Origin of Lithium–Potassium-Rich Brines in the Jianghan Basin, South China: Constraints by Water–Rock Reactions of Mesozoic–Cenozoic Igneous Rocks
Abstract
:1. Introduction
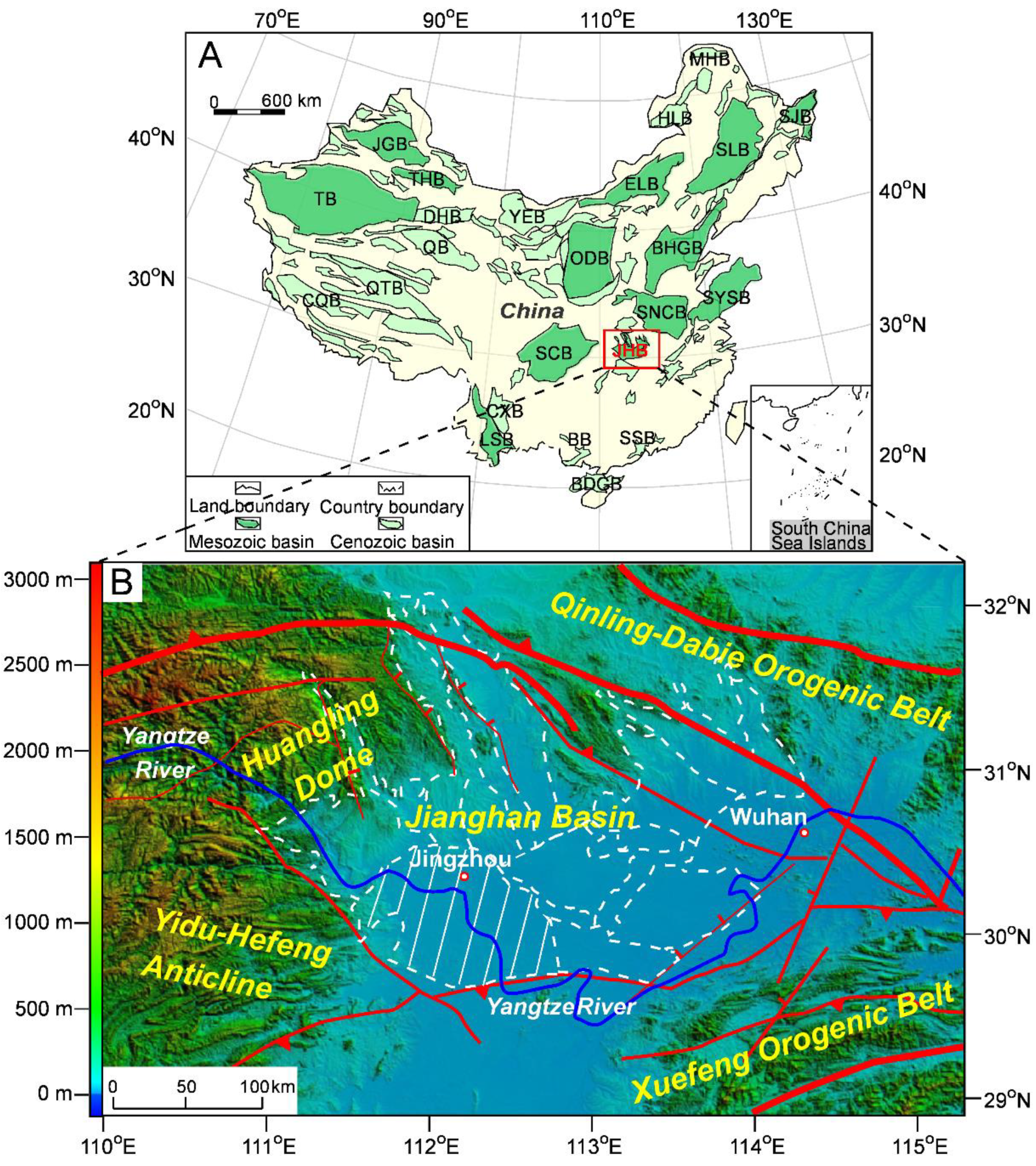
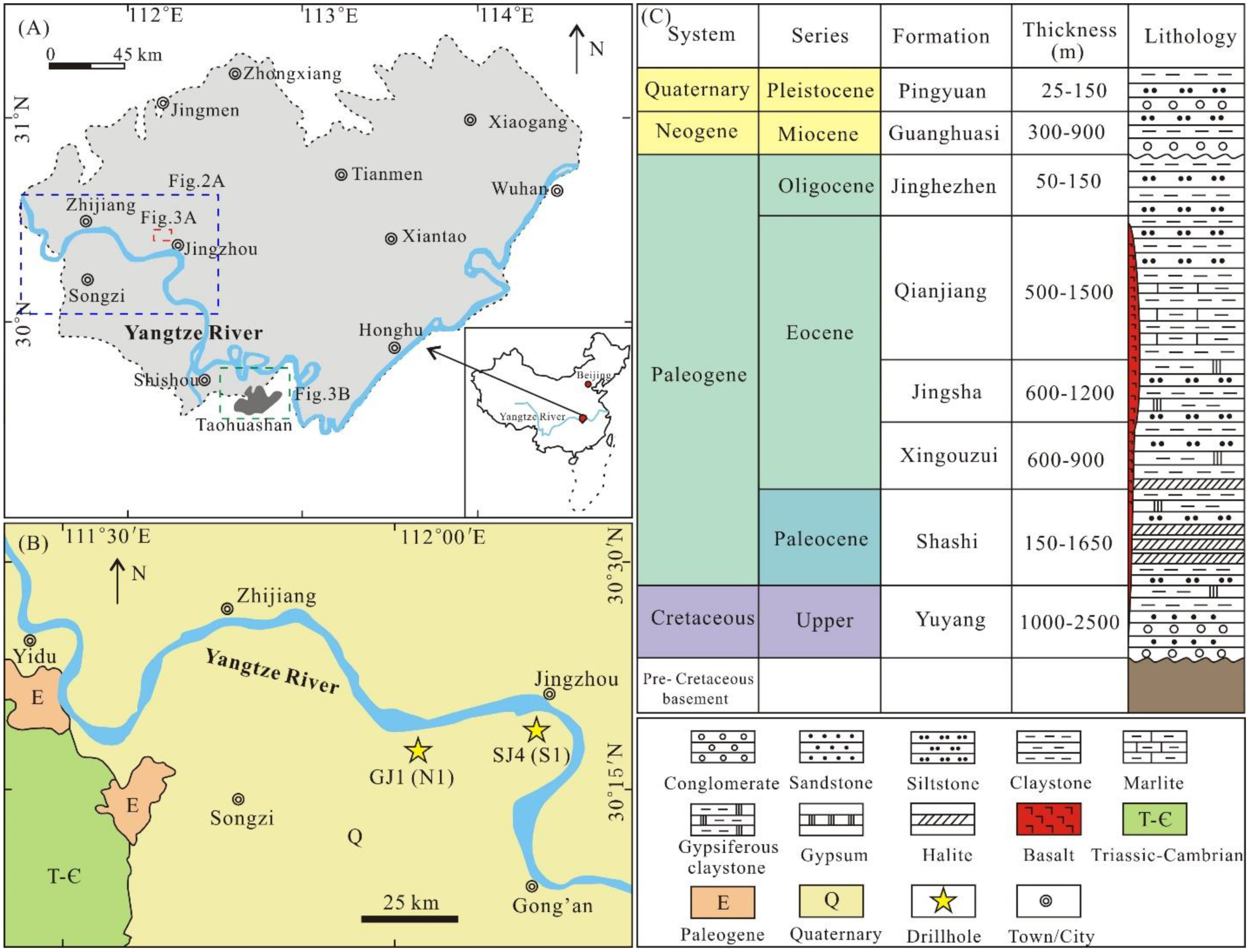
2. Geological Background
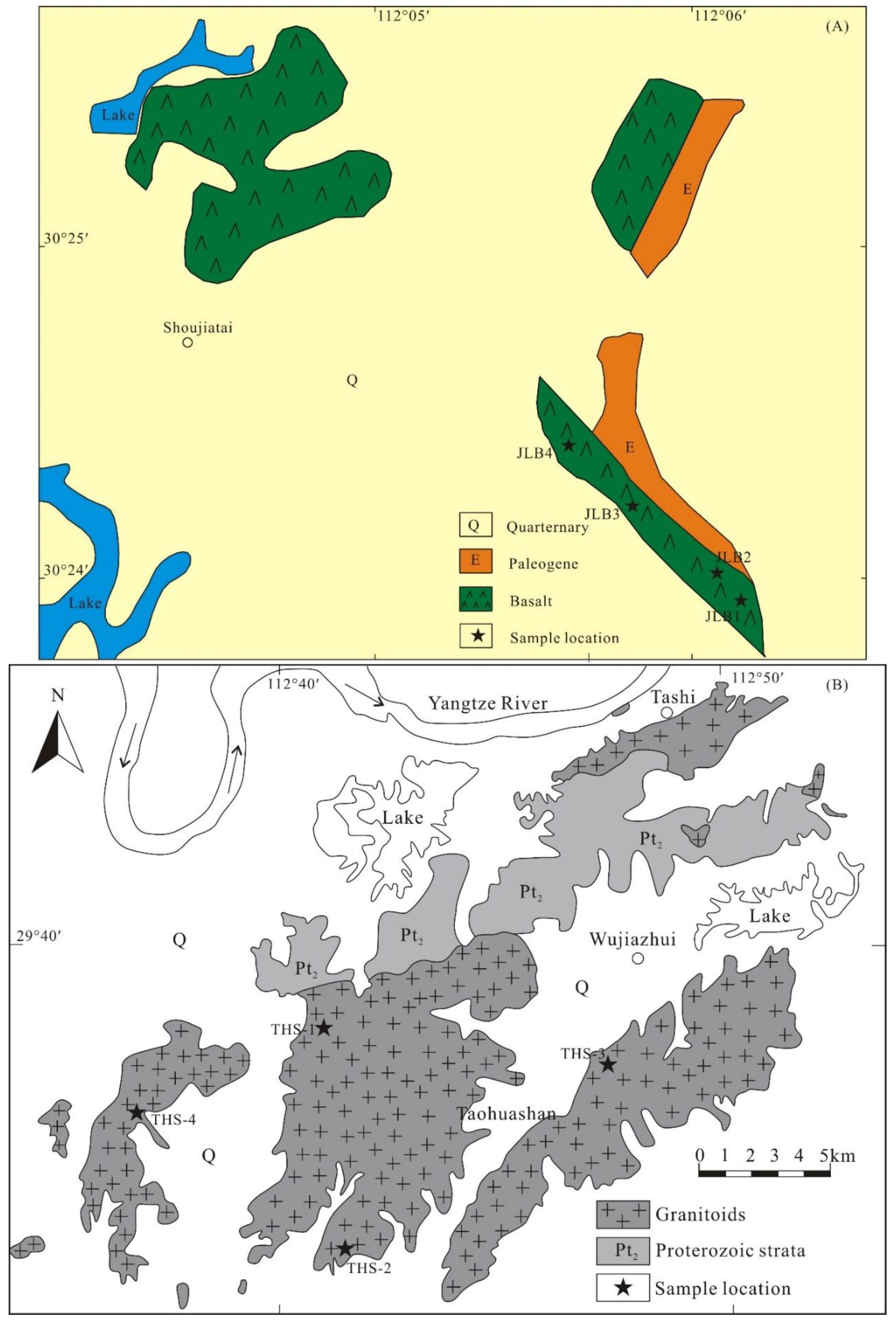
3. Samples and Methods
4. Results
4.1. Brine Composition
4.2. Petrology and Mineralogy
4.2.1. Basalt
4.2.2. Granite
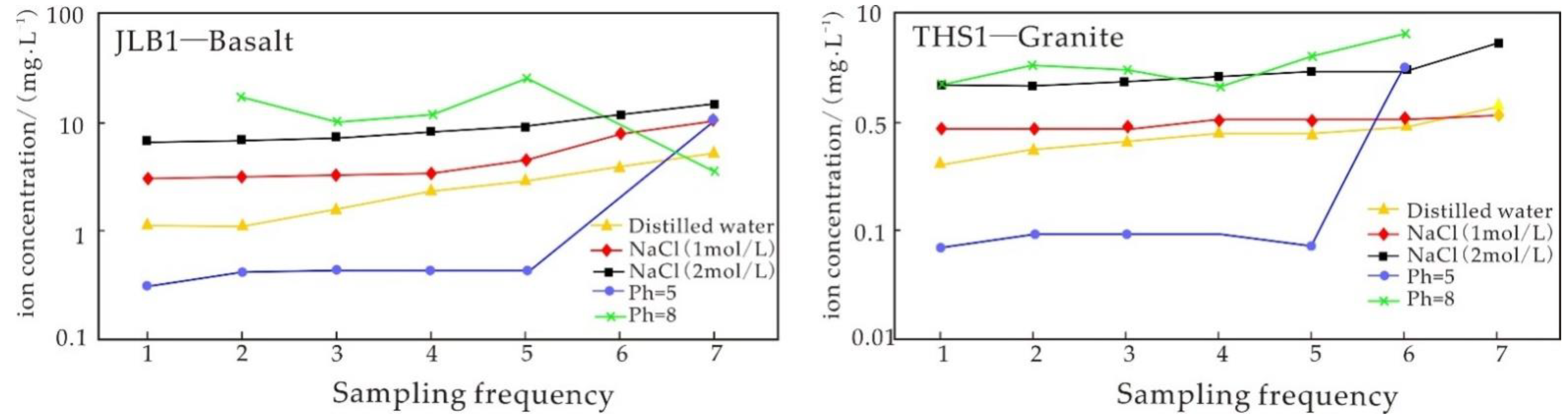
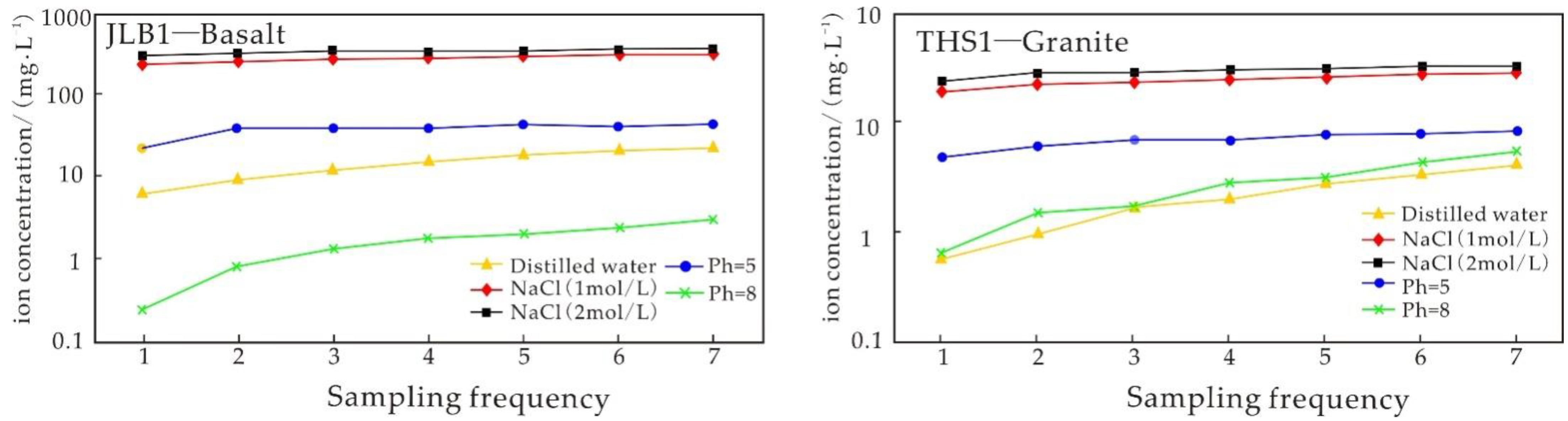
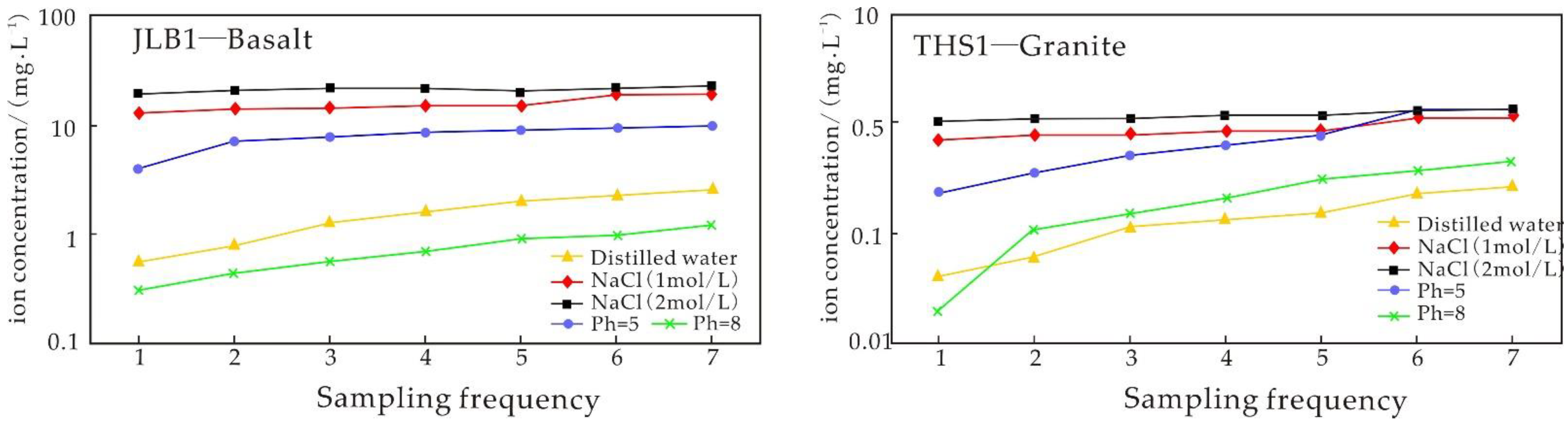
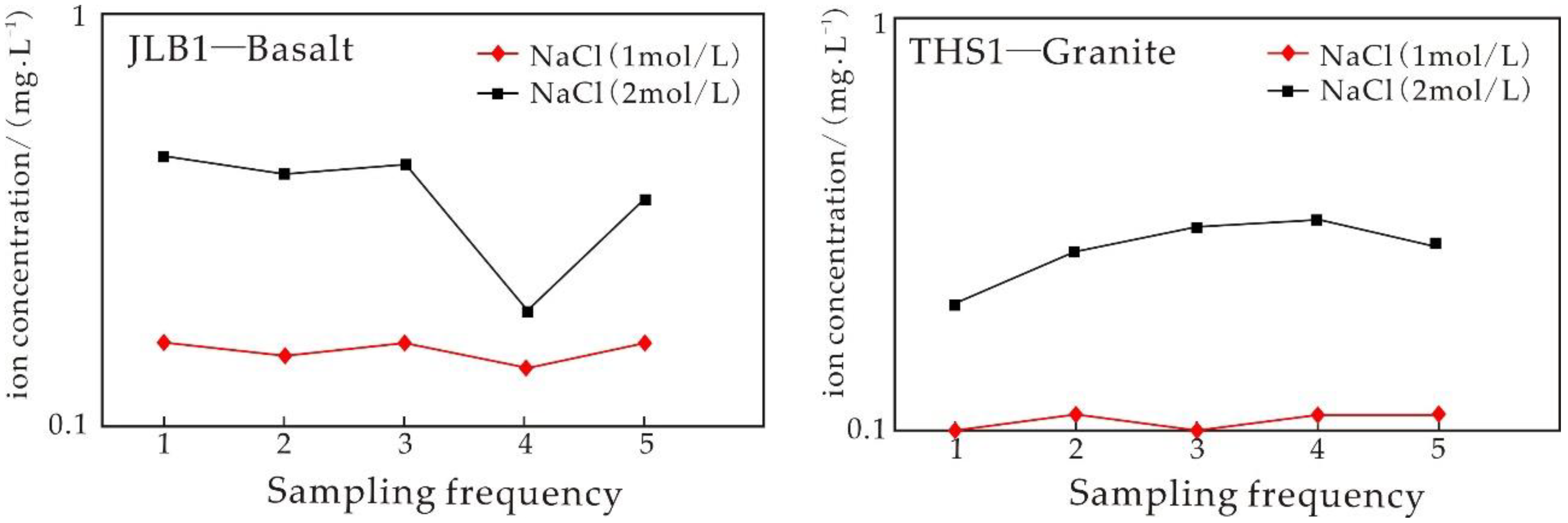
4.3. Experiment Results of the Water–Rock Reaction Due to Basalt Static Immersion
4.4. Results of High-Temperature Water–Rock Reactions
4.4.1. Experiment of Water–Rock Reactions over Time
