The Effect of pH on Stability of an Isolation Barrier Made of Dolomite Post-Floatation Waste
Abstract
:1. Introduction
2. Materials and Methods
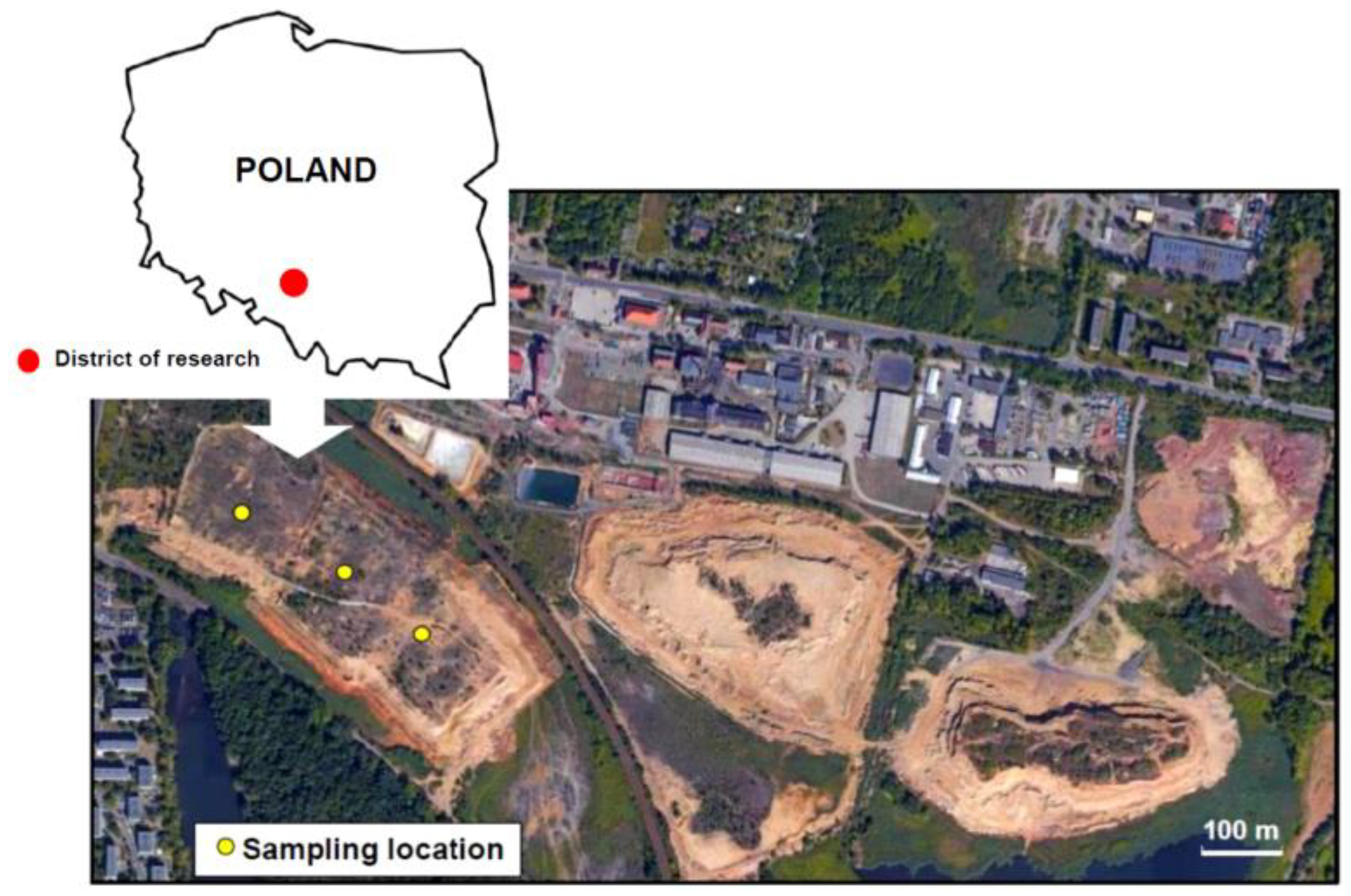
2.1. Research Materials
- The area of post-floatation tanks in Bytom is the largest among all Zn-Pb ore mining regions in Poland—the amount of waste deposited at the time of the