Detoxification of Arsenic-Containing Copper Smelting Dust by Electrochemical Advanced Oxidation Technology
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Methods
2.3. Characterization and Analysis
3. Results and Discussion
3.1. Characterization of the Spent Catalyst of the ARCD
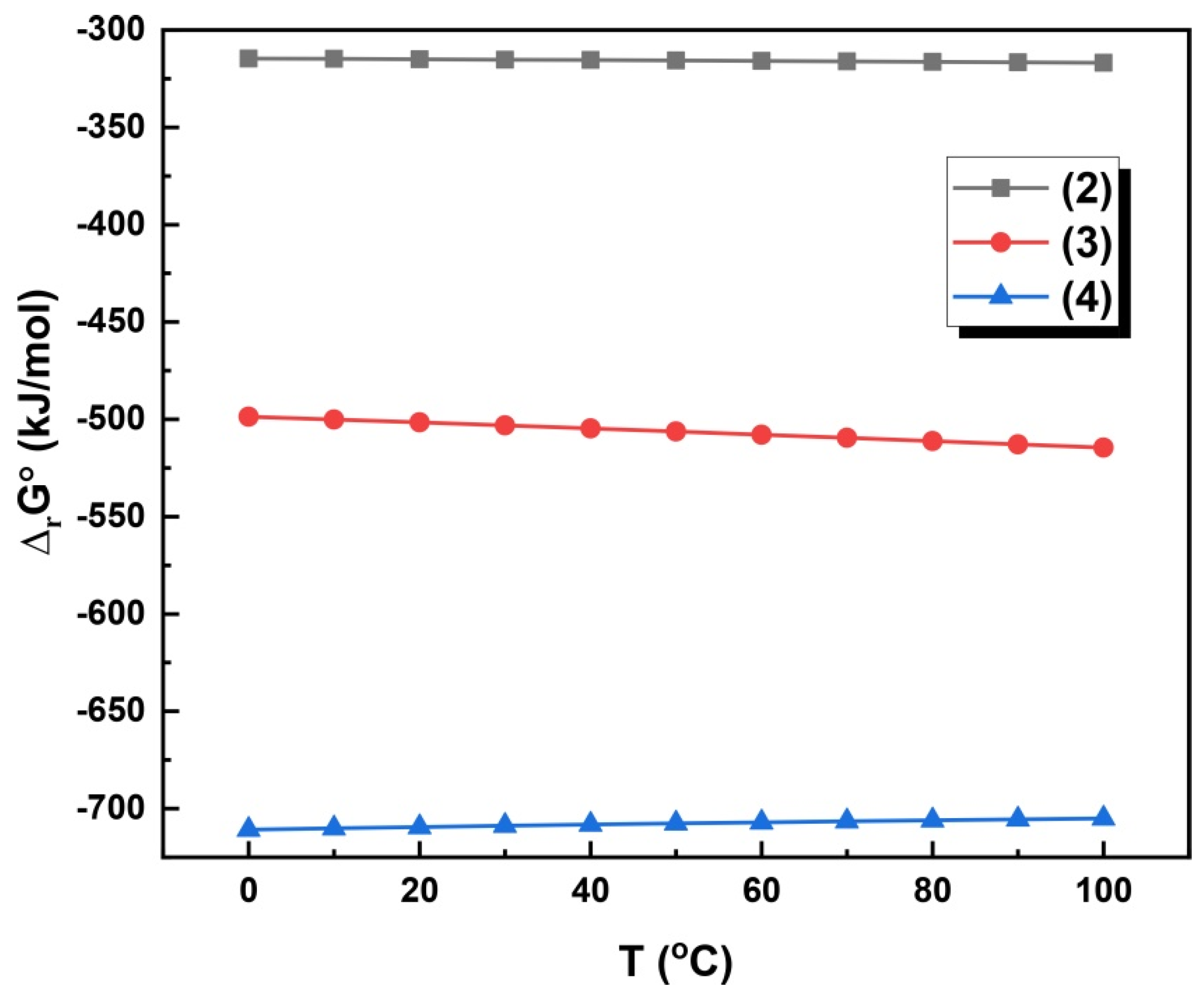
3.2. Alkaline Leaching Mechanisms
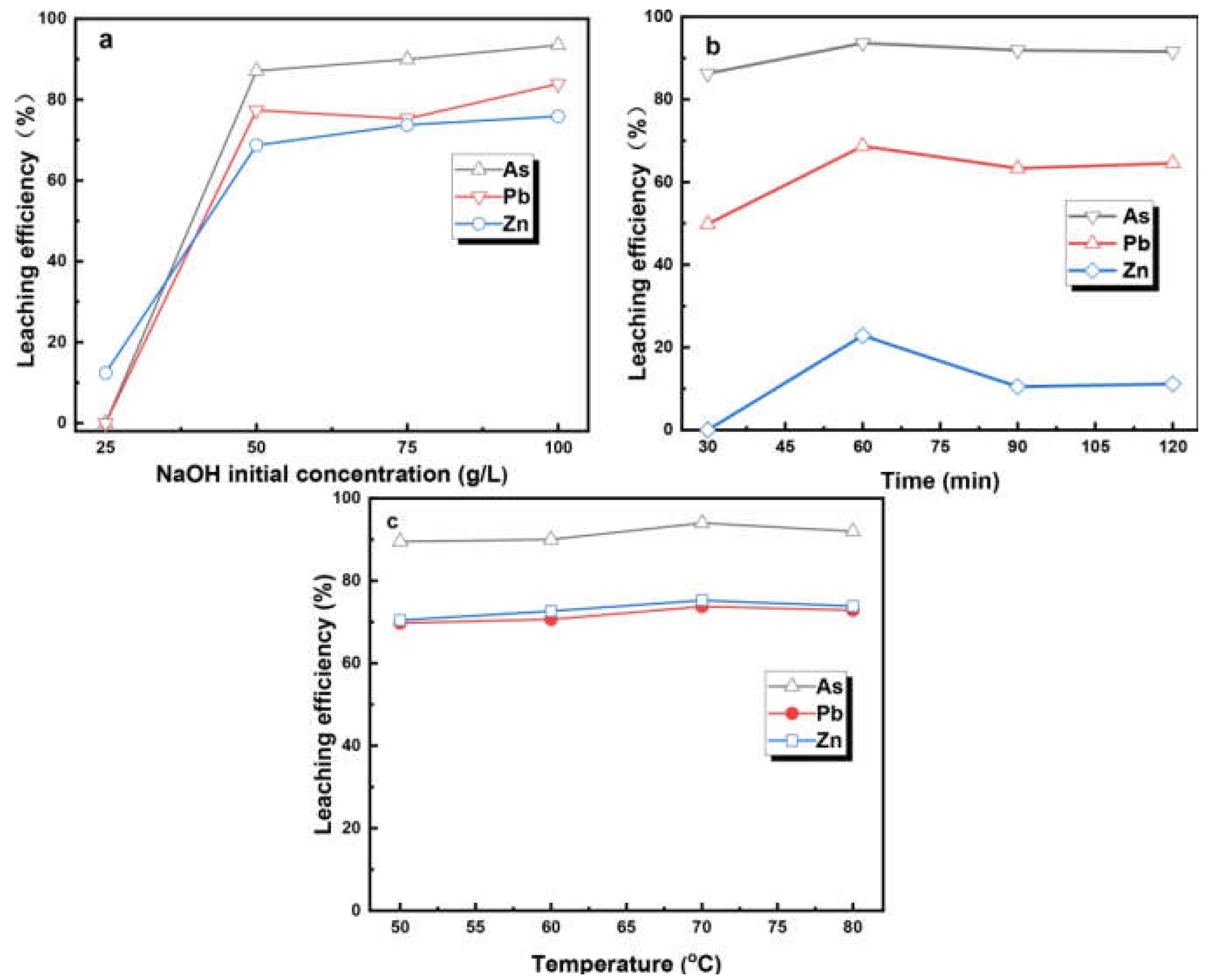
3.3. The Alkaline Leaching Process of the ARCD
3.4. Electrochemical Advanced Oxidation Treatment of ARCD
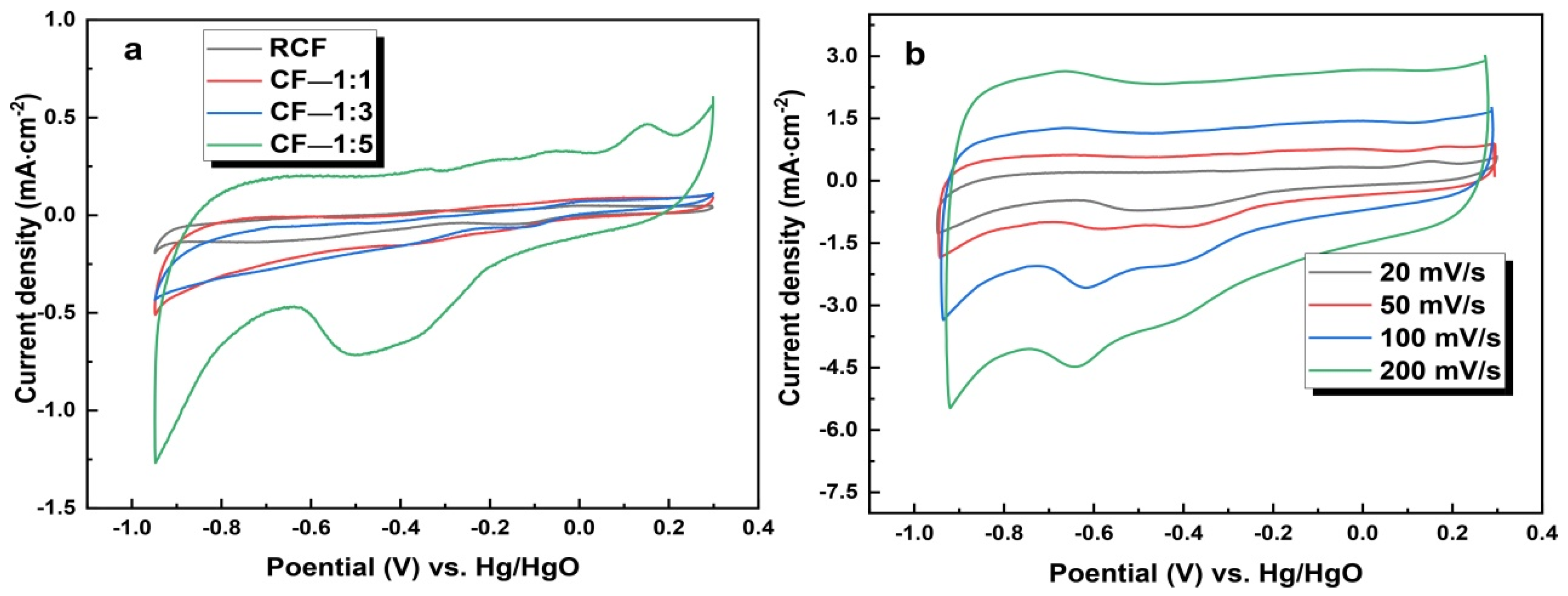
3.4.1. Characteristics of the Cf and Modified Carbon Felt
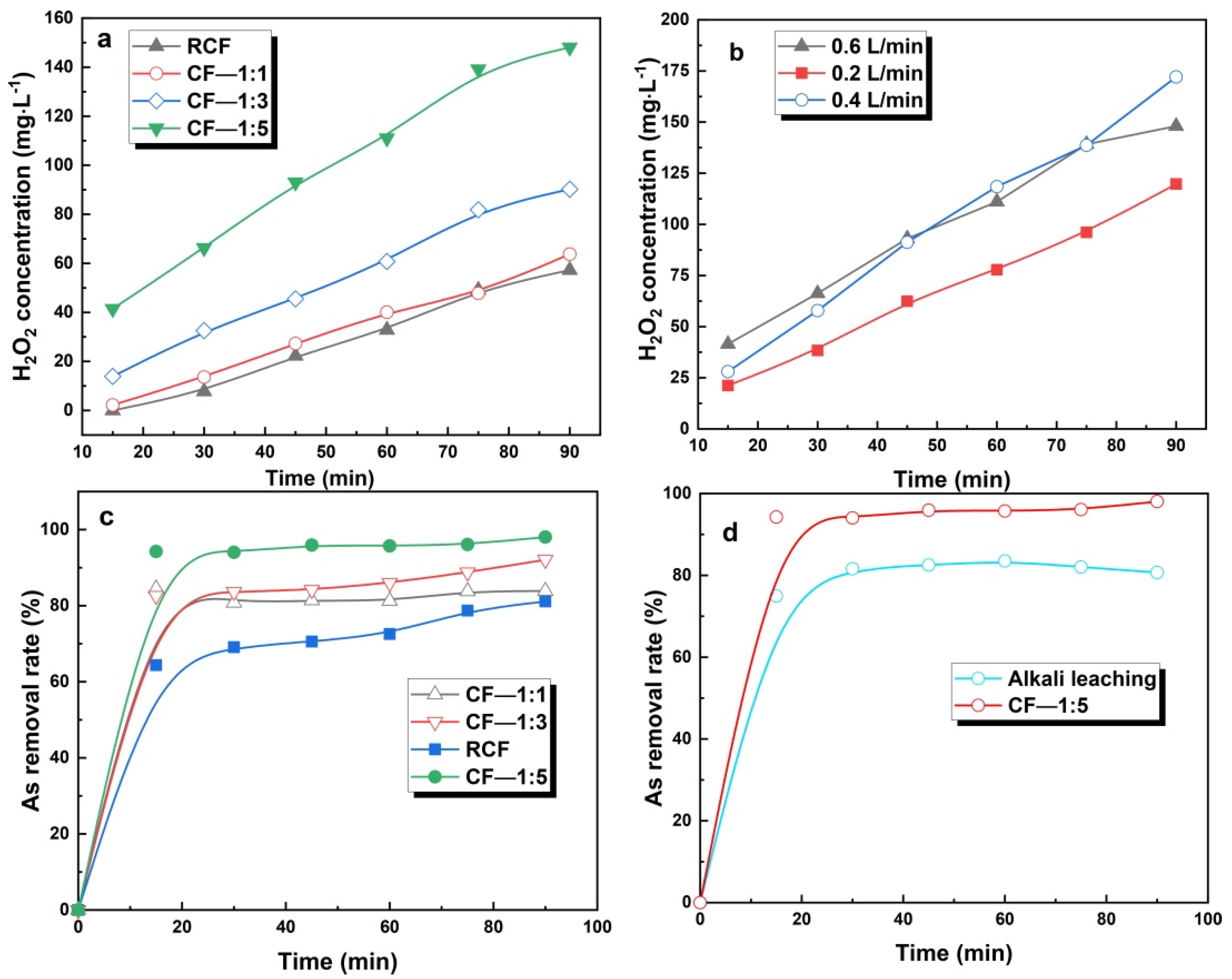
3.4.2. Performance of the Modified CF Electrode
3.4.3. Influencing Factors of Hydrogen Peroxide Generation
3.4.4. Performance of the Modified Electrode in Detoxification of ARCD
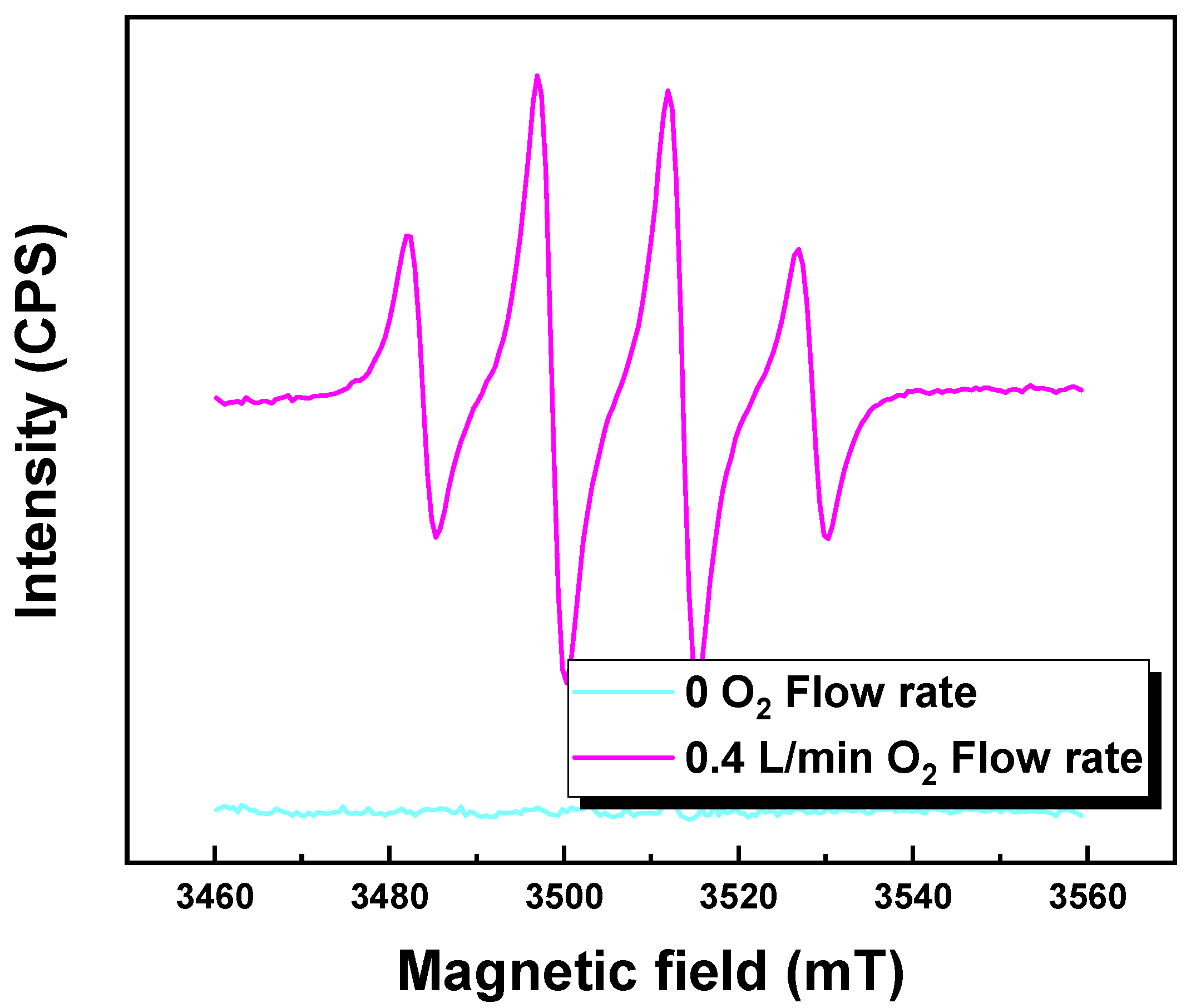
3.5. Electrochemical Oxidation Leaching Mechanisms
3.6. The Difference between Alkaline Leaching Process and Electrochemical Advanced Oxidation Leaching Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Zhang, G.L.; Deng, W.; Ma, J. Review of arsenic pollution and treatment progress in nonferrous metallurgy industry. Adv. Mater. Res. 2013, 634–638, 3239–3243. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, M.; Yu, X. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim. Acta 2015, 163, 182–189. [Google Scholar] [CrossRef]