4.4.2. Experiment of Water–Rock Reactions with Variable Temperatures
5. Discussion
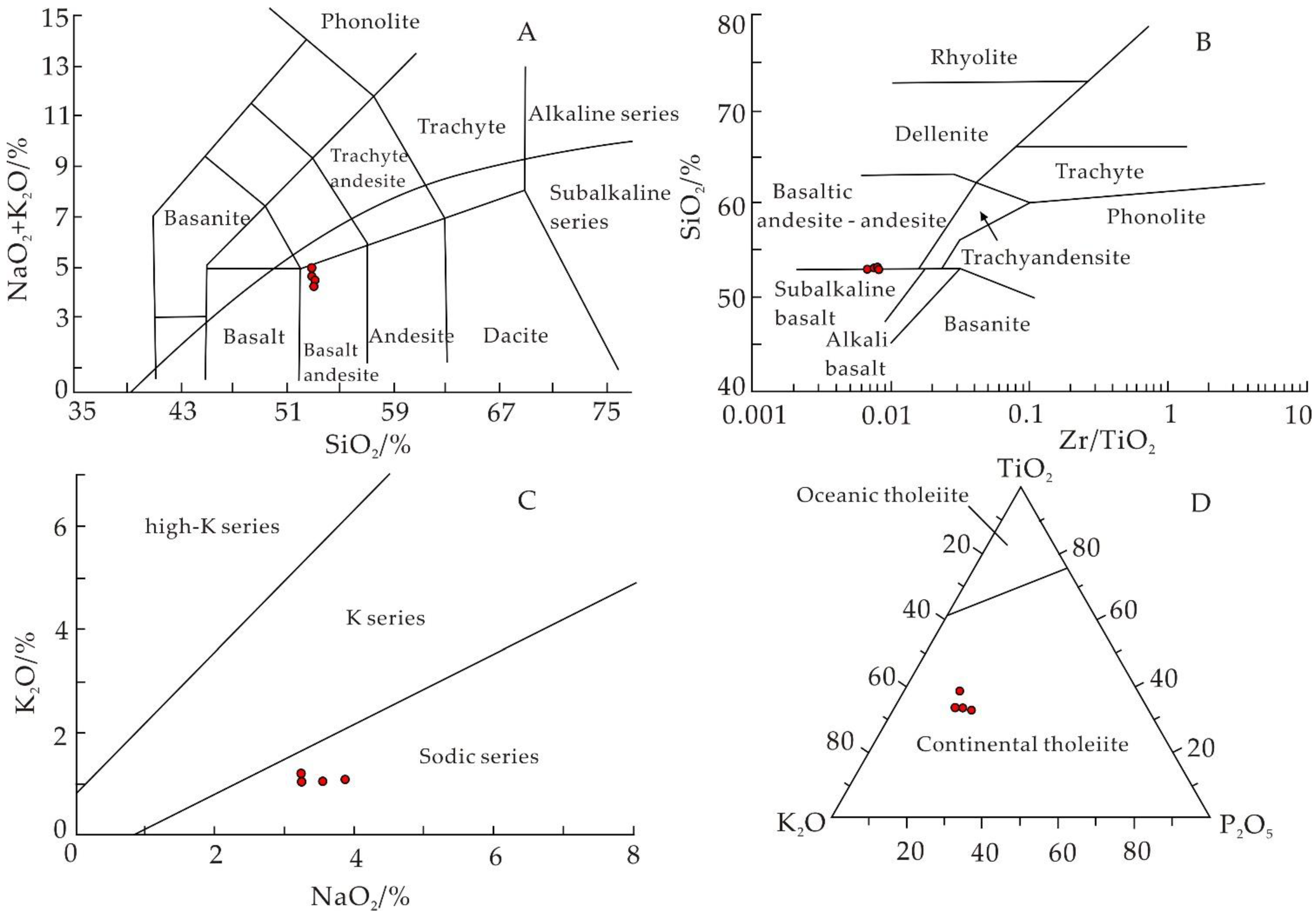
5.1. Geochemical Characteristics
5.2. Determination of the Starting and Stopping Temperatures of the Water–Rock Reaction
5.3. Discussion on the Genesis of Lithium–Potassium-Rich Brine
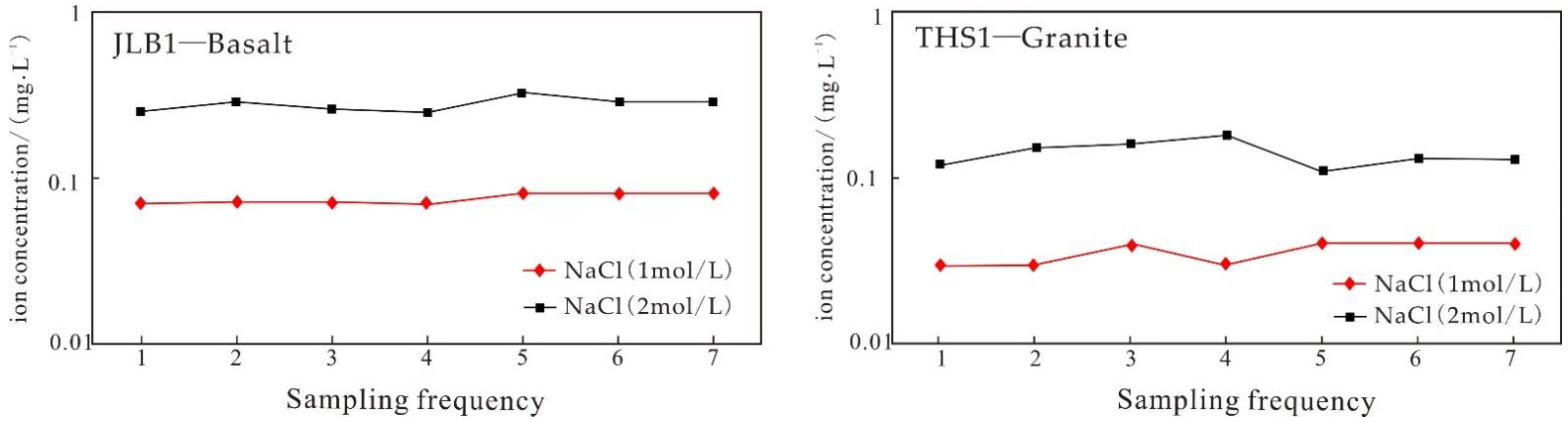
5.3.1. Analysis of Static Immersion Experimental Results
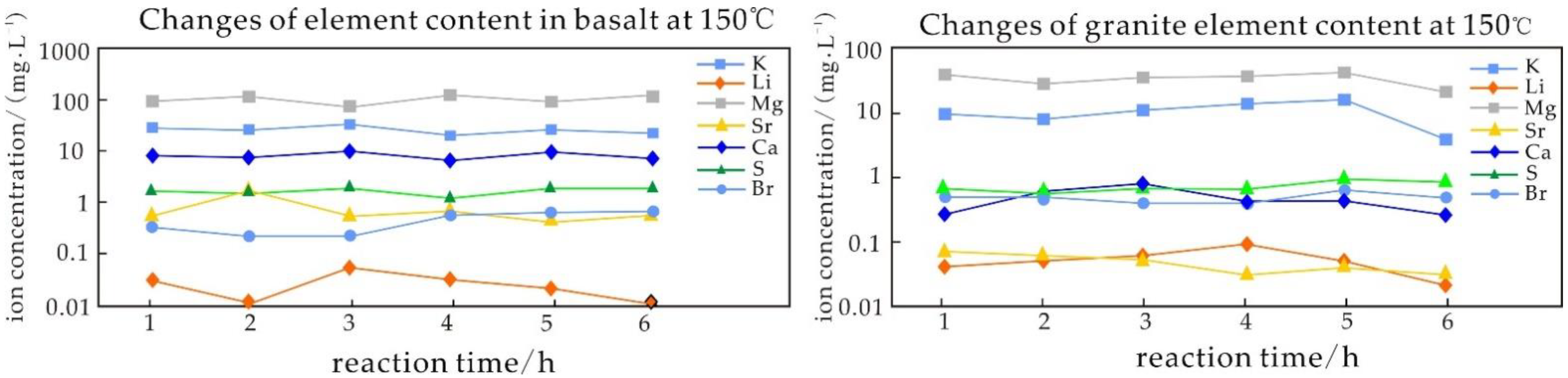
5.3.2. Influence of Time on the Igneous Rock Water–Rock Reaction
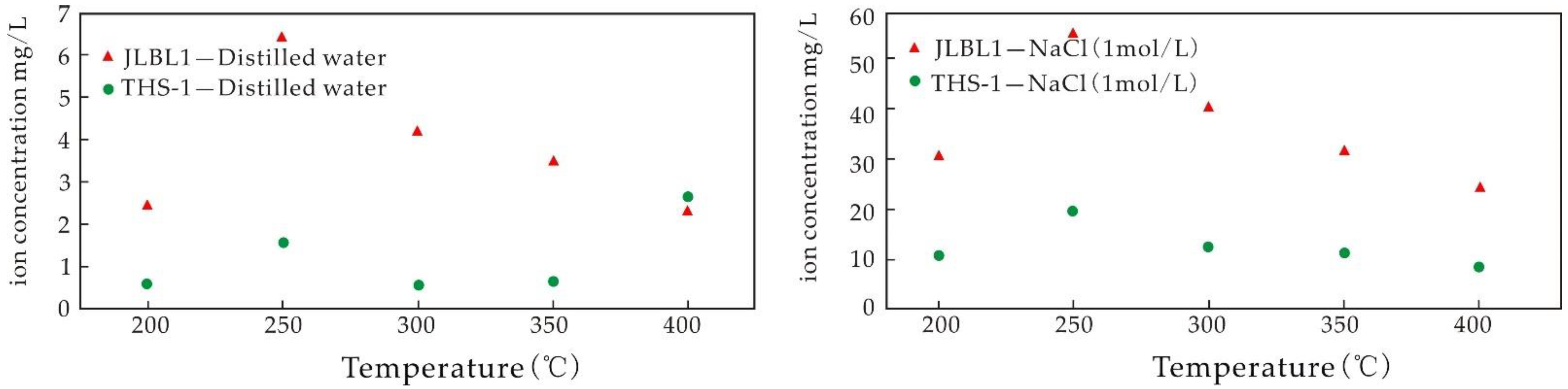
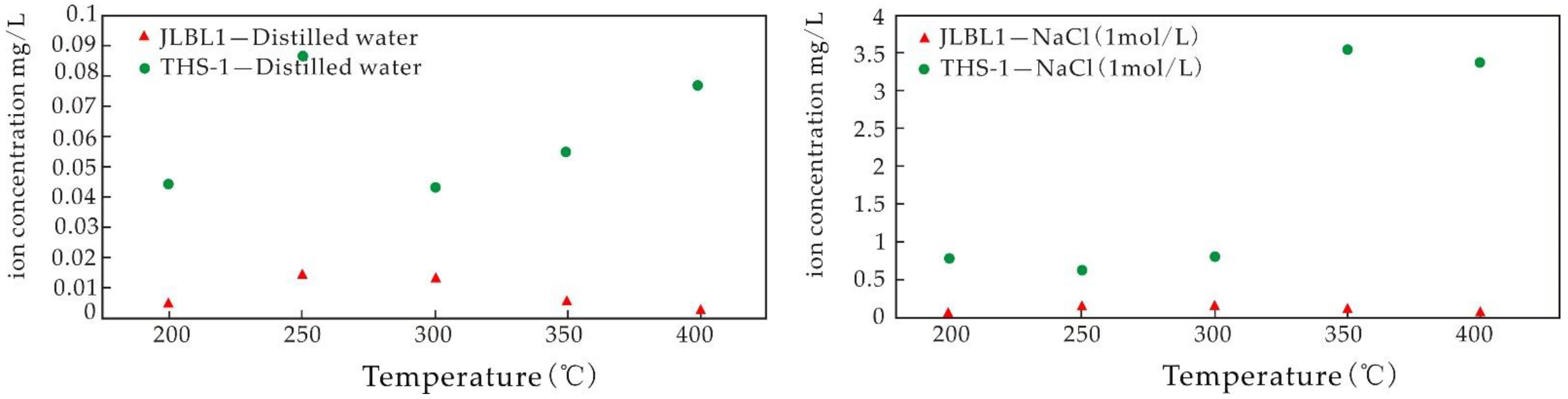
5.3.3. Influence of Temperature on the Igneous Rock Water–Rock Reaction
5.3.4. Influence of Fluid Composition on the Igneous Rock Water–Rock Reaction
5.3.5. Geotectonics
5.3.6. Paleocene Paleoclimate and Paleo-Lake Composition
6. Conclusions
- (1)
- The field evidence shows that Mesozoic and Cenozoic igneous rocks in the Jiangling depression are mainly basalt and granite, and their distribution area is more than half of the basin area. The magma is differentiated to different degrees, which could have provided lithium and potassium for the brine.
- (2)
- The static immersion experiment at room temperature shows that saline fluid is more likely to activate K ions in basalt. The weak alkaline solution more easily dissolves K. During the whole experiment, Li was not dissolved, indicating that the concentration of the solution was not the factor affecting the dissolution of lithium, and the main controlling factor was temperature.
- (3)
- The experiments of water–rock reaction with high temperature show that the dissolution rates of Ca, Mg, and Sr decrease with the increase in temperature, while the dissolution rates of K and Li first increase and then decrease with the increase in temperature. The dissolution of K and Li is easier when saline fluid reacts with volcanic rock. The dissolution rate of K in basalt is higher than that of Li, and that of Li in granite is higher than that of K. Compared with the results at normal temperatures, the ability of the fluid to leach elements is significantly enhanced at higher temperatures, the temperature is the main factor controlling the ability to leach elements, and the high-salinity fluid is the main migration carrier of ore-forming elements.