discontinuation of the use of tanks, i.e., in the early 1990s, was about 36 million Mg [41];
- The waste was produced in a short period of time, in the same treatment processes, and the ore subjected to floatation came exclusively from the deposits in Bytom, which guarantees its mineralogical homogeneity.
2.2. Research Procedure and Analytical Methods Used
2.2.1. Physical and Chemical Analyses
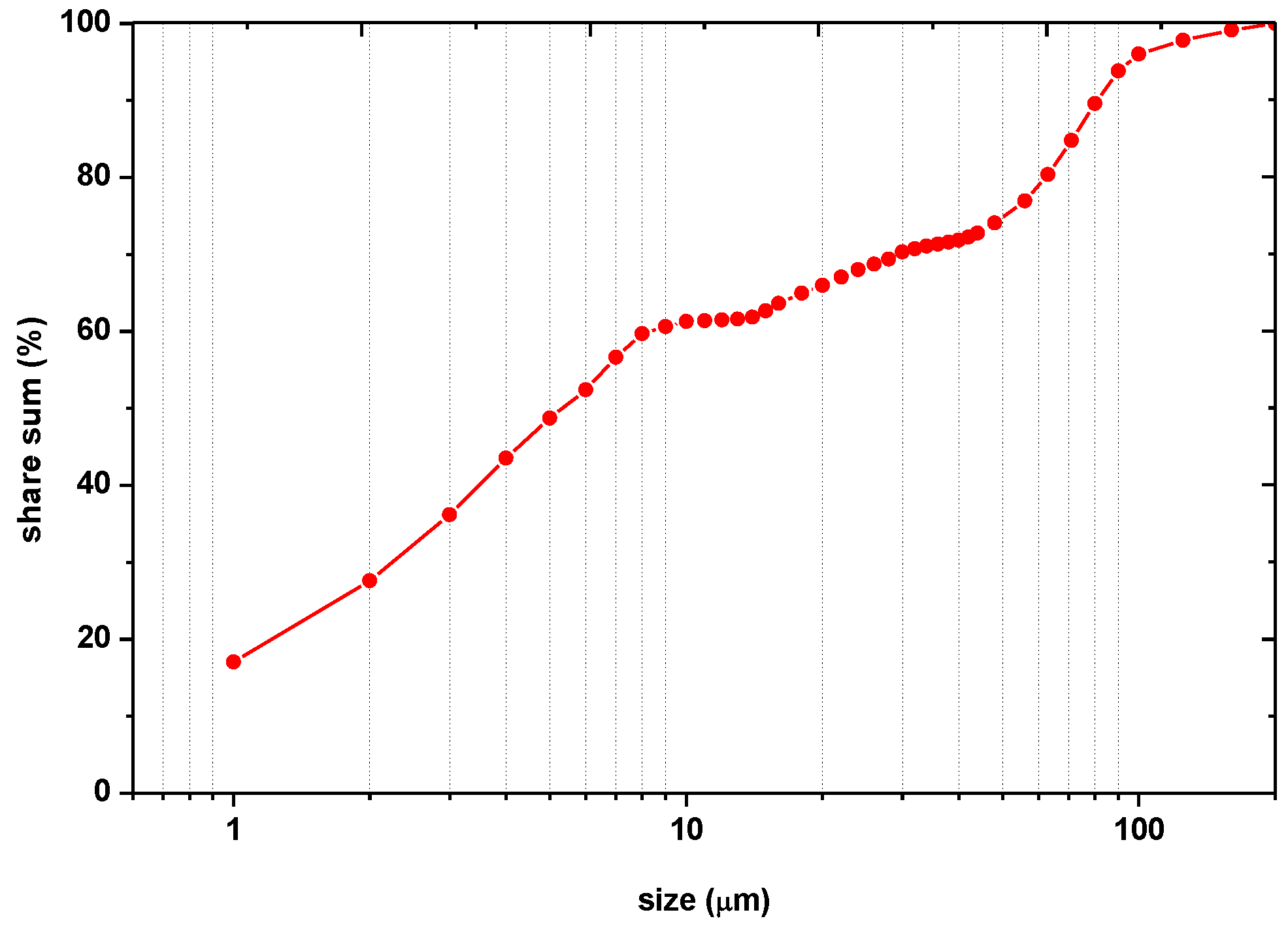
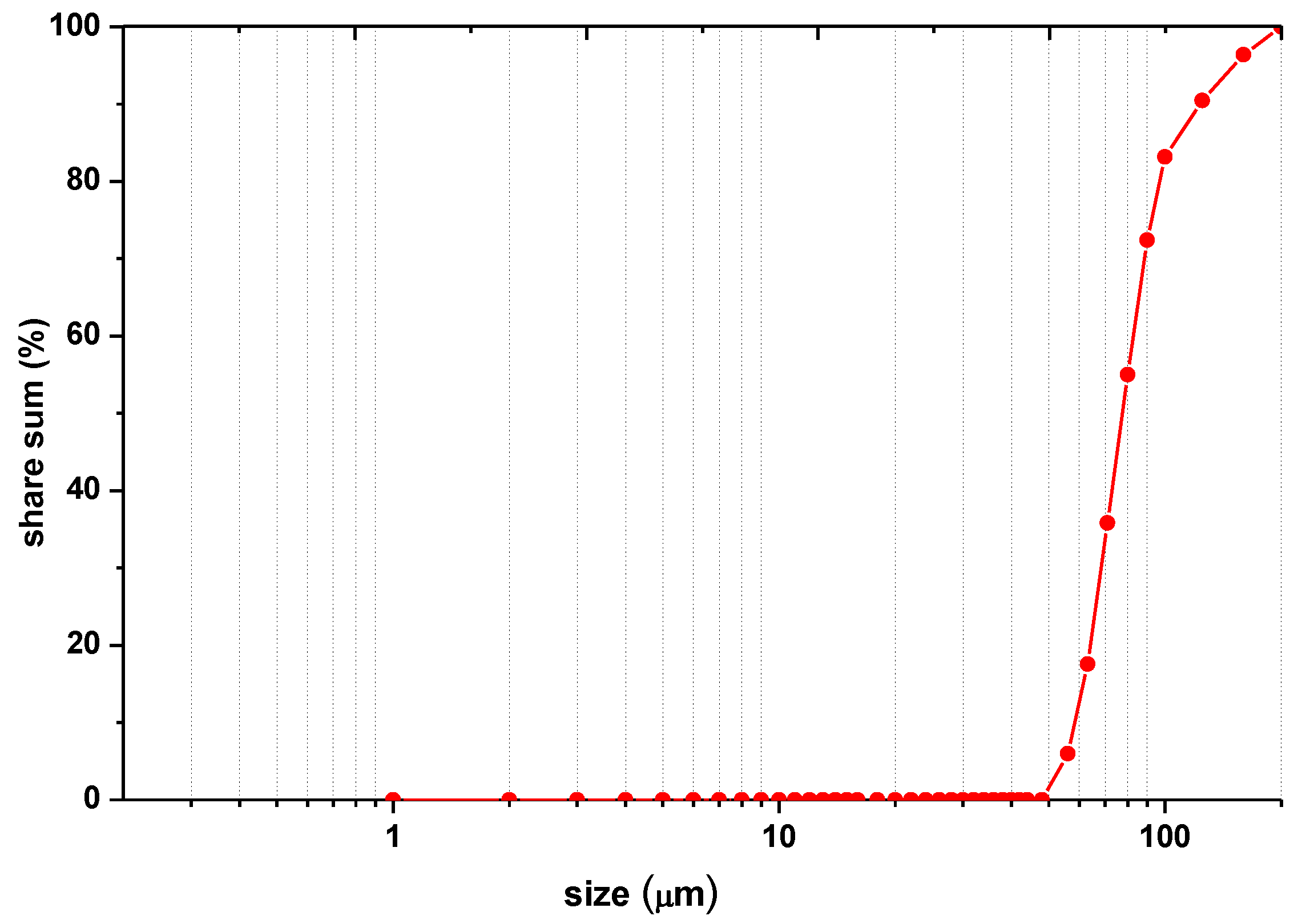
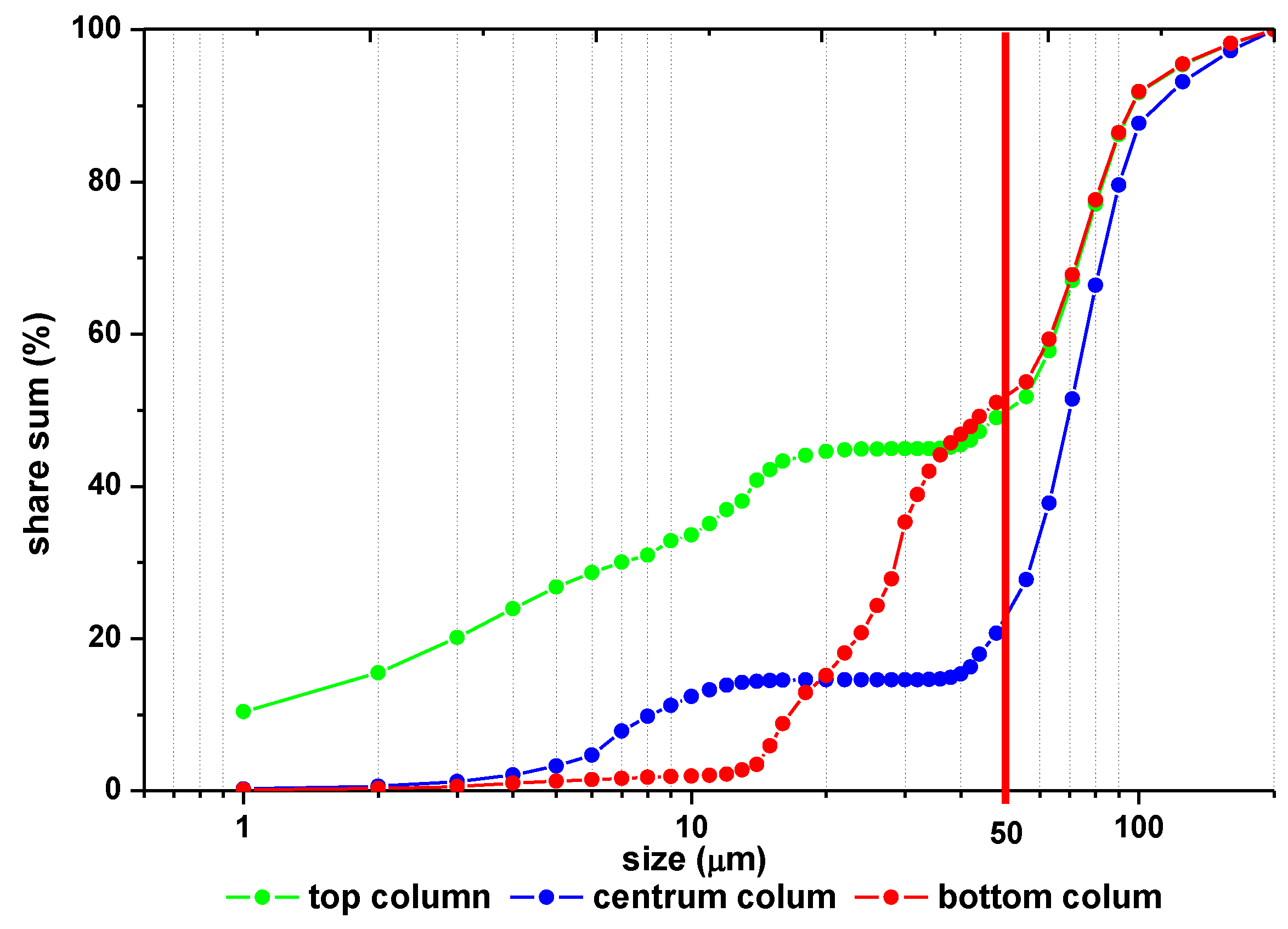
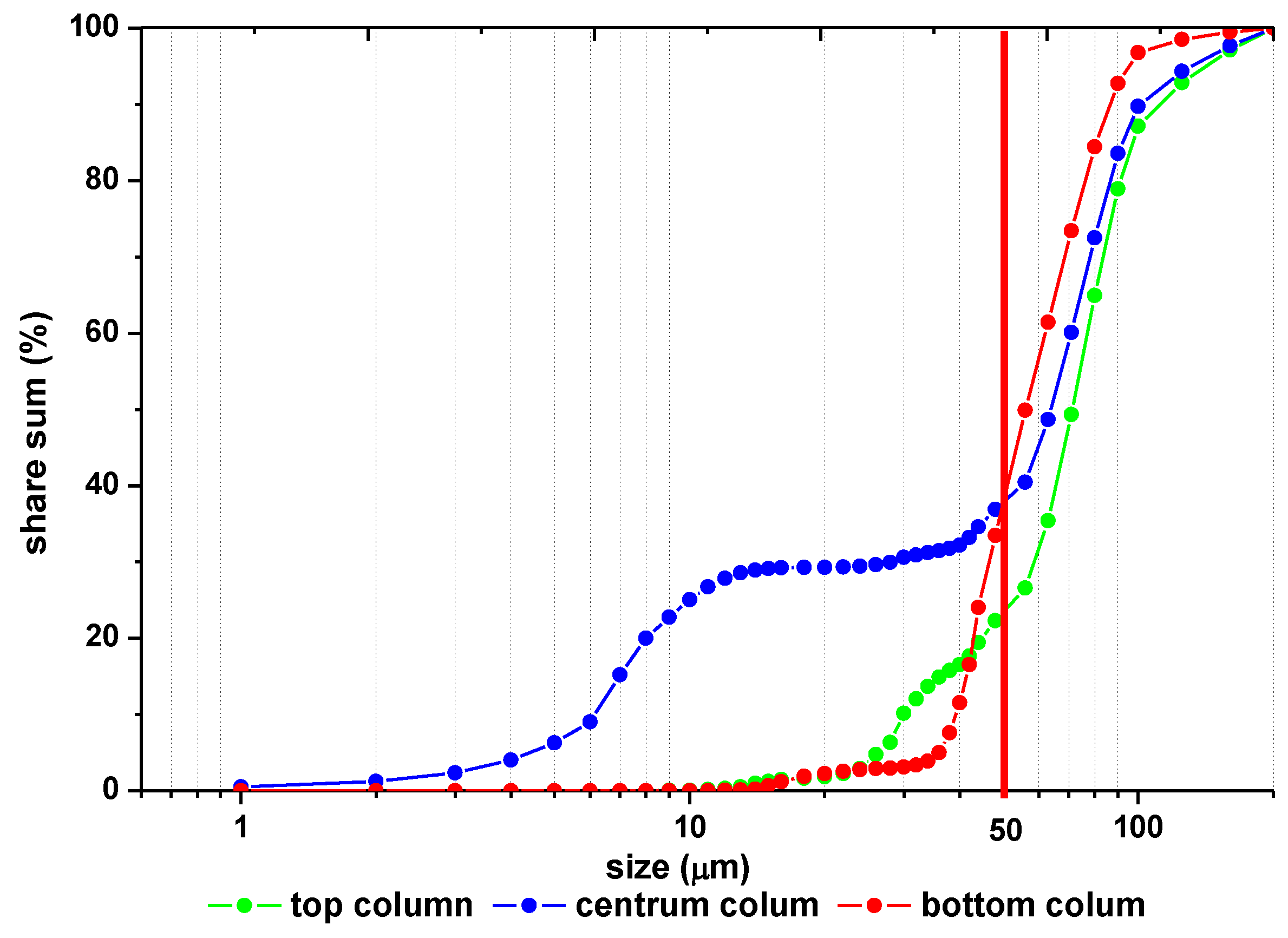
2.2.2. Column Tests
- Sample 1 was obtained from the top of the column;
- Sample 2 was obtained from the center of the column;
- Sample 3 was obtained from the bottom of the column.
