Influence of the Addition of Blast Furnace Slag to Alkali-Activated Mixtures Based on Natural Zeolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
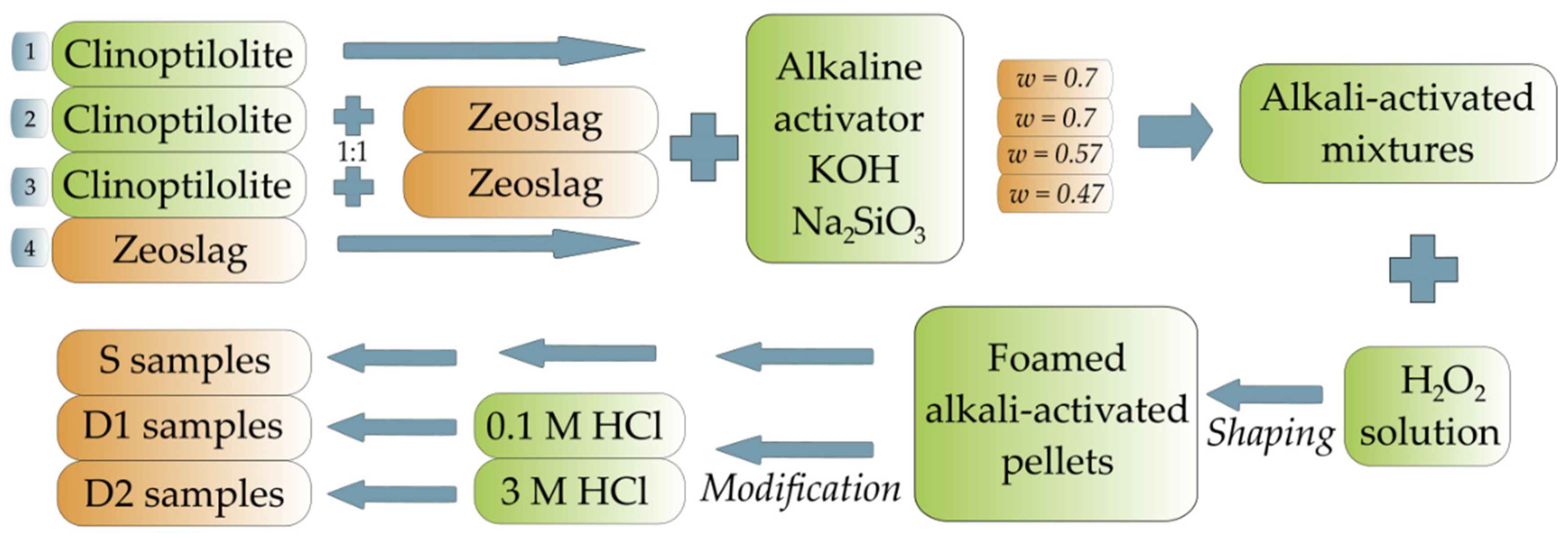
2.2. Synthesis of Alkali-Activated Zeolite Foams
2.3. Post-Synthesis Modifications of Alkali-Activated Zeolite Foams
2.4. Characterization Techniques
3. Results and Discussion
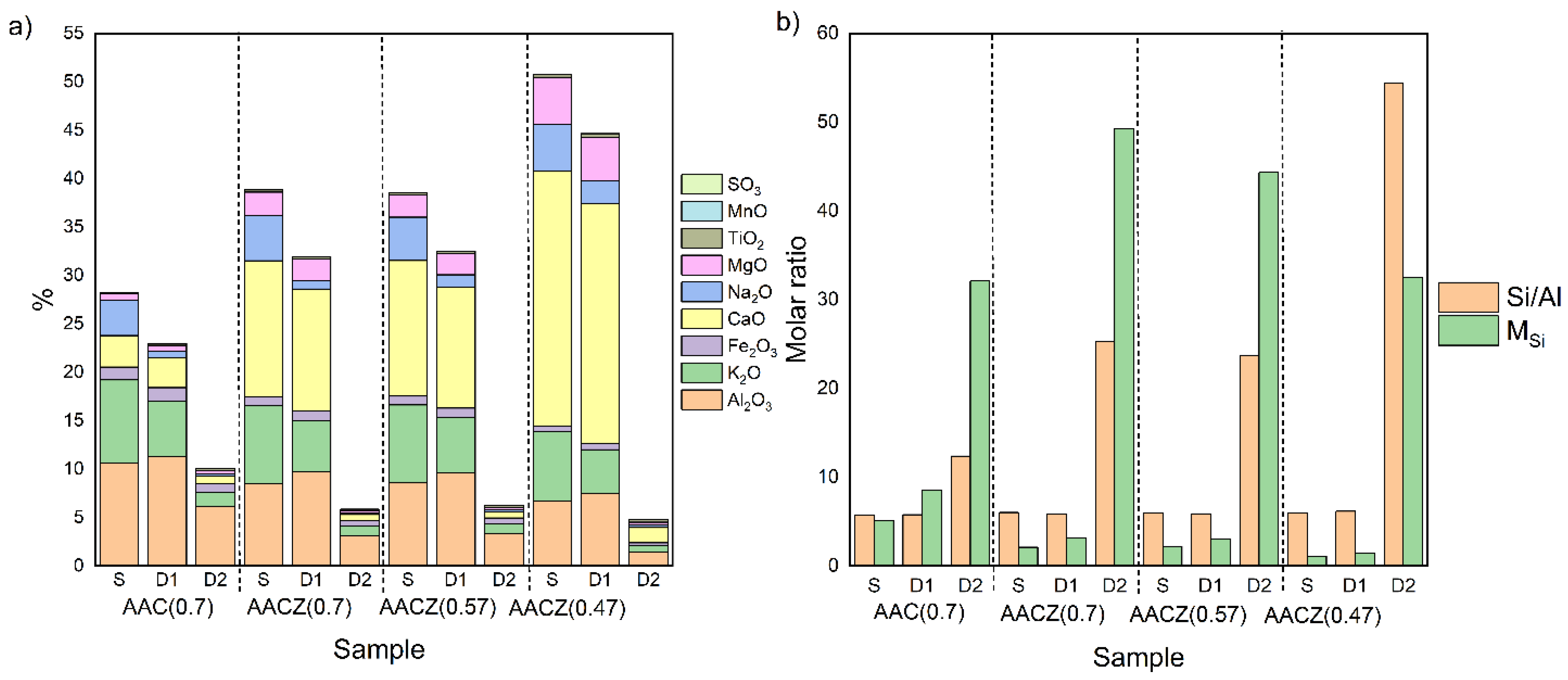
3.1. Elemental Composition
X-ray Fluorescence Analysis (XRF)
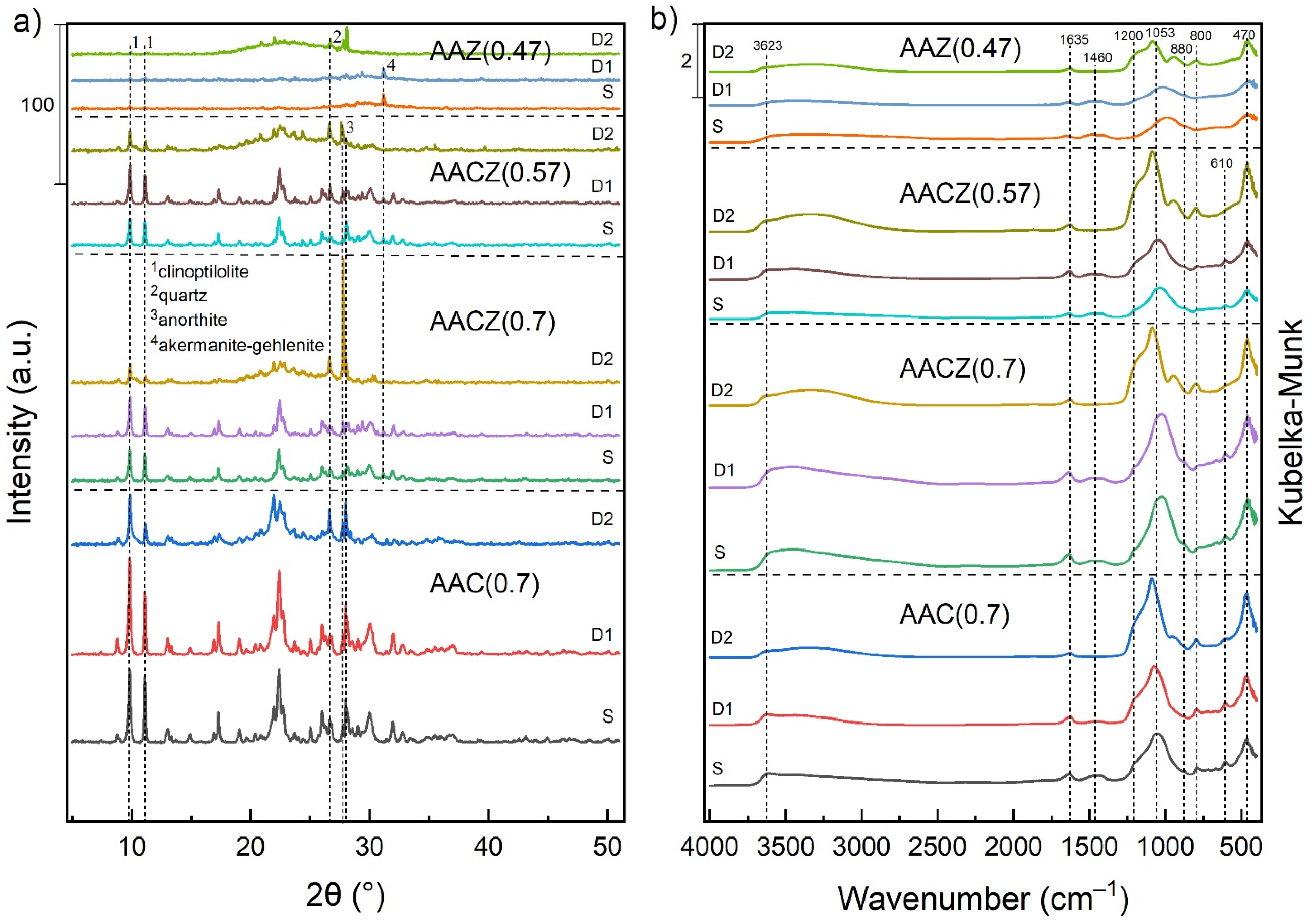
3.2. Crystalline Structure
3.2.1. X-ray Powder Diffraction (XRD)
3.2.2. Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFT)
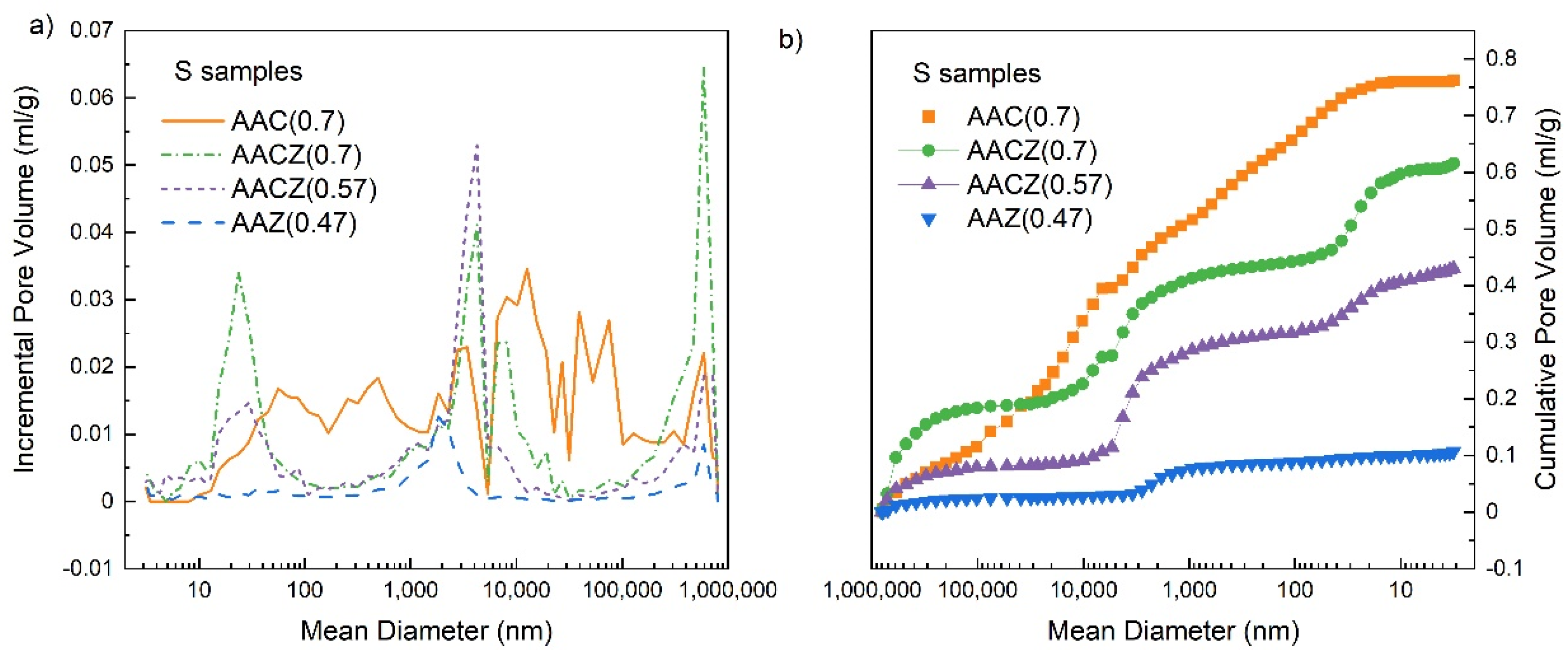
3.3. Porosity and Strength
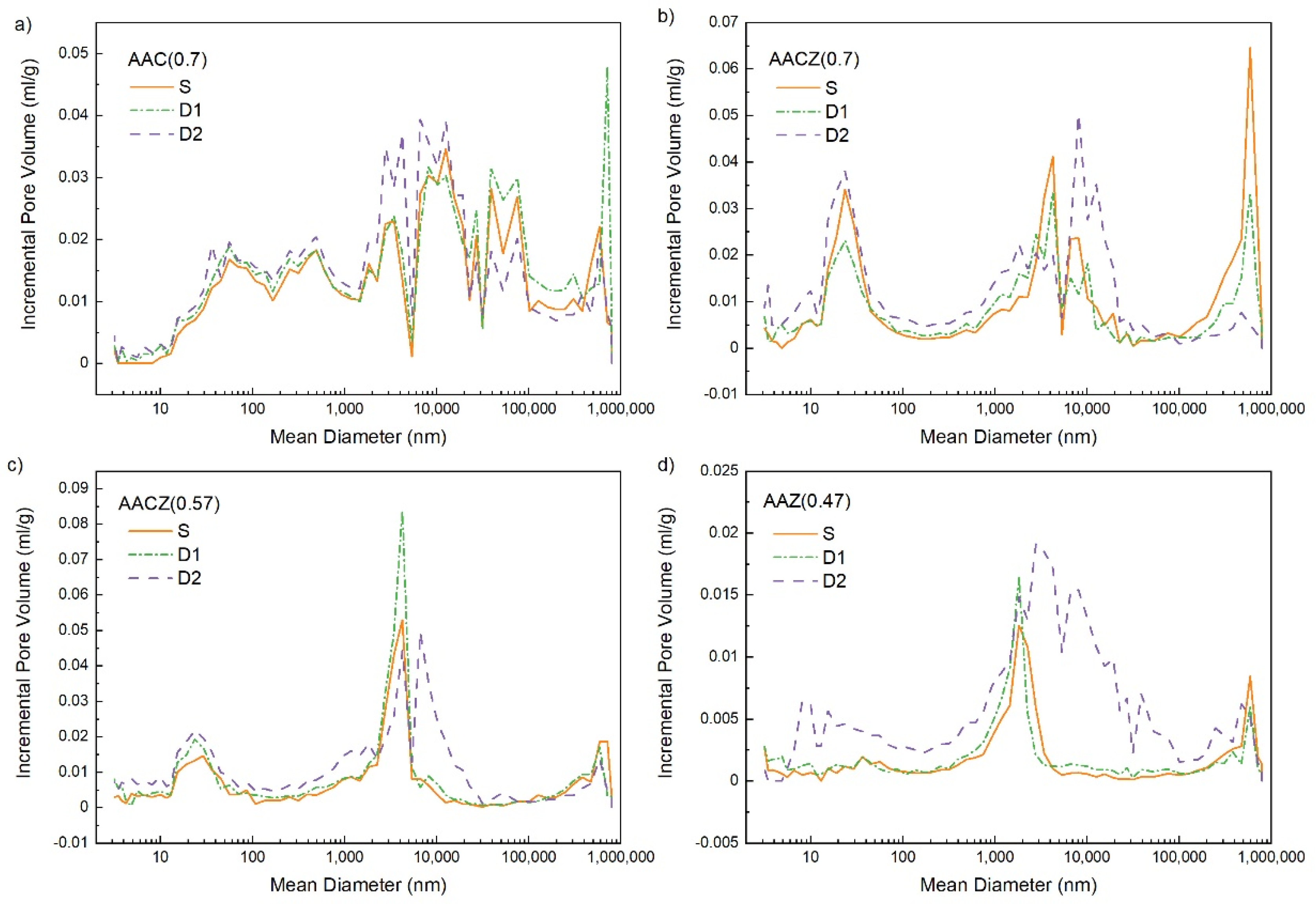
3.3.1. Mercury Porosimetry (Hg Porosimetry)
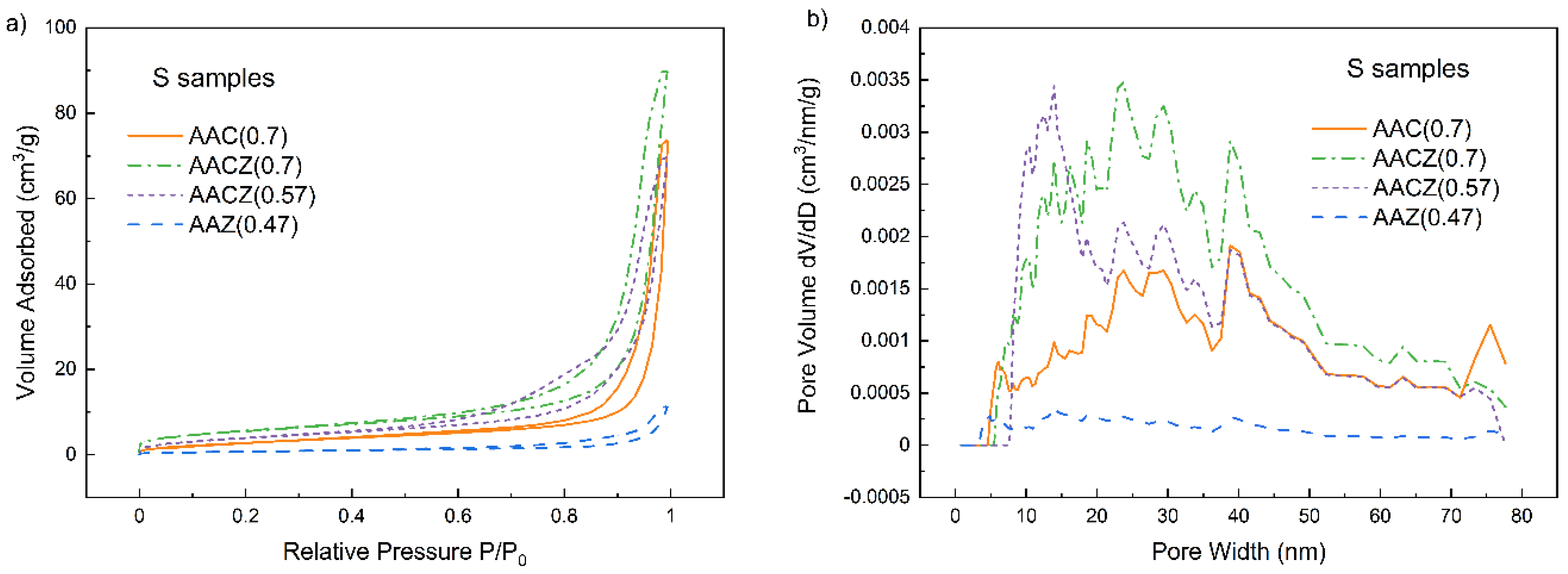
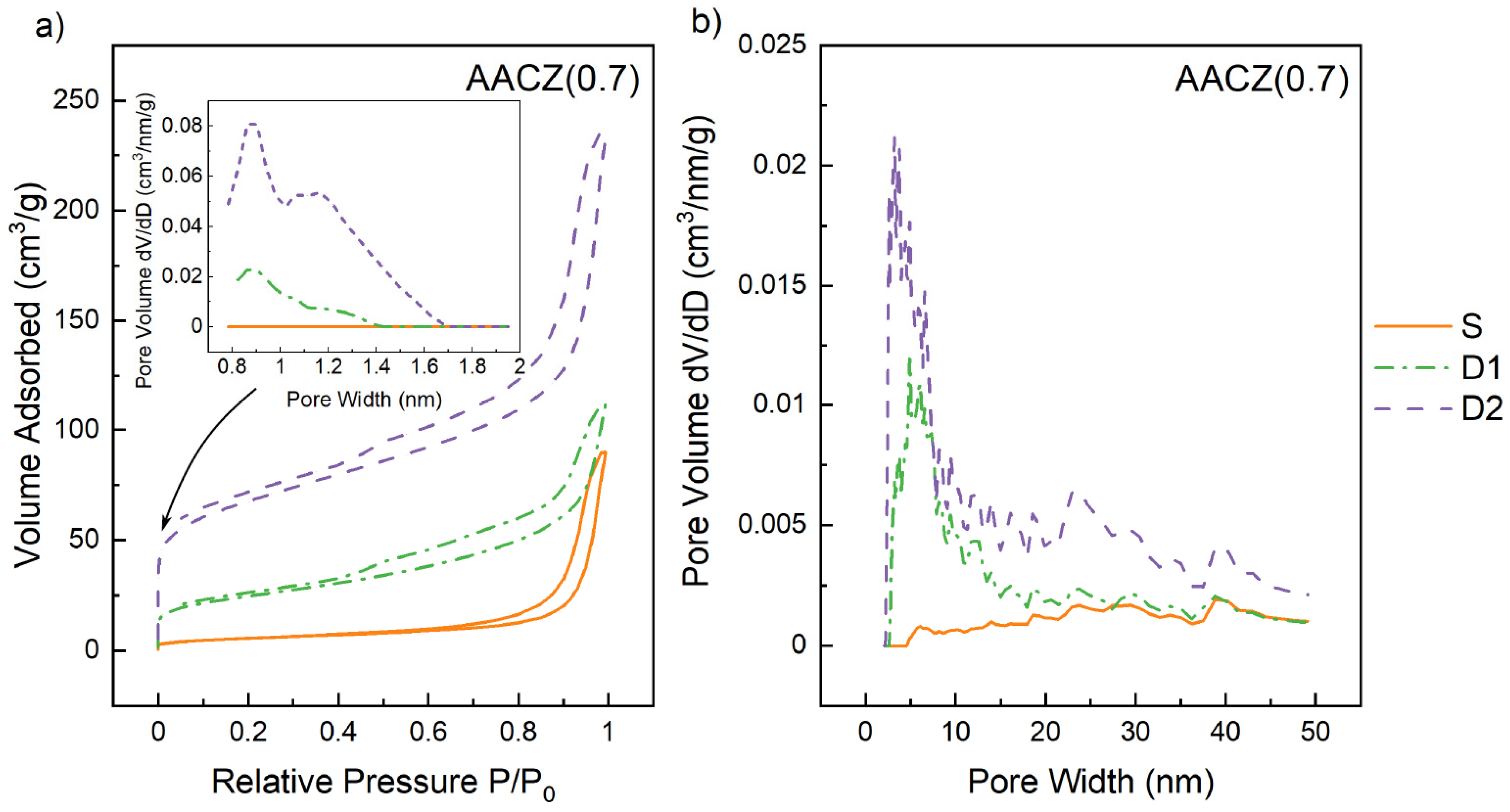
3.3.2. N2 Physisorption
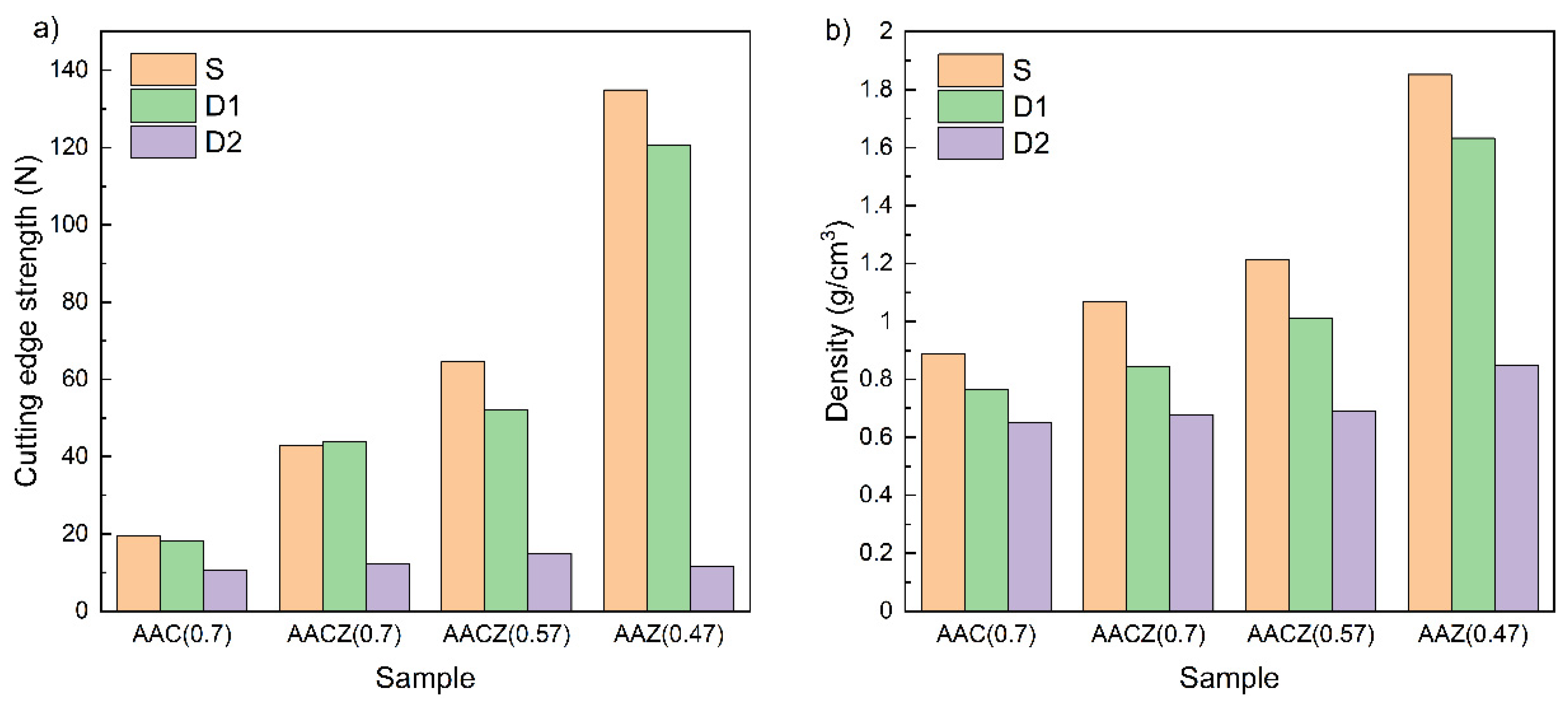
3.3.3. Cutting Edge Strength
3.4. Adsorption Properties
Thermogravimetric Analysis (TGA) CO2 Adsorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sample | SiO2 | Al2O3 | K2O | Fe2O3 | CaO | Na2O | MgO | TiO2 | SUM | Si/Al 1 | MSi 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AAC (0.7)/S | 71.60 | 10.60 | 8.60 | 1.34 | 3.25 | 3.61 | 0.67 | 0.16 | 99.83 | 5.73 | 5.13 |
| AAC (0.7)/D1 | 76.70 | 11.30 | 5.68 | 1.43 | 3.05 | 0.67 | 0.65 | 0.20 | 99.68 | 5.76 | 8.48 |
| AAC (0.7)/D2 | 89.90 | 6.17 | 1.44 | 0.89 | 0.76 | 0.26 | 0.32 | 0.19 | 99.93 | 12.36 | 32.11 |
| AACZ (0.7)/S | 60.30 | 8.51 | 8.00 | 0.91 | 14.10 | 4.69 | 2.38 | 0.23 | 99.12 | 6.01 | 2.11 |
| AACZ (0.7)/D1 | 67.50 | 9.77 | 5.22 | 1.01 | 12.60 | 0.83 | 2.24 | 0.25 | 99.42 | 5.86 | 3.16 |
| AACZ (0.7)/D2 | 93.90 | 3.15 | 0.93 | 0.63 | 0.60 | 0.14 | 0.20 | 0.21 | 99.75 | 25.29 | 49.28 |
| AACZ (0.57)/S | 60.60 | 8.56 | 8.06 | 0.91 | 14.00 | 4.48 | 2.31 | 0.23 | 99.15 | 6.01 | 2.14 |
| AACZ (0.57)/D1 | 66.90 | 9.64 | 5.67 | 0.97 | 12.50 | 1.28 | 2.17 | 0.28 | 99.41 | 5.89 | 3.06 |
