Abstract
The paper describes the possibilities of simple and effective modification of calcium sorbents used for flue gas desulfurization with a size between of 125–250 µm. The additives to the sorbents in the amount of 0.5% and 1.0% were inorganic sodium and lithium compounds. The research on the reactivity of sorbents was analyzed in the process of simultaneous calcination and sulfation at the temperature of 850 °C. The type of Na+ or Li+ cations and the inorganic salt anions have an influence on the modification of calcium sorbents in order to improve the efficiency of the calcination and sulfation process. Modification of calcium sorbents by adding inorganic sodium and lithium compounds, regardless of the amount, changes the reactivity coefficient RI [mol/mol] and the absolute sorption coefficient CI [g S/kg sorbent]. In the case of inorganic sodium salt (Additive 1), regardless of the amount of modifier added, there was a visible improvement in the reactivity of the sorbent: 1.0% of the additive caused an increase in the RI coefficient in relation to the raw sorbent by over 14%, and in the case of the CI coefficient by over 24%. Additional research was the analysis of the limestone behavior mechanism during the simultaneous calcination and sulfation (SCS) process under conditions of elevated temperature and with variable CO2 and O2 contents in the flue gas. The behavior of sorbents with a size distribution of 125–250 µm was assessed on the basis of the change in mass of the samples by determining the reactivity coefficient RI, [mol/mol] and the absolute sorption coefficient CI, [g S/kg sorbent]. Using the mercury porosimetry technique, the change in sorbent porosity in the subsequent stages of the simultaneous calcination and sulfation process was investigated. The process was carried out in the temperature range corresponding to the oxy-combustion (i.e., from 850 °C to 1000 °C).
1. Introduction
As a member of the EU, Poland must comply with the laws in force in the member states. Implementation of Directive 2001/80/EC resulted in the need to reduce the emissions of pollutants introduced into the air [1]. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 also introduced new restrictive SO2, NOx, and dust emission standards, with particular emphasis on fired sources of carbon [2]. The data included in Table 1 presenting the amount of pollutant emissions in the years 2012–2020 indicate that the emissions have decreased [3]. The introduction of new legal regulations should result in a further, significant reduction in pollutant emissions. Taking into account the important role of sorbents in the process of exhaust gas purification, research on the use of new sorbents or their modification is constantly carried out. As a result, these activities are leading to desulfurization products being obtained with better possibilities for their safe storage and a reduction in SO2 and NOx emissions so that they meet the standards on emissions industrial—the IED (Integrated Pollution Prevention and Control) [2]. Table 1 presents the results of the analysis carried out by the National Center for Emission Management and Balancing, and concerns the projected emissions from large fuel combustion sources by 2020 [4].

Table 1.
Projected emissions from large combustion plants (as defined in the IED Directive). Own study based on [4], TSP-total suspended particulate.
1.1. The Use and Application of Calcium Sorbents in Polish Power Plants
Limestones are sedimentary rocks consisting mainly of calcite—CaCO3 [5]. Due to their binding properties, limestone has been used since antiquity, mainly gypsum and calcium carbonate. Calcium is a widely distributed element and ranks fifth in terms of its content in the Earth’s crust [6]. There are numerous types of limestone in Poland, except the noblest varieties of sculptural and architectural marble. For the lime industry, limestone deposits are mainly found in the Świętokrzyskie Voivodeship (60% of total resources, mainly Devonian and Jurassic limestones), Łódzkie, Opolskie, and Śląskie voivodeships, their total resources, located in 120 deposits, amounted to 5591 million Mg at the end of 2014 [5,7,8]. Considering the age of the limestone, Jurassic limestones are the most important, they constitute over 59% of the resources, the next are Cretaceous limestones accounting for 21% of the resources, Devonian limestones about 8%, Triassic limestones about 8%, Tertiary limestones about 3%, and a small number of resources are limestones of the Carboniferous, Cambrian, and Precambrian ages [5,9]. High-quality limestone is used for the production of lime sorbents, which are used to reduce SO2 emissions. Limestones that provide good flue gas desulfurization effects are characterized by high chemical purity, content of CaCO3 of at least 94%, Fe2O3 most often below 0.4%, and MgO < 0.1% with a variable SiO2 content [5,10].
All methods of removing sulfur dioxide from flue gas consist of introducing a sorbent into the system, which reacts with gaseous SO2 to form solid compounds with it (CaSO3, CaSO4 mainly, CaSO4·2H2O, which is formed after the CaSO4 hydration reaction), while the reaction products are removed from the system. The most commonly used sorbents are limestone flour, hydrated lime, and ground quicklime. Less commonly used are ground dolomite, calcined magnesite, and sodium carbonate. Industrial waste (e.g., carbide lime), is used sporadically. The most numerous group of reagents used in flue gas desulfurization installations are lime sorbents; in Poland, the wet lime method and fluidized bed boilers are mainly used, less often in semi-dry and dry methods. Lime sorbents in the Polish power industry are used in 99% of the existing FGDs (flue gas desulfurization) installation due to the widespread availability, low purchase cost, and easy disposal of some products (e.g., synthetic gypsum obtained in the case of using the wet lime method) [5,11,12].
1.2. Modification Methods Used to Improve the Reactivity of Calcium Sorbents
The reduction in pollutants from the combustion of solid fuels, especially in the case of sulfur compounds, is achieved by binding them with calcium sorbents. The methods of improving the performance of sorbents (i.e., their reactivity) consist, in particular, in improving the quality of natural sorbents and reusing solid combustion products (Table 2). Depending on their origin, sorbents used for flue gas desulfurization differ in their reactivity, which is their efficiency in binding SO2 [13,14].

Table 2.
Methods of improving the reactivity of calcium sorbents [15,16,17].
There are also many methods of improving the degree of utilization, in which fly and bottom ashes are properly prepared and conditioned before re-introducing into the furnace chamber of the boiler. These methods are called ash reactivation methods, and the main ones are:
- grinding ashes, which causes grains to break and new surfaces to form, in the case of larger sorbent grains;
- hydration of ashes, under the influence of water or water vapor, as a result of which the CaSO4 structure is broken and sorbent grain fragmentation [16]; and
- pelletization of ashes and mixtures with fresh limestone or fuel [18].
The main reaction that takes place during the hydration process is (1):
CaO + H2O → Ca(OH)2
As a result of the reaction, the structure of Ca(OH)2 is broken and grains are fragmented. When introduced into the combustion chamber, it decomposes according to the reaction (2):
Ca(OH)2 → CaO + H2O
Too long a hydration time may lead to the reaction of the used sorbent with ash components, which will remove active Ca(OH)2. To avoid this undesirable phenomenon, sorbent reactivation technologies should be carried out under strictly controlled conditions [18]. Pelletization consists of combining the above methods, extending the residence time of the reactive material and obtaining high porosity (macropores), which improves the contact of SO2 with CaO grains, which leads to a high degree of calcium utilization [15,18]. The study in [16] presents an analysis of the possibility of pelleting mixtures of wet ash mass together with fuel, sorbent, or other solid materials (e.g., fly ash or incinerated waste). Thus, a reactive dry product that was easy to transport was obtained. Calcination releases CO2 from calcium carbonate, resulting in the formation of a porous product (i.e., CaO). As a result of high temperature, the formed CaO may sinter, creating eutectics with sorbent impurities [17]. The presence of impurities (additives) in the sorbent has a positive effect on the sulfation process. According to Fuertes and Fernande [19], additives, most often in the form of carbonates, increase the reactivity of sorbents in the following order (the most common compounds in sorbents are given):
Na2CO3 > Li2CO3 > K2CO3 > Na2SO4 >….> NaCl >…. > Al2(SO4)3
The presence of these compounds in calcium sorbents affects the reactivity through their catalytic effect on the process of calcium carbonate sulfation or lowering the decomposition temperature of calcium sulfate (VI). The thermal stability of CaSO4 is an important aspect taking into account the fact that calcium sorbents are introduced into the combustion chamber during the combustion process. Therefore, to optimize flue gas desulfurization along with the reactivity of sorbents, it is required that the sulfates produced as a result of the process are stable and exhibit temperature stability. The reactivity of sorbents may be determined by their natural properties, resulting from the deposit they come from, and this information, in turn, allows us to indicate new possibilities of modifying sorbents. In this context, the literature offers numerous examples of increasing the sorption of SO2 onto CaCO3 by adding various types of additives, mainly alkaline compounds [20,21,22]. The presence of impurities influences the texture characteristics (porosity and surface area) of CaO formed during calcination of limestone particles due to the sintering of the resulting CaO grains, accelerated by the presence of foreign ions. Consequently, the modification of calcium sorbents occurs by changing the textural properties during the calcination stage and modification of the internal structure of CaCO3.
1.3. The Role of Calcium Sorbents in the Oxy-Fuel Combustion-OFC Process
Oxyfuel (oxy-fuel combustion, OFC) is a promising technology aimed at reducing greenhouse gas emissions. The application of this process makes it possible to capture and store carbon dioxide. The course of this process is that during a typical oxygen combustion process, the exhaust gases are recirculated and mixed with high purity oxygen. Almost pure CO2 is obtained in the flue gas, which makes it possible to directly store this gas without the need for the special capture of CO2 from the flue gases, as is the case with flue gases from traditional boilers. As a result of exhaust gas recirculation, the concentration of carbon dioxide in the furnace chamber is increased by up to 95% by volume [23]. As a large proportion of the exhaust gas is recirculated, the concentration of pollutants such as SO2/SO3 also increases. To eliminate the problems associated with the presence of sulfur oxides, the exhaust gas has to be cleaned. In conventional air combustion, sulfur removal with calcium carbonate includes calcination and sulfation processes [24,25]. The course of the calcination process according to reaction (4) depends on the partial pressure of CO2 and the temperature of CaCO3 particles [26].
CaCO3 → CaO + CO2
The sulfation process takes place according to the processes (5–6):
CaO + SO2 + 1/2O2→ CaSO4
CaCO3 + SO2 + 1/2O2→ CaSO4 + CO2
The process (5) describes the so-called indirect sulfation process. Direct sulfation taking place according to reaction (6) is the basis for analyzing the mechanism of sulfur capture processes during oxygen combustion. As shown in the literature, the process described by reaction (6) has the greatest effect on sulfation under conditions of increased O2/CO2 concentration. In this case, gas diffusion plays an important role, which is the element controlling the processes. Based on the important role of sorbents for flue gas desulfurization in the process of traditional combustion and oxy-fuel combustion, the influence of various additives (inorganic salts) on increasing the amount of sulfur dioxide that can be adsorbed by the sorbent was investigated. Moreover, the influence of the presence of inorganic salts on the efficiency of the calcination and desulfurization process was investigated by analyzing the reactivity coefficient RI and the absolute sorption coefficient (CI). Knowing the initial and final mass as well as sulfur content in the initial sample and after the sulfation process, it is possible to determine the absolute sorption coefficient (CI) of the sorbent expressed in units of grams of sulfur bound by kg of sorbent. The following formula was used to determine this coefficient
where CI is the absolute sorption coefficient, g S/kg; Sp, Sk is the sulfur content in the initial and final sample, %; and mp, mk is the initial sample weight, final sample weight, g.
