Genesis of the Weizigou Au Deposit, Heilongjiang Province, NE China: Constraints from LA-ICP-MS Trace Element Analysis of Magnetite, Pyrite and Pyrrhotite, Pyrite Re-Os Dating and S-Pb Isotopes
Abstract
:1. Introduction
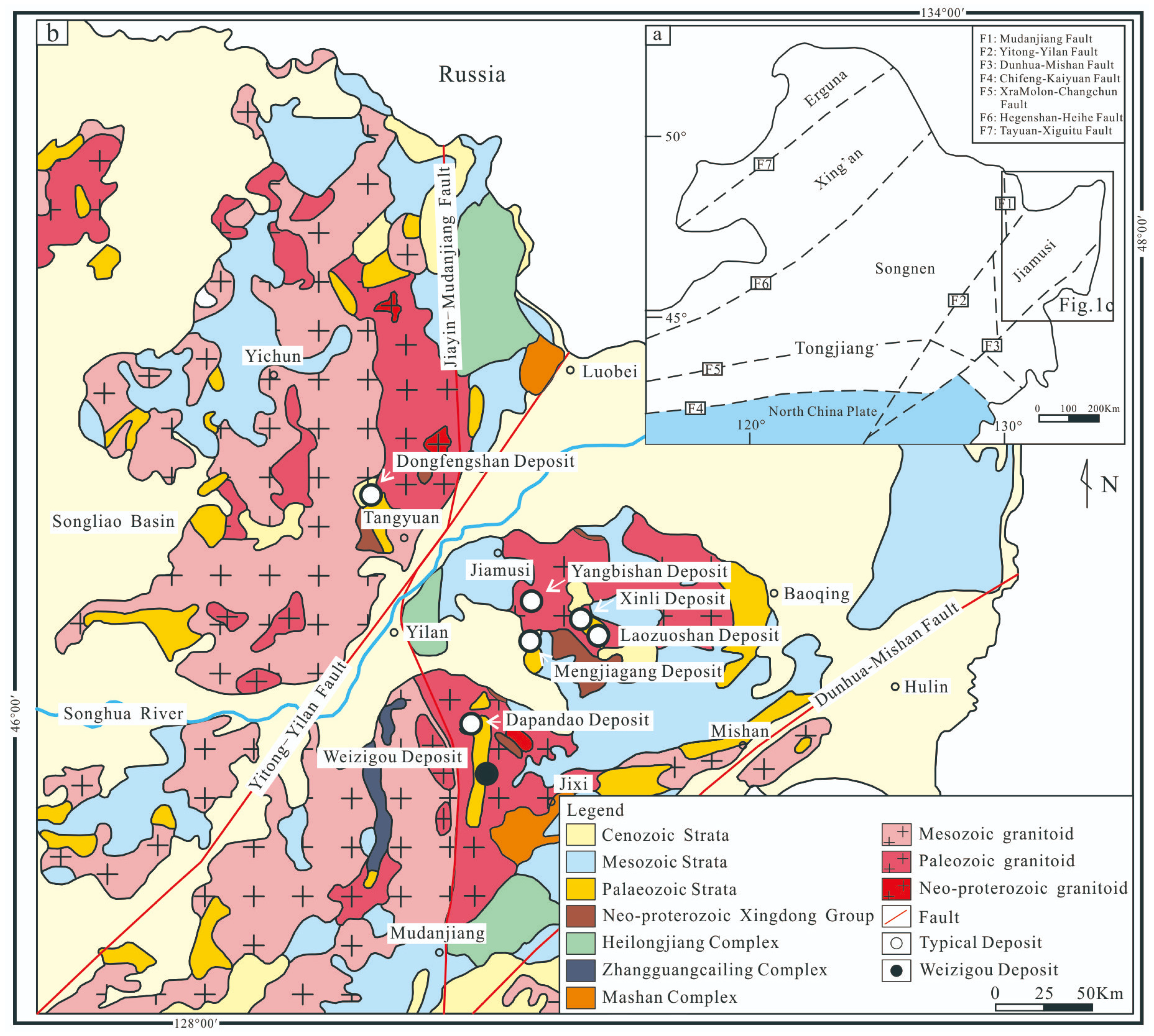
2. Geological Background
3. Deposit Geology
4. Samples and Analytical Methods
4.1. Pyrite Re-Os Dating
4.2. LA-ICP-MS Trace Element Analysis of Magnetite
4.3. LA-ICP-MS Trace Element Analysis of Pyrite and Pyrrhotite
4.4. S-Pb Isotope
5. Results
5.1. Pyrite Re-Os Dating
5.2. LA-ICP-MS Trace Element Analysis of Magnetite
5.3. LA-ICP-MS Trace Element Analysis of Pyrite and Pyrrhotite
5.4. Sulfur and Lead Isotopes
6. Discussion
6.1. Magnetite Genetic Type
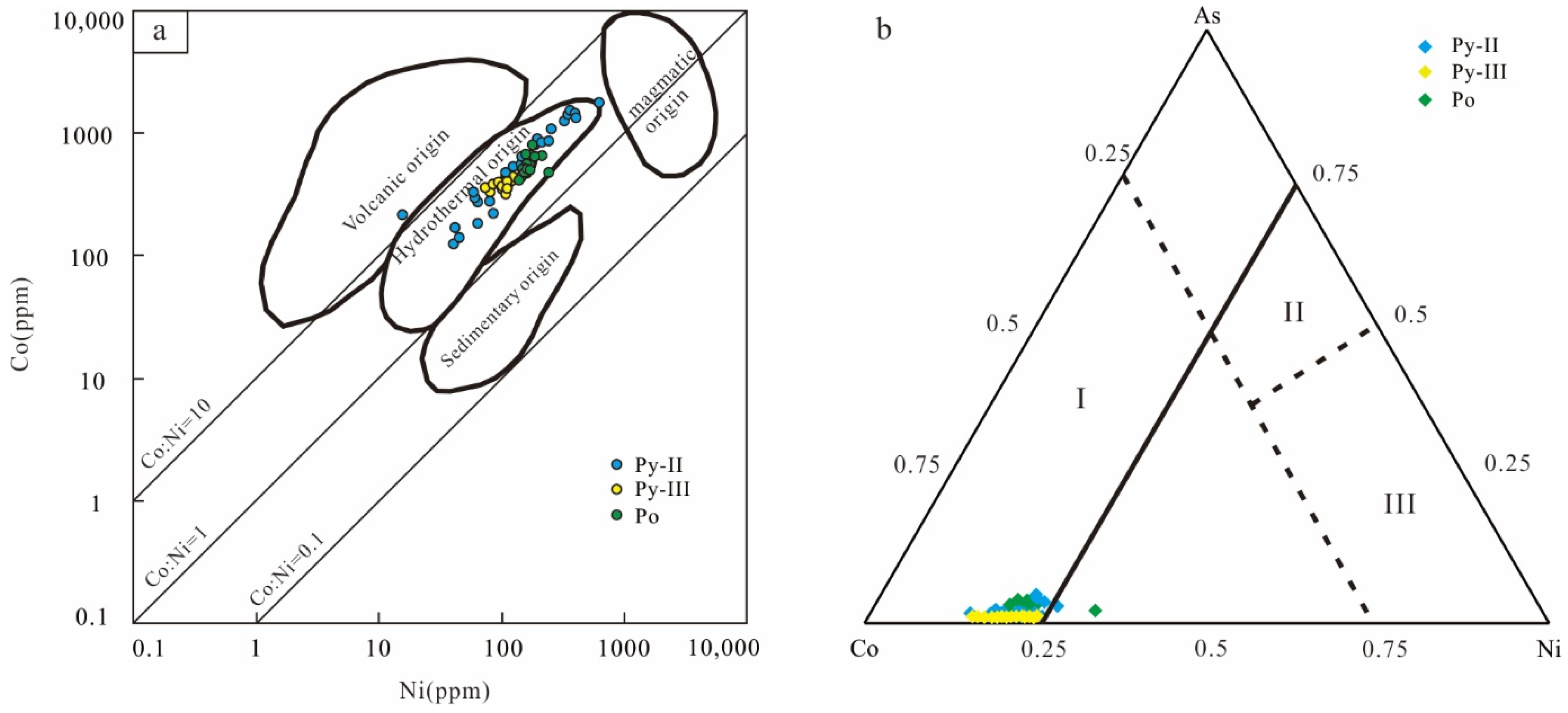
6.2. Pyrite and Pyrrhotite Genetic Type and S-Pb Sources
6.3. Ore Deposit Mineralization Age and Genetic Type
7. Conclusions
- There are four ore bodies in the Weizigou deposit, all of which are vein-like closely co-existing with meta-gabbro. The mineralization of gold and iron only develops in meta-gabbro. Wall rock alteration is developed, mainly chlorite, epidote and biotite. The main metal minerals developed in the deposit include magnetite, pyrite, pyrrhotite and chalcopyrite.
