Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks
Abstract
:1. Introduction
- Applications for recovering and removing metals and metalloids from different sources (feed solution);
- Aspects regarding chemical reactions and reactor design (precipitation reactor);
- Sulfide reagent sources (sulfide sources);
- Characteristics of precipitates (precipitate suspension);
- Advances in solid–liquid separation (clarification);
- Future perspectives.
2. Applications for Recovering and Removing Metals and Metalloids from Different Sources
2.1. Acid Mine Drainages (AMD)
2.2. Industrial Wastewater
2.3. Leachates from Catalysts, Electronic Waste, and Battery Waste
2.4. Smelting Leachates and Effluents
2.5. Leachates Solutions from Ores and Tailings
3. Features of Chemical Reactions and Reactor Design
3.1. Reaction Time
3.2. Reactor Type
3.3. Supersaturation Features
3.4. Effect of Excess Sulfide
4. Sulfide Reagent Sources
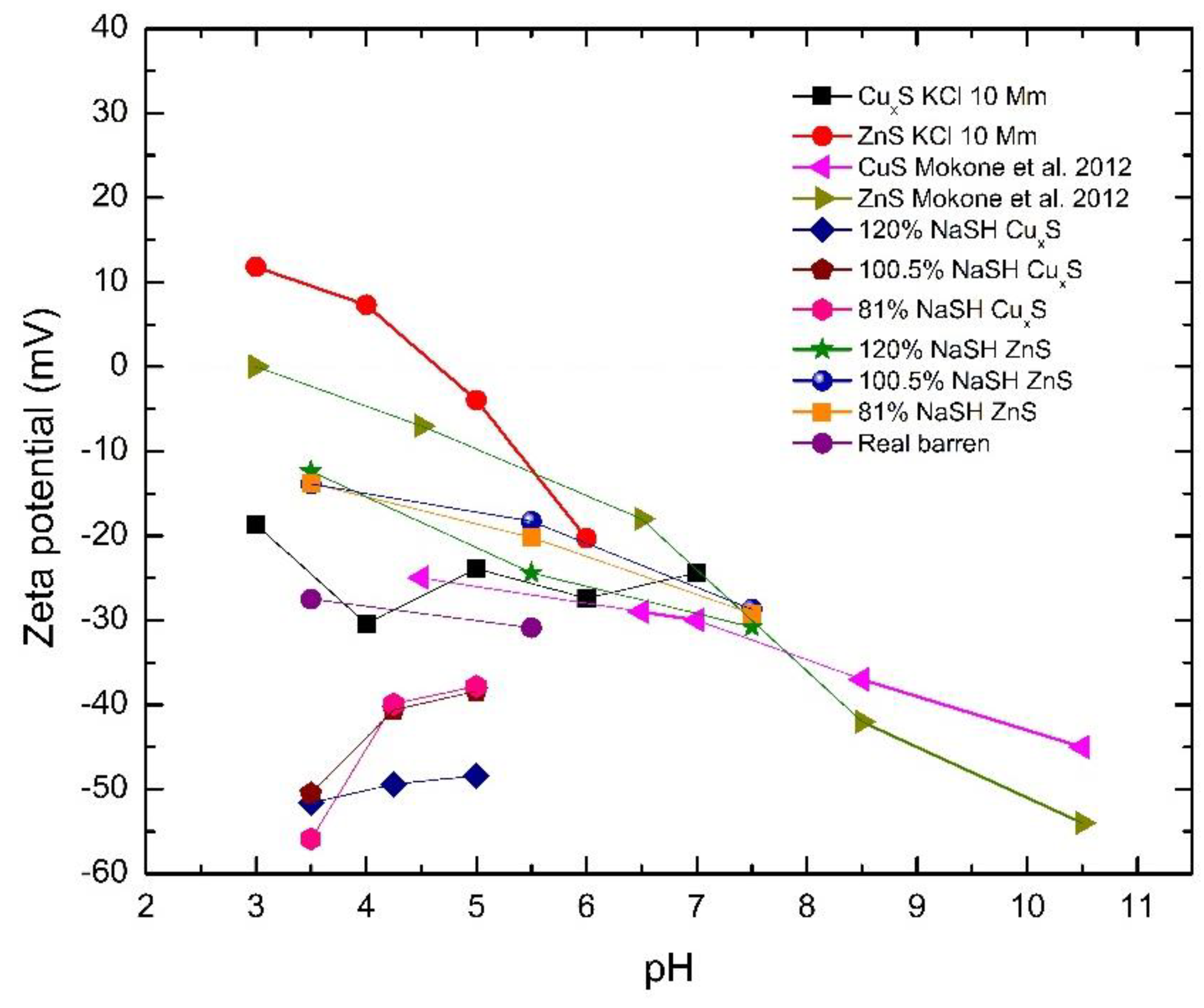
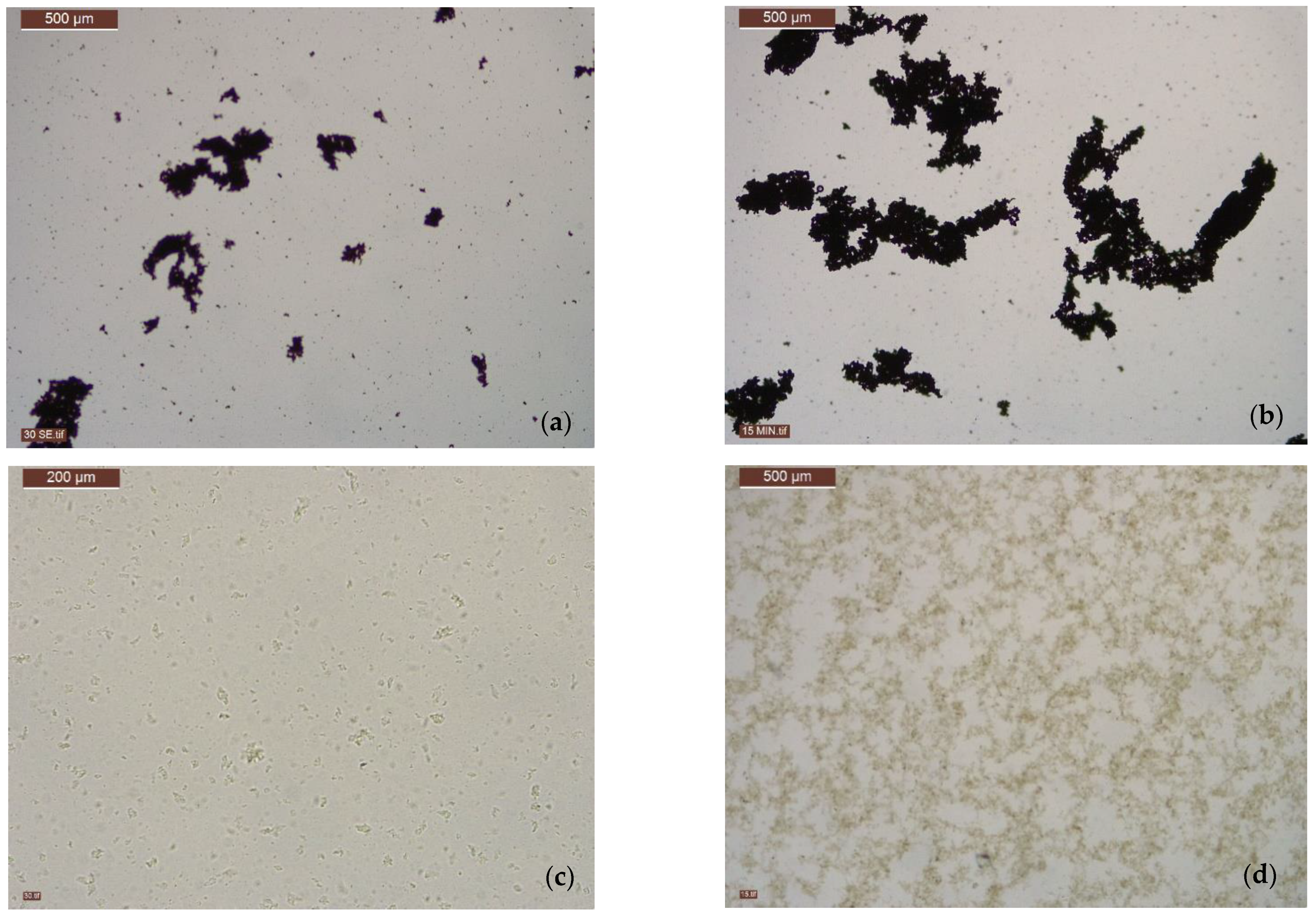
5. Characteristics of Precipitates
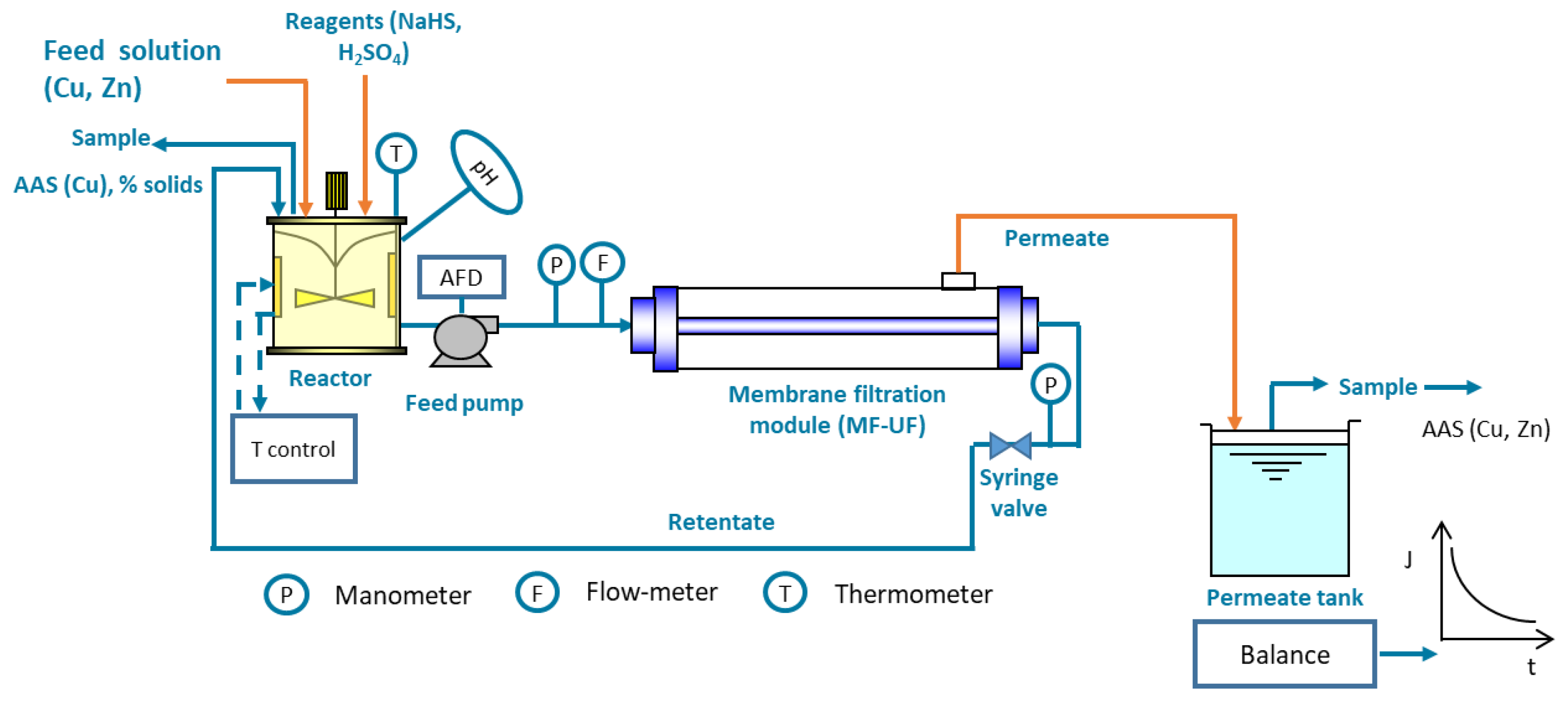
6. Latest Breakthroughs in Solid-Liquid Separation
7. Future Perspectives
7.1. Selective Precipitation and Recovery
7.2. Kinetic Studies
7.3. Reactor Type and Supersaturation Control
7.4. Solid-Liquid Separation
7.5. Stabilility of Precipitates for Disposal
7.6. Nanoparticle Production
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Monhemius, A.J. Precipitation diagrams for metal hydroxides, sulphides, arsenates and phosphates. Trans. Inst. Min. Metall. C 1977, 86, 202–206. [Google Scholar]
- Estay, H. Designing the SART process—A review. Hydrometallurgy 2018, 176, 147–165. [Google Scholar] [CrossRef]
- Kratochvil, D.; Ye, S.; López, O. Commercial Case Studies of Life Cycle Cost Reduction of ARD Treatment with Sulfide Precipitation. In Proceedings of the 10th International Conference on Acid Rock Drainage & IMWA Annual Conference (10th ICARD IMWA 2015), Santiago, Chile, 21–24 April 2015; Available online: https://www.imwa.info/docs/imwa_2015/IMWA2015_Kratochvi_020.pdf (accessed on 20 July 2021).