- (4)
- According to the experiments of water–rock reaction, the mineral composition of the ancient brine in the Jiangling depression during the Paleogene is consistent with that of the brine found today, indicating that the Jiangling depression in the Paleogene was influenced by hot and dry climate. However, these two compositions are different by a few orders of magnitude, indicating that the formation of lithium–potassium-rich brines requires a long period of time. The water–rock reaction is one of the important processes for brine formation, and surface evaporation and concentration are the main mechanisms for brine mineralization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pl | Plagioclase |
| Px | Pyroxene |
References
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Godfrey, L.V.; Chan, L.H.; Alonso, R.N.; Lowenstein, T.K.; McDonough, W.F.; Houston, J.; Li, J.; Bobst, A.; Jordan, T.E. The role of climate in the accumulation of lithium-rich brine in the central Andes. Appl. Geochem. 2013, 38, 92–102. [Google Scholar] [CrossRef]
- Wang, C.L.; Liu, C.L.; Liu, B.K.; Shen, L.J.; Cai, X.L.; Yu, X.C.; Xie, T.X.; Wang, L.C.; Zhao, Y.J.; Xuan, Z.Q. The discovery of carnallite in Paleocene Jiangling Depression and its potash searching significance. Acta Geol. Sin. 2015, 89, 129–136. [Google Scholar]
- Yu, X.C.; Wang, C.L.; Liu, C.L.; Zhang, Z.C.; Xu, H.M.; Huang, H.; Xie, T.X.; Li, H.N.; Liu, J.L. Sedimentary characteristics and depositional model of a Paleocene-Eocene salt lake in the Jiangling Depression, China. Chin. J. Oceanol. Limn. 2015, 33, 1426–1435. [Google Scholar] [CrossRef]
- Liu, C.L.; Yu, X.C.; Zhao, Y.J.; Wang, J.Y.; Wang, L.C.; Xu, H.M.; Li, J.; Wang, C.L. Atentative discussion on regional metallogenicbackground and mineralization mechanism of subterranean brinesrich in potassium and lithiumin South China Block. Miner. Depos. 2016, 35, 1119–1143. [Google Scholar]
- Wang, C.L.; Liu, C.L.; Yu, X.C.; Li, H.N.; Liu, J.L. The extremely hot and dry climatic events and potash enrichment in salt lakes. Acta Geol. Sin.-Engl. 2016, 90, 769–770. [Google Scholar]
- Zeng, F.M.; Xiang, S.Y. Geochronology and Mineral Composition of the Pleistocene Sediments in Xitaijin air Salt Lake Region, Qaidam Basin: Preliminary Results. J. Earth Sci. 2017, 8, 622–627. [Google Scholar] [CrossRef]
- Wang, D.H.; Liu, L.J.; Dai, H.Z.; Liu, S.B.; Hou, J.L.; Wu, X.S. Discussion on particularity and prospecting direction of large andsuper-large spodumene deposits. Earth Sci. 2017, 42, 2243–2257. [Google Scholar]
- Shen, C.B.; Mei, L.F.; Min, K.; Jonckheere, R.; Ratschbacher, L.; Yang, Z.; Peng, L.; Liu, Z. Multi-chronometric dating of the Huarong granitoids from the middle Yangtze Craton: Implications for the tectonic evolution of eastern China. J. Asian Earth Sci. 2012, 52, 73–87. [Google Scholar] [CrossRef]
- Li, R.Q.; Liu, C.L.; Jiao, P.C.; Wang, J.Y. The tempo-special characteristics and forming mechanism of Lithium-rich brines in China. China Geol. 2018, 1, 72–83. [Google Scholar] [CrossRef]
- Munk, L.A.; Boutt, D.F.; Hyneck, S.; Moran, B. Hydrogeochemical fluxes and processes contributing to the formation of lithium-enriched brinesina hyper-arid continental basin. Chem. Geol. 2018, 493, 37–57. [Google Scholar] [CrossRef]
- Li, R.Q.; Liu, C.L.; Xu, H.M.; Jiao, P.C.; Hu, Y.F.; Li, F.; Sun, X.H. Genesis of glauberite sedimentation in LopNur Salt Lake—Constraints from thermo dynamics imulation of the shallow ground water in the Tarim River Basin, China. Chem. Geol. 2020, 537, 119461. [Google Scholar] [CrossRef]
- Rosenthal, E.; Möller, P.; Buch-Leviatan, O.; Politi, M. The Hydrogeochemical Stratigraphy of Brinesand Its Implications on Water Management in the Central Jordan-Dead SeaRift Valley, Israel. Geofluids 2020, 17, 9812597. [Google Scholar]
- Godfrey, L.V.; Álvarez-Amado, F. Volcanic and Saline Lithium Inputs to the Salarde Atacama. Minerals 2000, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.L.; Liu, L.H.; Li, Q.; Meng, L.Y.; Liu, C.L.; Zhang, Y.Y.; Wang, J.Y.; Yu, X.C.; Yan, K. Petrogeochemical characteristics and genetic analysis of the source area of brinety pelithium-potassium ore in Jitai Basin of Jiangxi province. Acta Petrol. Mineral. 2020, 39, 65–84. [Google Scholar]
- Liu, C.L.; Wang, M.L.; Jiao, P.C.; Chen, Y.Z. The exploration experiences of pot ash deposits in the world and probing of countermeasures of China’s future pot ash-deposits investigation. Geol. Chem. Miner. 2006, 28, 1–8. [Google Scholar]
- Munk, L.A.; Hynek, S.A.; Bradley, D.C.; Boutt, D.; Labay, K.; Jochens, H. Lithium Brines: A Global Perspective. Rev. Econ. Geol. 2016, 18, 339–365. [Google Scholar]
- Lowenstein, T.K.; Spencer, R.J.; Zhang, P.X. Origin of Ancient Potash Evaporites: Clues from the Modern Nonmarine Qaidam Basin of Western China. Science 1989, 245, 1090–1092. [Google Scholar] [CrossRef]
- Holmearda, J.G.; Hutchinsan, R.W. Potash-bearing evaporates in the Danakil area, Etiopia. Econ. Geol. 1968, 63, 129–132. [Google Scholar]
- Arod, A. Mineral spring and saline lake of the western rift Valley, Uganda. Geochim. Cosmochim. Acta 1969, 10, 1152–1162. [Google Scholar] [CrossRef]
- Yu, X.C.; Liu, C.L.; Wang, C.L.; Zhao, J.X.; Wang, J.Y. Origin of geothermal waters from the Upper Cretaceous to Lower Eocene strata of the Jiangling Basin, SouthChina: Constraints by multi-isotopic tracers and water-rock interactions. Appl. Geochem. 2020, 124, 104810. [Google Scholar] [CrossRef]
- Munk, L.; Chamberlain, C.P. Lithium Brine Resources: A Predictive Exploration Model, U.S. Geological Survey, Mineral Resources External Research Program-Final Technical Report: G10AP00056.2011. Available online: http://minerals.usgs.gov/mrerp/reports/Munk-G10AP00056 (accessed on 8 November 2021).
- Hofstra, A.H.; Todorov, T.I.; Mercer, C.N.; Adams, D.T.; Marsh, E.E. Silicate melt inclusion evidence for extremepre-eruptive enrichment and post-eruptive depletion of lithium in silicic volcanic rocks of the western United States: Implications for the origin of lithium-rich brines. Econ. Geol. 2013, 108, 1691–1701. [Google Scholar] [CrossRef]
- Von Strandmann, P.A.P.; Frings, P.J.; Murphy, M.J. Lithium isotope behavior during weathering in the Ganges Alluvial Plain. Geochim. Cosmochim. Acta 2017, 198, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Price, J.G.; Lechler, P.J.; Lear, M.B.; Giles, T.F. Possible Volcanic Source of Lithium in Brines in Clayton Valley, Nevada. Geology and Ore Deposits 2000: The Great Basin and Beyond Proceedings: Geological Society of Nevada Symposium Proceedings: Nevada, USA, 2000. pp. 241–248. Available online: https://www.gsnv.org/shop/geology-and-ore-deposits-2000-the-great-basin-and-beyond-symposium-proceedings/ (accessed on 23 November 2021).