- Liu, D.G.; Min, X.B.; Ke, Y.; Chai, L.Y.; Liang, Y.J.; Li, Y.C.; Yao, L.W.; Wang, Z.B. Co-treatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker. Environ. Sci. Pollut. Res. 2018, 25, 7600–7607. [Google Scholar] [CrossRef]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choong, T.; Chuah, T.G.; Robiah, Y.; Koay, F.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Xu, B.; Ma, Y.; Gao, W.; Yang, J.; Jiang, T. A review of the comprehensive recovery of valuable elements from copper smelting open-circuit dust and arsenic treatment. JOM 2020, 72, 3860–3875. [Google Scholar] [CrossRef]
- Jaroíková, A.; Ettler, V.; Mihaljevi, M.; Drahota, P.; Culka, A.; Racek, M. Characterization and pH-dependent environmental stability of arsenic trioxide-containing copper smelter flue dust. J. Environ. Manag. 2018, 209, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Yi, Y.; Shi, J.; Tian, Q.H. Leaching behavior of metals from high-arsenic dust by NaOH–Na2S alkaline leaching. Trans. Nonferrous Met. Soc. China 2016, 26, 575–580. [Google Scholar] [CrossRef]
- Liu, W.; Han, J.; Ou, Z.; Wu, D.; Qin, W. Arsenic and antimony extraction from high arsenic smelter ash with alkaline pressure oxidative leaching followed by Na2S leaching. Sep. Purif. Technol. 2019, 222, 53–59. [Google Scholar]
- Chen, Y.; Liao, T.; Li, G.; Chen, B.; Shi, X. Recovery of bismuth and arsenic from copper smelter flue dusts after copper and zinc extraction. Miner. Eng. 2012, 39, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; Deng, P.; Rong, Z.; Cao, S. Study on thermodynamic model of arsenic removal from oxidative acid leaching. J. Mater. Res. Technol. 2020, 9, 3208–3218. [Google Scholar] [CrossRef]
- Zhong, D.P.; Li, L.; Tan, C. Separation of arsenic from the antimony-bearing dust through selective oxidation using CuO. Metall. Mater. Trans. B 2017, 48, 1308–1314. [Google Scholar] [CrossRef]
- Zhong, D.P.; Lei, L.I. Separation of arsenic from arsenic—Antimony-bearing dust through selective oxidation—Sulfidation roasting with CuS. Trans. Nonferrous Met. Soc. China 2020, 30, 223–235. [Google Scholar] [CrossRef]
- Shan, C.; Tong, M. Efficient removal of trace arsenite through oxidation and adsorption by magnetic nanoparticles modified with Fe-Mn binary oxide. Water Res. 2013, 47, 3411–3421. [Google Scholar] [CrossRef]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. High concentration of arsenate removal by electrocoagulation with calcium. Sep. Purif. Technol. 2014, 126, 7–14. [Google Scholar] [CrossRef]