2.2.3. Statistical Analyses
3. Results and Discussion
3.1. Physical and Chemical Analyses
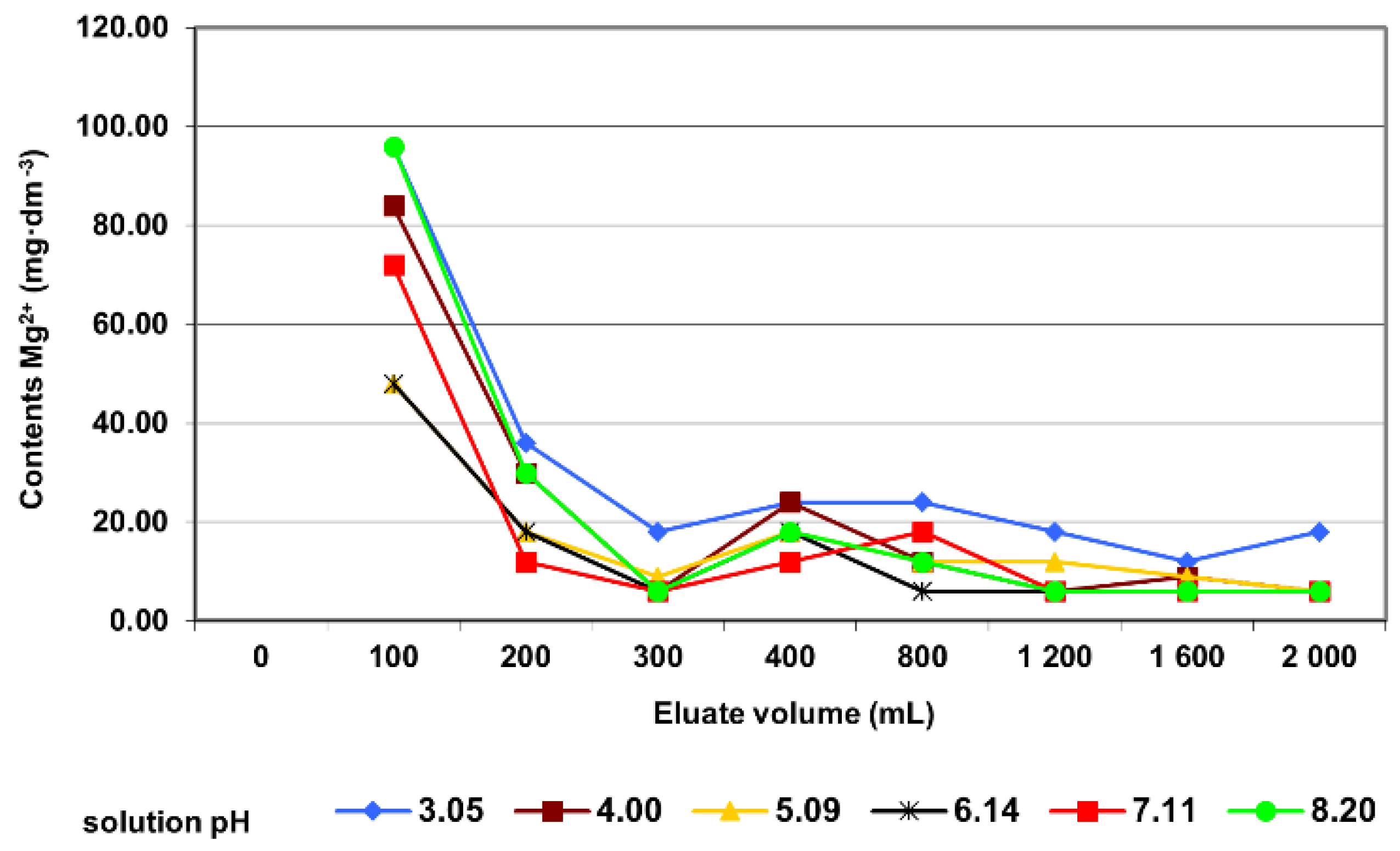
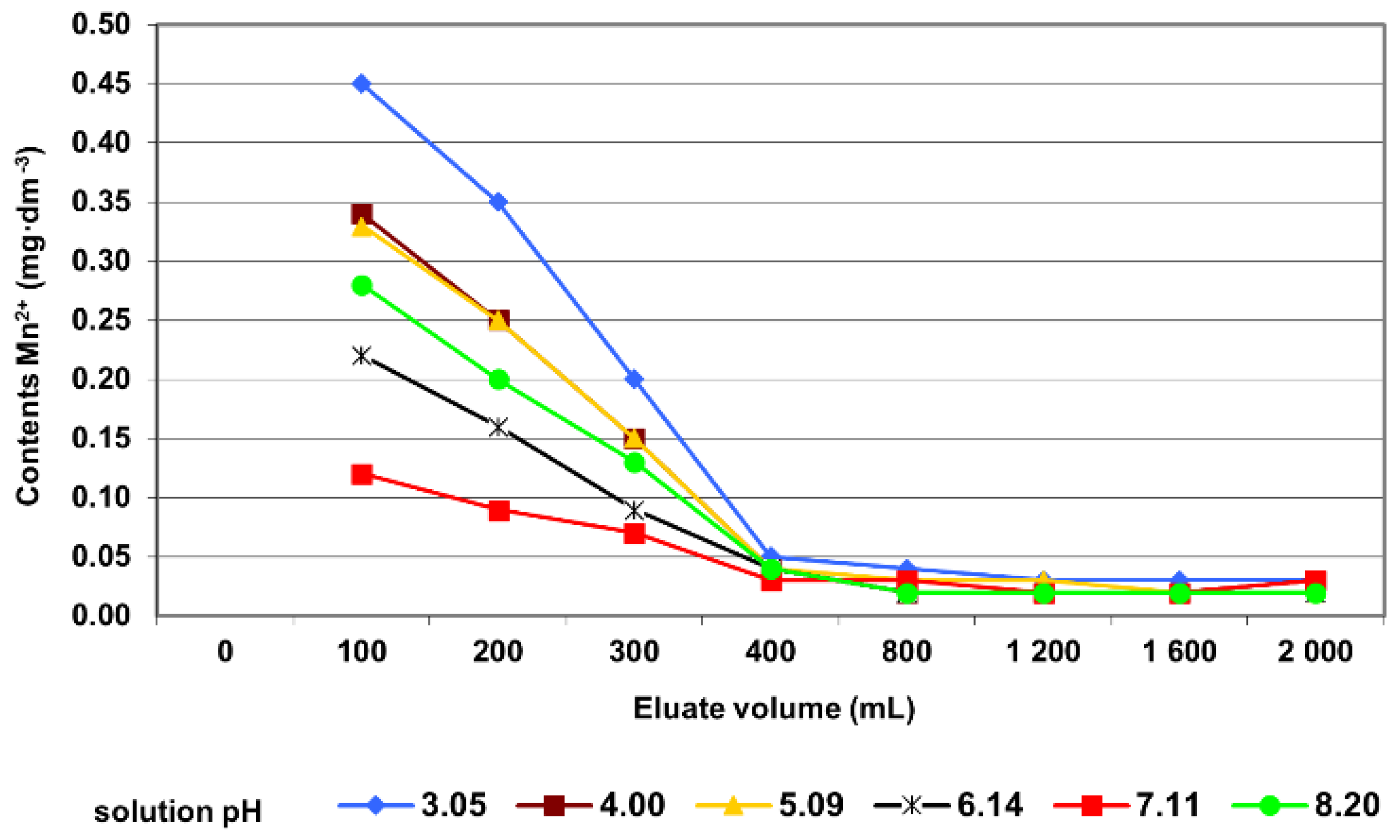
3.2. Column Tests
🠛
hydrozincite
3.3. Statistical Analysis
- Was statistically significantly, negatively, and very strongly related to the percentage content of grains from the clay fraction;
- Was statistically significantly, positively, and very strongly related to the percentage content of grains from the fine sand fraction;
- Was negatively and strongly correlated at the level of statistical tendency with the percentage content of grains from the silt fraction.
- Was statistically significantly, negatively, and very strongly related to the percentage content of grains from the clay and slit fractions;
- Was statistically significantly, positively, and very strongly correlated with the percentage content of grains from the fine sand fraction.
4. Conclusions
- The dolomite post-floatation waste proposed for the isolation barrier have supra-additive characteristics and combine the advantages of a chemical and physical barrier;
- Buffering properties of dolomites stabilize the pH of solutions infiltrating through them, thus limiting leaching of heavy metals;
- With its low filtration coefficient, this waste can be considered an impermeable material;
- The grain degradation occurring with migrating solutions leads to sealing of the barrier consisting of this waste.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daniel, D.E. Landfills for solid and liquid wastes. Environ. Geotech. 1998, 4, 1231–1246. [Google Scholar]
- Benson, C.H.; Daniel, D.E.; Boutwell, G.P. Field Performance of Compacted Clay Liners. J. Geotech. GeoEnviron. Eng. 1999, 125, 390–403. [Google Scholar] [CrossRef]
- Sobik-Szołtysek, J.; Siedlecka, E. Analysis of sorptive capabilities of post-flotation dolomites used in insulation barriers con-struction of dumping sites. Desalin. Water Treat. 2014, 52, 3775–3782. [Google Scholar] [CrossRef]
- Sobik-Szołtysek, J.; Jabłońska, B. Possibilities of joint management of sewage sludge and dolomite post-flotation waste. Ecol. Chem. Eng. S 2010, 17, 149–159. [Google Scholar]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, D.; Luo, X.; Li, Y.; Zhao, Q.; Li, M.; Zhao, Y.; Sun, T.; Ma, C. Simultaneous determination of trace Cd (II), Pb (II) and Cu (II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-L-lysine nanocom-posite modified glassy carbon electrode. J. Colloid Interface Sci. 2017, 490, 11–22. [Google Scholar] [CrossRef]
- Wang, W.-J.; Cai, Y.-L.; Li, B.-C.; Zeng, J.; Huang, Z.-Y.; Chen, X.-M. A voltammetric sensor for simultaneous determination of lead, cadmium and zinc on an activated carbon fiber rod. Chin. Chem. Lett. 2018, 29, 111–114. [Google Scholar] [CrossRef]
- Väänänena, K.; Leppänenb, M.T.; Chenc, X.; Akkanena, J. Metal bioavailability in ecological risk assessment of freshwater ecosystems: From science to environmental management. Ecotoxicol. Environ. Saf. 2018, 147, 430–446. [Google Scholar] [CrossRef]
- Jin, X.; Li, Y.; Yu, C.; Ma, Y.; Yang, L.; Hu, H. Synthesis of novel inorganic–organic hybrid materials for simultaneous adsorption of metal ions and organic molecules in aqueous solution. J. Hazard. Mater. 2011, 198, 247–256. [Google Scholar] [CrossRef]
- Srivastava, V.; Sharma, Y.C.; Sillanpää, M. Application of nano-magnesso ferrite(n-MgFe2O4) for the removal of Co2+ions from synthetic wastewater: Kinetic, equilibrium and thermodynamic studies. Appl. Surf. Sci. 2015, 338, 42–54. [Google Scholar] [CrossRef]