To determine the reactivity coefficient (RI), which is the number of moles of calcium needed to bind a mole of sulfur, expressed in units of mol Ca/mol S, the following formula was used:
where Ca0 is the calcium content in the sorbent, %.
Additionally, the behavior of CaCO3 during the aerobic combustion of solid fuels has been described. Tests were carried out on the reactivity of calcium sorbents under conditions simulating oxygen combustion, where the CO2 content gradually increases (from 20% to 70%) with a constant SO2 content in the temperature range from 850 °C to 1000 °C. A diagram of changes in the porosity of the limestone particle was proposed based on structural changes for given process conditions. The mechanism of this process was described based on the analysis of changes in the porosity of limestone particles at individual stages of the simultaneous calcination and sulfation process during oxygen combustion.
2. Materials and Methods
2.1. Research Material
The research used the sorbent fraction 125–250 µm, which was separated from the fraction 90–400 µm, which is commonly used in the Polish power industry. The composition of the sorbent is shown in Table 3.

Table 3.
Chemical composition of the sorbent used in the tests, %wt.
2.2. Apparatus
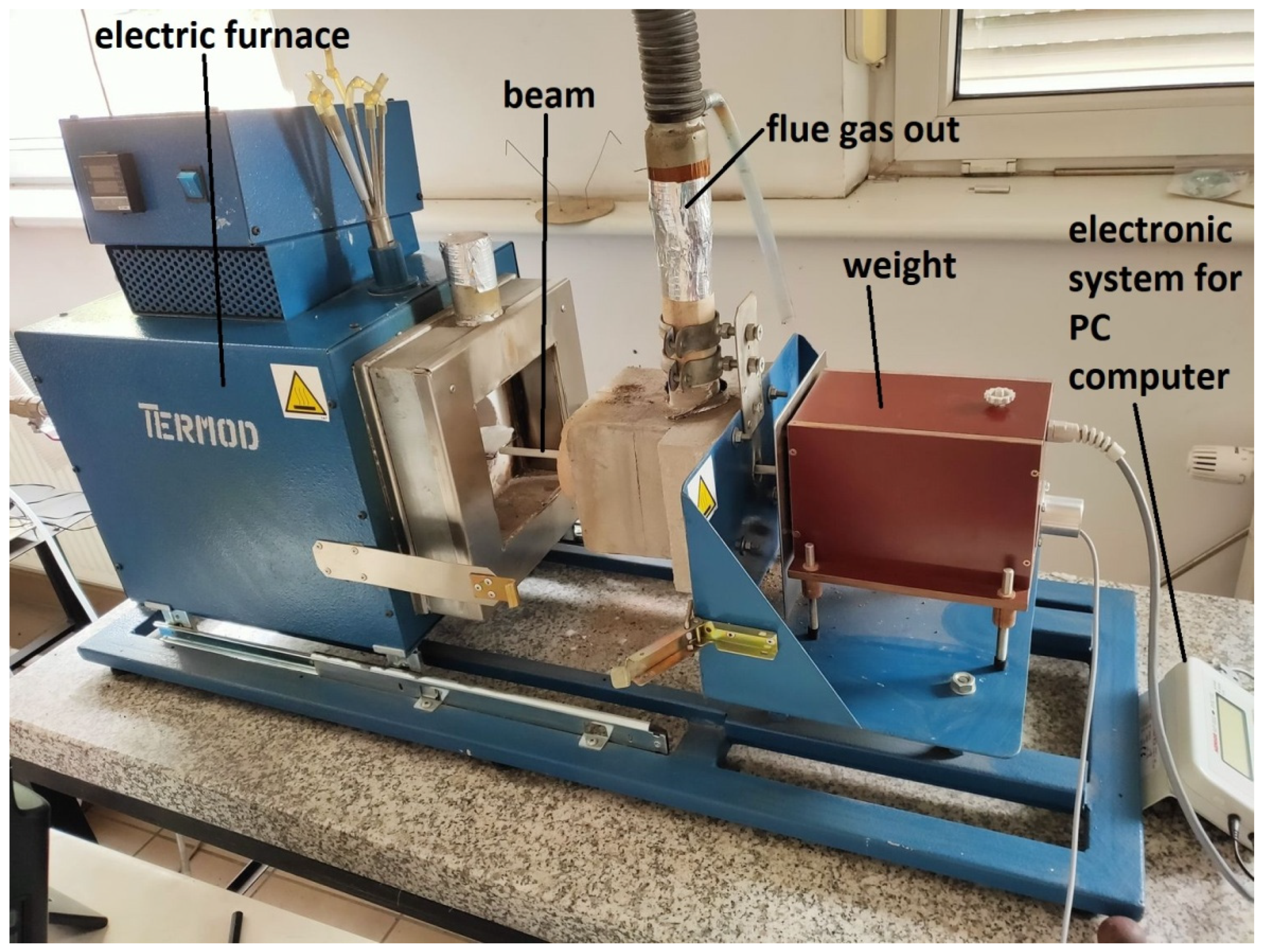
The reactivity tests were carried out on a stand specially prepared to test the reactivity of the sorbents. The configuration of the system and the organization of the gas flow allowed for the tracking and registration of changes in the mass of sorbent samples, not disturbed by the gas flow. The main part of the stand is a sealed laboratory furnace, electrically heated (heaters power 1.8 kW) and enabling the temperature inside the range to be kept up to 1100 °C. The accuracy of the temperature control was ±10 °C. The length of the furnace chamber was 220 mm and its diameter was 110 mm. A thermocouple was installed near the tested sample to control the temperature of the process. The exhaust gases were led to the environment through the exhaust gas duct. Indications of the measuring instruments were archived in a PC using an analog-to-digital converter. The layout of the stand is shown in Figure 1.
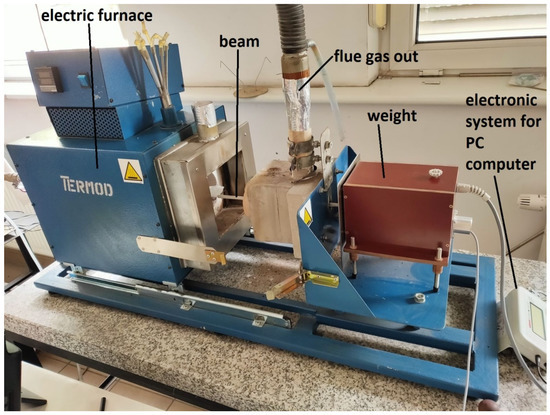
Figure 1.
Measuring stand for the sorbent reactivity tests [14,27].
2.3. Methodology
Synthetic flue gases were supplied to the furnace interior, the composition of which was selected and maintained during the measurement at the present level using automatic O2, CO2, and SO2 flow controllers. The synthetic flue gas stream was 5 L/min. The test was carried out for the concentration of gases provided in the test of the reactivity of calcium sorbents: CO2: 16%; SO2: 1870 ppm; O2: 3%; the rest was N2. Before the sorbent sample was introduced into the furnace, the exhaust gas components were pre-mixed in a mixer. The sorbent was tested, the chemical composition of which is presented in Table 4. The initial weight of the sorbent sample was 0.10 ± 0.02 g. The sorbent sample, granulated in accordance with the requirements of the reactivity test 125–250 μm, was placed on the arm of an electronic balance (see Figure 2) [14]. The balance arm was terminated with a specially made nickel mesh, constituting support for a cuboid made of porous glass wool, Al2O3 (Sibral®). The use of Al2O3 with high porosity was aimed at ensuring a high degree of dispersion of the sorbent grains to eliminate restrictions in the access of gaseous substrates to the surface of the grains during the reaction and product effluent (e.g., CO2). After the glass wool with the sample was placed on the balance arm, it was then introduced into the furnace heated to the preset temperature (850 °C). The sample was fed into the reactor by moving the furnace on the running gear (Figure 2).

Table 4.
Experimental conditions used for the analysis of sorbent properties during oxy-combustion.

Figure 2.
Method of placing a test sample on an electronic weighing pan [12].
In Table 4, the experimental conditions under which the analysis of the weight changes of the calcium sorbent samples were carried out to estimate the reactivity coefficient RI, [mol/mol] and the absolute sorption coefficient CI, [gS/kg sorbent] are presented. The formulas according to which the coefficients were calculated are presented in the publication [14,28].
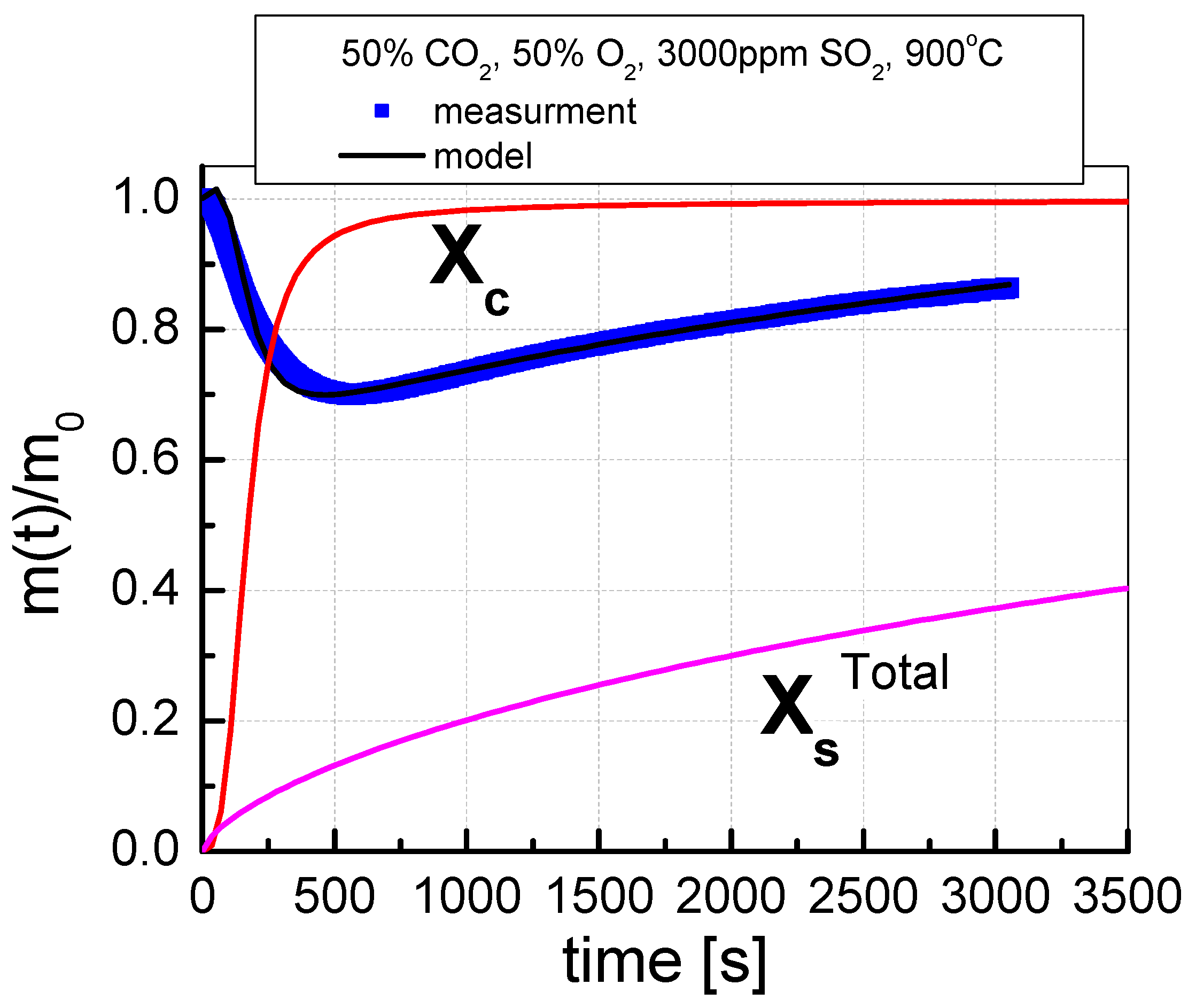
The course of the simultaneous calcination and sulfation (SCS) test consisted in placing the sorbent in a furnace heated to the appropriate temperature for 60 min in a mixture of gases, the composition of which is given in Table 5. The tests of the sorbent reactivity were carried out for various shares of CO2 and O2 (20/80%, 50/50% and 70/30%), with a constant SO2 concentration of 3000 ppm. The SCS process was carried out at selected temperatures from 850 °C to 1000 °C for the range of 50 °C. The test results are presented as changes in the degree of calcination (Xc) and sulfation (XsTotal) over time. The degree of calcination and the degree of sulfation of sorbents were calculated using the formulas included in the publication [28]. An exemplary graph of the change in sorbent mass during the SCS test is presented in Figure 3.