| AACZ (0.57)/D2 | 93.40 | 3.34 | 0.98 | 0.63 | 0.65 | 0.20 | 0.24 | 0.23 | 99.67 | 23.73 | 44.31 |
| AAZ (0.47)/S | 47.50 | 6.72 | 7.17 | 0.55 | 26.30 | 4.84 | 4.88 | 0.32 | 98.27 | 6.00 | 1.06 |
| AAZ (0.47)/D1 | 54.20 | 7.48 | 4.53 | 0.59 | 24.80 | 2.36 | 4.53 | 0.35 | 98.84 | 6.15 | 1.40 |
| AAZ (0.47)/D2 | 94.30 | 1.47 | 0.67 | 0.25 | 1.58 | 0.26 | 0.29 | 0.31 | 99.12 | 54.43 | 32.53 |
References
- Tišler, Z.; Horáček, J.; Šafář, J.; Velvarská, R.; Pelíšková, L.; Kocik, J.; Gherib, Y.; Marklova, K.; Bulanek, R.; Kubicka, D. Clinoptilolite foams prepared by alkali activation of natural zeolite and their post-synthesis modifications. Microporous Mesoporous Mater. 2019, 282, 169–178. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mohamed, A.S. Adsorption Removal of Safranin Dye Contaminants from Water Using Various Types of Natural Zeolite. Silicon 2019, 11, 1635–1647. [Google Scholar] [CrossRef]
- Margeta, K.; Zabukovec Logar, N.; Šiljeg, M.; Farkaš, A. Natural Zeolites in Water Treatment—How Effective is Their Use. In Water Treatment; Elshorbagy, W., Kabir Chowdhury, R., Eds.; inTech: London, UK, 2013; pp. 81–112. [Google Scholar]
- Thi Tran, Y.; Lee, J.; Kumar, P.; Kim, K.H.; SooLee, S. Natural zeolite and its application in concrete composite production. Compos. Part B Eng. 2019, 165, 354–364. [Google Scholar] [CrossRef]
- Pavelić, S.K.; Medica, J.S.; Gumbarević, D.; Filošević, A.; Przulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of Natural Zeolite (Clinoptilolite) in Agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [Green Version]
- Hailu, Y.; Tilahun, E.; Brhane, A.; Resky, H.; Sahu, O. Ion exchanges process for calcium, magnesium and total hardness from ground water with natural zeolite. Groundw. Sustain. Dev. 2019, 8, 457–467. [Google Scholar] [CrossRef]
- Vocciante, M.; D’Auris, A.D.F.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Adsorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Yan, W.; Shi, W.; Xu, R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M.; Malinska, K. Removal of Ammonia by Clinoptilolite. Glob. Nest J. 2010, 12, 256–261. [Google Scholar] [CrossRef]