- Four pyrite samples yielded a Re-Os icochron age of 197 ± 11 Ma and Osi values are similar to that of the lower crust.
- Sulfur maybe derived from the granitic gneiss and meta-gabbro exposed in the orefield and lead has a mixed source. Carbon mainly derived from the granitic gneiss.
- The in situ LA-ICP-MS trace element analysis results show that the formation of magnetite is closely related to IOCG-type mineralization, and the formation of pyrite and pyrrhotite is related to magmatic hydrothermal fluid.
- The Weizigou Au deposit experienced two-period mineralization, namely the IOCG-type mineralization in the Late Permian and the magmatic hydrothermal mineralization in the Early Jurassic.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, J.W.; Yu, J.J.; Yuan, S.D.; Cheng, Y.B.; Xie, G.Q.; Hou, K.J.; Xiang, J.F.; Yang, Z.X. Iron oxide-copper-gold deposits: Characteristics, present research situation and ore prospecting. Miner. Depos. 2008, 6, 267–278. (In Chinese) [Google Scholar]
- Zhao, X.F.; Zhou, M.F. Fe-Cu deposits in the Kangdian region, SW China: A Proterozoic IOCG (iron-oxide-copper-gold) metallogenic province. Miner. Depos. 2011, 46, 731–747. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.F.; Li, X.C.; Zhou, M.F. An overview on the characteristics and origin of iron-oxide copper gold (IOCG) deposits in China. Acta Petrol. Sin. 2019, 35, 99–118. (In Chinese) [Google Scholar]
- Roberts, D.E.; Hudson, G.R.T. The Olympic Dam copper-uranium-gold deposit, Roxby Downs, South Australia. Econ. Geol. 1983, 78, 799–822. [Google Scholar] [CrossRef]
- Hitzman, M.W.; Oreskes, N.; Einaudi, M.T. Geological characteristics and tectonic setting of proterozoic iron oxide (Cu-U-Au-REE) deposits. Precambrian Res. 1992, 58, 241–287. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Iron oxide-copper-gold deposits: An Andean view. Miner. Depos. 2003, 38, 787–812. [Google Scholar] [CrossRef]
- Zhang, X.C. The characteristics of the overseas iron-oxide Cu-Au deposits and the present situation of the studies. Adv. Earth Sci. 2003, 18, 551–560. (In Chinese) [Google Scholar]
- Oliver, N.H.S.; Cleverley, J.S.; Mark, G.; Pollard, P.J.; Fu, B.; Marshall, L.J.; Rubenach, M.J.; Williams, P.J.; Baker, T. Modeling the role of sodic alteration in the genesis of iron oxide-copper-gold deposits, Eastern Mount Isa block, Australia. Econ. Geol. 2004, 99, 1145–1176. [Google Scholar] [CrossRef]
- Williams, P.J.; Barton, M.; Johnson, D.A.; Fontboté, L.; de Haller, A.; Mark, G.; Oliver, N.H.S.; Marschik, R. Iron oxide copper-gold deposits: Geology, space-time distribution, and possible modes of origin. Econ. Geol. 2005, 100, 371–405. [Google Scholar]
- Pollard, P.J. An intrusion-related origin for Cu-Au mineralization in iron oxide-copper-gold (IOCG) provinces. Miner. Depos. 2006, 41, 179–187. [Google Scholar] [CrossRef]
- Nie, F.J.; Jiang, S.H.; Lu, Y.M. Geological features, ore-forming processes and prospecting model of iron oxide-copper-gold deposits. Geol. China 2008, 35, 1074–1087. (In Chinese) [Google Scholar]
- Groves, D.I.; Bierlein, F.P.; Meinert, L.D.; Hitzman, M.W. Iron Oxide Copper-Gold (IOCG) Deposits through Earth History: Implications for Origin, Lithospheric Setting, and Distinction from Other Epigenetic Iron Oxide Deposits. Econ. Geol. 2010, 105, 641–654. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y. Externa sulphur in IOCG mineralization: Implications on difinition and classification of the IOCG clan. Ore Geol. Rev. 2013, 51, 74–78. [Google Scholar] [CrossRef]
- Zhao, X.F.; Zhou, M.F.; Su, Z.K.; Li, X.C.; Chen, W.T.; Li, J.W. Geology, Geochronology, and Geochemistry of the Dahongshan Fe-Cu-(Au-Ag) Deposit, Southwest China: Implications for the Formation of Iron Oxide Copper-Gold Deposits in Intracratonic Rift Settings. Econ. Geol. 2017, 112, 603–628. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikon, W.; Robert, F.; Danyushevsky, L.V.; Chang, Z.S. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi Log deposit, Lena gold province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Large, R.R.; Halpin, J.A.; Danyushevsky, L.V.; Maslennikov, V.V.; Bull, S.W.; Long, J.A.; Gregory, D.D.; Lounejev, E.; Lyons, T.W.; Sack, P.J. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean-atmosphere evolution. Earth Planet. Sci. Lett. 2014, 389, 209–220. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and as in pyrite from hydrothermal ore deposits. Geochem. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef] [Green Version]
- Franchini, M.; Mcfarlane, C.; Maydagán, L.; Reich, M.; Lentz, D.R.; Meinert, L.; Bouhier, V. Trace metals in pyrite and marcasite from the Agua Rica porphyry-high sulfidation epithermal deposit, Catamarca, Argentina: Textural features and metal zoning at the porphyry to epithermal transition. Ore Geol. Rev. 2015, 66, 366–387. [Google Scholar] [CrossRef]
- Ye, T.; Li, N. The In-situ LA-ICPMS analyses of minor and trace elements in pyrite and its implication in Au deposit. Chin. J. Geol. 2015, 50, 1178–1199. (In Chinese) [Google Scholar]
- Belousov, I.; Large, R.R.; Meffre, S.; Danyushevsky, L.V.; Steadman, J.; Beardsmore, T. Pyrite compositions from VHMS and orogenic Au deposits in the Yilgarn Craton, Western Australia: Implications for gold and copper exploration. Ore Geol. Rev. 2016, 79, 474–499. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in Orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.; Chryssoulis, S.; Li, J.W.; Ma, C.Q.; Parada, M.A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochim. Cosmochim. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lan, T.G.; Wang, H.; Tang, Y.W.; Dai, Z.H. LA-ICP-MS trace element characteristics of magnetite from the Zhangjiawa iron deposit, Laiwu and constraints on metallogenic processes. Earth Sci. Front. 2018, 25, 32–49. [Google Scholar]