- Araoka, D.; Kawahata, H.; Takagi, T.; Watanabeet, Y.; Nishimura, K.; Nishioal, Y. Lithium and strontium isotopic system atic s in playas in Nevada, USA: Constraints on the origin of lithium. Miner. Depos. 2013, 49, 371–379. [Google Scholar] [CrossRef]
- Li, R.Q.; Liu, C.L.; Chen, X.; Chen, Y.Z.; Wang, C.L. Salting law by cooling deep potassium-bearing brine in Jiangling depression. J. Salt Lake Res. 2013, 21, 1–6. [Google Scholar]
- Li, R.Q.; Chen, X.; Liu, C.L.; Ma, L.C. Study on loss mechanism of rubidium during potassiumcry stallization from potassium-rich brine of Jiangling sunken area. Inorg. Chem. Ind. 2014, 46, 18–20, 26. [Google Scholar]
- Peccerillo, R.; Taylor, S.R. Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, NorthernTurkey. Contrib. Mineral. Petr. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Risacher, F.; Alonso, H.; Salazar, C. The origin of brines and salts in Chilean salars: A hydrochemical review. Earth-Sci. Rev. 2003, 63, 249–293. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, C.L.; Han, J.J.; Tang, L.W.; Cao, Q.; Li, T. Some problems related to the evaluation of subsurface brine resources in deep-seated aquifers in sedimentary basins. Acta Geol. Sin. 2013, 34, 610–616. [Google Scholar]
- Gilder, S.A.; Keller, G.R.; Luo, M.; Goodell, P.C. Timing and spatial distribution of rifting in China. Tectonophysics 1991, 197, 225–243. [Google Scholar] [CrossRef]
- Yu, X.Q.; Shu, L.S.; Deng, P.; Wang, B.; Zu, F.P. The sedimentary features of the Jurassic-Tertiary terrestrial strata in southeast China. J. Stratigr. 2003, 27, 224–263. [Google Scholar]
- Zhang, Y.Q.; Zhao, Y.; Dong, S.W.; Yang, N. Tectonic evolution stages of the Early Cretaceous rift basins in Eastern China and adjacent areas and their geodynamic background. Front. Earth Sci.-Front. 2004, 11, 123–133. [Google Scholar]
- Xu, L.X.; Yan, C.D.; Yu, H.L.; Wang, B.Q.; Yu, F.Q.; Wang, D.F. Chronology of Paleogene volcanic rocks in Jianghan Basin. Oil Gas Geol. 1995, 16, 132–137. [Google Scholar]
- Schellart, W.P.; Lister, G.S. The role of the East Asian active margin in widespread extensional and strike-slip deformation in East Asia. J. Geol. Soc. Lond. 2005, 162, 959–972. [Google Scholar] [CrossRef]
- Zhu, R.X.; Xu, Y.G.; Zhu, G.; Zhang, H.F.; Zheng, T.Y. Destruction of the North China Craton. Sci. China (Ser. D) 2012, 55, 1565–1587. [Google Scholar] [CrossRef]
- Liu, S.F.; Steel, R.; Zhang, G.W. Mesozoic sedimentary basin development and tectonic implication, northern Yangtze Block, eastern China: Record of continent–continent collision. J. Asian Earth Sci. 2005, 25, 9–27. [Google Scholar] [CrossRef]
- Shen, C.B.; Donelick, R.A.; O’Sullivan, P.B.; Jonckheere, R.; Yang, Z.; She, Z.B.; Miu, X.L.; Ge, X. Provenance and hinterland exhumation from LA-ICP-MS zircon U–Pb and fission-track double dating of Cretaceous sediments in the Jianghan Basin, Yangtze block, central China. Sediment. Geol. 2012, 281, 194–207. [Google Scholar] [CrossRef]
- Teng, X.H.; Fang, X.M.; Kaufman, A.J.; Liu, C.L.; Wang, J.Y.; Zan, J.B.; Yang, Y.B.; Wang, C.L.; Xu, H.M.; Schulte, R.F.; et al. Sedimentological and mineralogical records from drill core SKD1 in the Jianghan Basin, Central China, and their implications for late Cretaceous–early Eocene climate change. J. Asian Earth Sci. 2019, 182, 103936. [Google Scholar] [CrossRef]
- Wang, C.L.; Meng, L.Y.; Liu, C.L.; Yu, X.C.; Yan, K.; Liu, S.H.; You, C.; Li, K.K.; Teng, X.H. Study on the genesis of Paleocene underground brineboron deposit in Jiangling Depression. Acta Petrol. Mineral. 2021, 40, 1–13. [Google Scholar]
- Wang, B.J.; Lin, C.S.; Chen, Y.; Lu, M.G.; Liu, J.Y. Episodic tectonic movement and evolutional character in Jianghan basin. Oil Geophys. Prospect. 2006, 41, 226–230. [Google Scholar]
- Wang, C.L.; Liu, C.L.; Wang, J.Y.; Yu, X.C.; Yan, K. Palynology and stratigraphy of the thick evaporate-bearing Shashi Formation in Jiangling Depression, Jianghan Basin of South China, and its paleoclimate change. China Geol. 2020, 2, 283–291. [Google Scholar]
- Shen, C.B.; Mei, L.F.; Peng, L.; Chen, Y.Z.; Yang, Z.; Hong, G.F. LA-ICPMS U–Pb zircon age constraints on the provenance of Cretaceous sediments in the Yichang area of the Jianghan Basin, central China. Cretac. Res. 2012, 34, 172–183. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, P.Z.; Lease, R.O.; Zheng, D.; Wan, J.; Wang, W.; Zhang, H. Eocene onset and late Miocene acceleration of Cenozoic intracontinental extension in the North Qinling range–Weihe graben: Insights from apatite fission track thermochronology. Tectonophysics 2013, 584, 281–296. [Google Scholar] [CrossRef]
- Xu, Y.G. Recycled oceanic crust in the source of 90–40 Ma basalts in North and Northeast China: Evidence, provenance and significance. Geochim. Cosmochim. Acta 2014, 143, 49–67. [Google Scholar] [CrossRef]
- Sun, J.X.; Li, N.; Zhang, W.Q. Petrogenesis and characteristics of the mantle source for the Quaternary Datong basalt: Research on the major, trace elements and Sr-Nd-Pb-Hf isotopes. Acta Petrol. Sin. 2020, 36, 3331–3345. [Google Scholar]
- Liu, F.; Cao, F.; Zhang, Z.X.; Li, Q. Chronology and geochemistry of the granite near the Keketuohai No3 pegmatite in Xinjiang. Acta Petrol. Sin. 2014, 30, 1–15. [Google Scholar]
- Li, J.K.; Liu, X.F.; Wang, D.H. The metallogenetic regularity of lithium deposit in China. Acta Geol. Sin. 2014, 88, 2269–2283. [Google Scholar]
- Middlemost, E.A.K. Naming Materialsinthe Magma/Igneous Rock System. Earth-Sci. Rev. 1994, 37, 215–244. [Google Scholar] [CrossRef]
- Yu, H.L.; Yan, C.D.; Yu, F.Q.; Wang, D.F. Geochemical characteristics and tectonic environment of Eogene basalts of the Jiangling hollow. Geol. Rev. 1996, 42, 44–51. [Google Scholar]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1997, 20, 325–343. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.F. Geological conditions for the formation of oil and gas in the second subsidence zone of the New Cathaysia Series. J. Daqing Petrol. Inst. 1997, 2, 7–21. [Google Scholar]
- Xu, Y.G.; Fan, Q.C. Cenozoic Volcanismin Eastern China: Reviewand Perspectives. Bull. Mineral. Petrol. Geochem. 2015, 34, 682–689. [Google Scholar]
- Lin, W.; Wang, Q.C. Late Mesozoic extensional tectonics in the North China block: A crustal response to subcontinental mantle removal? Bull. Soc. Geol. Fr. 2006, 117, 287–297. [Google Scholar] [CrossRef]
- Zhai, M.; Fan, Q.; Zhang, H.; Sui, J.; Shao, J.A. Lower crustal processes leading to Mesozoic lithospheric thinning beneath eastern North China: Underplating, replacement and delamination. Lithos 2007, 96, 36–54. [Google Scholar] [CrossRef]
- Mao, J.W.; Xie, G.Q.; Li, X.F.; Zhang, Z.H.; Wang, Y.T.; Wang, Z.L.; Zhao, C.S.; Yang, F.Q.; Li, H.M. Geodynamic process and metallogeny: History and present research trend, with a special discussion on continental accretion and related metallogeny throughout geological history in South China. Miner. Depos. 2005, 24, 193–205. [Google Scholar]