- Kannan, A.D.; Parameswaran, P. Ammonia adsorption and recovery from swine wastewater permeate using naturally occurring clinoptilolite. J. Water Process. Eng. 2021, 43, 10223. [Google Scholar] [CrossRef]
- Bilim, C. Properties of cement mortars containing clinoptilolite as a supplementary cementitious material. Constr. Build. Mater. 2011, 25, 3175–3180. [Google Scholar] [CrossRef]
- Burris, L.E.; Juenger, M.C.G. Milling as a pretreatment method for increasing the reactivity of natural zeolites for use as supplementary cementitious materials. Cem. Concr. Compos. 2016, 65, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Burris, L.E.; Juenger, M.C.G. Effect of calcination on the reactivity of natural clinoptilolite zeolites used as supplementary cementitious materials. Constr. Build. Mater. 2020, 258, 11998. [Google Scholar] [CrossRef]
- Faghihian, H.; Talebi, M.; Pirouzi, M. Adsorption of nitrogen from natural gas by clinoptilolite. J. Iran. Chem. Soc. 2008, 5, 394–399. [Google Scholar] [CrossRef]
- Hao, X.; Hu, H.; Li, Z.; Wu, L.; Liu, X.; Zhang, Y. Adsorption Properties of Modified Clinoptilolite for Methane and Nitrogen. Materials 2018, 11, 2024. [Google Scholar] [CrossRef] [Green Version]
- Wahono, S.K.; Prasetyo, D.J.; Jatmiko, T.H.; Pratiwi, D.; Suwanto, A.; Hernawan; Vasilev, K. Multi-stage dealumination for characteristic engineering of mordenite-clinoptilolite natural zeolite. AIP Conf. Proc. 2019, 2085, 020044. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Nikolov, A.; Rostovsky, I.; Nugteren, H. Geopolymer materials based on natural zeolite. Case Stud. Constr. Mater. 2017, 6, 198–205. [Google Scholar] [CrossRef]
- Tišler, Z.; Klegová, A.; Svobodová, E.; Šafář, J.; Strejcová, K.; Kohout, J.; Šlang, S.; Pacultová, K.; Rodríguez-Padrón, D.; Bulánek, R. Cobalt Based Catalysts on Alkali-Activated Zeolite Foams for N2O Decomposition. Catalysts 2020, 10, 1398. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Nikolov, A.; Nugteren, H.; Rostovsky, I. Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr. Build. Mater. 2020, 243, 11825. [Google Scholar] [CrossRef]