- Altun, M.; Sahinkaya, E.; Durukan, I.; Bektas, S.; Komnitsas, K. Arsenic removal in a sulfidogenic fixed-bed column bioreactor. J. Hazard. Mater. 2014, 269, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yang, Z.; He, Z.; Wu, L.; Hu, C.; Wu, W.; Qu, J. Treatment of strongly acidic wastewater with high arsenic concentrations by ferrous sulfide (FeS): Inhibitive effects of S(0)-enriched surfaces. Chem. Eng. J. 2016, 304, 986–992. [Google Scholar] [CrossRef]
- Wang, H.; Chen, F.; Mu, S.; Zhang, D.; Pan, X.; Lee, D.; Chang, J. Removal of antimony (Sb(V)) from Sb mine drainage: Biological sulfate reduction and sulfide oxidation-precipitation. Bioresour. Technol. 2012, 146, 799–802. [Google Scholar] [CrossRef]
- Xingyu, L.; Gang, Z.; Xiaoqiang, W.; Laichang, Z.; Jiankang, W.; Renman, R.; Dianzuo, W. A novel low pH sulfidogenic bioreactor using activated sludge as carbon source to treat acid mine drainage (AMD) and recovery metal sulfides: Pilot scale study. Miner. Eng. 2013, 48, 51–55. [Google Scholar] [CrossRef]
- Macingova, E.; Luptakova, A. Recovery of Metals from Acid Mine Drainage. Chem. Eng. Trans. 2012, 28, 109–114. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. Process development for the recovery of rare earth elements and critical metals from an acid mine leachate. Miner. Eng. 2020, 153, 106382. [Google Scholar] [CrossRef]
- Ying, Z.; Zhijing, F.; Xiaofei, C.; Guoyan, Z. Bioremediation Process and Bioremoval Mechanism of Heavy Metal Ions in Acidic Mine Drainage. Chem. Res. Chin. Univ. 2018, 34, 33–38. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Lin, N.; Ou, C.; Wang, X.; Zhang, L.; Jiang, F. Removal of heavy metals using a novel sulfidogenic AMD treatment system with sulfur reduction: Configuration, performance, critical parameters and economic analysis. Environ. Int. 2020, 136, 105457. [Google Scholar] [CrossRef]
- Kumar, M.; Pakshirajan, K. Continuous removal and recovery of metals from wastewater using inverse fluidized bed sulfidogenic bioreactor. J. Clean. Prod. 2021, 284, 124769. [Google Scholar] [CrossRef]
- Costa, J.M.; Rodriguez, R.P.; Sancinetti, G.P. Removal sulfate and metals Fe+2, Cu+2, and Zn+2 from acid mine drainage in an anaerobic sequential batch reactor. J. Environ. Chem. Eng. 2017, 5, 1985–1989. [Google Scholar] [CrossRef]
- Colipai, C.; Southam, G.; Oyarzún, P.; González, D.; Díaz, V.; Contreras, B.; Nancucheo, I. Synthesis of Copper Sulfide Nanoparticles Using Biogenic H2S Produced by a Low-pH Sulfidogenic Bioreactor. Minerals 2017, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Mokone, T.P.; van Hille, R.P.; Lewis, A.E. Effect of solution chemistry on particle characteristics during metal sulfide precipitation. J. Colloid Interface Sci. 2010, 351, 10–18. [Google Scholar] [CrossRef]
- Castro-Neto, E.S.; Aguiar, A.B.S.; Rodriguez, R.P.; Sancinetti, G.P. Acid mine drainage treatment and metal removal based on a biological sulfate reducing process. Braz. J. Chem. Eng. 2018, 35, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Sahinkaya, E.; Yucesoy, Z. Biotreatment of acidic zinc- and copper-containing wastewater using ethanol-fed sulfidogenic anaerobic baffled reactor. Bioprocess Biosyst. Eng. 2010, 33, 989–997. [Google Scholar] [CrossRef]
- Leal-Gutiérrez, M.J.; Cuéllar-Briseño, R.; Castillo-Garduño, A.M.; Bernal-González, M.; Chávez-Castellanos, A.E.; Solís-Fuentes, J.A.; Durán-Domínguez-de-Bazúa, M.; Bazúa-Rueda, E.R. Precipitation of Heavy Metals Ions (Cu, Fe, Zn and Pb) from Mining Flotation Effluents Using a Laboratory-Scale Upflow Anaerobic Sludge Blanket Reactor. Water Air Soil Pollut. 2021, 197, 232. [Google Scholar] [CrossRef]