- Wu, L.L.; Mei, L.F.; Patonb, D.A. Late Cretaceous-Cenozoic intraplate extension and tectonic transitions in eastern China: Implications for intraplate geodynamic origin. Mar. Petrol. Geol. 2020, 117, 104379. [Google Scholar] [CrossRef]
- Yang, C.Q.; Chen, K.Q.; Chen, Z.Q.; Zhan, H.J. Constituent evolution and exploration potential in Jiangling depression. Nat. Gas Ind. 2003, 23, 51–54. [Google Scholar]
- Xu, Y.G.; Ma, J.L.; Frey, F.A.; Feigenson, M.D.; Liu, J.F. Role of lithosphere interaction in the genesis of Quaternary alkali and tholeiitic basalts from Datong, western North China Craton. Chem. Geol. 2005, 224, 247–271. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.X.; Graham, D.; Su, S.; Deng, J. Geochemistry of Cenozoic basalts and mantle xenoliths in Northeast China. Lithos 2007, 96, 108–126. [Google Scholar] [CrossRef]
- Palmer, M.R. Boron-isotope systematics of Halmahera arc (Indonesia) lavas: Evidence for involvement of the subducted slab. Geology 1991, 19, 215–217. [Google Scholar] [CrossRef]






| Sample | TDS (g/L) | pH | K (g/L) | Na (g/L) | Ca (g/L) | Mg (g/L) | Cl (g/L) | SO4 (g/L) | B (mg/L) | Li (mg/L) | Sr (mg/L) | Br (mg/L) | I (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1-1 | 331 | 5.90 | 10.18 | 111.87 | 4.16 | 0.22 | 183.48 | 0.41 | 950.66 | 104.71 | 409.73 | 461.92 | 31.91 |
| N1-2 | 335 | 5.93 | 10.26 | 113.93 | 4.23 | 0.21 | 184.59 | 0.32 | 971.99 | 105.58 | 406.38 | 440.30 | 31.75 |
| N1-3 | 332 | 5.99 | 10.35 | 112.82 | 4.35 | 0.22 | 182.68 | 0.54 | 960.62 | 107.41 | 402.02 | 433.02 | 31.95 |
| S1-1 | 359 | 6.52 | 6.99 | 106.10 | 13.56 | 0.04 | 202.02 | 0.26 | 588.23 | 48.61 | 1031.70 | 227.60 | 39.05 |
| S1-2 | 352 | 6.58 | 6.41 | 105.20 | 12.46 | 0.05 | 197.70 | 0.24 | 651.40 | 44.25 | 955.85 | 206.65 | 36.15 |
| S1-3 | 351 | 6.50 | 6.32 | 102.97 | 12.37 | 0.06 | 194.42 | 0.28 | 642.18 | 44.15 | 954.50 | 203.30 | 35.65 |
| Samples | SiO2 | TiO2 | Al2O3 | TFe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | Li (μg/g) | Zr (μg/g) | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JLB1 | 53.04 | 2.00 | 14.00 | 9.24 | 0.18 | 8.32 | 6.37 | 3.25 | 1.03 | 0.20 | 1.89 | 15.0 | 147 | 99.82 |
| JLB2 | 53.10 | 1.95 | 13.03 | 9.64 | 0.10 | 8.29 | 6.23 | 3.24 | 1.20 | 0.22 | 1.85 | 29.6 | 152 | 99.95 |
| JLB3 | 52.94 | 2.03 | 13.25 | 8.98 | 0.10 | 7.49 | 8.55 | 3.56 | 1.05 | 0.22 | 1.63 | 17.9 | 159 | 99.81 |
| JLB4 | 52.86 | 2.26 | 13.86 | 8.82 | 0.17 | 6.82 | 8.21 | 3.87 | 1.09 | 0.29 | 1.63 | 19.0 | 149 | 100.09 |
| 18DT10 | 49.77 | 2.28 | 14.77 | 12.65 | 0.15 | 7.29 | 8.84 | 3.08 | 0.82 | 0.37 | 0 | 7.26 | 158 | 100.02 |
| 18DT12 | 51.68 | 1.99 | 14.01 | 12.33 | 0.16 | 7.34 | 8.05 | 3.21 | 0.63 | 0.26 | 0 | 5.43 | 136 | 99.66 |
| 18DT13 | 53.45 | 1.82 | 14.14 | 11.95 | 0.16 | 6.71 | 7.83 | 3.28 | 0.75 | 0.21 | 0 | 5.34 | 124 | 100.28 |
| 18DT14 | 52.28 | 1.89 | 14.03 | 12.23 | 0.16 | 7.18 | 8.03 | 3.09 | 0.49 | 0.21 | 0 | 4.87 | 128 | 99.58 |
| Samples | SiO2 | TiO2 | Al2O3 | TFe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | Li (μg/g) | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THS-1 | 73.05 | 0.15 | 14.10 | 1.50 | 0.04 | 0.30 | 1.26 | 3.62 | 5.35 | 0.08 | 0.48 | 90.6 | 100.12 |
| THS-2 | 70.33 | 0.35 | 15.14 | 2.23 | 0.04 | 0.63 | 2.04 | 2.88 | 4.64 | 0.13 | 1.06 | 82.0 | 99.85 |
| THS-3 | 71.30 | 0.17 | 14.39 | 1.89 | 0.04 | 0.48 | 1.88 | 3.17 | 5.19 | 0.11 | 0.89 | 102.0 | 99.78 |
| THS-4 | 70.78 | 0.36 | 15.10 | 1.96 | 0.05 | 0.51 | 1.84 | 3.12 | 4.96 | 0.09 | 0.77 | 82.0 | 99.82 |
| KKTH117 | 73.45 | 0.18 | 14.22 | 1.74 | 0.06 | 0.37 | 2.05 | 3.29 | 3.26 | 0.04 | 0.63 | 72 | 99.93 |
| KKTH118 | 72.96 | 0.23 | 14.24 | 2.16 | 0.05 | 0.52 | 2.33 | 3.11 | 3.13 | 0.05 | 0.64 | 82 | 100.14 |
| KKTH119 | 70.69 | 0.18 | 15.74 | 1.68 | 0.04 | 0.35 | 2.13 | 3.06 | 4.94 | 0.05 | 0.53 | 80.8 | 99.87 |
| KKTH120 | 72.30 | 0.19 | 14.96 | 1.86 | 0.05 | 0.42 | 2.21 | 3.06 | 3.91 | 0.07 | 0.80 | 67.6 | 100.46 |
| KKTH121 | 73.81 | 0.21 | 14.00 | 2.15 | 0.05 | 0.49 | 2.43 | 3.36 | 2.62 | 0.06 | 0.60 | 83.1 | 100.24 |
| Samples | Lithology | Fluid | Time (Days) | Ion Concentration (mg·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Sr | B | Br | I | ||||
| JLB1 | Basalt | Distilled water | 1 | 1.1 | 5. 63 | 0.55 | 0.04 | - | - | - |
| JLB1 | Basalt | Distilled water | 5 | 1.12 | 8.62 | 0.79 | 0.05 | - | - | - |
| JLB1 | Basalt | Distilled water | 11 | 1.56 | 11.58 | 1.29 | 0.06 | - | - | - |
| JLB1 | Basalt | Distilled water | 19 | 2.28 | 14.80 | 1.59 | 0.07 | - | - | - |
| JLB1 | Basalt | Distilled water | 29 | 2.88 | 17.82 | 1.98 | 0.08 | - | - | - |
| JLB1 | Basalt | Distilled water | 41 | 3.82 | 19.99 | 2.21 | 0.09 | - | - | - |
| JLB1 | Basalt | Distilled water | 55 | 5.12 | 21.92 | 2.65 | 0.11 | - | - | - |
| JLB1 | Basalt | 1 mol/L NaCl | 1 | 3.06 | 226.91 | 12.40 | 1.25 | 0.07 | 3.23 | 0.16 |
| JLB1 | Basalt | 1 mol/L NaCl | 5 | 3.15 | 245.69 | 13.95 | 1.33 | 0.07 | 3.48 | 0.15 |
| JLB1 | Basalt | 1 mol/L NaCl | 11 | 3.28 | 259.68 | 14.41 | 1.39 | 0.07 | 3.46 | 0.16 |
| JLB1 | Basalt | 1 mol/L NaCl | 19 | 3.36 | 275.64 | 14.82 | 1.34 | 0.07 | 3.39 | 0.14 |
| JLB1 | Basalt | 1 mol/L NaCl | 29 | 4.40 | 279.88 | 14.65 | 1.41 | 0.08 | 3.16 | 0.16 |
| JLB1 | Basalt | 1 mol/L NaCl | 41 | 7.97 | 288.28 | 18.66 | 1.52 | 0.08 | 3.98 | - |
| JLB1 | Basalt | 1 mol/L NaCl | 55 | 10.90 | 299.40 | 18.97 | 1.59 | 0.08 | 4.87 | - |
| JLB1 | Basalt | 2 mol/L NaCl | 1 | 6.77 | 272.96 | 18.87 | 1.55 | 0.25 | 8.45 | 0.45 |
| JLB1 | Basalt | 2 mol/L NaCl | 5 | 6.98 | 301.85 | 20.35 | 1.68 | 0.28 | 7.99 | 0.41 |
| JLB1 | Basalt | 2 mol/L NaCl | 11 | 7.42 | 321.52 | 20.81 | 1.72 | 0.26 | 8.68 | 0.43 |
| JLB1 | Basalt | 2 mol/L NaCl | 19 | 8.51 | 322.65 | 20.95 | 1.74 | 0.25 | 7.55 | 0.19 |
| JLB1 | Basalt | 2 mol/L NaCl | 29 | 8. 98 | 324.84 | 20.54 | 1.73 | 0.32 | 8.52 | 0.35 |
| JLB1 | Basalt | 2 mol/L NaCl | 41 | 11.93 | 328.15 | 21.58 | 1.78 | 0.28 | 10.28 | - |
| JLB1 | Basalt | 2 mol/L NaCl | 55 | 14.86 | 343.45 | 22.79 | 1.90 | 0.28 | 10.13 | - |
| JLB1 | Basalt | Ph = 5 | 1 | 0.31 | 22.51 | 4.03 | 0.12 | |||
| JLB1 | Basalt | Ph = 5 | 5 | 0.42 | 36.98 | 7.10 | 0.19 | |||
| JLB1 | Basalt | Ph = 5 | 11 | 0.43 | 39.05 | 7.96 | 0.19 | |||
| JLB1 | Basalt | Ph = 5 | 19 | 0.42 | 38.54 | 8.45 | 0.20 | |||
| JLB1 | Basalt | Ph = 5 | 29 | 0.44 | 40.72 | 8.91 | 0.21 | |||
| JLB1 | Basalt | Ph = 5 | 41 | 0 | 38.64 | 9.35 | 0.20 | |||
| JLB1 | Basalt | Ph = 5 | 55 | 10.44 | 40.69 | 10.08 | 0.20 | |||
| JLB1 | Basalt | Ph = 8 | 1 | 0 | 0.24 | 0.31 | 0 | |||
| JLB1 | Basalt | Ph = 8 | 5 | 16.96 | 0.79 | 0.44 | 0 | |||
| JLB1 | Basalt | Ph = 8 | 11 | 10.55 | 1.28 | 0.56 | 0 | |||
| JLB1 | Basalt | Ph = 8 | 19 | 12.42 | 1.73 | 0.69 | 0.01 | |||
| JLB1 | Basalt | Ph = 8 | 29 | 26.81 | 1.99 | 0.88 | 0.01 | |||
| JLB1 | Basalt | Ph = 8 | 41 | 0 | 2.38 | 0.96 | 0.01 | |||
| JLB1 | Basalt | Ph = 8 | 55 | 3.57 | 2.93 | 1.18 | 0.01 | |||