Table 5.
Values of the reactivity coefficient RI and the absolute sorption coefficient CI.
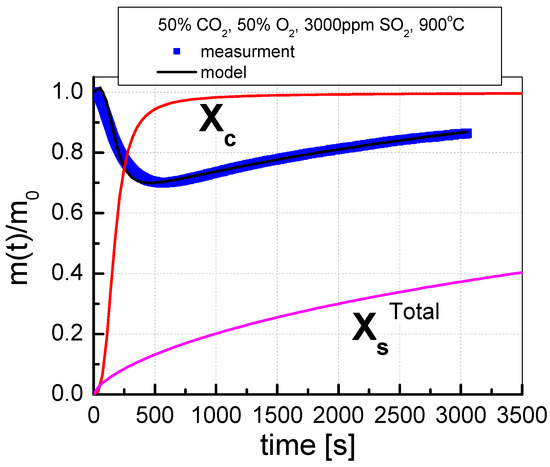
Figure 3.
An example of the mass change process during the SCS test, indicating the results of the approximation of the calcination and sulfation processes.
The change in sorbent mass recorded during the test was approximated by the formulas [26,27] based on the determined approximation equation, the calcination degree (Xc), and the sulfation degree (XsTotal) were distinguished. Assuming the degree of calcination as the amount of calcium carbonate related to the total content of calcium carbonate in the sample, and that all carbon compounds are decomposed and it is possible to have bound carbon in the form of calcium carbonate, the formula for the degree of calcination can be derived according to Equation (9)
where Xc is the degree of calcination; MCa, MC is the molar mass of calcium and carbon, g/mol; mk, m0—final and initial sample weight, g; η is the sorbent purity; Ck is the carbon content in the final sample, %; and Ca0 is the calcium content in the initial sample, %.
Similarly, assuming the degree of sulfation as the amount of calcium sulfate relative to the total conversion of CaO to CaSO4 and assuming that after entering the furnace, the only sulfur compound present in the sorbent is CaSO4, the conversion rate of CaO to CaSO4 can be calculated according to formula (10)
where XsTotal is the degree of sulfation; Ms is the molar mass of sulfur, g/mol; and Sk is the carbon content in the final sample, %.
During the SCS process, calcination may occur throughout the test, but will be most intense at the beginning of the process. The course of calcination and its rate strongly depends on the temperature. The sulfation process begins almost immediately after the sorbent is introduced into the station, as soon as CaO becomes available for SO2, resulting from the calcination process. The speed and rate of the sorbent sulfation process are indirectly temperature dependent through a calcination process that limits the amount of CaO available.
Measurements of changes in the porosity of calcium sorbents during the process were carried out using a mercury porosimeter PoreMaster 33 equipped with Quantachrome Poremaster for Windows software 7.01. The tests with the use of a mercury porosimeter were carried out three times for each of the tested sorbent samples.
3. Results and Discussion
3.1. The Effect of Additives Modifying Sorbents
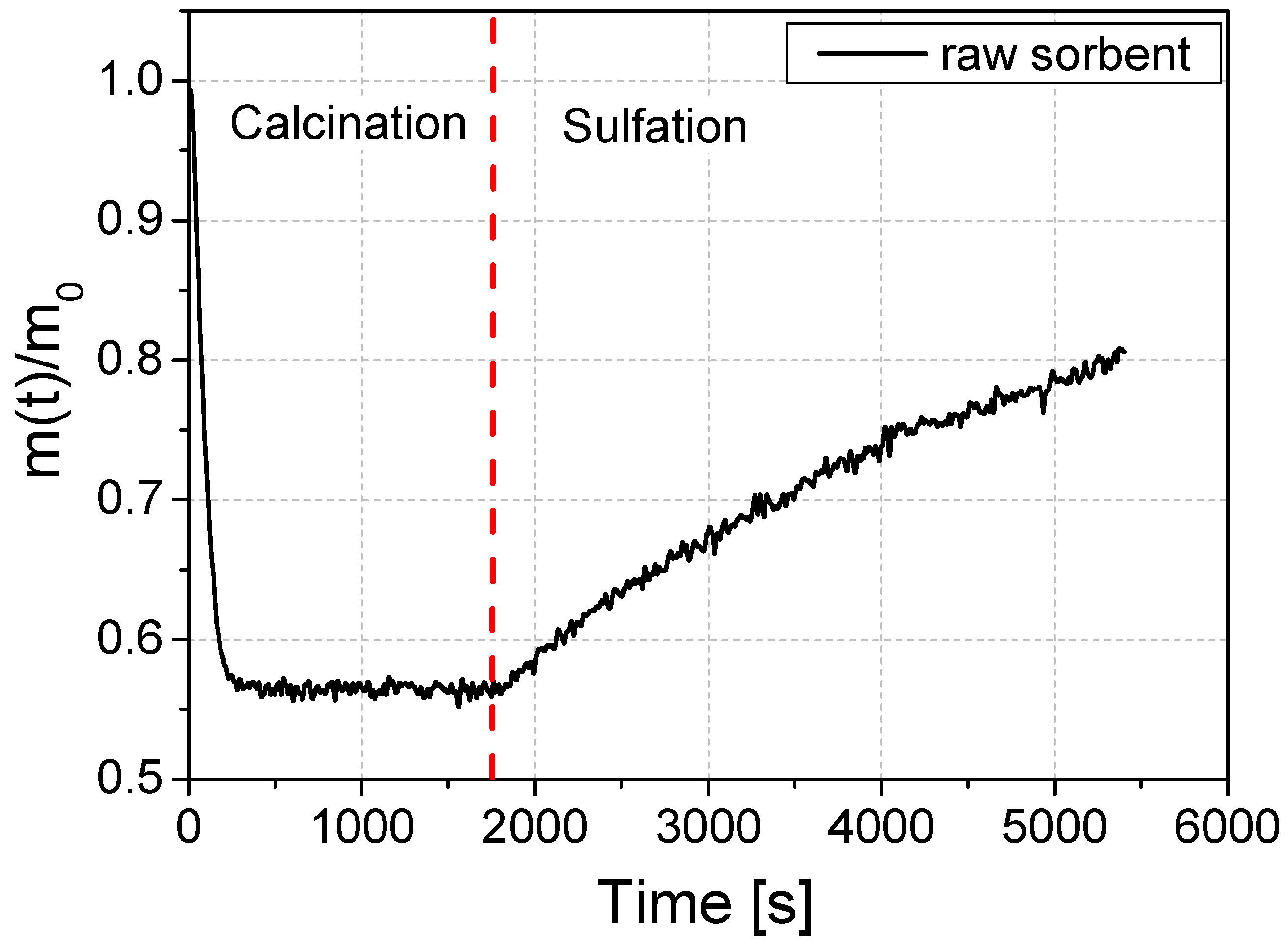
The reactivity of the raw sorbent (i.e., the one that is mined in a limestone mine) was initially tested. The course of the loss of mass is shown in Figure 4. The graph shows the change in the mass of the sorbent sample during the reactivity test. In the first phase of the test, the sorbent sample is calcined. The sorbent sample is exposed to high temperature in an atmosphere of N2, CO2, and O2 gases. After calcination is complete, SO2 is added to the atmosphere in which the sample is located and the sulfation step is started, which is seen as the weight of the sample increases. The sulfation process took 60 min. In Figure 4, an exemplary mass change curve during the reactivity test using the example of an unmodified sorbent is shown. The initial sample weight and the final sample weight are needed to calculate the reactivity coefficient RI [mol/mol] and the absolute sorption coefficient CI [g S/kg sorbent].
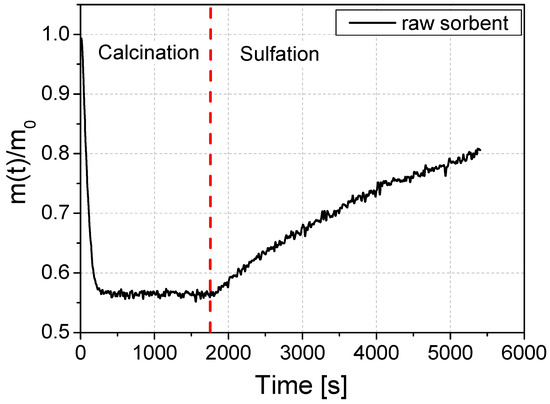
Figure 4.
An example of a course of mass change during the reactivity test on the example of a raw sorbent sample.