- Dora, M.L.; Upadhyay, D.; Randive, K.R.; Shareef, M.; Baswani, S.R.; Ranjan, S. Trace element geochemistry of magnetite and pyrite and sulfur isotope geochemistry of pyrite and barite from the Thanewasna Cu-(Au) deposit, western Bastar Craton, central India: Implication for ore genesis. Ore Geol. Rev. 2020, 117, 103262. [Google Scholar] [CrossRef]
- Liang, P.; Wu, C.; Hu, X.; Xie, Y.L. Textures and geochemistry of magnetite: Indications for genesis of the Late Paleozoic Laoshankou Fe-Cu-Au deposit NW China. Ore Geol. Rev. 2020, 124, 103632. [Google Scholar] [CrossRef]
- Zhang, Y.; Lian, Y.L.; Jin, E.M. Geological features and prospecting orientation of the Weizigou gold deposit in Heilongjiang Province. Geol. Resour. 2011, 20, 426–429. (In Chinese) [Google Scholar]
- Cheng, J. Geological Characteristics and Prospecting Direction of the Weizigou Gold Deposit in the Jixi County, Heilongjiang. Master’s Thesis, Jilin University, Changchun, China, 2013. (In Chinese). [Google Scholar]
- Bo, J.W. Metallogenic characteristics and comprehensive information prediction of Fe and Au deposits in Jiantang area Heilongjiang Province. Master’s Thesis, Jilin University, Changchun, China, 2016. (In Chinese). [Google Scholar]
- Wang, F.; Xu, W.L.; Xu, Y.G.; Gao, F.H.; Ge, W.C. Late Triassic bimodal igneous rocks in eastern Heilongjiang Province, NE China: Implications for the initiation of subduction of the Paleo-Pacific Plate beneath Eurasia. J. Asian Earth Sci. 2015, 97, 406–423. [Google Scholar] [CrossRef]
- Heilongjiang Bureau of Geology and Mineral Resources. Regional Geology of Heilongjiang Province; Geological Publishing House: Beijing, China, 1993; pp. 347–418. (In Chinese) [Google Scholar]
- Bai, J.W.; Wang, W.X.; Zhang, H.R. Character of glaucophane schists in metamorphic zone in Yilan, Mudanjiang, Heilongjiang. Acta Petrol. Mineral. 1988, 7, 298–308. (In Chinese) [Google Scholar]
- Wu, F.Y.; Yang, J.H.; Lo, C.H.; Wilde, S.A.; Sun, D.Y.; Jahn, B.M. The Heilongjiang Group: A Jurassic accretionary complex in the Jiamusi Massif at the western Pacific margin of northeastern China. Isl. Arc 2007, 16, 156–172. [Google Scholar] [CrossRef]
- Zhou, J.B.; Pu, X.G.; Hou, H.S.; Han, W.; Cao, J.L.; Li, G.Y. The Mesozoic accretionary complex in NE China and its tectonic implications for the subduction of the Paleo-Pacific plate beneath the Eurasia. Acta Petrol. Sin. 2018, 34, 2845–2856. [Google Scholar]
- Li, W.M.; Liu, Y.J.; Zhao, Y.L.; Feng, Z.Q.; Zhou, J.P.; Wen, Q.B.; Liang, C.Y.; Zhang, D. Tectonic evolution of the Jiamusi Block, NE China. Acta Petrol. Sin. 2020, 36, 665–684. (In Chinese) [Google Scholar]
- Wu, F.Y.; Sun, D.Y.; Ge, W.C.; Zhang, Y.B.; Grant, M.L.; Wilde, S.A.; Jahn, B.M. Geochronology of the Phanerozoic granitoids in northeastern China. J. Asian Earth Sci. 2011, 41, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.S. Regional metamorphism and evolution of Mashan khondalite series. Acta Petrol. Mineral. 1992, 11, 97–100. (In Chinese) [Google Scholar]
- Jiang, J.S. Geochemistry of Mashan-Group khondalite series. Geochemica 1993, 2, 363–372. (In Chinese) [Google Scholar]
- Guo, X.Z.; Takasu, A.; Liu, Y.J.; Li, W.M. Zn-rich spinel in association with quartz in the Al-rich metapelites from the Mashan Khondalite series, NE China. J. Earth Sci. 2014, 25, 207–223. [Google Scholar] [CrossRef]
- Li, W.M.; Takasu, A.; Liu, Y.J.; Genser, J.; Neubauer, F.; Guo, X.Z. 40Ar/39Ar ages of the high-P/T metamorphic rocks of the Heilongjiang Complex in the Jiamusi Massif, northeastern China. J. Mineral. Petrol. Sci. 2009, 104, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Li, W.M.; Takasu, A.; Liu, Y.J.; Genser, J.; Zhao, Y.L.; Han, G.; Guo, X.Z. U-Pb and 40Ar/39Ar age constrains on protolish and high-P/T type metamorphism of the Heilongjiang Complex in the Jiamusi Massif, NE China. J. Mineral. Petrol. Sci. 2010, 106, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.B.; Wilde, S.A.; Zhao, G.C.; Zhang, X.Z.; Zheng, C.Q.; Wang, Y.J.; Zhang, X.H. The onset of Pacific margin accretion in NE China: Evidence form the Heilongjiang high-pressure metamorphic belt. Tectonophysics 2009, 478, 230–246. [Google Scholar] [CrossRef]
- Wang, C.W.; Jin, W.; Zhang, X.Z.; Ma, Z.H.; Chi, X.G.; Liu, Y.J.; Li, N. New understanding of the late Paleozoic tectonics in northeastern China and adjacent areas. J. Stratigr. 2008, 32, 119–136. [Google Scholar]
- Ma, Y.P.; Ren, Y.S.; Hao, Y.J.; Lai, K.; Zhao, H.L.; Liu, J. Genesis and material source of scheelite of Yangbishan iron-tungsten deposit in Heilongjiang, NE China. J. Jilin Univ. Earth Sci. Ed. 2018, 48, 105–117. (In Chinese) [Google Scholar]
- Sun, M.D. Late Mesozoic Magmatism and Its Tectonic Implication for the Jiamusi Block and Adjacent Areas of NE China. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2013. (In Chinese). [Google Scholar]
- Sun, M.D.; Xu, Y.G.; Wilde, S.A.; Chen, H.L.; Yang, S.F. The Permian Dongfanghong island-arc gabbro of the Wandashan Orogen, NE China: Implications for Paleo-Pacific subduction. Tectonophysics 2015, 659, 122–136. [Google Scholar] [CrossRef]