- Oguz, E. Removal of phosphate from aqueous solution with blast furnace slag. J. Hazard. Mater. 2004, 114, 131–137. [Google Scholar] [CrossRef]
- Pal, S.C.; Mukherjee, A.; Pathak, S.R. Investigation of hydraulic activity of ground granulated blast furnace slag in concrete. Cem. Concr. Res. 2003, 33, 1481–1486. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Shahmansouri, A.A.; Yazdani, M.; Ghanbari, S.; Akbarzadeh Bengar, H.; Jafari, A.; Ghatte, H.F. Artificial neural network model to predict the compressive strength of eco-friendly geopolymer concrete incorporating silica fume and natural zeolite. J. Clean. Prod. 2021, 279, 123697. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. A novel conversion process for waste slag: Synthesis of a hydrotalcite-like compound and zeolite from blast furnace slag and evaluation of adsorption capacities. J. Mater. Chem. 2010, 20, 5052–5062. [Google Scholar] [CrossRef]
- Hrachovcová, K.; Tišler, Z.; Svobodová, E.; Šafář, J. Modified Alkali Activated Zeolite Foams with Improved Textural and Mechanical Properties. Minerals 2020, 10, 483. [Google Scholar] [CrossRef]
- Beers, A.E.W.; van Bokhoven, J.A.; de Lathouder, K.M.; Kapteijn, F.; Moulijn, J.A. Optimization of zeolite Beta by steaming and acid leaching for the acylation of anisole with octanoic acid: A structure–activity relation. J. Catal. 2003, 2182, 239–248. [Google Scholar] [CrossRef]
- Stanciakova, K.; Ensing, B.; Göltl, F.; Bulo, R.E.; Weckhuysen, B.M. Cooperative Role of Water Molecules during the Initial Stage of Water-Induced Zeolite Dealumination. ACS Catal. 2019, 9, 5119–5135. [Google Scholar] [CrossRef]
- van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev.-Sci. Eng. 2003, 45, 297–319. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.A.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite-Clinoptilolite-As a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar] [CrossRef]