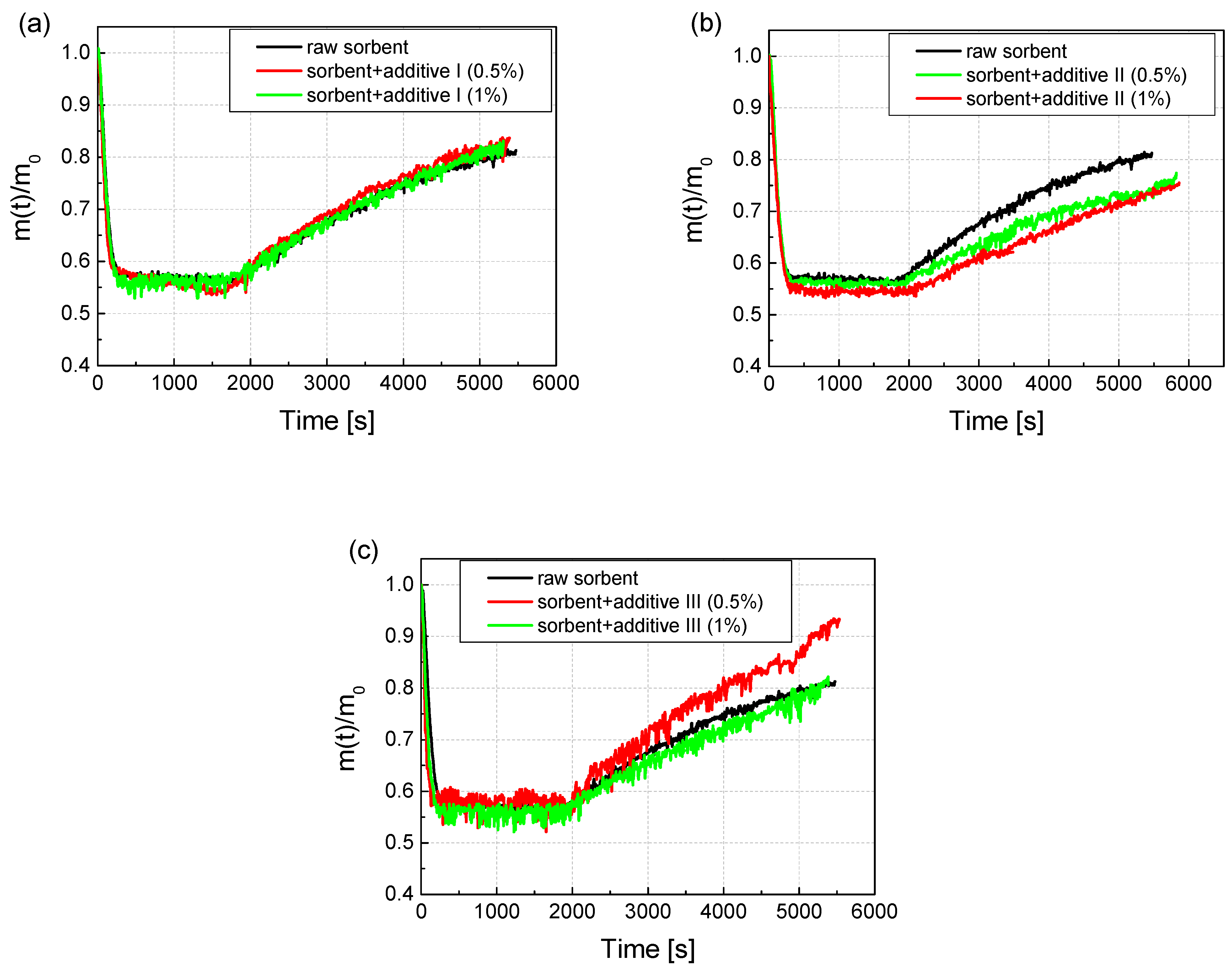
The raw sorbent samples were modified by adding Additive 1 in the amount of 0.5% and 1%. Additive 1 was Na2CO3, Additive 2 was LiOH, and Additive 3 was Li2CO3. The test was carried out for the concentration of gases provided in the test of the reactivity of calcium sorbents: CO2: 16%; SO2: 1870 ppm; O2: 3%; the rest was N2. The furnace was heated to the temperature of 850 °C. After the addition of the additive, the sorbents were intensively mixed, and the samples obtained in this way were subjected to the reactivity test as in the case of the raw sorbent sample. The weight change results recorded during the reactivity test are shown in Figure 5a. It can be concluded from the course of the test that the addition of Additive 1 in the amount of 0.5% and 1% improved the reactivity of the sorbent. The RI ratio increased relative to the raw sorbent by more than 14% with the addition of 1%, and by more than 15% with the addition of Additive 1 in the amount of 0.5%. In the case of the CI coefficient, this increase was even higher and amounted to over 24% for the additive in the amount of 1% and almost 18% for the additive in the amount of 0.5%. The addition of Additive 2 to the sorbent in the amount of both 0.5% and 1% resulted in a smaller increase in the mass of the sample during the sulfation phase, which is visible in Figure 5b. The lower weight gain caused by the lower amount of adsorbed SO2 resulted in a reduction in the RI coefficient relative to the raw sorbent by 10% for the addition of 1% and by 2% for the addition of 0.5%. For Additive 2, the results of the absolute sorption coefficient CI also decreased relative to the raw sorbent by 2.1% and 5.5%, respectively, for the additives 1% and 0.5%. In the case of Additive 3, the recorded changes in weight showed that depending on the amount of additive, the obtained results may differ significantly (Figure 5c). In the case of Additive 3 in the amount of 0.5%, the amount of adsorbed sulfur increased significantly by over 11%, which is visible in Figure 5c. However, in the case of adding 1% of Additive 3, the amount of adsorbed practical sulfur did not change (increase by 0.8%). Additionally, the CI increased by more than 20% with 0.5% of Additive 3, and with 1% of Additive 3, the increase was much smaller at 7.7%.
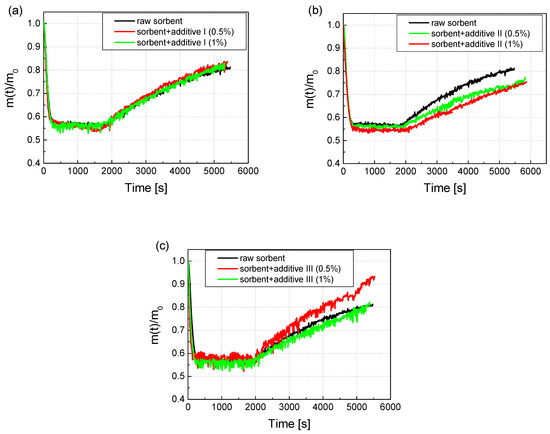
Figure 5.
The course of mass changes during the reactivity test for the raw sorbent, and (a) the sorbent modified with Additive 1 in the amount of 0.5% and 1%; (b) sorbent modified with Additive 2 in an amount of 0.5% and 1%; (c) sorbent modified with Additive 3 in an amount of 0.5% and 1%.
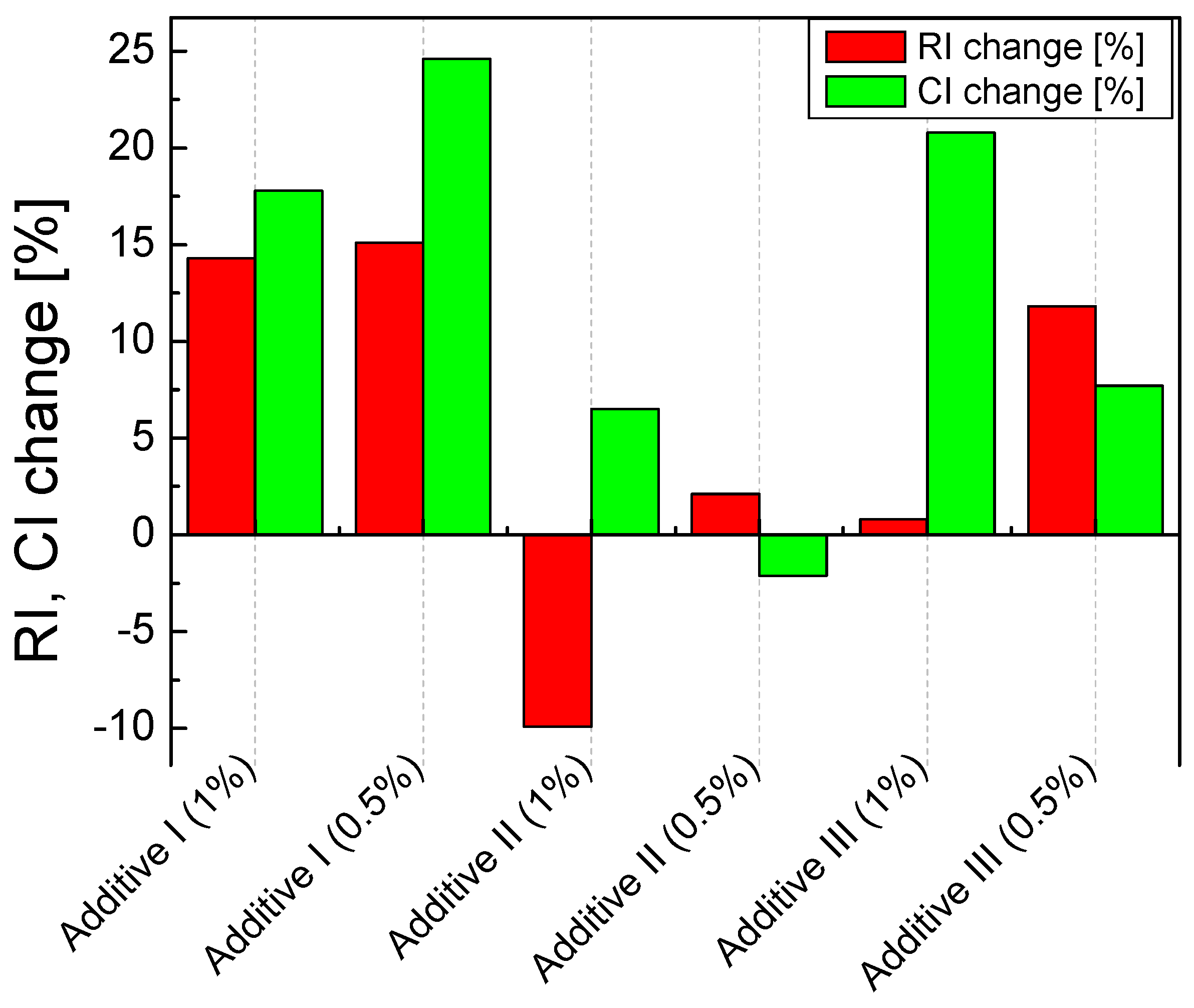
In Figure 6, the change in the RI coefficients values is shown, where the largest was associated with Additive 1 both in the amounts of 0.5 (by 15.1%) and 1% (by 14.3%). Then, the sorbent with Additive 3 in the amount of 0.5% (by 11.8%) also reacted positively. The same addition, but in the amount of 1%, practically did not change the RI index. The addition of Additive 2 to the sorbent decreased its reactivity. Similar changes in values were observed in the case of the absolute sorption coefficient CI. Additionally, in this case, Additive 1 performed the best, both in the amount of 0.5 (by 17.8%) and 1% (by 24.6%). Additive 3 also significantly improved the CI by 0.5% (by 20.8%). Additive 3 in the amount of 1% improved the CI only by 7.7%, while Additive 2 in both 0.5 and 1% resulted in a deterioration of the absolute sorption.