- Bi, J.H.; Ge, W.C.; Yang, H.; Zhao, G.C.; Yu, J.J.; Zhang, Y.L.; Wang, Z.H.; Tian, D.X. Petrogenesis and tectonic implications of Early Paleozoic granitic magmatism in the Jiamusi Massif, NE China: Geochronological, geochemical and Hf isotopic evidence. J. Asian Earth Sci. 2014, 96, 308–331. [Google Scholar] [CrossRef]
- Yang, H.; Ge, W.C.; Zhao, G.C.; Yu, J.J.; Zhang, Y.L. Early Permian-Late Triassic granitic magmatism in the Jiamusi-Khanka Massif eastern segment of the Central Asian Orogenic Belt and its implications. Gondwana Res. 2015, 27, 1509–1533. [Google Scholar] [CrossRef]
- Yang, H.; Ge, W.C.; Bi, J.H.; Wang, Z.H.; Tian, D.X.; Dong, Y.; Chen, H.J. The Neoproterozoic-Early Paleozoic evolution of the Jiamusi Block, NE China and its East Gondwana connection: Geochemical and zircon U-Pb-Hf isotopic constraints from the Mashan Complex. Gondwana Res. 2018, 54, 102–121. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.J.; Wilde, S.A.; Zhou, J.B.; Wang, M.; Ge, M.H.; Wang, J.M.; Ling, Y.Y. Initial subduction of the Paleo-Pacific Oceanic plate in NE China: Constraints from whole0rock geochemistry and zircon U-Pb and Lu-Hf isotopes of the Khanka Lake granitoids. Lithos 2017, 274–275, 254–270. [Google Scholar] [CrossRef]
- Tang, J.; Xu, W.L.; Wang, F.; Ge, W.C. Subduction history of the Paleo-Pacific slab beneath Eurasian continent: Mesozoic-Paleogene magmatic records in Northeast Asia. Sci. China Earth Sci. 2018, 61, 527–559. [Google Scholar] [CrossRef]
- Shirey, S.B.; Walker, R.J. Carius Tube Digestion for Low-Blank Rhenium-Osmium Analysis. Anal. Chem. 1995, 67, 2136–2141. [Google Scholar] [CrossRef]
- Du, A.D.; Zhao, D.M.; Wang, S.X.; Sun, D.Z.; Liu, D.Y. Precise Re-Os Dating for Molybdenite by ID-NTIMS with Carius Tube Sample Preparation. Rock Miner. Anal. 2001, 20, 247–252. (In Chinese) [Google Scholar]
- Li, C.; Yang, X.; Zhao, H.; Zhou, L.M.; Du, A.D.; Li, X.W.; Qu, W.J. High Precise Isotopic Measurements of pg-ng Os by Negative Ion Thermal Ionization Mass Spectrometry. Rock Miner. Anal. 2015, 34, 392–398. [Google Scholar]
- Zong, K.Q.; Klemd, R.; Yuan, Y.; He, Z.Y.; Guo, J.L.; Shi, X.L.; Liu, Y.S.; Hu, Z.C.; Zhang, Z.M. The assembly of Rodinia: The correlation of early Neoproterozoic (ca. 900 Ma) high-grade metamorphism and continental arc formation in the southern Beishan Orogen, southern Central Asian Orogenic Belt (CAOB). Precambrian Res. 2017, 290, 32–48. [Google Scholar] [CrossRef]
- Hu, H.; Lentz, D.; Li, J.W.; McCarron, T.; Zhao, X.F.; Hall, D. Reequilibration processes in magnetite from iron skarn deposits. Econ. Geol. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Jiang, S.Y.; Ciobanu, C.L.; Yang, T.; Cook, N.J. Sulfur isotope fractionation in pyrite during laser ablation: Implications for laser ablation multiple collector inductively coupled plasma mass spectrometry mapping. Chem. Geol. 2017, 450, 223–234. [Google Scholar] [CrossRef]
- Broughm, S.G.; Hanchar, J.M.; Tornos, F. Mineral chemistry of magnetite from magnetite-apatite mineralization and their host rocks: Examples from Kiruna, Sweden, and El Laco, Chile. Miner. Depos. 2017, 52, 1223–1244. [Google Scholar] [CrossRef]
- Huang, X.W.; Beaudoin, G. Textures and chemical composition of magnetite from iron oxide-copper-gold (IOCG) and Kiruna-type iron oxide-apatite (IOA) deposits and their implications for ore genesis and magnetite classification schemes. Econ. Geol. 2019, 114, 953–979. [Google Scholar] [CrossRef]
- Klemm, D.D.; Henckel, J.; Dehm, R.M.; Von Gruenewaldt, G. The geochemistry of titanomagnetite in magnetite layers and their host rocks of the eastern Bushveld Complex. Econ. Geol. 1985, 80, 1075–1088. [Google Scholar] [CrossRef]
- Nadoll, P.; Koenig, A.E. LA-ICP-MS of magnetite: Methods and reference materials. J. Anal. At. Spectrom. 2011, 26, 1872–1877. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Hayes, T.S.; Koenig, A.E.; Box, S.E. Geochemistry of magnetite from hydrothermal ore deposits and host rocks of the Mesoproterozoic Belt Supergroup, United States. Econ. Geol. 2012, 107, 1275–1292. [Google Scholar] [CrossRef]
- Carew, M.J.; Mark, G.; Oliver, N.H.S.; Pearson, N. Trace element geochemistry of magnetite and pyrite in Fe oxide (+/−Cu-Au) mineralised systems: Insights into the geochemistry of ore-forming fluids. Geochim. Cosmochim. Acta 2006, 70, A83. [Google Scholar] [CrossRef]
- Reguir, E.P.; Chakhmouradian, A.R.; Halden, N.M.; Yang, P.; Zaitsev, A.N. Early magmatic and reaction-induced trends in magnetite from the carbonatites of Kerimasi. Can. Mineral. 2008, 46, 879–900. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Galicki, M.; Marshall, D.; Staples, R.; Thorkelson, D.; Downie, C.; Gallagher, C.; Enkin, R.; Davis, W. Iron oxide ± Cu ± Au deposits in the Iron Range, Purcell Basin, southeastern British Columbia. Econ. Geol. 2012, 107, 1293–1301. [Google Scholar] [CrossRef]
- Angerer, T.; Hagemann, S.G.; Danyushevsky, L. High-grade iron ore at Windarling, Yilgarn Craton: A product of syn-orogenic deformation, hypogene hydrothermal alteration and supergene modification in an Archean BIF-basalt lithostratigraphy. Mineral. Depos. 2013, 48, 697–728. [Google Scholar] [CrossRef]
- Huang, X.; Qi, L.; Meng, Y. The element geochemistry of magnetite from Fe(-Cu) deposits in the Hami Region, Eastern Tianshan Orogenic Belt, NW China. Acta Geol. Sin. Engl. Ed. 2014, 88, 176–195. [Google Scholar] [CrossRef]