- Radziemska, M. Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. CATENA 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C.H.; Al-Muhtaseb, A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Kinetic and thermody-namics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Duffy, A.; Walker, G.; Allen, S. Investigations on the adsorption of acidic gases using activated dolomite. Chem. Eng. J. 2006, 117, 239–244. [Google Scholar] [CrossRef]
- Tozsin, G. Inhibition of acid mine drainage and immobilization of heavy metals from copper flotation tailings using a marble cutting waste. Int. J. Miner. Met. Mater. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Baran, P.; Wdowin, M.; Franus, W. Waste dolomite powder as a waste dolomite powder as an ad-sorbent of Cd, Pb (II), and Zn from aqueous solutions. Environ. Earth Sci. 2017, 79, 521. [Google Scholar] [CrossRef]
- Stefaniak, E.; Dobrowolski, R.; Staszczuk, P. Adsorption on the adsorption of Chromium (VI) ions on dolomite and dolomitic sorbents. Adsorp. Sci. Technol. 2000, 18, 107–115. [Google Scholar] [CrossRef]
- Tangviroon, P.; Noto, K.; Igarashi, T.; Kawashima, T.; Ito, M.; Sato, T.; Mufalo, W.; Chirwa, M.; Nyambe, I.; Nakata, H.; et al. Immobilization of Lead and Zinc Leached from Mining Residual Materials in Kabwe, Zambia: Possibility of Chemical Immobilization by Dolomite, Calcined Dolomite, and Magnesium Oxide. Minerals 2020, 10, 763. [Google Scholar] [CrossRef]
- Irani, M.; Amjadi, M.; Mousavian, M.A. Comparative study of lead sorption onto natural perlite, dolomite and diatomite. Chem. Eng. J. 2011, 178, 317–323. [Google Scholar] [CrossRef]
- Lee, S.; Dyer, J.A.; Sparks, N.L.; Scrivner, N.C.; Elzinga, E.J. A multi-scale assessment of Pb(II) sorption on dolomite. J. Colloid Interface Sci. 2006, 298, 20–30. [Google Scholar] [CrossRef]
- Kocaoba, S. Comparison of Amberlite IR 120 and dolomite’s performances for removal of heavy metals. J. Hazard. Mater. 2007, 147, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, G.; Mehawej, M. Adsorption of arsenate on untreated dolomite powder. J. Hazard. Mater. 2007, 148, 259–266. [Google Scholar] [CrossRef]
- Pehlivan, E.; Özkan, A.M.; Dinç, S.; Parlayici, S. Adsorption of Cu2+ and Pb2+ ion on dolomite powder. J. Hazard. Mater. 2009, 167, 1044–1049. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ghaemi, A.; Torab-Mostaedi, M.; Asadollahzadeh, M.; Hemmati, A. Adsorption of cadmium and nickel from aqueous solutions using dolomite powder. Desalin. Water Treat. 2015, 53, 149–157. [Google Scholar] [CrossRef]
- Yamkate, N.; Chotpantarat, S.; Sutthirat, C. Removal of Cd2+, Pb2+ and Zn2+ from contaminated water using dolomite powder. Hum. Ecol. Risk. Assess. 2017, 23, 5. [Google Scholar] [CrossRef]
- Farmaki, S.; Vorrisi, E.; Karakasi, O.K.; Moutsatsou, A. Effect of limestone and dolomite tailings’ particle size on potentially toxic elements adsorption. Open Geosci. 2018, 10, 726–739. [Google Scholar] [CrossRef]
- Marque´s, M.J.; Martinez-Conde, E.; Rovira, J.V.; Ordonez, S. Heavy metals pollution of aquatic ecosystems in the vicinity of a recently closed underground lead–zinc mine (Basque Country, Spain). Environ. Geol. 2001, 40, 1125–1137. [Google Scholar]
- Zhang, G.; Liu, C.-Q.; Yang, Y.; Wu, P. Characterization of Heavy Metals and Sulphur Isotope in Water and Sediments of a Mine-Tailing Area Rich in Carbonate. Water Air Soil Pollut. 2004, 155, 51–62. [Google Scholar] [CrossRef]
- Ospina-Alvarez, N.; Głaz, Ł.; Dmowski, K.; Krasnodębska-Ostręga, B. Mobility of toxic elements in carbonate sediments from a mining area in Poland. Environ. Chem. Lett. 2014, 12, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, K.; Bartzas, G.; Paspaliaris, I. Efficiency of limestone and red mud barriers: Laboratory column studies. Miner. Eng. 2004, 17, 183–194. [Google Scholar] [CrossRef]