- Menzel, K.; Barros, L.; García, A.; Ruby-Figueroa, R.; Estay, H. Metal sulfide precipitation coupled with membrane filtration process for recovering copper from acid mine drainage. Sep. Purif. Technol. 2021, 270, 118721. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Long, J.; Zhang, P.; Chen, Y. Combined Fenton process and sulfide precipitation for removal of heavy metals from industrial wastewater: Bench and pilot scale studies focusing on in-depth thallium removal. Front. Environ. Sci. Eng. 2019, 13, 49. [Google Scholar] [CrossRef]
- Wang, L.P.; Chen, Y.J. Sequential Precipitation of Iron, Copper, and Zinc from Wastewater for Metal Recovery. J. Environ. Eng. 2019, 145, 04018130. [Google Scholar] [CrossRef]
- Janyasuthiwong, S.; Rene, E.R.; Esposito, G.; Lens, P.N.L. Effect of pH on Cu, Ni and Zn removal by biogenic sulfide precipitation in an inversed fluidized bed bioreactor. Hydrometallurgy 2015, 158, 94–100. [Google Scholar] [CrossRef]
- Bilgin, A.; Jaffé, P.R. Precipitation of Copper (II) in a Two-Stage Continuous Treatment System Using Sulfate Reducing Bacteria. Waste Biomass Valorization 2018, 10, 2907–2914. [Google Scholar] [CrossRef]
- De Alburquerque, V.F.; de Barros, A.L.; Lopes, A.C.; dos Santos, A.B.; do Nascimento, R.F. Removal of the metal ions Zn2+, Ni2+, and Cu2+ by biogenic sulfide in UASB reactor and speciation studies. Desalination Water Treat. 2013, 51, 2093–2101. [Google Scholar] [CrossRef]
- Guo, L.; Du, Y.; Yi, Q.; Li, D.; Cao, L.; Du, D. Efficient removal of arsenic from “dirty acid” wastewater by using a novel immersed multi-start distributor for sulphide feeding. Sep. Purif. Technol. 2015, 142, 209–214. [Google Scholar] [CrossRef]
- Nanusha, M.Y.; Carlier, J.D.; Carvalho, G.I.; Costa, M.C.; Paiva, A.P. Separation and recovery of Pd and Fe as nanosized metal sulphides by combining solvent extraction with biological strategies based on the use of sulphate-reducing bacteria. Sep. Purif. Technol. 2019, 212, 747–756. [Google Scholar] [CrossRef]
- Yatim, S.R.M.; Zainuddin, N.A.; Mokhtar, N.S.; Syahjidan, H.N.; Kamsuri, S.N.H. Competitiveness in removing copper, zinc and chromium trivalent in plating industrial effluent by using hydroxide precipitation versus sulphide precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012084. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, R.; Deng, W.; Li, J. Highly efficient removal of Cu(II), Zn(II), Ni(II) and Fe(II) from electroplating wastewater using sulphide from sulphidogenic bioreactor effluent. Environ. Technol. 2012, 33, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.A.; Mamat, T.A.R.; Maarof, H.I.; Puasa, S.W.; Yatim, S.R.M. Removal of Nickel, Zinc and Copper from Plating Process Industrial Raw Effluent Via Hydroxide Precipitation Versus Sulphide Precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 012122. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Liang, Z.; Sun, J.; Qiu, Y.; Qiu, C.; Liang, X.; Zhu, Y.; Wang, P.; Li, Y.; Jiang, F. A pilot-scale sulfur-based sulfidogenic system for the treatment of Cu-laden electroplating wastewater using real domestic sewage as electron donor. Water Res. 2021, 195, 116999. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.C.; Guibal, E. Metal valorization from the waste produced in the manufacturing of Co/Mo catalysts: Leaching and selective precipitation. J. Mater. Cycles Waste Manag. 2019, 21, 525–538. [Google Scholar] [CrossRef]