- Boutroy, E.; Dare, S.A.S.; Beaudoin, G.; Barnes, S.J.; Lightfoot, P.C. Magnetite composition in Ni-Cu-PGE deposits worldwide and its application to mineral exploration. J. Geochem. Explor. 2014, 145, 64–81. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhou, M.F.; Gao, J.F.; Hu, R. Geochemistry of magnetite from Fe-Cu deposits in the Kangdian metallogenic province, SW China. Miner. Depos. 2015, 50, 795–809. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D.H. Iron-titanium oxide minerals and synthetic equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Toplis, M.; Carroll, M. An experimental study of the influence of oxygen fugacity on Fe-Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J. Petrol. 1995, 36, 1137–1170. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhou, M.F.; Li, X.; Gao, J.F.; Hou, K. In-situ LA-ICP-MS trace elemental analyses of magnetite: Cu—(Au, Fe) deposits in the Khetri copper belt in Rajasthan Province, NW India. Ore Geol. Rev. 2015, 65, 929–939. [Google Scholar] [CrossRef]
- Van Baalen, M.R. Titanium mobility in metamorphic systems: A review. Chem. Geol. 1993, 100, 233–249. [Google Scholar] [CrossRef]
- Liu, P.P.; Zhou, M.F.; Chen, W.T.; Gao, J.F.; Huang, X.W. In-situ LA-ICP-MS trace elemental analyses of magnetite: Fe-Ti-(V) oxide-bearing mafic-ultramafic layered intrusions of the Emeishan Large Igneous Province, SW China. Ore Geol. Rev. 2015, 65, 853–871. [Google Scholar] [CrossRef]
- Huang, X.W.; Gao, J.F.; Qi, L.; Meng, Y.M.; Wang, Y.C.; Dai, Z.H. In-situ LA-ICP-MS trace elements analysis of magnetite: The Fenghuangshan Cu-Fe-Au deposit, Tongling, Eastern China. Ore Geol. Rev. 2016, 72, 746–759. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhou, M.F. In-situ LA-ICP-MS trace elemental analyses of magnetite: The Mesozoic Tengtie skarn Fe deposit in the Nanling Range, South China. Ore Geol. Rev. 2015, 65, 872–883. [Google Scholar] [CrossRef]
- Ding, M.P.; Tang, H.S.; Chen, Y.J.; Dong, L.H.; Li, J.H.; Qu, X.; Li, Q.G.; Sun, X.H.; Zhou, Z.J.; Shi, G.H. Genesis of the Erik iron ore deposit in the Taxkorgan area of the West Kunlun, Xinjiang: Constraints from ore deposit geology and in situ LA-ICP-MS analysis of magnetite. Earth Sci. 2018, 43, 3169–3185. (In Chinese) [Google Scholar]
- Chung, D.; Zhou, M.F.; Gao, J.F.; Chen, W.T. In-situ LA-ICP-MS trace elemental analyses of magnetite: The late Palaeoproterozoic Sokoman Iron Formation in the Labrador Trough, Canada. Ore Geol. Rev. 2015, 65, 917–928. [Google Scholar] [CrossRef]
- Balan, E.; Villiers, J.P.R.D.; Eeckhout, S.G.; Glatzel, P.; Toplis, M.J.; Fritsch, E.; Allard, T.; Galoisy, L.; Calas, G. The oxidation state of vanadium in titanomagnetite from layered basic intrusions. Am. Mineral. 2006, 91, 953–956. [Google Scholar] [CrossRef]
- Bordage, A.; Balan, E.; Villiers, J.; Cromarty, R.; Juhin, A.; Carvallo, C.; Calas, G.; Raju, P.V.S.; Glatzel, P. V oxidation state in Fe-Ti oxides by high-energy resolution fluorescence-detected x-ray absorption spectroscopy. Phys. Chem. Miner. 2011, 38, 449–458. [Google Scholar] [CrossRef]
- Sievwright, R.H.; Wilkinson, J.J.; O’Neill, H.S.C.; Berry, A.J. Thermodynamic controls on element partitioning between titanomagnetite and andesiti-dacitic silicate melts. Contrib. Mineral. Petrol. 2017, 172, 62. [Google Scholar] [CrossRef]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. Mineral. Petrol. 2002, 144, 22–37. [Google Scholar] [CrossRef]
- Hu, Z.C.; Zhang, W.; Liu, Y.S.; Gao, S.; Li, M.; Zong, K.Q.; Chen, H.H.; Hu, S.H. “Wave” Signal-Smoothing and Mercury-Removing Device for Laser Ablation Quadrupole and Multiple Collector ICPMS Analysis: Application to Lead Isotope Analysis. Anal. Chem. 2015, 87, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Ilton, E.S.; Eugster, H.P. A base metal exchange between magnetite and chloriderich hydrothermal fluid. Geochem. Cosmichim. Acta 1989, 53, 291–301. [Google Scholar] [CrossRef]
- Wen, G.; Li, J.W.; Hofstra, A.H.; Koenig, A.E.; Lowers, H.A.; Adams, D. Hydrothermal reequilibration of igneous magnetite in altered granitic plutons and its implications for magnetite classification schemes: Insights from the Handan-Xingtai iron district, North China Craton. Geochim. Cosmochim. Acta 2017, 213, 255–270. [Google Scholar] [CrossRef]
- Salazar, E.; Barra, F.; Reich, M.; Simon, A.; Leisen, M.; Palma, G.; Romero, R.; Rojo, M. Trace element geochemistry of magnetite from the Cerro Negro Norte iron oxide−apatite deposit, northern Chile. Miner. Depos. 2020, 55, 409–428. [Google Scholar] [CrossRef]
- Craig, J.R.; Vokes, F.M.; Solberg, T.N. Pyrite: Physical and chemical textures. Mineralium Deposita 1998, 34, 82–101. [Google Scholar] [CrossRef]
- Zhou, T.F.; Zhang, L.J.; Yuan, F.; Fan, Y.; David, R.C. LA-ICP-MS in situ trace element analysis of pyrite from the Xinqiao Cu-Au-S deposit in Tongling Anhui and its constraints on the ore genesis. Earth Sci. Front. 2010, 17, 306–319. (In Chinese) [Google Scholar]
- Yan, Y.T.; Li, S.R.; Jia, B.J.; Zhang, N.; Yan, L.N. Composition typomorphic characteristics and statistic analysis of pyrite in gold deposits of different genetic types. Earth Sci. Front. 2012, 19, 214–226. (In Chinese) [Google Scholar]
- Zhang, J.; Deng, J.; Chen, H.Y.; Yang, L.Q.; Cooke, D.; Danyushevsky, L.; Gong, Q.J. LA-ICP-MS trace element analysis of pyrite from the Chang’an gold deposit, Sanjiang region, China: Implication for ore-forming process. Gondwana Res. 2014, 26, 557–575. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y. Multi-stage pyrite and hydrothermal mineral assemblage of the Hatu gold district (west Junggar, Xinjiang, NW China): Implications for metallogenic evolution. Ore Geol. Rev. 2015, 69, 243–267. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Wu, K.X.; Hu, R.Z.; Bi, X.W.; Peng, J.T.; Tang, Q.L. Ore Lead isotopes as a tracer for ore-forming material sources: A review. Geol.-Geochem. 2002, 30, 73–81. (In Chinese) [Google Scholar]