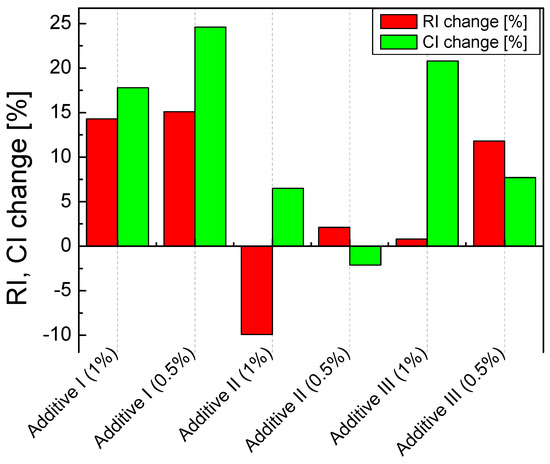
Figure 6.
Changes in the RI and CI coefficient depend on the type and amount of the additive.
The promoting effect of additives can be better understood given the role that contaminants play in the sulfation reaction. Slaughter et al. [29] explained the positive effect of the presence of sodium compounds, which react with CaO, causing physical changes in the structure and increasing the degree of surface development, thus increasing the number of contact points of calcium oxide with exhaust gases. On the other hand, a possible mechanism to promote the calcination and sulfation process proposed by Wang et al. [24] is based on the volatilization of alkali, the formation of sulfates, transport to the surface of the sorbent, and the formation of a fused layer due to the formed eutectic. According to these authors, the transport of SO2 and O2 to and through the melt was faster, which favors the improvement of the sulfation rate. Another possible interpretation of the effect of promoting pollutants (additives) was given by Borgwardt [20]. The author believes that the effect is a consequence of increased solid-state diffusion by foreign ions (improved ion exchange). This causes network defects and promotes the movement of ions.
3.2. Study on the Behavior of Sorbents under Simultaneous Calcination and Sulfation (SCS)
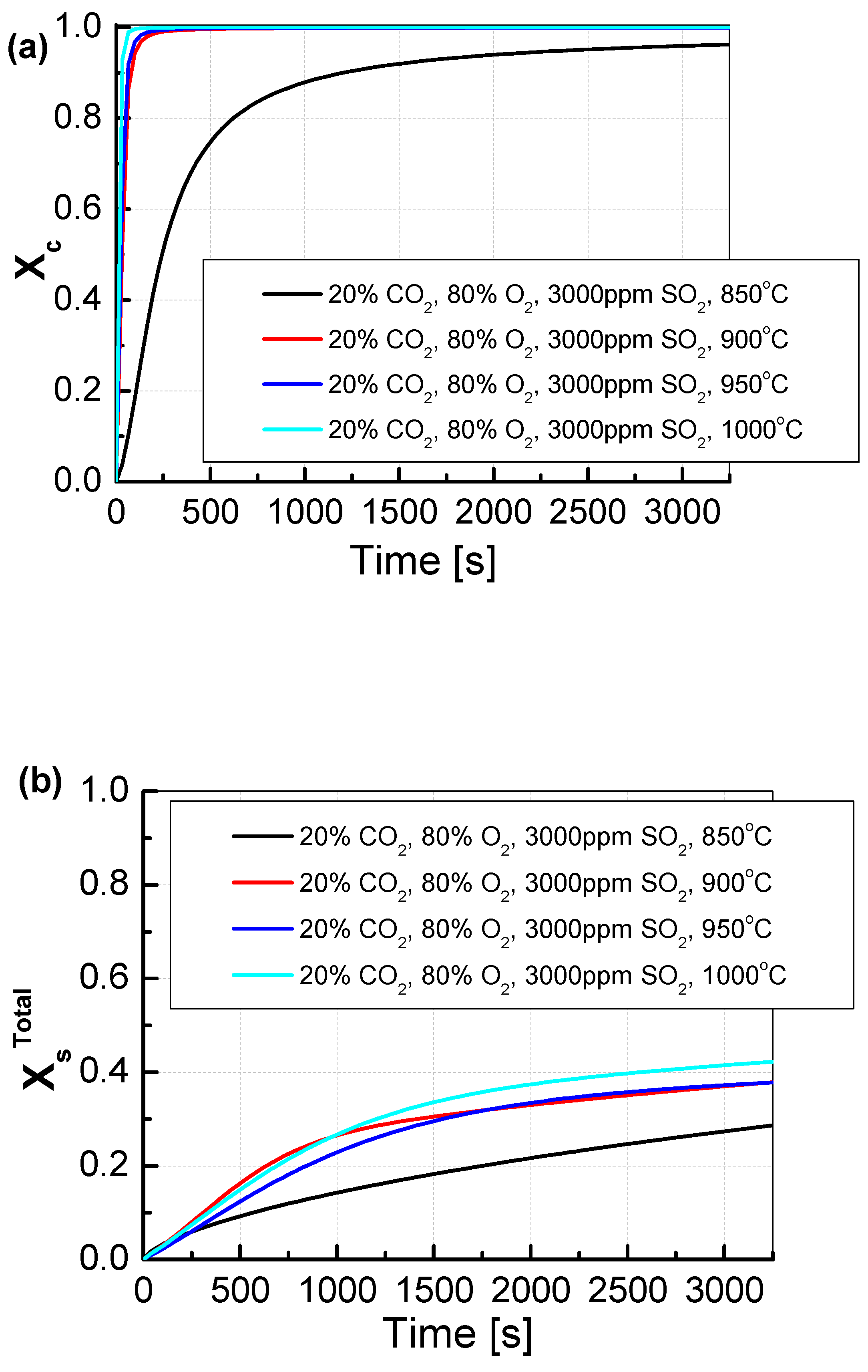
The sorbents were tested under conditions of simultaneous calcination and sulfation (SCS). In this test, in contrast to the classic test of the reactivity of calcium sorbents, during which the calcination and sulfation processes are carried out separately, the reactivity of sorbents in the exhaust gas containing SO2 and CO2 was examined. The simultaneous calcination and sulfation test allowed for more accurate reproduction of the conditions prevailing in a fluidized bed boiler, where the sorbent is fed directly with the fuel to the combustion chamber, and the calcination and sulfation processes run simultaneously [30]. For CO2/O2 shares of 20/80% (Figure 7a), it can be observed that the Xc calcination for the temperature of 850 °C proceeds practically throughout the entire test. As the calcination front proceeded, the sulfation front also ran, which is shown in Figure 7b. Sulfation proceeded at the same pace throughout the test and reached approximately 30%. At higher temperatures, different behaviors of the sorbent could be observed. Already at a temperature of 900 °C, the sorbent calcined in 100% in less than 500 s, which was also reflected in the sulfation process, which took place faster than at lower temperatures and reached a maximum of about 38%. At the temperatures of 950 °C and 1000 °C, the calcination process was even faster than at the temperature of 900 °C, in less than 100 s. The degree of sulfation for the temperature of 950 °C was very similar to that for the temperature of 900 °C, with higher dynamics in the range of 500–1500 s. For the temperature of 1000 °C, the degree of sulfation up to 1000 was the same as for lower temperatures, and after this time, it reached higher values and the maximum sulfation was 42%.
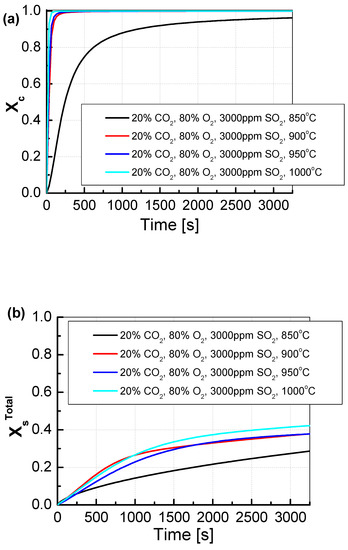
Figure 7.
Comparison of the results of approximation of (a) the degree of calcination Xc and (b) the total degree of XsTotal sulfation determined depending on the volume fraction of CO2 in synthetic exhaust containing 20% CO2 and 3000 ppm SO2.
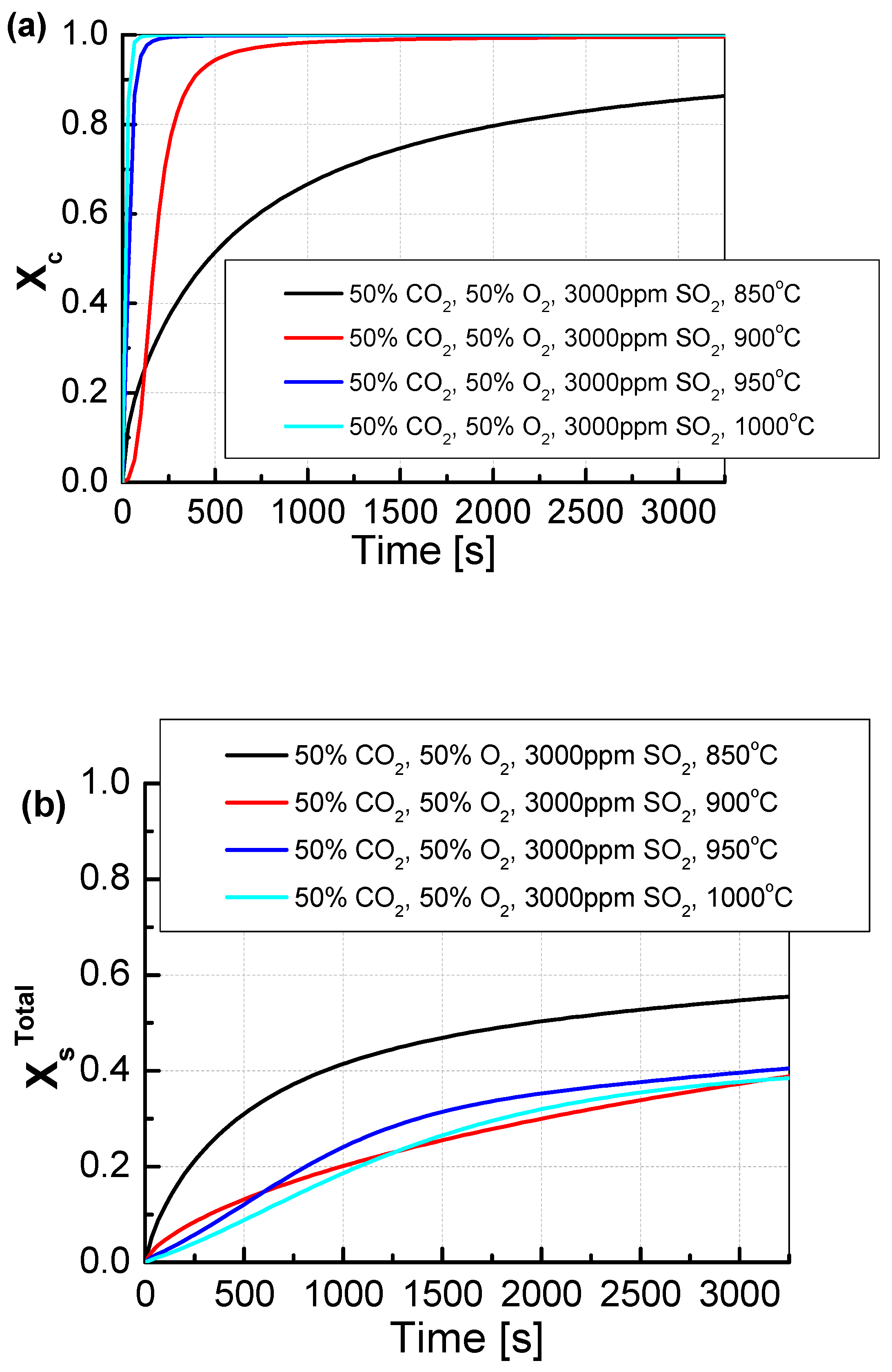
The change in CO2 and O2 shares from 20/80 to 50/50% resulted in a change in the mechanism in both the calcination and sulfation of the tested sorbent samples (Figure 8). The increase in CO2 concentration at the temperature of 850 °C caused a decrease in the dynamics of calcination and the maximum degree of sorbent calcination, which was approximately 85%. The decrease in the calcination dynamics was caused by the increased concentration of CO2 in the atmosphere surrounding the sorbent grains. The change in calcination dynamics did not cause the deterioration of the sulfation degree, which increased to 58%. The change in calcination dynamics was particularly noticeable at the temperature of 900 °C, as the sorbent reached its maximum calcination only after 2000 s. In contrast to the temperature of 850 °C at 900 °C, the degree of sulfation practically did not change and at the end of the measurement, it was about 40%. At the two higher temperatures, 950 °C and 1000 °C, the calcination was slightly slower than for the lower CO2 concentration. Furthermore, the degree of sulfation changed slightly.