- Tišler, Z.; Hrachovcová, K.; Svobodová, E.; Šafář, J.; Pelíšková, L. Acid and Thermal Treatment of Alkali-Activated Zeolite Foams. Minerals 2019, 9, 719. [Google Scholar] [CrossRef] [Green Version]
- Elaiopoulos, K.; Perraki, T.; Grigoropoulou, E. Monitoring the effect of hydrothermal treatments on the structure of a natural zeolite through a combined XRD, FTIR, XRF, SEM and N2-porosimetry analysis. Microporous Mesoporous Mater. 2010, 134, 29–43. [Google Scholar] [CrossRef]
- Valdés, H.; Riquelme, A.L.; Solar, V.A.; Azzolina-Jury, F.; Thibault-Starzyk, F. Removal of chlorinated volatile organic compounds onto natural and Cu-modified zeolite: The role of chemical surface characteristics in the adsorption mechanism. Sep. Purif. Technol. 2021, 258, 11808. [Google Scholar] [CrossRef]
- Belmokhtar, N.; Ammari, M.; Brigui, J.; Ben Allal, L. Comparison of the microstructure and the compressive strength of two geopolymers derived from Metakaolin and an industrial sludge. Constr. Build. Mater. 2017, 146, 621–629. [Google Scholar] [CrossRef]
- Catauroa, M.; Papale, F.; Lamanna, G.; Bollino, F. Geopolymer/PEG Hybrid Materials Synthesis and Investigation of the Polymer Influence on Microstructure and Mechanical Behavior. Mater. Res. 2015, 18, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Perná, I.; Šupová, M.; Hanzlíček, T. The characterization of the Ca-K geopolymer/solidified fluid fly-ash interlayer. Ceramics-Silikáty 2017, 61, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Cakicioglu-Ozkan, F.; Ulku, S. The effect of HCl treatment on water vapor adsorption characteristics of clinoptilolite rich natural zeolite. Microporous Mesoporous Mater. 2005, 77, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Krivácsy, Z.; Hlavay, J. A simple method for the determination of clinoptilolite in natural zeolite rocks. Zelites 1995, 15, 551–555. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic polymers from alkali activation of metakaolin: Effect of setting and curing on structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B.B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transfer 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Schneider, D.; Attallah, A.G.; Wassersleben, S.; Wenzel, M.; Matysik, J.; Krause-Rehberg, R.; Enke, D. Advanced textural characterization of biogenic silica by nitrogen physisorption, positron annihilation lifetime spectroscopy and hyperpolarized 129Xe NMR spectroscopy. Microporous Mesoporous Mater. 2020, 307, 11051. [Google Scholar] [CrossRef]
- Thommes, K.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Jiang, Z.; Li, Z.; Gao, Z.; Bai, Y.; Zhao, S.; Feng, J. The effect of the variation in material composition on the heterogeneous pore structure of high-maturity shale of the Silurian Longmaxi formation in the southeastern Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2015, 23, 464–473. [Google Scholar] [CrossRef]
- Rachman, R.A.; Martia, U.T.I.; Aulia, W.; Iqbal, R.M.; Widiastuti, N.; Kurniawan, F. Combination of microbial fuel cell and zeolite Na-Y adsorption for chromium removal. AIP Conf. Proc. 2018, 2049, 020073. [Google Scholar] [CrossRef]
- Strejcová, K.; Tišler, Z.; Svobodová, E.; Velvarská, R. Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use. Molecules 2020, 25, 4989. [Google Scholar] [CrossRef]
- Silva, J.A.C.; Schumann, K.; Rodrigues, A.E. Sorption and kinetics of CO2 and CH4 in binderless beads of 13X zeolite. Microporous Mesoporous Mater. 2012, 158, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, H.N. Zinc hydroxide nitrate nanosheets conversion into hierarchical zeolitic imidazolate frameworks nanocomposite and their application for CO2 sorption. Mater. Today Chem. 2020, 15, 10022. [Google Scholar] [CrossRef]
- Tišler, Z.; Šafář, J.; Velvarská, R.; Pelíšková, L. Modifikované alkalicky aktivované zeolitové pěny: Příprava a charakterizace. Chem. Listy 2019, 113, 111–116. [Google Scholar]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef] [Green Version]