- White, W.M. Geochemistry; John Wiley & Sons Inc.: New York, NY, USA, 2013; pp. 406–409. [Google Scholar]
- Loftus-Hills, G.; Solomon, M. Cobalt, nickel and selenium in sulphides as indicators of ore genesis. Miner. Depos. 1967, 2, 228–242. [Google Scholar] [CrossRef]
- Bralia, A.; Sabatini, G.; Troja, F. A revaluation of the Co/Ni ratio in pyrite as geochemical tool in ore genesis problems. Miner. Depos. 1979, 14, 353–374. [Google Scholar] [CrossRef]
- Brill, B. Trace-element contents and partitioning of elements in ore minerals from the CSA Cu-Pb-Zn deposit, Australia. Can. Mineral. 1989, 27, 263–274. [Google Scholar]
- Duan, S.G.; Dong, M.H.; Zhang, Z.H.; Jiang, Z.S.; Li, F.M. A LA-ICP-MS analysis of element in magnetite from Dunde iron deposit in Western Tianshan Mountains, Xijiang: Constraints on genesis of the deposit. Miner. Depos. 2014, 33, 1325–1337. (In Chinese) [Google Scholar]
- Gong, L.; Ma, G. The characteristic typomorphic composition of pyrite and its indicative meaning to metal deposits. Contrib. Geol. Miner. Resour. Res. 2011, 26, 162–166. (In Chinese) [Google Scholar]
- Rye, R.O.; Ohmoto, H. Sulfur and Carbon Isotopes and Ore Genesis: A Review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Chen, J.F. Geochemistry of Stable Isotopes; Science Press: Beijing, China, 2000; pp. 38–73. (In Chinese) [Google Scholar]
- Stacey, J.S.; Hedlund, D.C. Lead-isotopic compositions of diverse igneous rocks and ore deposits from southwestern New Mexico and their implications for early Proterozoic crustal evolution in the western United States. Geol. Soc. Am. Bull. 1983, 94, 1558–1567. [Google Scholar] [CrossRef]
- Zartman, R.E.; Doe, B.R. Plumbotectonics-the model. Tectonophysics 1981, 75, 135–162. [Google Scholar] [CrossRef]
- Hao, Y.J.; Ren, Y.S.; Duan, M.X.; Zhao, X.; Yang, Q.; Tong, K.Y.; Li, C. Mineralization time and tectonic setting of the Zhengguang Au deposit in the Duobaoshan ore field, Heilongjiang Province, NE China. Arab. J. Geosci. 2016, 9, 655. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, Y.S.; Hao, Y.J.; Wang, B.; Sun, Z.M.; Li, J.M. Ore fluid, geochronology and tectonic setting of mesothermal gold metallogeny in southeastern Jilin Province, Northeast China: A case study of the Shajingou gold deposit. Ore Geol. Rev. 2019, 109, 229–252. [Google Scholar] [CrossRef]
- Li, C.; Qu, W.J.; Wang, D.H.; Chen, Z.H.; Du, A.D. Advances in the study of the Re-Os isotopic system of organic-rich sample. Acta Petrol. Mineral. 2010, 29, 421–430. (In Chinese) [Google Scholar]
- Maslennikov, V.V.; Ayupova, N.R.; Herrington, R.J.; Danyushevskiy, L.V.; Large, R.R. Ferruginous and manganiferous haloes around massive sulphide deposits of the Urals. Ore Geol. Rev. 2012, 47, 5–41. [Google Scholar] [CrossRef]
- Knipping, J.L.; Bilenker, L.D.; Simon, A.C.; Reich, M.; Barra, F.; Deditius, A.P.; Wälle, M.; Heinrich, C.A.; Holtz, F.; Munizaga, R. Trace elements in magnetite from massive iron oxide-apatite deposits indicate a combined formation by igneous and magmatic-hydrothermal processes. Geochim. Cosmochim. Acta 2015, 171, 15–38. [Google Scholar] [CrossRef] [Green Version]











| Samples No. | Samples Weight (g) | Re Conc (ppb) | Os Conc (ppb) | 187Re Conc (ppb) | 187Os Conc (ppb) | 187Re/188Os | 187Os/188Os | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | σ | Value | σ | Value | σ | Value | σ | Value | σ | Value | σ | ||
| WZG7-4 | 0.65037 | 2.222 | 0.017 | 0.0249 | 0.0002 | 1.396 | 0.010 | 0.0401 | 0.0003 | 432 | 4.6 | 12.40 | 0.03 |
| WZG7-5 | 0.65044 | 1.775 | 0.013 | 0.0012 | 0.0000 | 1.116 | 0.008 | 0.0055 | 0.0000 | 6894 | 77 | 33.59 | 0.17 |
| WZG7-6 | 0.65056 | 3.669 | 0.027 | 0.0127 | 0.0001 | 2.306 | 0.017 | 0.0199 | 0.0002 | 1396 | 15 | 12.06 | 0.03 |
| WZG7-7 | 0.65038 | 1.600 | 0.012 | 0.0012 | 0.0000 | 1.006 | 0.007 | 0.0051 | 0.0000 | 6370 | 74 | 32.23 | 0.18 |
| WZG7-8 | 0.30063 | 1.663 | 0.012 | 0.0037 | 0.0000 | 1.045 | 0.008 | 0.0052 | 0.0000 | 2189 | 23 | 10.92 | 0.04 |
| WZG7-9 | 0.65026 | 9.463 | 0.072 | 0.0382 | 0.0003 | 5.951 | 0.045 | 0.0758 | 0.0006 | 1198 | 12 | 15.26 | 0.03 |
| WZG7-10 | 0.65020 | 5.267 | 0.039 | 0.0230 | 0.0002 | 3.310 | 0.025 | 0.0676 | 0.0005 | 1108 | 11 | 22.63 | 0.04 |
| WZG10-2 | 0.65065 | 0.383 | 0.003 | 0.0010 | 0.0000 | 0.241 | 0.002 | 0.0017 | 0.0006 | 1946 | 20 | 13.36 | 0.04 |
| Sample | Types | No. | Mg | Al | Si | Ca | Ti | V | Cr | Mn | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WZG-12-2 | Mag-II-A | 1 | 159.51 | 3154.18 | 1512.28 | 19.29 | 798.7 | 18.5 | 288.33 | 168.92 | 9.93 | 13.35 | 0 | 81.52 |
| 2 | 348.74 | 4651.57 | 2538.21 | 72.11 | 922.18 | 16.73 | 185.87 | 240.33 | 11.59 | 11.2 | 0.73 | 325.28 | ||
| 3 | 119.82 | 3060.06 | 1872.74 | 0 | 834.55 | 16.07 | 77.62 | 174.57 | 8.91 | 31.97 | 0 | 21.92 | ||
| 4 | 213.89 | 5422.75 | 1634.58 | 15.4 | 902.78 | 16.18 | 189.91 | 274.49 | 12.61 | 55.85 | 0.48 | 189.19 | ||
| 5 | 168.37 | 3987.95 | 1912.87 | 76.27 | 934.76 | 18.33 | 145.52 | 203.33 | 11.35 | 22.9 | 0 | 60.44 | ||
| 6 | 208.78 | 4372.47 | 1383.45 | 630.53 | 906.17 | 19 | 148.72 | 228.49 | 11.52 | 38.38 | 0 | 119.77 | ||
| 7 | 113.41 | 2918.67 | 2260.75 | 0 | 787.47 | 19.56 | 123.73 | 172.03 | 10.92 | 17.28 | 0 | 45.44 | ||
| 8 | 64.34 | 2427.54 | 1552.11 | 0 | 753.3 | 18.95 | 172.48 | 161.65 | 10.31 | 0 | 1.56 | 8.57 | ||
| 9 | 208.17 | 4169.59 | 2246.09 | 0 | 923.04 | 18.11 | 113.86 | 151.09 | 10.77 | 29.74 | 0 | 105.48 | ||