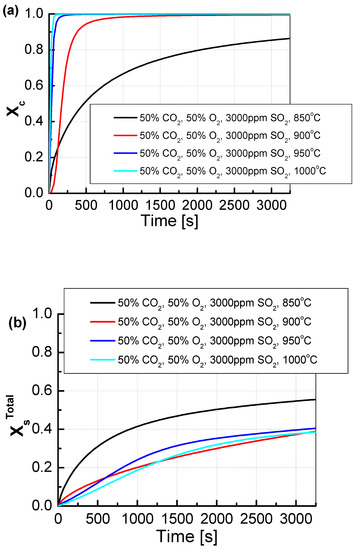
Figure 8.
Comparison of the results of the approximation of (a) the degree of calcination Xc and (b) the total degree of XsTotal sulfation determined depending on the volume fraction of CO2 in synthetic exhaust containing 50% CO2 and 3000 ppm SO2.
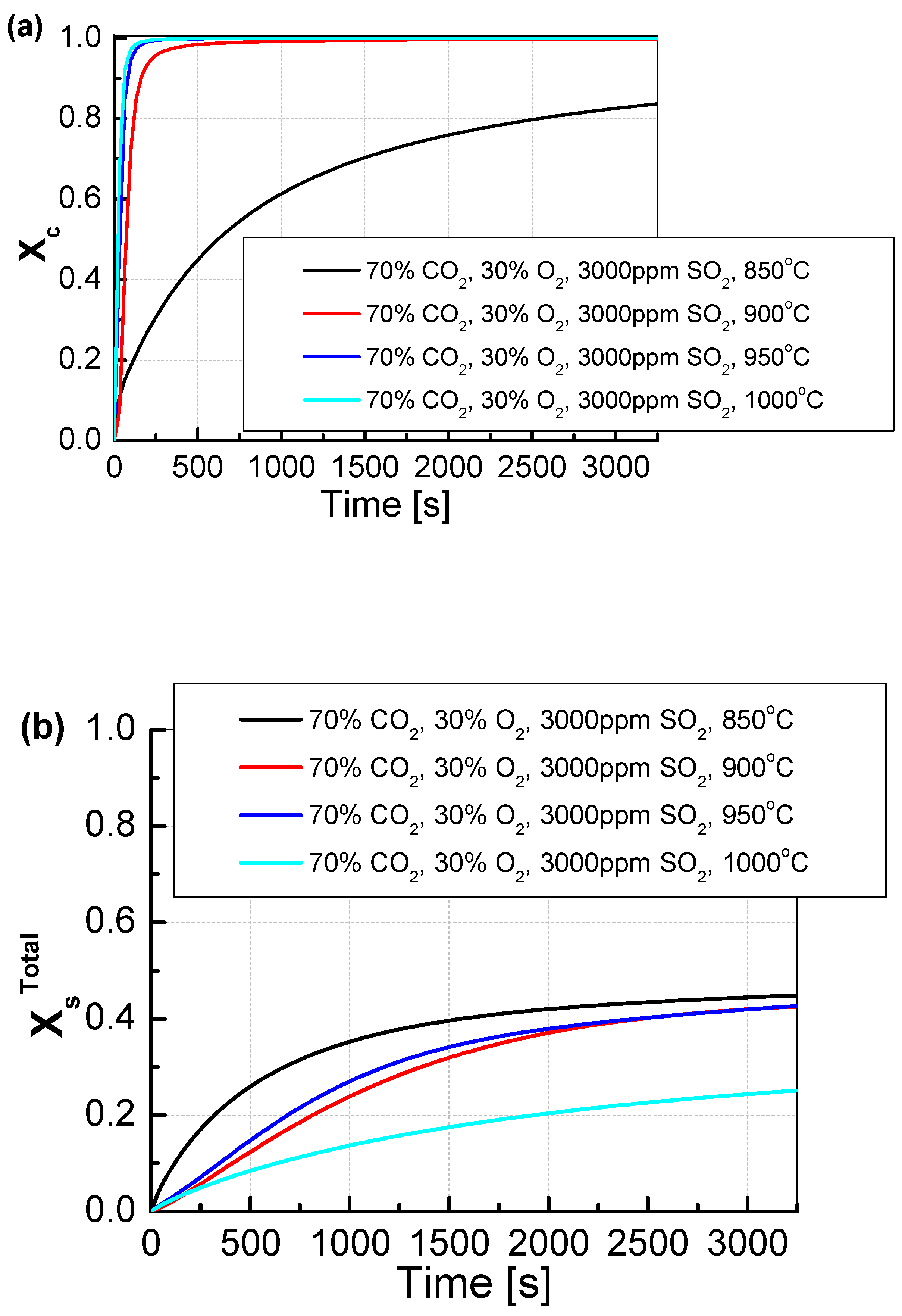
For a CO2/O2 concentration of 70/30%, the degree of calcination, regardless of temperature, practically did not change (Figure 9). The degree of sulfation only changed for the two extreme temperatures of 850 °C and 1000 °C. In both of these cases, there was a decrease in the degree of sulfation, which was most likely caused by a very high degree of CO2 concentration in the pores of the sorbent. In the case of high measurement temperature (here 1000 °C), the change in the open porosity of the sorbent, which probably resulted from the appearance of sinters and the limitation of the contact surface of the sorbent with the flue gas, had an additional impact on the results of sulfation. The degree of sulfation for the temperatures of 900 °C and 950 °C did not change significantly.
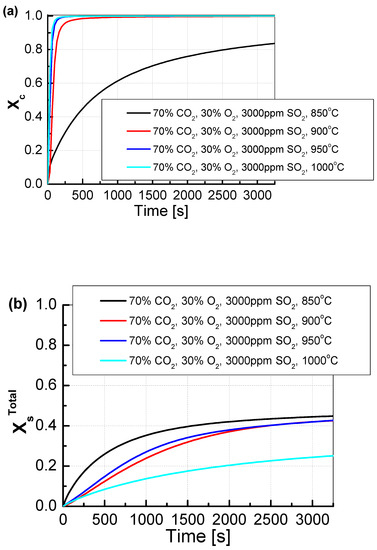
Figure 9.
Comparison of the results of the approximation of (a) the degree of calcination Xc and (b) the total degree of XsTotal sulfation determined depending on the volume fraction of CO2 in synthetic exhaust containing 70% CO2 and 3000 ppm SO2.
In Table 6, the results of the calculations of reactivity and absolute sorption for the tested sorbents are presented. For the CO2 concentration of 20%, the RI coefficient decreased with increasing temperature: for the temperature of 850 °C, it was 3.06, and for the temperature of 1000 °C, it was 2.37. Along with the decrease in the RI coefficient, the CI coefficient increased from 102.8 (850 °C) to 132.4 (1000 °C). The improvement in reactivity could be explained by the faster and more complete calcination of the sorbent, which allowed more SO2 to be adsorbed. For the concentration of 50/50% CO2/O2, the lowest RI result was achieved for the temperature of 850 °C—1.7 (CI—185.2), at the next tested temperature of 900 °C, the RI coefficients were significantly lower and amounted to 2.66 (CI—118.1), in subsequent temperatures, this result decreased and for 1000 °C, it amounted to 2.55 (CI—123.3). In the highest tested CO2 concentration, 70%, the RI coefficient again reached the lowest value for the lowest temperature of 850 °C (RI—1.86, CI—169.2); in subsequent temperatures, the RI values increased to reach the RI value of 4.33 at the highest temperature (CI—72.5), which corresponded to the very low degree of sulfation visible in Figure 8. It can be assumed that the reason for this was the high temperature that melted the outer surface of the sorbent grains, and at the same time, the high concentration of CO2 in the vicinity of the sample hindered the process of sorbent calcination.

Table 6.
Calculated coefficients of reactivity and absolute sorption for the tested sorbent samples.
To understand the factors influencing the mechanism of the simultaneous calcination and sulfation process, the analysis of the changes in the porosity of sorbents after the SCS process and in the subsequent time intervals of the process for the selected temperature of 900 °C was performed. The intensity of adsorption processes is determined by the micropores (d < 0.002 µm) and mesopores (d = 0.002–0.050 µm) [30]. An important factor in adsorption processes is open porosity, which consists of intergranular and intra-grain porosity [31,32]. Table 7 presents the values of the parameters estimated for the tested sorbent in its initial (raw) state and the subsequent stages of the calcination process with simultaneous sulfation under oxy-combustion conditions. The conditions of 20% CO2 + 80% O2 + 3000 ppm (at T = 900 °C and for comparison at T = 850 °C, 950 °C, 1000 °C) were selected for the analysis of morphological changes of the sorbent. The increase in the porosity of sorbents after the SCS process at the tested temperatures (up to 950 °C) is a partial result of the decomposition of CaCO3, according to reaction (4). This was confirmed by the courses of changes in the SCS process presented in Figure 7b, based on which it was observed that increasing the process temperature to 900 °C and 950 °C accelerated the sulfation process compared to 850 °C. The positive effect of the binding of sulfur oxides (acceleration of reactions (5) and (6)) results from the catalyzing effect of temperature, which, by accelerating the decomposition of limestone, contributes to the surface changes of the sorbent by increasing the proportion of open pores, particularly the inter-grain porosity. The value of inter-grain porosity, defined as macropores occurring between micrometric single grains, is of decisive importance in sorption processes, especially where physical adsorption takes place [33]. At the temperature of 1000 °C, the post-process porosity value decreased to approximately 16%, of which only about 1.15% was the intergrain porosity. It was probably the result of creating a compact surface layer in the form of sinters. Regardless of the process temperature, mixture composition and changes in the surface layer caused by chemical and physical processes, the average particle diameter of the sorbents did not change and was above 140 µm (Table 7).