| THS-1 | Granite | Distilled water | 1 | 0.42 | 0.56 | 0.04 | 0.01 | - | - | - |
| THS-1 | Granite | Distilled water | 5 | 0.52 | 0.93 | 0.06 | 0.01 | - | - | - |
| THS-1 | Granite | Distilled water | 11 | 0.64 | 1.65 | 0.11 | 0.02 | - | - | - |
| THS-1 | Granite | Distilled water | 19 | 0.83 | 1.98 | 0.13 | 0.02 | - | - | - |
| THS-1 | Granite | Distilled water | 29 | 0.74 | 2.77 | 0.15 | 0.03 | - | - | - |
| THS-1 | Granite | Distilled water | 41 | 0.91 | 3.29 | 0.23 | 0.03 | - | - | - |
| THS-1 | Granite | Distilled water | 55 | 1.49 | 4.11 | 0.26 | 0.04 | - | - | - |
| THS-1 | Granite | 1 mol/L NaCl | 1 | 0.88 | 18.33 | 0.69 | 0.07 | 0.03 | 2.22 | 0.10 |
| THS-1 | Granite | 1 mol/L NaCl | 5 | 0.9 | 21.48 | 0.78 | 0.08 | 0.03 | 2.19 | 0.11 |
| THS-1 | Granite | 1 mol/L NaCl | 11 | 0.95 | 22.69 | 0.78 | 0.08 | 0.04 | 2.25 | 0.10 |
| THS-1 | Granite | 1 mol/L NaCl | 19 | 1.02 | 23.94 | 0.81 | 0.08 | 0.03 | 2.15 | 0.11 |
| THS-1 | Granite | 1 mol/L NaCl | 29 | 1.03 | 24.49 | 0.84 | 0.08 | 0.04 | 3.14 | 0.11 |
| THS-1 | Granite | 1 mol/L NaCl | 41 | 1.08 | 27.06 | 1.11 | 0.09 | 0.04 | 3.13 | - |
| THS-1 | Granite | 1 mol/L NaCl | 55 | 1.18 | 27.87 | 1.15 | 0.10 | 0.04 | 3.16 | - |
| THS-1 | Granite | 2 mol/L NaCl | 1 | 2.18 | 22.66 | 1.01 | 0.09 | 0.12 | 5.32 | 0.20 |
| THS-1 | Granite | 2 mol/L NaCl | 5 | 2.26 | 26.68 | 1.06 | 0.09 | 0.15 | 5.65 | 0.27 |
| THS-1 | Granite | 2 mol/L NaCl | 11 | 2.4 | 27.67 | 1.08 | 0.09 | 0.16 | 5.22 | 0.31 |
| THS-1 | Granite | 2 mol/L NaCl | 19 | 2.66 | 28.99 | 1.13 | 0.10 | 0.18 | 4.96 | 0.32 |
| THS-1 | Granite | 2 mol/L NaCl | 29 | 2.88 | 30.14 | 1.14 | 0.10 | 0.11 | 5.86 | 0.28 |
| THS-1 | Granite | 2 mol/L NaCl | 41 | 3.15 | 30.85 | 1.22 | 0.11 | 0.13 | 7.23 | - |
| THS-1 | Granite | 2 mol/L NaCl | 55 | 5.29 | 30.67 | 1.22 | 0.11 | 0.13 | 7.44 | - |
| THS-1 | Granite | Ph = 5 | 1 | 0.07 | 4.55 | 0.24 | 0.12 | |||
| THS-1 | Granite | Ph = 5 | 5 | 0.09 | 5.89 | 0.36 | 0.15 | |||
| THS-1 | Granite | Ph = 5 | 11 | 0.09 | 6.63 | 0.51 | 0.18 | |||
| THS-1 | Granite | Ph = 5 | 19 | 0.09 | 6.85 | 0.63 | 0.21 | |||
| THS-1 | Granite | Ph = 5 | 29 | 0.07 | 7.46 | 0.75 | 0.22 | |||
| THS-1 | Granite | Ph = 5 | 41 | 3.19 | 7.64 | 1.23 | 0.27 | |||
| THS-1 | Granite | Ph = 5 | 55 | - | 8.06 | 1.26 | 0.34 | |||
| THS-1 | Granite | Ph = 8 | 1 | 2.34 | 0.63 | 0.02 | 0 | |||
| THS-1 | Granite | Ph = 8 | 5 | 3.14 | 1.47 | 0.11 | 0 | |||
| THS-1 | Granite | Ph = 8 | 11 | 3.20 | 1.60 | 0.15 | 0.01 | |||
| THS-1 | Granite | Ph = 8 | 19 | 2.12 | 2.81 | 0.21 | 0.01 | |||
| THS-1 | Granite | Ph = 8 | 29 | 4.23 | 3.13 | 0.30 | 0.01 | |||
| THS-1 | Granite | Ph = 8 | 41 | 6.83 | 4.20 | 0.37 | 0.01 | |||
| THS-1 | Granite | Ph = 8 | 55 | - | 5.42 | 0.43 | 0.02 | |||
| Samples | Lithology | Fluid (NaCl) | T (°C) | Time (h) | Ion Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Li | Mg | Sr | Ca | S | Br | |||||
| JLB1 | Basalt | 1 mol/L | 150 | 1 | 28.59 | 0.03 | 102.3 | 0.52 | 7.99 | 1.61 | 0.31 |
| JLB1 | Basalt | 1 mol/L | 150 | 2 | 25.98 | 0.01 | 117.57 | 1.67 | 7.74 | 1.51 | 0.21 |
| JLB1 | Basalt | 1 mol/L | 150 | 3 | 33.50 | 0.05 | 73.11 | 0.51 | 9.97 | 1.92 | 0.22 |
| JLB1 | Basalt | 1 mol/L | 150 | 4 | 19.66 | 0.03 | 125.52 | 0.68 | 6.42 | 1.23 | 0.55 |
| JLB1 | Basalt | 1 mol/L | 150 | 5 | 27.04 | 0.02 | 89.52 | 0.40 | 9.32 | 1.91 | 0.61 |
| JLB1 | Basalt | 1 mol/L | 150 | 6 | 22.13 | 0.01 | 124.28 | 0.57 | 6.85 | 1.87 | 0.60 |
| THS-1 | Granite | 1 mol/L | 150 | 1 | 9.76 | 0.04 | 39.37 | 0.07 | 0.28 | 0.67 | 0.49 |
| THS-1 | Granite | 1 mol/L | 150 | 2 | 8.05 | 0.05 | 28.47 | 0.06 | 0.62 | 0.58 | 0.47 |
| THS-1 | Granite | 1 mol/L | 150 | 3 | 11.32 | 0.06 | 35.51 | 0.05 | 0.84 | 0.69 | 0.41 |
| THS-1 | Granite | 1 mol/L | 150 | 4 | 14.21 | 0.09 | 38.04 | 0.03 | 0.39 | 0.66 | 0.43 |
| THS-1 | Granite | 1 mol/L | 150 | 5 | 16.68 | 0.05 | 44.27 | 0.04 | 0.46 | 0.96 | 0.63 |
| THS-1 | Granite | 1 mol/L | 150 | 6 | 3.87 | 0.02 | 21.66 | 0.03 | 0.25 | 0.86 | 0.49 |
| Samples | Lithology | Fluid/Solution | T (°C) | Ion Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Sr | Li | Br | ||||
| JLB1 | Basalt | Distilled water | 200 | 2.423 | 17.047 | 1.300 | - | 0.005 | 0.184 |
| JLB1 | Basalt | Distilled water | 250 | 6.424 | 13.181 | 0.541 | - | 0.014 | 0.197 |
| JLB1 | Basalt | Distilled water | 300 | 4.172 | 1.136 | 0.172 | - | 0.013 | - |
| JLB1 | Basalt | Distilled water | 350 | 3.455 | 0.625 | 0.054 | - | 0.005 | - |
| JLB1 | Basalt | Distilled water | 400 | 2.344 | 0.385 | 0.020 | - | 0.002 | - |
| JLB1 | Basalt | 1 mol/L NaCl | 200 | 30.517 | 764.660 | 12.202 | 4.472 | 0.042 | 0.676 |
| JLB1 | Basalt | 1 mol/L NaCl | 250 | 54.629 | 687.422 | 0.857 | 4.229 | 0.131 | 0.640 |
| JLB1 | Basalt | 1 mol/L NaCl | 300 | 40.015 | 459.802 | 0.319 | 2.509 | 0.152 | - |
| JLB1 | Basalt | 1 mol/L NaCl | 350 | 31.343 | 198.168 | 0.122 | 0.785 | 0.091 | - |
| JLB1 | Basalt | 1 mol/L NaCl | 400 | 24.114 | 156.936 | 0.050 | 0.042 | 0.035 | - |
| THS-1 | Granite | Distilled water | 200 | 0.579 | - | - | - | 0.044 | 0.115 |
| THS-1 | Granite | Distilled water | 250 | 1.572 | 0.047 | - | - | 0.087 | 0.142 |
| THS-1 | Granite | Distilled water | 300 | 0.559 | 0.025 | - | - | 0.043 | 0.179 |
| THS-1 | Granite | Distilled water | 350 | 0.656 | - | - | - | 0.055 | - |
| THS-1 | Granite | Distilled water | 400 | 2.631 | 1.949 | 0.023 | - | 0.077 | - |
| THS-1 | Granite | 1 mol/L NaCl | 200 | 10.643 | 6.127 | - | 4.216 | 0.764 | 0.761 |
| THS-1 | Granite | 1 mol/L NaCl | 250 | 19.490 | 5.408 | - | 4.306 | 0.626 | 0.613 |
| THS-1 | Granite | 1 mol/L NaCl | 300 | 12.472 | 3.065 | - | 2.561 | 0.781 | 0.580 |
| THS-1 | Granite | 1 mol/L NaCl | 350 | 11.186 | 1.441 | - | 1.322 | 3.525 | - |
| THS-1 | Granite | 1mol/L NaCl | 400 | 8.705 | 0.723 | 0.151 | 0.361 | 3.373 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yu, X.; Li, R.; Liu, L.; Yan, K.; You, C. Origin of Lithium–Potassium-Rich Brines in the Jianghan Basin, South China: Constraints by Water–Rock Reactions of Mesozoic–Cenozoic Igneous Rocks. Minerals 2021, 11, 1330. https://doi.org/10.3390/min11121330
Wang C, Yu X, Li R, Liu L, Yan K, You C. Origin of Lithium–Potassium-Rich Brines in the Jianghan Basin, South China: Constraints by Water–Rock Reactions of Mesozoic–Cenozoic Igneous Rocks. Minerals. 2021; 11(12):1330. https://doi.org/10.3390/min11121330
Chicago/Turabian StyleWang, Chunlian, Xiaocan Yu, Ruiqin Li, Lihong Liu, Kai Yan, and Chao You. 2021. "Origin of Lithium–Potassium-Rich Brines in the Jianghan Basin, South China: Constraints by Water–Rock Reactions of Mesozoic–Cenozoic Igneous Rocks" Minerals 11, no. 12: 1330. https://doi.org/10.3390/min11121330
APA StyleWang, C., Yu, X., Li, R., Liu, L., Yan, K., & You, C. (2021). Origin of Lithium–Potassium-Rich Brines in the Jianghan Basin, South China: Constraints by Water–Rock Reactions of Mesozoic–Cenozoic Igneous Rocks. Minerals, 11(12), 1330. https://doi.org/10.3390/min11121330