- Jin, Y.; Fei, L.; Tong, M.; Hou, Y. Removal of arsenate by cetyltrimethylammonium bromide modified magnetic nanoparticles. J. Hazard. Mater. 2012, 227–228, 461–468. [Google Scholar] [CrossRef]
- Bui, T.H.; Kim, C.; Hong, S.P.; Yoon, J. Effective adsorbent for arsenic removal: Core/shell structural nano zero-valent iron/manganese oxide. Environ. Sci. Pollut. Res. 2017, 24, 24235–24242. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, J.; Liu, W.; Qin, W.; Cai, L. Arsenic removal from lead-zinc smelter ash by NaOH-H2O2 leaching. Sep. Purif. Technol. 2019, 209, 128–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, W. Arsenic oxidation and removal from flue gas using H2O2/Na2S2O8 solution. Fuel Process. Technol. 2017, 167, 355–362. [Google Scholar] [CrossRef]
- Tubić, A.; Agbaba, J.; Dalmacija, B.; Rončević, S.; Ivančev-Tumbas, I. Effects of O3, O3/H2O2 and coagulation on natural organic matter and arsenic removal from typical northern Serbia source water. Sep. Sci. Technol. 2010, 45, 2453–2464. [Google Scholar] [CrossRef]
- Kim, D.H.; Bokare, A.D.; Min, S.K.; Choi, W. Heterogeneous catalytic oxidation of As(III) on nonferrous metal oxides in the presence of H2O2. Environ. Sci. Technol. 2015, 49, 3506. [Google Scholar] [CrossRef]