- Costello, C. Acid Mine Drainage: Innovative Treatment Technologies; National Network of Environmental Management Studies Fellow for U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response Technology Innovation Office: Washington, DC, USA, 2003. [Google Scholar]
- Singh, T.N.; Singh, S.K.; Mishra, A.; Singh, P.K.; Singh, V.K. Effect of acidic water on physico-mechanical behavior of rock. Indian J. Eng. Mater. Sci. 1999, 6, 66–72. [Google Scholar]
- Singh, T.N.; Sharma, P.K.; Khandelwal, M. Effect of pH on the physico-mechanical properties of marble. Bull. Eng. Geol. Environ. 2006, 66, 81–87. [Google Scholar] [CrossRef]
- Gupta, V.; Ahmed, I. The effect of pH of water and mineralogical properties on the slake durability (degradability) of different rocks from the Lesser Himalaya, India. Eng. Geol. 2007, 95, 79–87. [Google Scholar] [CrossRef]
- Abora, K.; Beleña, I.; Bernal, S.A.; Dunster, A.; Nixon, P.A.; Provis, J.L.; Tagnit-Hamou, A.; Winnefeld, F. Durability and Testing—Chemical Matrix Degradation Processes. In Alkali Activated Materials; Provis, J., van Deventer, J., Eds.; RILEM State-of-the-Art Reports; Springer: Dordrecht, The Netherlands, 2014; Volume 13. [Google Scholar]
- Franklin, J.; Chandra, R. The slake-durability test. Int. J. Rock Mech. Min. Sci. 1972, 9, 325–328. [Google Scholar] [CrossRef]
- Ghobadi, M.H.; Kapelehe, M. The Influence of pH of Water and Chemical Composition on the Durability of Different Rocks from the Qom Formation, East and Northeast of Hamedan, Iran. J. Eng. Geol. 2017, 10, 3699–3718. [Google Scholar] [CrossRef] [Green Version]
- Yagiz, S. The Effect of pH of the Testing Liquid on the Degradability of Carbonate Rocks. Geotech. Geol. Eng. 2018, 36, 2351–2363. [Google Scholar] [CrossRef]
- Tejashwi, L.; Ananthkumar, M.; Chinnaiyan, P.; Bharath Krishnaa, A.C.; Sasi, M. Dolomite rock sand as fine aggregate re-placement in construction activities: A comparative study. Conf. Paper Mater. Today Proc. 2021, 46, 5148–5152. [Google Scholar]
- Agrawal, Y.; Gupta, T.; Siddique, S.; Sharma, R.K. Potential of dolomite industrial waste as construction material: A review. Innov. Infrastruct. Solut. 2021, 6, 1–15. [Google Scholar] [CrossRef]
- Girczys, J.; Sobik-Szołtysek, J. Waste of the Zinc-Lead Industry; Monograph Series Nr. 87; Publisher of the Częstochowa University of Technology: Czestochowa, Poland, 2002. (In Polish) [Google Scholar]
- Polish Committee for Standarization. PN-EN ISO 17892-3:2016-03—Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 3: Determination of Particle Density; Polish Committee for Standarization: Warsaw, Poland, 2016. [Google Scholar]
- Polish Committee for Standarization. PN-EN ISO 17892-2:2015-02—Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 2: Determination of Bulk Density; Polish Committee for Standarization: Warsaw, Poland, 2015. [Google Scholar]
- Al-Abed, S.R.; Jegadeesan, G.; Purandare, J.; Allen, D. Leaching behavior of mineral processing waste: Comparison of batch and column investigations. J. Hazard. Mater. 2008, 153, 1088–1092. [Google Scholar] [CrossRef]
- Polish Committee for Standarization. PN-ISO 6332:2001—Water Quality—Determination of Iron—Spectrometric Method Using 1,10-Phenanthroline; Polish Committee for Standarization: Warsaw, Poland, 2001. (In Polish) [Google Scholar]
- Polish Committee for Standarization. PN-ISO 9280:2002. Water Quality—Determination of Sulphates (VI)—Gravimetric Method with Barium Chloride; Polish Version; Polish Committee for Standarization: Warsaw, Poland, 2002. [Google Scholar]
- Aral, İ.F.; Cihan, M.T. Investigation of properties of mortars containing waste stone powder instead of sand under freez-ing-thawing effect. IOP Conf. Ser. Earth Environ. Sci. 2019, 362, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.; Peled, A.; Bar-Nes, G. Dolomite-based quarry-dust as a substitute for fly-ash geopolymers and cement pastes. J. Clean. Prod. 2019, 235, 910–919. [Google Scholar] [CrossRef]