| 10 | 552.32 | 5066.33 | 3009.29 | 225.03 | 914.92 | 17.45 | 144.26 | 249.5 | 11.51 | 33.42 | 1.13 | 314.78 | ||
| 11 | 140.13 | 4483.02 | 1871.34 | 333.65 | 912.78 | 18.86 | 159.39 | 223.25 | 12.26 | 0 | 1.91 | 117.63 | ||
| 12 | 145.44 | 5146.71 | 1812.78 | 0 | 928.44 | 16.58 | 126.84 | 240.81 | 13.07 | 0 | 0 | 141.2 | ||
| 13 | 104.15 | 3950.7 | 1716.89 | 0 | 769.1 | 16.41 | 134.97 | 195.37 | 12.43 | 35.13 | 0 | 72.41 | ||
| 14 | 483.24 | 4569.37 | 2147.74 | 306.6 | 920.09 | 17.44 | 115.8 | 249.36 | 12.99 | 39.05 | 1.04 | 302.79 | ||
| 15 | 349.52 | 4805.98 | 2204.55 | 1718.48 | 1073.44 | 21.41 | 104.51 | 229.64 | 14.12 | 23.57 | 1.34 | 159.92 | ||
| WZG-1-2 | Mag-II-B | 1 | 397.38 | 12,372.09 | 669.4 | 36.28 | 4107.69 | 80.54 | 9.88 | 1368.67 | 15.87 | 1.7 | 0.26 | 474.38 |
| 2 | 228.06 | 8196.11 | 1977.29 | 70.82 | 4216.18 | 86.01 | 13.27 | 1147.33 | 13.34 | 2.02 | 0 | 267.43 | ||
| 3 | 218.16 | 9657.02 | 1504.31 | 3.04 | 4120.99 | 82.91 | 12.13 | 1206.15 | 13.82 | 1.24 | 0 | 357.02 | ||
| 4 | 332.15 | 10,675.81 | 2537.01 | 50.74 | 4153.37 | 81.92 | 12.91 | 1286.17 | 15.39 | 1.55 | 0 | 390.26 | ||
| 5 | 243.77 | 9173.97 | 2208.96 | 59.39 | 4279.17 | 82.21 | 13.92 | 1138.65 | 14.52 | 1.73 | 0.16 | 443.5 | ||
| 6 | 382.92 | 3802.58 | 4123.46 | 138.47 | 4055.02 | 87.77 | 25.83 | 786.59 | 9.59 | 0.98 | 0.4 | 72.53 | ||
| 7 | 370.23 | 5423.39 | 3755.84 | 26.67 | 4036.41 | 82.1 | 22.08 | 978.09 | 10.8 | 1.08 | 0 | 94.83 | ||
| 8 | 389.56 | 9109.79 | 2259.98 | 99.33 | 4200.97 | 80.18 | 15.18 | 1228.83 | 14.06 | 1.5 | 0.15 | 429.13 | ||
| 9 | 312.84 | 8380.16 | 2143.38 | 25.89 | 4206.48 | 87.2 | 15.47 | 1015.38 | 12.94 | 1.7 | 0.1 | 409.35 | ||
| 10 | 114.97 | 6194.97 | 1693.53 | 0 | 4280.88 | 95.66 | 14.27 | 774.05 | 10.79 | 1.19 | 0.2 | 201.16 |
| Types | Sample | No. | Ti | V | Cr | Mn | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Py-II | WZG-1-2 | 1 | 9.25 | 0.18 | 0.31 | 92.58 | 217.64 | 15.34 | 12.67 | 3.63 | 1.82 | 1.19 | 17.33 | 16.6 | 8.44 | 41.31 |
| 2 | 7.86 | 0.08 | 0.43 | 63.76 | 170.77 | 41.61 | 15.84 | 2.2 | 0.09 | 1.25 | 6.8 | 0 | 7.22 | 5.87 | ||
| 3 | 1.91 | 0 | 2.17 | 29.04 | 298.15 | 59.85 | 8.62 | 0.94 | 0.07 | 1.23 | 2.6 | 0 | 5.16 | 4.16 | ||
| WZG-6-5 | 4 | 12.93 | 0.09 | 0 | 0.87 | 891.72 | 196.49 | 0.33 | 0.83 | 0 | 9.05 | 6.41 | 4.14 | 0.95 | 0.01 | |
| 5 | 13.62 | 0 | 0 | 0.93 | 649.65 | 145.4 | 4.36 | 0.78 | 0 | 9.61 | 6.17 | 5.75 | 0.92 | 0.03 | ||
| 6 | 13.3 | 0 | 0 | 0.91 | 886.46 | 200.16 | 3.08 | 1.99 | 0.03 | 9.16 | 5.18 | 4.85 | 4.47 | 0.02 | ||
| 7 | 12.45 | 0.09 | 0 | 0 | 396.65 | 112.78 | 0 | 0.64 | 0.1 | 9.4 | 6.32 | 0 | 0.72 | 0.02 | ||
| 8 | 14.72 | 0.12 | 0 | 1.9 | 220.36 | 84.55 | 9.27 | 4 | 0.06 | 9.8 | 6.24 | 4.35 | 2.13 | 1.09 | ||
| 9 | 12.98 | 0.12 | 0 | 1.11 | 124.98 | 40.05 | 20.7 | 3.03 | 0.03 | 9.92 | 5.81 | 0 | 0.44 | 0.08 | ||
| 10 | 12.48 | 0.13 | 1.61 | 0.88 | 579.92 | 175.69 | 0 | 0.95 | 0.02 | 9.79 | 5.42 | 4.81 | 0.51 | 0.03 | ||
| 11 | 11.13 | 0.12 | 0 | 0 | 441.03 | 144.49 | 0.3 | 2.93 | 0.02 | 9.72 | 5.38 | 3.97 | 0.51 | 0.03 | ||
| 12 | 11.01 | 0 | 0 | 4.58 | 809.54 | 184.71 | 11.14 | 1.16 | 0.06 | 9.54 | 6.14 | 5.28 | 4.61 | 0.12 | ||
| 13 | 12.59 | 0 | 0 | 34.56 | 548.17 | 140.23 | 812.33 | 4.74 | 0.27 | 9.99 | 11.34 | 0 | 8.02 | 0.13 | ||
| 14 | 12.5 | 0.1 | 0 | 1.08 | 393.18 | 103.34 | 2.52 | 0.39 | 0.07 | 9.52 | 6.95 | 5.24 | 0.77 | 0.04 | ||
| 15 | 13.25 | 0.09 | 0 | 0.91 | 654.48 | 171.1 | 9.76 | 0 | 0 | 9.3 | 4.85 | 5.04 | 3.04 | 0.03 | ||
| 16 | 13.2 | 0 | 0 | 0 | 864.18 | 241.04 | 0.99 | 0 | 0.04 | 9.48 | 6.49 | 6.27 | 6.34 | 0.03 | ||
| 17 | 14.1 | 0.13 | 4.78 | 0 | 140.23 | 44.42 | 33.08 | 4.82 | 0 | 9.51 | 7.58 | 4.32 | 3.92 | 4.57 | ||
| 18 | 24.6 | 0.84 | 0 | 449 | 184.86 | 63.72 | 46.16 | 5.87 | 1.62 | 10.29 | 6.94 | 0 | 9.74 | 15.78 | ||
| Py-II | WZG-6-5 | 19 | 11.03 | 0.14 | 0 | 1.37 | 1333.6 | 329.65 | 13.14 | 2.68 | 0.08 | 9.37 | 7.43 | 7.62 | 9.9 | 3.88 |
| 20 | 26.53 | 0.84 | 20.87 | 79.57 | 807.36 | 187.54 | 42.84 | 14.76 | 2.44 | 9.64 | 10.13 | 4.91 | 9.83 | 21.08 | ||
| 21 | 14.6 | 0 | 0 | 4.53 | 1053 | 255.12 | 3.92 | 1.56 | 0.1 | 9.85 | 6.94 | 6.55 | 4.15 | 2.48 | ||
| 22 | 13.19 | 0 | 3.11 | 5.98 | 612.34 | 150.6 | 3.09 | 1.27 | 0.12 | 9.19 | 6.73 | 5.12 | 2.71 | 1.2 | ||
| 23 | 9.14 | 0.52 | 17.4 | 44.78 | 529.46 | 122.74 | 10.78 | 7.56 | 1.67 | 9.63 | 5.59 | 5.64 | 5.31 | 13.98 | ||
| 24 | 11.03 | 0 | 0 | 0 | 845.52 | 208.81 | 1.99 | 0.42 | 0.02 | 9.49 | 4.73 | 5.62 | 5.66 | 0.06 | ||
| 25 | 11.18 | 0 | 0 | 0.73 | 1531.9 | 360.42 | 10.97 | 1.02 | 0 | 9.37 | 4.98 | 11.47 | 38.93 | 0.23 | ||
| 26 | 11.2 | 0.08 | 0 | 1.51 | 1346.88 | 395.66 | 5.75 | 0.87 | 0.07 | 9.27 | 6.52 | 8.81 | 5.73 | 4.01 | ||
| 27 | 10.12 | 0.14 | 1.75 | 8.57 | 1431.22 | 408.41 | 26.74 | 1.67 | 0.3 | 8.82 | 7.33 | 6.11 | 9.53 | 24.58 | ||
| 28 | 9.43 | 0 | 0 | 0.99 | 1253.1 | 320.06 | 1.91 | 0 | 0 | 8.28 | 5.64 | 6.75 | 2.68 | 0.17 | ||
| 29 | 10.34 | 0 | 9.48 | 1.14 | 1777.16 | 620.72 | 10.67 | 1.52 | 0.07 | 9.05 | 6.53 | 8.1 | 12.51 | 0.56 | ||
| 30 | 12.73 | 0 | 0 | 1.19 | 464.61 | 107.1 | 4.49 | 1.84 | 0.05 | 9.43 | 8.29 | 3.76 | 3.61 | 1.19 | ||
| 31 | 8.9 | 0.08 | 0 | 0.78 | 331.08 | 59.12 | 0.52 | 0.58 | 0 | 8.68 | 3.26 | 1.93 | 0.73 | 0.01 | ||
| 32 | 11.3 | 0.08 | 1.57 | 0 | 278.04 | 79.78 | 0.8 | 0.28 | 0.03 | 9.01 | 4.19 | 2.26 | 1.03 | 0.07 | ||
| 33 | 13.14 | 0.19 | 0 | 0 | 273.81 | 63.42 | 1.14 | 1.3 | 0.03 | 9.53 | 4.04 | 4.76 | 0.95 | 0.06 | ||
| Py-III | WZG-12-2 | 1 | 2.05 | 0.02 | 10.44 | 7.75 | 423.13 | 105.83 | 0.43 | 1.84 | 0.06 | 1.25 | 0.25 | 1.52 | 2.23 | 4.54 |