- Papa, E.; Medri, V.; Paillard, C.; Contri, B.; Natali Murri, A.; Vaccari, A.; Landi, E. Geopolymer-hydrotalcite composites for CO2 capture. J. Clean. Prod. 2019, 237, 11773. [Google Scholar] [CrossRef]
- Minelli, M.; Medri, V.; Papa, E.; Miccio, F.; Landi, E.; Doghieri, F. Geopolymers as solid adsorbent for CO2 capture. Chem. Eng. Sci. 2016, 148, 267–274. [Google Scholar] [CrossRef]
- Rodríguez, E.; García, R. Low-cost hierarchical micro/macroporous carbon foams as efficient sorbents for CO2 capture. Fuel Process. Technol. 2017, 156, 235–245. [Google Scholar] [CrossRef]

| SiO2 | Al2O3 | K2O | Fe2O3 | CaO | Na2O | MgO | TiO2 | MnO | SO3 | SUM | Si/Al | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLI | 66.80 | 10.98 | 4.23 | 1.97 | 4.23 | 0.22 | 0.65 | 0.24 | / | / | 99.29 | 5.16 |
| Zeoslag | 49.00 | 9.28 | 1.39 | 0.62 | 30.90 | 0.44 | 5.89 | 0.36 | 0.43 | 1.30 | 97.88 | 4.48 |
| Sample | Intensity 9.9° 2θ [020] (%) 1 | Intensity 11.2° 2θ [200] (%) 1 | Intensity Ratio 11.2°/9.9° | |
|---|---|---|---|---|
| S | 100 | 100 | 0.91 | |
| AAC (0.7) | D1 | 133.7 | 95.1 | 0.65 |
| D2 | 69.0 | 30.5 | 0.40 | |
| S | 42.2 | 42.4 | 0.91 | |
| AACZ (0.7) | D1 | 53.6 | 42.5 | 0.72 |
| D2 | 25.7 | 9.6 | 0.34 | |
| S | 36.7 | 37.2 | 0.92 | |
| AACZ (0.57) | D1 | 53.1 | 43.5 | 0.75 |
| D2 | 25.7 | 13.7 | 0.48 | |
| S | 4.0 | 3.1 | 0.69 | |
| AAZ (0.47) | D1 | 3.6 | 3.7 | 0.93 |
| D2 | 3.2 | 2.6 | 0.73 |
| Mesopore Volume in the Range of 3–50 nm (mL/g) | Total Intrusion Volume (mL/g) | |||||
|---|---|---|---|---|---|---|
| Sample | S | D1 | D2 | S | D1 | D2 |
| AAC (0.7) | 0.058 | 0.080 | 0.094 | 0.763 | 0.873 | 0.881 |
| AACZ (0.7) | 0.160 | 0.143 | 0.252 | 0.616 | 0.513 | 0.729 |
| AACZ (0.57) | 0.102 | 0.126 | 0.177 | 0.431 | 0.512 | 0.664 |
| AAZ (0.47) | 0.016 | 0.023 | 0.049 | 0.107 | 0.117 | 0.348 |
| Sample | Surface Area SBET (m2/g) 1 | Total Pore Volume (cm3/g) 2 | Mesopore Volume (cm3/g) 2 | Micropore Volume (cm3/g) 2 | |
|---|---|---|---|---|---|
| AAC (0.7) | S | 11 | 0.114 | 0.051 | 0.000 |
| D1 | 52 | 0.162 | 0.088 | 0.003 | |
| AACZ (0.7) | S | 20 | 0.139 | 0.098 | 0.000 |