Table 7.
Change in the open porosity of calcium sorbents during the calcination process with simultaneous sulfation.
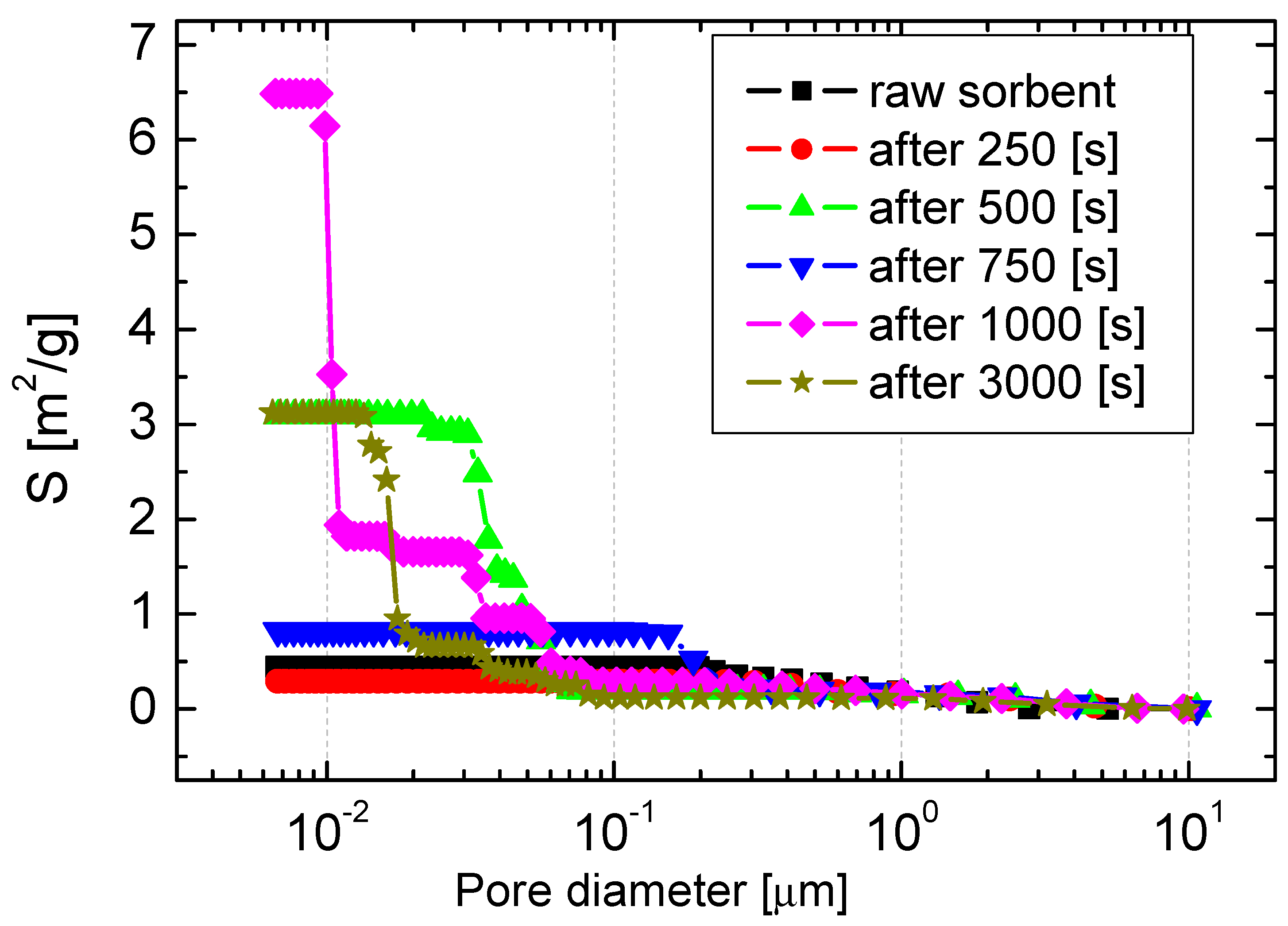
Figure 10 shows the distribution of the specific surface area of sorbents after exposure to the atmosphere: 20% CO2 + 80% O2 + 3000 ppm SO2, T = 900 °C. The highest value of the specific surface area was observed at 1000 s in the duration of the process. After 250 s, when the porosity increased almost four times, the surface area was reduced from 0.44 to 0.31 m2/g. After 1000 s, the process also showed the highest differentiation of the sorbent surface in terms of the size of the pores.
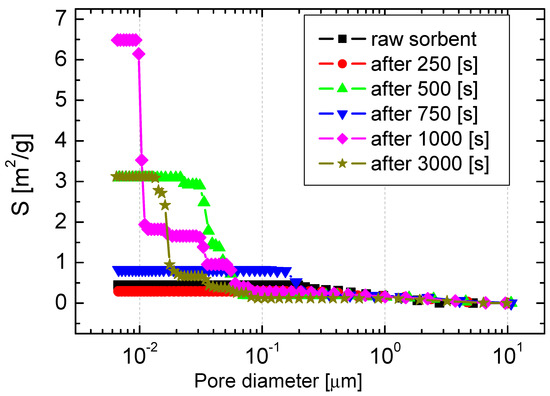
Figure 10.
The distribution of the specific surface area of sorbents after the simultaneous calcination and sulfation process.
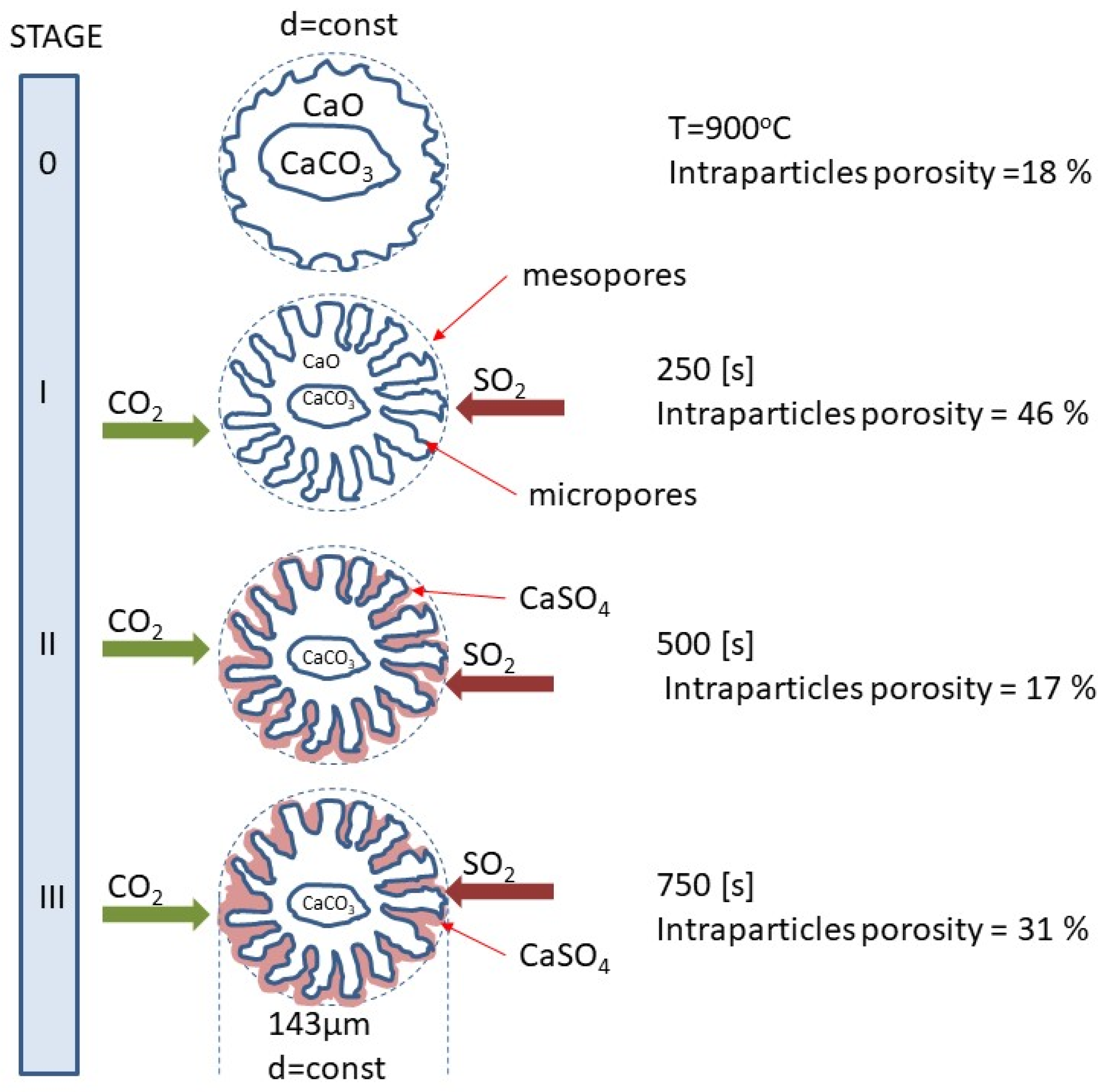
Figure 11 shows a diagram of the surface changes of calcium sorbents occurring in the subsequent stages of the calcination and sulfation process during oxy-combustion, proposed based on the observed changes in porosity. By analyzing the changes that took place on the sorbent surface during the SCS process in a mixture of 20% CO2 + 80% O2 + 3000 ppm at T = 900 °C from the beginning of the process (raw) to approximately 750 s. An increase in open porosity and an initial increase in intergranular porosity were observed. This time range corresponds to the calcination process described by Equation (4) as the decomposition of limestone. In the next stage of the process, sulfation takes place, with the deposition of calcium sulfate on the top CaCO3 layer, which is accompanied by the closing of the pores and channels through which SO2 is supplied to the CaCO3 surface, which results in a decrease in the reactivity of the sorbent.
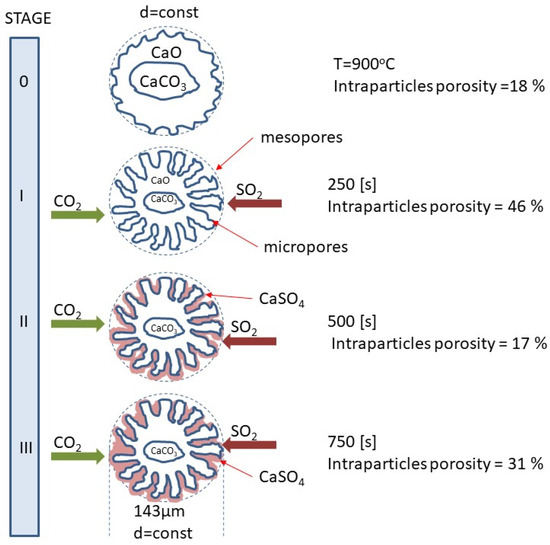
Figure 11.
Evolution of porosity changes in the sorbent grain during simultaneous calcination and sulfation under oxy-fuel combustion.