- Oturan, M.A. Electrochemical advanced oxidation technologies for removal of organic pollutants from water. Environ. Sci. Pollut. Res. 2014, 21, 8333–8335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wu, Y.; Huang, C.; Xiao, D.; Chu, P.K. Graphite felt incorporated with MoS2/rGO for electrochemical detoxification of high-arsenic fly ash. Chem. Eng. J. 2019, 382, 122763. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, Y.; Wei, J. Reinforced As(III) oxidation by the in-situ electro-generated hydrogen peroxide on MoS2 ultrathin nanosheets modified carbon felt in alkaline media. Electrochim. Acta 2017, 252, 245–253. [Google Scholar]
- Wang, X.Q.; Liu, C.P.; Yuan, Y.; Li, F.B. Arsenite oxidation and removal driven by a bio-electro-Fenton process under neutral pH conditions. J. Hazard. Mater. 2014, 275, 200–209. [Google Scholar] [CrossRef]
- Xia, G.; Lu, Y.; Xu, H. Electrogeneration of hydrogen peroxide for electro-Fenton via oxygen reduction using polyacrylonitrile-based carbon fiber brush cathode. Electrochim. Acta 2015, 158, 390–396. [Google Scholar] [CrossRef]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvori Ca, F.I.; Pinson, J.; Oturan, M.A. Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Tian, J.; Olajuyin, A.M.; Mu, T.; Yang, M.; Xing, J. Efficient degradation of rhodamine B using modified graphite felt gas diffusion electrode by electro-Fenton process. Environ. Sci. Pollut. Res. 2016, 23, 11574–11583. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Feng, H.; Zhou, Y.; Zeng, G.; Lu, Y.; Yu, J.; Ren, X.; Peng, B.; Liu, X. Carbon felt cathodes for electro-Fenton process to remove tetracycline via synergistic adsorption and degradation. Sci. Total. Environ. 2019, 670, 921–931. [Google Scholar] [CrossRef]
- Liu, X.; Yang, D.; Zhou, Y.; Zhang, J.; Lin, T. Electrocatalytic properties of N-doped graphite felt in electro-Fenton process and degradation mechanism of levofloxacin. Chemosphere 2017, 182, 306–315. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.; Li, J.; Wan, Z. Effects of N mono- and N/P dual-doping on H2O2, OH generation, and MB electrochemical degradation efficiency of activated carbon fiber electrodes. Chemosphere 2018, 193, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Le, T.H.; Bechelany, M.; Esposito, G.; Hullebusch, E.V.; Oturan, M.A.; Cretin, M. A hierarchical cofe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Camacho, J.; Wee, H.Y.; Kramer, T.A.; Autenrieth, R. Arsenic stabilization on water treatment residuals by calcium addition. J. Hazard. Mater. 