| D1 | 88 | 0.172 | 0.127 | 0.012 | |
| D2 | 240 | 0.362 | 0.253 | 0.048 | |
| AACZ (0.57) | S | 15 | 0.108 | 0.075 | 0.000 |
| D1 | 69 | 0.148 | 0.113 | 0.004 | |
| AAZ (0.47) | S | 3 | 0.017 | 0.010 | 0.000 |
| D1 | 38 | 0.055 | 0.046 | 0.001 | |
| Samples | CO2 Adsorbed (wt%) | CO2 Adsorbed (mmol/g) | ||
|---|---|---|---|---|
| AAC (0.7) | D1 | 3.07 | 0.70 | |
| AACZ (0.7) | S | 1.93 | 0.44 | |
| D1 | 2.58 | 0.59 | ||
| D2 | 1.95 | 0.44 | ||
| AACZ (0.57) | D1 | 2.49 | 0.57 | |
| AAZ (0.47) | D1 | 1.36 | 0.31 | |
| Clinoptilolite | S | 1.84 | 0.42 | |
| Clinoptilolite | D1 | 2.12 | 0.48 | [50] |
| Clinoptilolite | D2 | 1.08 | 0.25 | |
| Geopolymer | 0.23 | 0.05 | [55] | |
| Geopolymer G23 | 2.51 | 0.57 | [56] | |
| Activated carbon | 5.28 | 1.2 | ||
| Silicate | 5.72 | 1.3 | ||
| Zeolite 13 X | 17.60 | 4.0 | ||
| Zeolite Naγ | 11.88 | 2.7 | ||
| Zeolite HZSM-5-280 | 8.36 | 1.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strejcová, K.; Tišler, Z.; Sharkov, N.; Michálková, M.; Peroutková, K.; Svobodová, E. Influence of the Addition of Blast Furnace Slag to Alkali-Activated Mixtures Based on Natural Zeolites. Minerals 2021, 11, 1307. https://doi.org/10.3390/min11121307
Strejcová K, Tišler Z, Sharkov N, Michálková M, Peroutková K, Svobodová E. Influence of the Addition of Blast Furnace Slag to Alkali-Activated Mixtures Based on Natural Zeolites. Minerals. 2021; 11(12):1307. https://doi.org/10.3390/min11121307
Chicago/Turabian StyleStrejcová, Kateřina, Zdeněk Tišler, Nikita Sharkov, Martina Michálková, Kateřina Peroutková, and Eliška Svobodová. 2021. "Influence of the Addition of Blast Furnace Slag to Alkali-Activated Mixtures Based on Natural Zeolites" Minerals 11, no. 12: 1307. https://doi.org/10.3390/min11121307
APA StyleStrejcová, K., Tišler, Z., Sharkov, N., Michálková, M., Peroutková, K., & Svobodová, E. (2021). Influence of the Addition of Blast Furnace Slag to Alkali-Activated Mixtures Based on Natural Zeolites. Minerals, 11(12), 1307. https://doi.org/10.3390/min11121307