- Vemic, M.; Bordas, F.; Comte, S.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Recovery of molybdenum, nickel and cobalt by precipitation from the acidic leachate of a mineral sludge. Environ. Technol. 2016, 37, 2231–2242. [Google Scholar] [CrossRef]
- Park, I.; Kim, D.; Kim, M.; Jeong, H. Recovery of Cadmium in Nickel-Cadmium Leaching Solution by Sulfide Precipitation Method. J. Korean Inst. Met. Mater. 2019, 57, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Oraby, E.; Eksteen, J. Recovery of copper and the deportment of other base metals from alkaline glycine leachates derived from printed circuit boards (WPCBs). Hydrometallurgy 2021, 199, 105540. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Ferella, F.; Beolchini, F.; Kopacek, B.; Vegliò, F. Recovery of yttrium from fluorescent powder of cathode ray tube, CRT: Zn removal by sulphide precipitation. Waste Manag. 2013, 33, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Cibati, A.; Cheng, K.Y.; Morris, C.; Ginige, M.P.; Sahinkaya, E.; Pagnanelli, F.; Kaksonen, A.H. Selective precipitation of metals from synthetic spent refinery catalyst leach liquor with biogenic H2S produced in a lactate-fed anaerobic baffled reactor. Hydrometallurgy 2013, 139, 154–161. [Google Scholar] [CrossRef]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef]
- Calvert, G.; Kaksonen, A.H.; Cheng, K.Y.; Van Yken, J.; Chang, B.; Boxall, N.J. Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide. Minerals 2019, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Yang, T.; Liu, W.; Zhang, D.; Chen, L. Removal of arsenic from acid wastewater via sulfide precipitation and its hydrothermal mineralization stabilization. Trans. Nonferrous Met. Soc. China 2019, 29, 2411–2421. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Jin, B. An effective separation process of arsenic, lead, and zinc from high arsenic-containing copper smelting ashes by alkali leaching followed by sulfide precipitation. Waste Manag. Res. 2020, 38, 1214–1221. [Google Scholar] [CrossRef]
- Hong, T.; Zheng, T.; Liu, M.; Mumford, K.A.; Stevens, G.W. Investigation on the recovery of rhenium in the high arsenite wash acid solution from the copper smelting process using reducing sulfide precipitation method. Hydrometallurgy 2020, 195, 105402. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Hu, Y.; Han, H.; Luo, X.; Sun, W.; Yue, T.; Wang, L.; Cao, X.; Zhou, H. Selective sulfide precipitation of copper ions from arsenic wastewater using monoclinic pyrrhotite. Sci. Total Environ. 2020, 705, 135816. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Kong, L.; Hu, X.; Peng, X. Recovery of Re(VII) from strongly acidic wastewater using sulphide: Acceleration by UV irradiation and the underlying mechanism. J. Hazard. Mater. 2021, 416, 126233. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, S.; Kermer, R.; Aubel, T.; Martin, M.; Schippers, A.; Johnson, D.B.; Janneck, E. Implementation of biological and chemical techniques to recover metals from copper-rich leach solutions. Hydrometallurgy 2018, 179, 274–281. [Google Scholar] [CrossRef]