- Nagarajan, P.; Shashikala, A.P. Development of Ground-Granulated Blast-Furnace Slag-Dolomite Geopolymer Concrete. ACI Mater. J. 2019, 116. [Google Scholar] [CrossRef]
- Barbhuiya, S. Effects of fly ash and dolomite powder on the properties of self-compacting concrete. Constr. Build. Mater. 2011, 25, 3301–3305. [Google Scholar] [CrossRef]
- Korjakins, A.; Gaidukovs, S.; Šahmenko, G.; Bajāre, D.; Pizele, D. Investigation of alternative dolomite filler properties and their application in concrete production. Sci. Proc. Riga Tech. Univ. Const. Sci. 2008, 2, 64–71. [Google Scholar]
- Mikhailova, O.; Yakovlev, G.; Maeva, I.; Senkov, S. Effect of dolomite limestone powder on the compressive strength of con-crete. Procedia Eng. 2013, 57, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Trafas, M. Changes in the properties of post-flotation wastes due to vegetation introduced during process of reclamation. Appl. Geochem. 1996, 11, 181–185. [Google Scholar] [CrossRef]
- Nowak, A.K.; Kowalski, Z.; Wzorek, Z.; Klamecki, G.; Gorazda, K. Chromium leaching from mine fillings containing chro-mium mud. Pol. J. Chem. Technol. 2004, 6, 33–35. [Google Scholar]
- Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste. Off. J. Eur. Communities 1999, 42, L182.
- Kuterasińska, J.; Król, A. Mechanical properties of alkali-activated binders based on copper slag, architecture civil engineering environment. ACEE 2015, 8, 61–67. [Google Scholar]
- Mizerna, K.; Król, A.; Mróz, A. Environmental assessment of applicability of mineral-organic composite for landfill area re-habilitation. International Conference Energy, Environment and Material Systems (EEMS 2017). E3S Web. Conf. 2017, 19, 02020. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.J.; Meeussen, J.C.L.; Comans, R.N.J. Leaching of heavy metals from contaminated soils: An experimental and mod-eling study. Environ. Sci. Technol. 2004, 38, 4390–4395. [Google Scholar] [CrossRef]
- Van der Sloot, H.A.; van Zomeren, A. Characterization leaching tests and associated geochemical speciation modeling to assess long term release behavior from extractive wastes. Mine Water Environ. 2012, 31, 92–103. [Google Scholar] [CrossRef]
- Van der Sloot, H.A. Horizontal standardization and harmonization of leaching test methods for waste, secondary raw mate-rials, construction materials and (contaminated) soil. In Proceedings Wascon 2003, Waste Materials in Construction; Ortiz de Urbina, G., Goumans, J.J.J.M., Eds.; ISCOWA: San Sebastian, Spain, 2003. [Google Scholar]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Sintorini, M.M.; Widyatmoko, H.; Sinaga, E.; Aliyah, N. Effect of pH on metal mobility in the soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 737. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, X.; Luo, H.; Deng, J.; Dai, L.; Ju, Z.; Zhu, Z.; Zou, L.; Ji, L.; Li, B.; et al. Release of Heavy Metals from the Pyrite Tailings of Huangjiagou Pyrite Mine: Batch Experiments. Sustainability 2016, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Górecka, E. Mineral sequence development in the Zn–Pb deposits of the Silesian-Cracow area, Poland. Works Natl. Geol. Instit. 1996, 154, 26–36. [Google Scholar]
- Communication from the Commission to the European Parliament, The Council, The European Economic and Social Com-mittee and the Committee of the Regions. A New Circular Economy Action Plan, Brussels, 11.3.2020, COM. 2020. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:9903b325-6388-11ea-b735-01aa75ed71a1.0017.02/DOC_1&format=PDF (accessed on 5 December 2021).






