yield of Zn and Mn from spent Zn-Mn batteries at high pulp density of 10. J. Hazard. Mater. 2015, 298, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Xin, B.; Pang, K.; Jiang, M.; Li, Z.; Zhao, J.; Zhang, M. Microwave assisted dissolution efficiency of bioleaching of spent alkaline zinc manganese battery. Chin. J. Environ. Eng. 2015, 9, 5199–5205. [Google Scholar] [CrossRef]
- Naseri, T.; Bahaloo-Horeh, N.; Mousavi, S.M. Bacterial leaching as a green approach for typical metals recovery from end-of-life coin cells batteries. J. Clean. Prod. 2019, 220, 483–492. [Google Scholar] [CrossRef]
- Chatterjee, A.; Das, R.; Abraham, J. Bioleaching of heavy metals from spent batteries using Aspergillus nomius JAMK1. Int. J. Environ. Sci. Technol. 2020, 17, 49–66. [Google Scholar] [CrossRef]
- Ruhatiya, C.; Gandra, R.; Kondaiah, P.; Manivas, K.; Samhith, A.; Gao, L.; Lam, J.S.L.; Garg, A. Intelligent optimization of bioleaching process for waste lithium-ion batteries: An application of support vector regression approach. Int. J. Energy Res. 2021, 45, 6152–6162. [Google Scholar] [CrossRef]
- Gazzo, D.V.; Reed, D.W. Optimization of a Lithium Ion Battery Bioleaching Process Utilizing Organic Acids Produced by Gluconobacter Oxydans; Idaho National Lab. (INL): Idaho Falls, ID, USA, 2019. [Google Scholar]
- Pourhossein, F.; Mousavi, S.M. A novel step-wise indirect bioleaching using biogenic ferric agent for enhancement recovery of valuable metals from waste light emitting diode (WLED). J. Hazard. Mater. 2019, 378, 120648. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Enhancement of copper, nickel, and gallium recovery from LED waste by adaptation of Acidithiobacillus ferrooxidans. Waste Manag. 2018, 79, 98–108. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, T.; Li, M.; Yan, N. Bio-leaching of heavy metals from electroplating sludge by Thiobacillus. Ecol. Environ. 2008, 17, 1787–1791. [Google Scholar]
- Marra, A.; Cesaro, A.; Rene, E.R.; Belgiorno, V.; Lens, P.N.L. Bioleaching of metals from WEEE shredding dust. J. Environ. Manag. 2018, 210, 180–190. [Google Scholar] [CrossRef]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A novel eco-friendly hybrid approach for recovery and reuse of copper from electronic waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Pant, D.; Joshi, D.; Upreti, M.K.; Kotnala, R.K. Chemical and biological extraction of metals present in E waste: A hybrid technology. Waste Manag. 2012, 32, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Yazici, E.Y.; Deveci, H. Ferric sulphate leaching of metals from waste printed circuit boards. Int. J. Miner. Process. 2014, 133, 39–45. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D. Leaching and Selective Recovery of Cu from Printed Circuit Boards. Metals 2019, 9, 1034. [Google Scholar] [CrossRef]
- Golsteijn, L.; Valencia Martinez, E. The Circular Economy of E-Waste in the Netherlands: Optimizing Material Recycling and Energy Recovery. J. Eng. 2017, 2017, 8984013. [Google Scholar] [CrossRef]
- EU Horizon 2020 CEWASTE Project. Available online: https://cewaste.eu/about-the-project/ (accessed on 3 October 2021).
- Schroeder, P.; Anggraeni, K.; Weber, U. The Relevance of Circular Economy Practices to the Sustainable Development Goals. J. Ind. Ecol. 2019, 23, 77–95. [Google Scholar] [CrossRef]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Bakhiyi, B.; Gravel, S.; Ceballos, D.; Flynn, M.A.; Zayad, J. Has the question of e-waste opened a Pandora’s box? An overview of unpredictable issues and challenges. Environ. Int. 2018, 110, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Huda, N. Reverse logistics and closed-loop supply chain of Waste Electrical and Electronic Equipment (WEEE)/E-waste: A comprehensive literature review. Resour. Conserv. Recycl. 2018, 137, 48–75. [Google Scholar] [CrossRef]
- Cárdenas, J.P.; Valdés, J.; Quatrini, R.; Duarte, F.; Holmes, D.S. Lessons from the genomes of extremely acidophilic bacteria and archaea with special emphasis on bioleaching microorganisms. Appl. Microbiol. Biotechnol. 2010, 88, 605–620. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.J.; Tettelin, H.; Blake, R.; Eisen, J.A.; Holmes, D.S. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Holmes, D.S. Comparative genome analysis of Acidithiobacillus ferrooxidans, A. thiooxidans and A. caldus: Insights into their metabolism and ecophysiology. Hydrometallurgy 2008, 94, 180–184. [Google Scholar] [CrossRef]
- Parida, B.K.; Panda, S.; Misra, N.; Panda, P.K.; Mishra, B.K. BBProF: An Asynchronous Application Server for Rapid Identification of Proteins Associated with Bacterial Bioleaching. Geomicrobiol. J. 2014, 31, 299–314. [Google Scholar] [CrossRef]
- Gönen, M.; Rodene, D.D.; Panda, S.; Akcil, A. Techno-economic Analysis of Boric Acid Production from Colemanite Mineral and Sulfuric Acid. Miner. Process. Extr. Metall. Rev. 2021, 1–9. [Google Scholar] [CrossRef]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Yim, M.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; et al. Techno-economic and Life Cycle Analysis for Bioleaching Rare-Earth Elements from Waste Materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).