- Chen, T.; Lei, C.; Yan, B.; Xiao, X. Metal recovery from the copper sulfide tailing with leaching and fractional precipitation technology. Hydrometallurgy 2014, 147–148, 178–182. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, B.; Zhang, D.; Chen, L.; Yang, T. Selective Separation of Similar Metals in Chloride Solution by Sulfide Precipitation Under Controlled Potential. JOM 2017, 69, 2358–2363. [Google Scholar] [CrossRef]
- Gim-Krumm, M.; Quilaqueo, M.; Rojas, V.; Seriche, G.; Ruby-Figueroa, R.; Cortés-Arriagada, D.; Romero, J.; Troncoso, E.; Estay, H. Impact of precipitate characteristics and precipitation conditions on the settling performance of a sulfide precipitation process: An exhaustive characterization of the aggregation behavior. Hydrometallurgy 2019, 189, 105150. [Google Scholar] [CrossRef]
- Estay, H.; Ruby-Figueroa, R.; Quilaqueo, M.; Seriche, G.; Cortés, I.; Gim-Krumm, M.; Barros, L. Enhancing the effectiveness of copper and cyanide recovery in gold cyanidation: A new integrated membrane process. Hydrometallurgy 2021, 202, 105606. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Gim-Krumm, M.; Ruby-Figueroa, R.; Troncoso, E.; Estay, H. Determination of size distribution of precipitation aggregates using non-invasive microscopy and semiautomated image processing and analysis. Minerals 2019, 9, 724. [Google Scholar] [CrossRef] [Green Version]
- Estay, H.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Barros, L.; Ruby-Figueroa, R.; Romero, J.; Troncoso, E. Optimizing the SART process: A critical assessment of its design criteria. Miner. Eng. 2020, 146, 106116. [Google Scholar] [CrossRef]
- Estay, H.; Ruby-Figueroa, R.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Díaz-Quezada, S.; Cortés, I.; Barros, L. Changing the conventional clarification method in metal sulfide precipitation by a membrane-based filtration process. J. Mater. Sci. Technol. 2021, 11, 693–709. [Google Scholar] [CrossRef]
- Barros, L.; Gim-Krumm, M.; Seriche, G.; Quilaqueo, M.; Castillo, C.; Ihle, C.F.; Ruby- Figueroa, R.; Estay, H. In-situ and real-time aggregation size evolution of copper sulfide precipitates using focused beam reflectance measurement (FBRM). Powder Technol. 2021, 380, 205–218. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. The sulfide precipitation behaviour of Cu and Au from their aqueous alkaline glycinate and cyanide complexes. Sep. Purif. Technol. 2019, 218, 181–190. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. Sulfide precipitation of copper from alkaline glycine-cyanide solutions: Precipitate characterisation. Miner. Eng. 2020, 145, 106102. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Papirio, S.; Villa-Gomez, D.K.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Acid mine drainage treatment in fluidized-bed bioreactors by sulfate-reducing bacteria: A critical review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2545–2580. [Google Scholar] [CrossRef]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Alam, R.; McPhedran, K. Applications of biological sulfate reduction for remediation of arsenic—A review. Chemosphere 2019, 222, 932–944. [Google Scholar] [CrossRef]
- Diez-Ercilla, M.; Sánchez-España, J.; Yusta, I.; Wendt-Potthoff, K.; Koschorreck, M. Formation of biogenic sulphides in the water column of an acidic pit lake: Biogeochemical controls and effects on trace metal dynamics. Biogeochemistry 2014, 121, 519–536. [Google Scholar] [CrossRef]
- Sethurajan, M.; Gaydardzhiev, S. Bioprocessing of spent lithium ion batteries for critical metals recovery—A review. Resour. Conserv. Recycl. 2021, 165, 105225. [Google Scholar] [CrossRef]