2009, 165, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Bindi, L.; Catelani, T.; Chelazzi, L.; Bonazzi, P. Reinvestigation of the crystal structure of lautite, CuAsS. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, i22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tian, J.; Han, H.; Sun, W.; Hu, Y.; Wang, T.; Yang, Y.; Cao, X.; Tang, H. Arsenic removal from arsenic-containing copper and cobalt slag using alkaline leaching technology and MgNH4AsO4 precipitation. Sep. Purif. Technol. 2020, 238, 116422. [Google Scholar] [CrossRef]
- Zhan, M.; Xi, Y.; Xian, Q.; Kong, L. Photosensitized degradation of bisphenol a involving reactive oxygen species in the presence of humic substances. Chemosphere 2006, 63, 378–386. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; Dimento, B.P.; Mcneill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Yu, X.; Zheng, S.; Du, H.; Dreisinger, D.; Yi, Z. A promising approach to recover a spent scr catalyst: Deactivation by arsenic and alkaline metals and catalyst regeneration. Chem. Eng. J. 2018, 342, 1–8. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
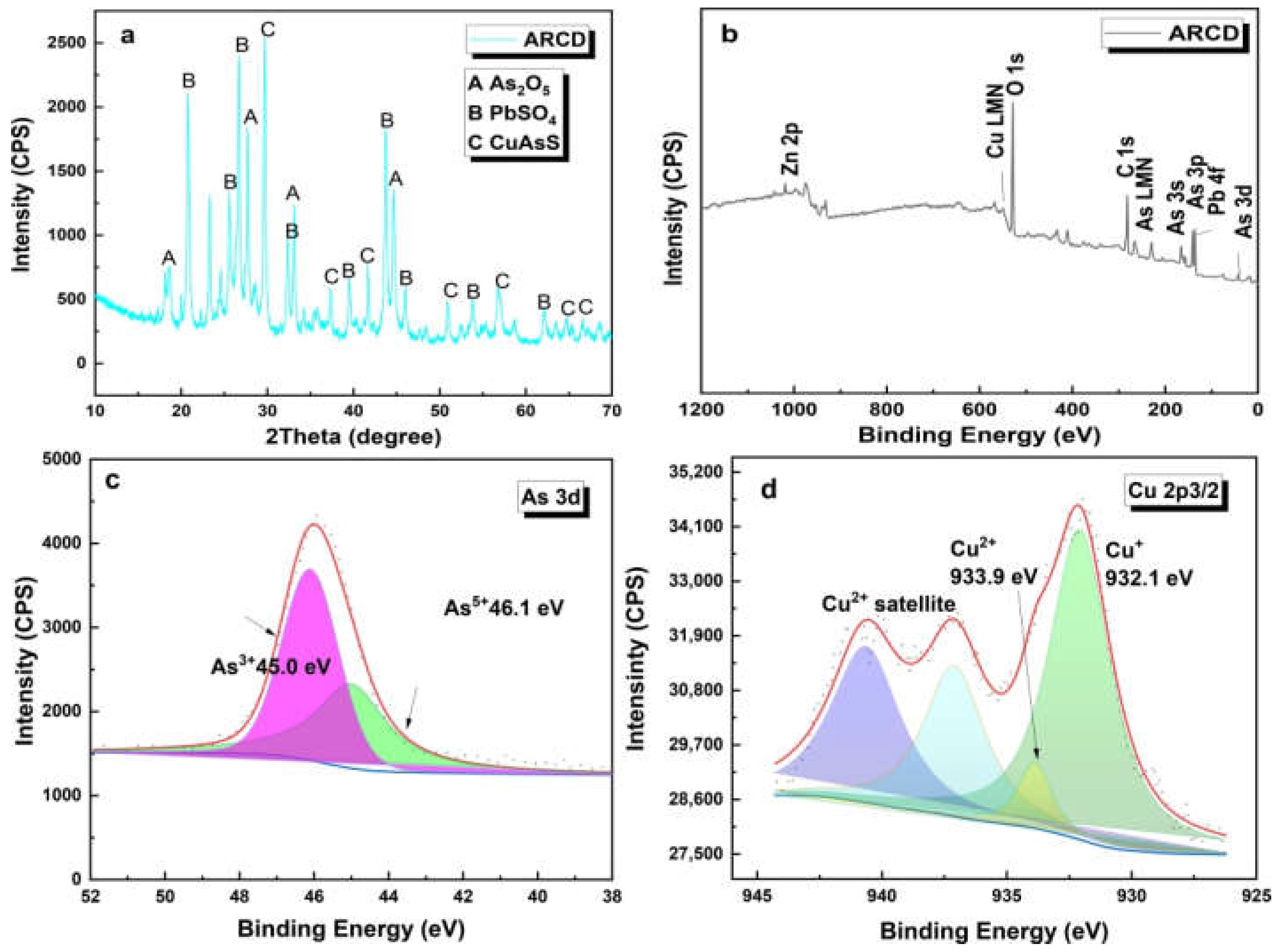