4. Conclusions
As part of the study of the reactivity of modified sorbents, an analysis of the effect of the quality of the additive and its quantity (percentage) in relation to the sorbent without (raw) additives was carried out. Modification of calcium sorbents by adding inorganic sodium and lithium compounds, regardless of the amount (0.5% or 1.0%), changes the reactivity coefficient RI [mol/mol], and the absolute sorption coefficient CI [g S/kg of sorbent]. The efficiency of the SCS process depends on the type of Na+ or Li+ cation and the anions forming the inorganic salt. Regardless of the amount of modifier added, in the case of the inorganic sodium salt (Additive 1), there was a visible improvement in the reactivity of the sorbent: 1.00% of the additive increased the RI index relative to the raw sorbent by over 14%, and in the case of the CI index by over 24%. The effect of additives that improve the desulfurization process, is to intensify the diffusion process taking place in the solid-state, which has a catalytic effect on the reactions.
Calcium sorbents also showed satisfactory reactivity in the SCS process, which better reflects the actual desulfurization process. The oxy-combustion process is a promising solution for the future management of CO2 captured from flue gas. The study analyzed the influence of the mixture composition and its temperature after recirculation on the reactivity of calcium sorbents. The process of simultaneous calcination and sulfation is a process controlled by chemical reactions (4–6) and physical phenomena, the intensity of which depends on the open porosity. As a result of the partial decomposition of CaCO3 after the SCS process in the studied temperature range, a positive effect of temperature on the surface changes in the sorbent was observed by increasing the open porosity, especially the increase in the share of intergrain porosity: increasing the process temperature to 900 °C and 950 °C accelerated the calcination and sulfation processes compared to 850 °C; at the temperature of 1000 °C, the calcination process took place in less than 100 s. An increase in CO2 concentration from 20% to 50% and 70% changed the dynamics of the calcination process, and its intensity decreased, which is probably related to the intensification of the reaction rate (4), during which an additional amount of CO2 is released, which inhibits the calcination process.
Author Contributions
Conceptualization, R.W. and M.W.; Methodology, M.W.; Formal analysis, R.W. and M.W.; Investigation, M.W. and R.W.; Writing—original draft preparation, R.W.; Writing—review and editing, M.W.; Visualization, M.W.; Supervision, R.W. All authors have read and agreed to the published version of the manuscript.
Funding
The scientific research was funded by the statute subvention of Czestochowa University of Technology, Faculty of Infrastructure and Environment. The research was funded by project no. BS/PB400/301/21.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| List of Symbols | |
| Ca0 | Calcium content in the sorbent, % |
| Ck | Carbon content in the final sample, % |
| η | Sorbent purity |
| MCa, MC | Molar mass of calcium and carbon, g/mol |
| mk, m0 | Final and initial sample weight, g |
| Ms | Molar mass of sulfur, g/mol |
| Sk | Carbon content in the final sample, % |
| Sp | Sulfur content in the initial sample, % |
| Xc | Degree of calcination |
| XsTotal | Degree of sulfation |
| Abbreviations | |
| CI | Absolute sorption coefficient |
| FGDs | Flue gas desulfurization |
| LOI | Loss on ignition |
| OFC | Oxy-fuel combustion |
| RI | Reactivity coefficient |
| SCS | Simultaneous calcination and sulfation |
| TSP | Total suspended particulate |
References
- Directive 2001/80/EC of the European Parliament and of the Council of 23 October 2001 on the Limitation of Emissions of Certain Pollutants into the Air from Large Combustion Plants, 27 January 2001. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2001.309.01.0001.01.ENG (accessed on 15 July 2021).
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control) (Recast), 17 November 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0075 (accessed on 15 July 2021).
- Pilch, M.; Skrzypek, J. Wykorzystanie wielosektorowych modeli makroekonomicznych w modelowaniu krajowych systemów energetycznych. Stud. Econ. Law 2017, CV, 311–339. (In Polish) [Google Scholar]
- Serwis Rzeczypospolitej Polskiej. Available online: https://www.gov.pl/web/srodowisko/czyste-powietrze (accessed on 15 July 2021).
- Galos, K.; Szlugaj, J.; Burkowicz, A. Sources of limestone sorbents for flue gas desulphurization in Poland in the context of the needs of domestic power industry. Polityka Energetyczna Energy Policy J. 2016, 19, 149–170. [Google Scholar]
- Szymanek, A. Odsiarczanie Spalin Metodami Suchymi. Available online: http://www.plan-rozwoju.pcz.pl/wyklady/ener_srod/ener_szy.pdf (accessed on 18 July 2021).
- Szuflickiego, M.; Malon, A.; Tymińskiego, M. (Eds.) Balance of Mineral. Resources in Poland as of 31 December 2014; Wyd. PIG-PIB: Warszawa, Poland, 2015. (In Polish) [Google Scholar]
- Sorbenty do Adsiarczania Spalin Metodami Wapienniczymi. Available online: http://phavi.wapno-info.pl/at/attachments/2012/1206/120054-sorbenty-do-odsiarczania-spalin-metodami-wapienniczymi.pdf (accessed on 20 July 2021).
- Wyszomirski, P.; Galos, K. Mineral and Chemical Raw Materials of the Ceramics Industry; AGH Scientific Educational Pub: Kraków, Poland, 2007. (In Polish) [Google Scholar]
- Szmigielska, E.; Głomba, M. Physico-chemical analysis of limestones used in energy flue gas desulphurization technologies. In Proceedings of the Materials of the 11th Conference POL-EMIS 2012, Atmospheric Air Protection, Sienna-Czarna Góra, Poland, 13–16 June 2012. (In Polish). [Google Scholar]
- Roszczynialski, W.; Gawlicki, M. Directions for the management of flue gas desulphurization products. In Proceedings of the 7th Conference on “Current Issues and Prospects of Mineral Resources Management”, Polanica Zdrój, Poland, 19–21 November 1997. (In Polish). [Google Scholar]
- Knura, P. Semi-dry flue gas desulphurization method using a pneumatic reactor integrated with a fabric filter (RP + FT method)—Directions of technology development, potential and possibilities. In Proceedings of the 2nd Conference of Electricity Producers, Skawina, Poland, 28–30 September 2011. (In Polish). [Google Scholar]
- Demir, I.; Hughes, R.E.; DeMaris, P.J. Formation and use of coal combustion residues from three types of power plants burning Illinois coals. Fuel 2001, 80, 659–1673. [Google Scholar] [CrossRef]
- Włodarczyk, R.; Wichliński, M. Flue gas desulphurization with the use of calcium sorbents modified with active metal salts. Przemysł Chem. 2019, 98, 1272–1275. (In Polish) [Google Scholar]
- Wu, Y.H.; Anthony, E.J.; Jia, L. Experimental Studies on Hydration of Partially Sulphated CFBC Ash. Can. J. Chem. Eng. 2003, 81, 1200–1214. [Google Scholar] [CrossRef]
- Wang, C.; Shen, X.; Xu, Y. Investigation on sulfation of modified Ca-based sorbent. Fuel Process. Technol. 2002, 79, 121–133. [Google Scholar] [CrossRef]
- Moe, T.A.; Mann, M.D.; Hajicek, D.H.; Weiss, A.J. Demonstration of pelletized fly ash reinjection for reduction of limestones consumption and ash disposal. In Proceedings of the 13th International Conference on Fluidized Bed Combustion: FBC, Technology of Choice, Orlando, Florida, 7–10 May 1995; Volume 2, pp. 1267–1274. [Google Scholar]
- Montagnaro, F.; Salatino, P.; Scala, F. The influence of temperature on limestone sulfation and attrition under fluidized bed combustion conditions. Exp. Therm. Fluid Sci. 2010, 34, 352–358. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Fernandez, M.J. The effect of metallic salt additives on direct sulfation of calcium carbonate and on decomposition of sulfated samples. Thermochim. Acta 1996, 276, 257–263. [Google Scholar] [CrossRef]
- Borgwardt, R.H.; Bruce, K.R.; Blake, J. An investigation of product-layer diffusivity for calcium oxide sulfation. Ind. Eng. Chem. Res. 1987, 26, 1993. [Google Scholar] [CrossRef]
- Davini, P.; DeMichele, G.; Ghetti, P. An investigation of the influence of sodium chloride on the desulphurization properties of limestone. Fuel 1992, 7l, 831. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Fernandez, M.J. Sulfation of dolomite particles at high CO2 partial pressures. Thermochim. Acta 1995, 254, 63–78. [Google Scholar] [CrossRef]
- Chen, J.; Yao, H.; Zhang, L. A study on the calcination and sulphation behaviour of limestone during oxy-fuel combustion. Fuel 2012, 102, 386. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Jia, L.; Tan, Y. Simultaneous calcination and sulfation of limestone in CFBB. Appl. Energy 2015, 155, 478. [Google Scholar] [CrossRef]
- Zhou, S.; Hossseini, T.; Zhang, X.; Haque, N.; Zhang, L. Selective removal of sodium and calcium from low-rank coal–Process integration, simulation and techno-economic evaluation. Fuel Process. Technol. 2018, 172, 13. [Google Scholar] [CrossRef]
- Ostergaard, M.B.; Petersen, R.R.; Konig, J.; Bockowski, M.; Yue, Y.J. Impact of gas composition on thermal conductivity of glass foams prepared via highpressure sintering. J. Non Cryst. Solids 2019, X1, 100014. [Google Scholar] [CrossRef]
- Włodarczyk, R.; Wichliński, M.; Bis, Z. Zmiana porowatości sorbentu wapniowego podczas jednoczesnej kalcynacji i siarczanowania w procesie oksyspalania. E3S Web Conf. 2018, 49, 00131. [Google Scholar] [CrossRef] [Green Version]
- Olas, M. Reactivity of Calcium Sorbents Subjected to Mechanical Activation. Ph.D. Thesis, Czestochowa University of Technology, Częstochowa, Poland, 2006. (In Polish). [Google Scholar]
- Slaughter, D.M.; Chen, S.L.; Seeker, W.R.; Pershing, D.W.; Kirchgessner, D.A. Increased SO2 removal with the addition of alkali metals and chromium to calcium-based sorbents. In Proceedings of the 22nd International Symposium on Combustion, The Combustion Institute, Seattle, WA, USA, 14–19 August 1988; p. 1155. [Google Scholar]
- Wichliński, M.; Włodarczyk, R. Porosity Changes in Sorbents during Simultaneous Calcination and Sulphation for Oxy-Fuel Combustion. Przemysł Chem. 2020, 99, 560–563. [Google Scholar]
- Liu, C.J.; Wang, G.X.; Sang, S.X.; Rudolph, V. Changes in pore structure of anthracite coal associated with CO2 sequestration process. Fuel 2010, 89, 2665. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry Home Page. Available online: www.iupac.org (accessed on 25 July 2021).
- Lacaze, J.; Arnal, A.; Dupuy, J.-L.; Poquillion, D. Separation of the intra-particle porosity in images of powder compacts. Image Anal. Stereol. 2002, 21, 183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).