| Chemical Component | Contents, % |
|---|---|
| CO2 | 31.90 ± 0.854 |
| CaO | 25.8 ± 0.252 |
| MgO | 12.813 ± 0.290 |
| Fe2O3 | 10.267 ± 0.368 |
| SO3 | 10.073 ± 0.284 |
| ZnO | 5.136 ± 0.103 |
| SiO2 | 2.327 ± 0.097 |
| PbO | 0.814 ± 0.006 |
| Al2O3 | 0.535 ± 0.007 |
| MnO | 0.424 ± 0.005 |
| As2O3 | 0.311 ± 0.002 |
| Na2O | 0.088 ± 0.006 |
| K2O | 0.046 ± 0.001 |
| Cl | 0.035 ± 0.004 |
| CdO | 0.020 ± 0.001 |
| P2O5 | 0.017 ± 0.0009 |
| Cr2O3 | 0.008 ± 0.0004 |
| Ref. Code | Mineral Name | Chemical Formula | SemiQuant, % |
|---|---|---|---|
| 01-081-8229 | Dolomite | CaMg(CO3)2 | 61.9 |
| 98-002-0179 | Calcite | CaCO3 | 5.6 |
| 04-006-2810 | Pyrite | FeS2 | 6.7 |
| 98-003-4652 | Bassanite | CaSO4(H2O)0.5 | 12.4 |
| 98-001-7789 | Sphalerite | Zn0.73Fe0.27S | 0.8 |
| 98-004-6153 | Smithsonite | ZnCO3 | 9.3 |
| 98-000-5729 | Quartz low | SiO2 | 3.3 |
| Colour | Particle Density, g·cm−3 | Bulk Density, g·cm−3 | pHH20, (-) | Filtration Coefficient, m·s−1 |
|---|---|---|---|---|
| Honey-rusty | 2.83 ± 0.087 | 1.67 ± 0.055 | 7.61 ± 0.08 | 6.52 × 10−9 ± 0.79 |
| Column Number | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Initial pH | 3.05 | 4.00 | 5.09 | 6.14 | 7.11 | 8.20 | |
| pH of the eluate after successive portions of the leaching solution | |||||||
| Successive portions of the leaching solution, mL | 0–100 | 7.52 | 7.80 | 7.72 | 7.80 | 7.67 | 7.75 |
| 100–200 | 7.65 | 7.83 | 7.78 | 7.80 | 7.56 | 7.82 | |
| 200–300 | 7.81 | 7.85 | 7.89 | 7.87 | 7.85 | 7.86 | |
| 300–400 | 7.90 | 7.89 | 7.89 | 7.96 | 7.96 | 7.97 | |
| 400–500 | 8.05 | 8.02 | 8.07 | 8.05 | 8.04 | 8.06 | |
| 500–600 | 7.97 | 7.99 | 7.98 | 7.98 | 7.99 | 7.97 | |
| 600–700 | 7.88 | 7.86 | 7.83 | 7.90 | 7.87 | 7.86 | |
| 700–800 | 7.80 | 7.82 | 7.84 | 7.82 | 7.83 | 7.84 | |
| 800–900 | 8.02 | 8.00 | 8.01 | 8.03 | 8.00 | 8.04 | |
| 900–1000 | 8.09 | 8.07 | 8.11 | 8.12 | 8.15 | 8.20 | |
| 1000–1100 | 8.17 | 8.18 | 8.14 | 8.18 | 8.14 | 8.16 | |
| 1100–1200 | 8.16 | 8.17 | 8.16 | 8.19 | 8.18 | 8.16 | |
| 1200–1300 | 8.11 | 8.09 | 8.07 | 8.04 | 8.09 | 8.12 | |
| 1300–1400 | 8.10 | 8.10 | 8.07 | 8.04 | 8.08 | 8.11 | |
| 1400–1500 | 8.14 | 8.14 | 8.15 | 8.17 | 8.15 | 8.17 | |
| 1500–1600 | 8.14 | 8.14 | 8.13 | 8.12 | 8.13 | 8.16 | |
| 1600–1700 | 8.13 | 8.10 | 8.11 | 8.14 | 8.12 | 8.14 | |
| 1700–1800 | 8.15 | 8.13 | 8.16 | 8.14 | 8.16 | 8.16 | |
| 1800–1900 | 8.14 | 8.10 | 8.09 | 8.12 | 8.10 | 8.11 | |
| 1900–2000 | 8.13 | 8.05 | 8.03 | 8.04 | 8.06 | 8.10 | |
| Type of Sample | Place of Sampling | % of Grains in the Range | ||
|---|---|---|---|---|
| <2 μm (Clay) | 2–20 μm (Silt) | 20–200 μm (Fine Sand) | ||
| Initial sample for testing | - | 0 | 0 | 100 |
| Column 1 (pH = 3.05) | Top column | 15.82 ± 0.81 | 30.1 ± 1.03 | 54.08 ± 1.24 |
| Center column | 0.68 ± 0.07 | 13.83 ± 0.37 | 85.49 ± 0.77 | |
| Bottom column | 0.37 ± 0.04 | 18.18 ± 0.58 | 81.45 ± 0.65 | |
| Column 3 (pH = 5.09) | Top column | 0 | 1.82 ± 0.04 | 98.18 ± 0.29 |
| Center column | 1.21 ± 0.07 | 28.22 ± 0.49 | 70.57 ± 0.62 | |
| Bottom column | 0 | 2.22 ± 0.17 | 97.78 ± 0.73 | |
| Column 6 (pH = 8.2—) | Top column | 0 | 7.98 ± 0.21 | 92.02 ± 0.99 |
| Center column | 0.74 ± 0.03 | 17.53 ± 0.31 | 81.73 ± 0.93 | |
| Bottom column | 0 | 2.97 ± 0.18 | 97.03 ± 0.83 | |
| Dependent Variable | Independent Variable—pH Initial Solution | |
|---|---|---|
| Zn2+ contents | Pearson’s r | −0.38 |
| Significance | 0.115 | |
| Fe2+ contents | Pearson’s r | −0.23 |
| Significance | 0.362 | |
| Ca2+ contents | Pearson’s r | −0.54 |
| Significance | 0.021 | |
| Mg2+ contents | Pearson’s r | −0.56 |
| Significance | 0.016 | |
| Mn2+ contents | Pearson’s r | −0.73 |
| Significance | <0.001 | |
| SO42− contents | Pearson’s r | −0.33 |
| Significance | 0.186 |
| Dependent Variable | Independent Variable—pH Initial Solution | |||
|---|---|---|---|---|
| Top Column | Center Column | Bottom Column | ||
| Percentage of the fraction: clay (˂2 µm) | Pearson’s r | −0.80 | −0.01 | −0.80 |
| Significance | 0.010 | 0.980 | 0.010 | |
| Percentage of the fraction: silt (2–20 µm) | Pearson’s r | −0.66 | 0.13 | −0.77 |
| Significance | 0.054 | 0.736 | 0.014 | |
| Percentage of the fraction: fine sand (20–200 µm) | Pearson’s r | 0.72 | −0.12 | 0.77 |
| Significance | 0.030 | 0.751 | 0.015 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobik-Szołtysek, J. The Effect of pH on Stability of an Isolation Barrier Made of Dolomite Post-Floatation Waste. Minerals 2021, 11, 1384. https://doi.org/10.3390/min11121384
Sobik-Szołtysek J. The Effect of pH on Stability of an Isolation Barrier Made of Dolomite Post-Floatation Waste. Minerals. 2021; 11(12):1384. https://doi.org/10.3390/min11121384
Chicago/Turabian StyleSobik-Szołtysek, Jolanta. 2021. "The Effect of pH on Stability of an Isolation Barrier Made of Dolomite Post-Floatation Waste" Minerals 11, no. 12: 1384. https://doi.org/10.3390/min11121384
APA StyleSobik-Szołtysek, J. (2021). The Effect of pH on Stability of an Isolation Barrier Made of Dolomite Post-Floatation Waste. Minerals, 11(12), 1384. https://doi.org/10.3390/min11121384