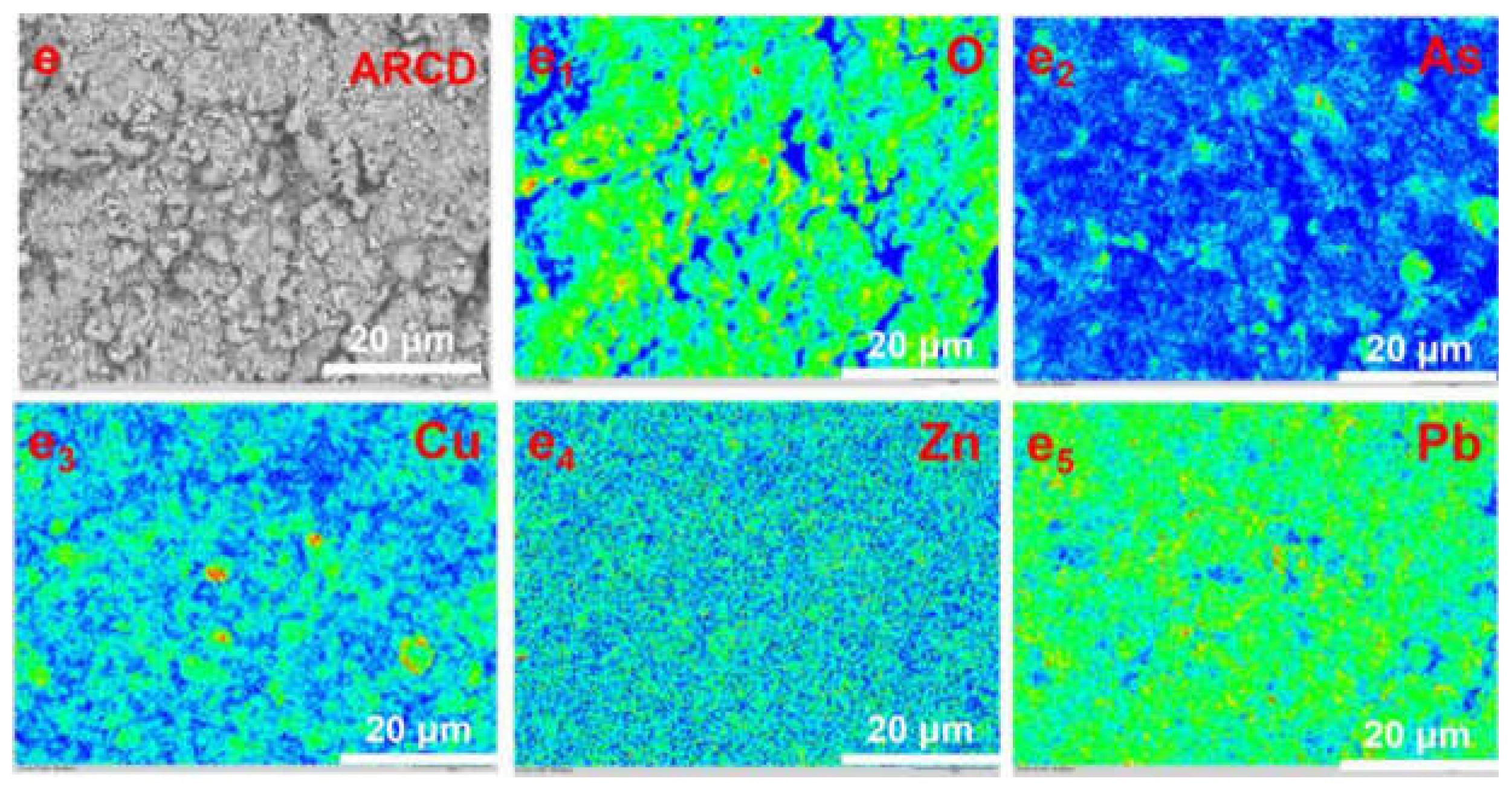





| Element | As2O3 | CuO | ZnO | PbO | Bi2O3 | Fe2O3 | * SO3 | Others |
|---|---|---|---|---|---|---|---|---|
| Content | 8.78 | 15.31 | 1.95 | 17.7 | 6.62 | 1.63 | 20.73 | 27.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yuan, J.; Liu, B.; Du, H.; Dreisinger, D.; Cao, Y.; Han, G. Detoxification of Arsenic-Containing Copper Smelting Dust by Electrochemical Advanced Oxidation Technology. Minerals 2021, 11, 1311. https://doi.org/10.3390/min11121311
Li M, Yuan J, Liu B, Du H, Dreisinger D, Cao Y, Han G. Detoxification of Arsenic-Containing Copper Smelting Dust by Electrochemical Advanced Oxidation Technology. Minerals. 2021; 11(12):1311. https://doi.org/10.3390/min11121311
Chicago/Turabian StyleLi, Meng, Junfan Yuan, Bingbing Liu, Hao Du, David Dreisinger, Yijun Cao, and Guihong Han. 2021. "Detoxification of Arsenic-Containing Copper Smelting Dust by Electrochemical Advanced Oxidation Technology" Minerals 11, no. 12: 1311. https://doi.org/10.3390/min11121311
APA StyleLi, M., Yuan, J., Liu, B., Du, H., Dreisinger, D., Cao, Y., & Han, G. (2021). Detoxification of Arsenic-Containing Copper Smelting Dust by Electrochemical Advanced Oxidation Technology. Minerals, 11(12), 1311. https://doi.org/10.3390/min11121311