| 2 | 2 | 0 | 2.36 | 4.24 | 398.84 | 105.99 | 0.23 | 1.67 | 0.02 | 1.4 | 0.16 | 4.5 | 1.31 | 4.25 | ||
| 3 | 2.11 | 0.02 | 4.82 | 598.26 | 437.75 | 120.94 | 0.55 | 1.84 | 0.19 | 1.1 | 0.59 | 20.9 | 1.49 | 2.98 | ||
| 4 | 1.86 | 0.01 | 3.21 | 64.7 | 365.09 | 72.14 | 0.08 | 1.61 | 0.03 | 1.07 | 0.38 | 4.65 | 0.61 | 2.19 | ||
| 5 | 2.15 | 0.01 | 25.11 | 7.71 | 410.13 | 117.09 | 0.33 | 2.22 | 0.01 | 1.45 | 0.49 | 0 | 3.56 | 8.09 | ||
| 6 | 1.84 | 0.01 | 1.18 | 38.73 | 383.77 | 112.18 | 0.84 | 7.55 | 0.02 | 1.09 | 0.4 | 4.55 | 0.79 | 1.77 | ||
| 7 | 1.93 | 0 | 1.18 | 354.36 | 363.93 | 106.07 | 0.2 | 1.52 | 0.13 | 0.99 | 0.52 | 12.14 | 3.96 | 6.68 | ||
| 8 | 1.73 | 0.01 | 1.75 | 15.11 | 387.2 | 88.66 | 0.09 | 1.57 | 0 | 1.19 | 0.09 | 1.98 | 0.7 | 1.52 | ||
| Po | WZG-6-5 | 18 | 11.13 | 0 | 0 | 0 | 484.55 | 156.2 | 3.25 | 1.24 | 0.06 | 10.97 | 13.47 | 6.88 | 0.91 | 1.15 |
| 19 | 6.86 | 0 | 0 | 2.94 | 525.22 | 159.17 | 5.87 | 1.36 | 0.06 | 10.98 | 13.62 | 8.75 | 0.82 | 0.51 | ||
| 20 | 14.99 | 0.28 | 0 | 0 | 520.98 | 161.27 | 6.57 | 15.81 | 0.1 | 12.23 | 16.71 | 11.52 | 0.84 | 0.6 | ||
| 21 | 9.01 | 0 | 4.65 | 0 | 506.15 | 161.23 | 7.33 | 1.92 | 0 | 11.29 | 13.38 | 5.42 | 1.01 | 0.62 | ||
| 22 | 6.57 | 0.27 | 0 | 3.07 | 533.83 | 160.21 | 5.29 | 0 | 0 | 12.39 | 13.16 | 5.82 | 1.16 | 0.91 | ||
| 23 | 8.02 | 0 | 0 | 0 | 539.96 | 164.98 | 7.81 | 0 | 0 | 12.42 | 15.13 | 7.94 | 0.72 | 0.51 | ||
| 24 | 7.52 | 0 | 0 | 0 | 498.43 | 158.24 | 5.7 | 7.74 | 0.07 | 11.18 | 15.14 | 7.91 | 1.05 | 1.15 | ||
| 25 | 15.32 | 0.33 | 0 | 0 | 510.59 | 159.13 | 2.05 | 0 | 0.09 | 10.7 | 14.63 | 6.34 | 1.35 | 1.57 |
| Sample No. | Mineral | δ34SV-CDT (‰) | 206Pb/204Pb | 2σ | 207Pb/204Pb | 2σ | 208Pb/204Pb | 2σ |
|---|---|---|---|---|---|---|---|---|
| WZG-7-4 | Pyrite | 11.8 | 18.634 | 0.002 | 15.638 | 0.001 | 38.591 | 0.004 |
| WZG-7-7 | Pyrite | 9.9 | 18.605 | 0.004 | 15.648 | 0.005 | 38.671 | 0.01 |
| WZG-7-8 | Pyrite | 7.9 | 19.963 | 0.003 | 15.653 | 0.003 | 39.129 | 0.007 |
| WZG-7-10 | Pyrite | 4.7 | 20.136 | 0.002 | 15.71 | 0.002 | 38.962 | 0.005 |
| WZG-10-2 | Pyrite | 9.9 | 18.718 | 0.002 | 15.637 | 0.002 | 38.534 | 0.004 |
| WZG-2-1 | Granitic gneiss | 7.44 | ||||||
| WZG-2-4 | Granitic gneiss | 8.44 | ||||||
| WZG-2-5 | Granitic gneiss | 7.99 | ||||||
| WZG-5-5 | Meta-gabbro | 4.74 | ||||||
| WZG-11-1 | Meta-gabbro | 10.54 | ||||||
| WZG-20-3 | Meta-gabbro | 4.37 |
| Sample No. | Mineral | δ34SV-CDT (‰) |
|---|---|---|
| WZG-7-1-01 | Pyrite | 11.58 |
| WZG-7-1-02 | Pyrite | 12.31 |
| WZG-7-1-03 | Pyrite | 11.9 |
| WZG-10-1-01 | Pyrite | 11.79 |
| WZG-10-1-02 | Pyrite | 12.89 |
| WZG-10-1-03 | Pyrite | 11.82 |
| WZG-10-1-01 | Chalcopyrite | 12.83 |
| WZG-10-1-02 | Chalcopyrite | 12.76 |
| WZG-10-1-03 | Chalcopyrite | 12.52 |
| WZG-10-1-04 | Chalcopyrite | 12.24 |
| WZG-10-1-05 | Chalcopyrite | 12.58 |
| WZG-12-1-01 | Pyrite | 11.41 |
| WZG-12-1-02 | Pyrite | 12.04 |
| WZG-12-1-03 | Pyrite | 12.19 |
| WZG-12-1-04 | Pyrite | 12.48 |
| WZG-12-1-05 | Pyrite | 12.14 |
| WZG-12-1-06 | Pyrite | 12.04 |
| WZG-10-1-01 | Pyrrhotite | 11.99 |
| WZG-10-1-02 | Pyrrhotite | 12.08 |
| WZG-10-1-03 | Pyrrhotite | 12.02 |
| WZG-12-1-01 | Pyrrhotite | 12.01 |
| WZG-12-1-02 | Pyrrhotite | 11.6 |
| WZG-12-1-03 | Pyrrhotite | 11.87 |
| Deposit Type | Typical Deposit | Analysis Method | Average Element Content (ppm) | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Al | Mn | Ti | Ca | V | Cr | Co | Ni | Zn | ||||
| Magmatic | Emeishan Taihe deposit | LA-ICP-MS | 7109 | 3617 | 3277 | 107,520 | 337.7 | 3758 | 23 | 22.5 | 80 | 205.5 | Liu et al. [78] |
| Emeishan Panzhihua deposit | LA-ICP-MS | 21,587 | 18,565 | 3218 | 85,668 | 62.9 | 3808 | 524.8 | 165.9 | 86.3 | 439.9 | ||
| Skarn | Fenghuangshan Cu-Fe-Au deposit | LA-ICP-MS | 760.6 | 1957 | 1713 | 432.4 | 2588 | 22.5 | — | 58.8 | — | 150.4 | Huang et al. [79] |
| Tengtie skarn Fe deposit | LA-ICP-MS | 77.2 | 1402 | 2057 | 587.2 | 173.3 | 59.7 | 31.1 | 88.5 | 27.3 | 1439 | Zhao et al. [80] | |
| VMS | Erik Fe deposit | LA-ICP-MS | 203.7 | 865.5 | 1019 | 632.2 | 143.4 | 3527 | 6.5 | 4.2 | 54.1 | 103.9 | Ding et al. [81] |
| BIF | Canada Sokoman Fe deposit | LA-ICP-MS | 138 | 162 | 390 | 19 | 73 | 26 | 6.6 | 6.5 | 1.5 | 7.7 | Chung et al. [82] |
| IOCG | Thanewasna Cu-(Au) deposit | LA-ICP-MS | 180.8 | 816.7 | 2543 | 341.7 | 958.3 | 25,337 | 1382 | 895.1 | 3548 | 839.3 | Dora et al. [24] |
| Weizigou deposit Mag-II-A | LA-ICP-MS | 225.3 | 4146 | 210.8 | 885.5 | 226.5 | 18 | 148.8 | 11.6 | 23.5 | 137.8 | ||
| Weizigou deposit Mag-II-B | LA-ICP-MS | 299 | 8299 | 1093 | 4166 | 51.1 | 84.7 | 15.5 | 13.1 | 1.5 | 314 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Hao, Y.; Lu, S. Genesis of the Weizigou Au Deposit, Heilongjiang Province, NE China: Constraints from LA-ICP-MS Trace Element Analysis of Magnetite, Pyrite and Pyrrhotite, Pyrite Re-Os Dating and S-Pb Isotopes. Minerals 2021, 11, 1380. https://doi.org/10.3390/min11121380
Gao Y, Hao Y, Lu S. Genesis of the Weizigou Au Deposit, Heilongjiang Province, NE China: Constraints from LA-ICP-MS Trace Element Analysis of Magnetite, Pyrite and Pyrrhotite, Pyrite Re-Os Dating and S-Pb Isotopes. Minerals. 2021; 11(12):1380. https://doi.org/10.3390/min11121380
Chicago/Turabian StyleGao, Yu, Yujie Hao, and Siyu Lu. 2021. "Genesis of the Weizigou Au Deposit, Heilongjiang Province, NE China: Constraints from LA-ICP-MS Trace Element Analysis of Magnetite, Pyrite and Pyrrhotite, Pyrite Re-Os Dating and S-Pb Isotopes" Minerals 11, no. 12: 1380. https://doi.org/10.3390/min11121380