- Hedjazi, F.; Monhemius, A.J. Copper-gold ore processing with ion exchange and SART technology. Miner. Eng. 2014, 64, 120–125. [Google Scholar] [CrossRef]
- Estay, H.; Gim-Krumm, M.; Quilaqueo, M. Two-stage SART process: A feasible alternative for gold cyanidation plants with high zinc and copper contents. Minerals 2018, 8, 392. [Google Scholar] [CrossRef] [Green Version]
- Medina, D.; Anderson, C.G. A review of the cyanidation treatment of copper-gold ores and concentrates. Metals 2020, 10, 897. [Google Scholar] [CrossRef]
- Uçar, D. Sequential Precipitation of Heavy Metals Using Sulfide-Laden Bioreactor Effluent in a pH Controlled System. Miner. Process. Extr. Metall. Rev. 2017, 38, 162–167. [Google Scholar] [CrossRef]
- Ostermeyer, P.; Bonin, L.; Folens, K.; Verbruggen, F.; García-Timermans, C.; Verbeken, K.; Rabaey, K.; Hennebel, T. Effect of speciation and composition on the kinetics and precipitation of arsenic sulfide from industrial metallurgical wastewater. J. Hazard. Mater. 2021, 409, 124418. [Google Scholar] [CrossRef] [PubMed]
- Gharabaghi, M.; Irannajad, M.; Azadmehr, A.R. Selective Sulphide Precipitation of Heavy Metals from Acidic Polymetallic Aqueous Solution by Thioacetamide. Ind. Eng. Chem. Res. 2012, 51, 954–963. [Google Scholar] [CrossRef]
- Tokuda, H.; Kuchar, D.; Mihara, N.; Kubota, M.; Matsuda, H.; Fukuta, T. Study on reaction kinetics and selective precipitation of Cu, Zn, Ni and Sn with H2S in single-metal and multi-metal systems. Chemosphere 2008, 73, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, B.; Zeng, W.; Li, K.; Liu, S.; Hu, H.; Guo, W. Design and analysis of continuous-flow reactors for copper sulfide precipitation process by a computational method. Environ. Sci. Pollut. Res. 2019, 26, 34531–34551. [Google Scholar] [CrossRef]
- Simons, A. Fundamental Study of Copper and Cyanide Recovery from Gold Tailing by Sulfidisation. Ph.D. Thesis, WA School of Mines, Curtin University, Perth, WA, USA, 2015. [Google Scholar]
- Van Hille, R.P.; Peterson, K.A.; Lewis, A.E. Copper sulphide precipitation in a fluidised bed reactor. Chem. Eng. Sci. 2005, 60, 2571–2578. [Google Scholar] [CrossRef]
- Mokone, T.P.; van Hille, R.P.; Lewis, A.E. Metal sulphides from wastewater: Assessing the impact of supersaturation control strategies. Water Res. 2012, 46, 2088–2100. [Google Scholar] [CrossRef]
- Villa-Gomez, D.; Ababneh, H.; Papirio, S.; Rousseau, D.P.L.; Lens, P.N.L. Effect of sulfide concentration on the location of the metal precipitates in inversed fluidized bed reactors. J. Hazard. Mater. 2011, 192, 200–207. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Riekkola-Vanhanen, M.L.; Puhakka, J.A. Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res. 2003, 37, 255–266. [Google Scholar] [CrossRef]
- Sampaio, R.M.M.; Timmers, R.A.; Kocks, N.; André, V.; Duarte, M.T.; van Hullebusch, E.D.; Farges, F.; Lens, P.N.L. Zn-Ni sulfide selective precipitation: The role of supersaturation. Sep. Purif. Technol. 2010, 74, 108–118. [Google Scholar] [CrossRef]
- Zeng, W.; Guo, W.; Li, B.; Wei, Z.; Dionysiou, D.D.; Xiao, R. Kinetics and mechanistic aspects of removal of heavy metal through gas-liquid sulfide precipitation: A computational and experimental study. J. Hazard. Mater. 2021, 408, 124868. [Google Scholar] [CrossRef] [PubMed]
- Kiran, M.G.; Pakshirajan, K.; Das, G. An overview of sulfidogenic biological reactors for the simultaneous treatment of sulfate and heavy metal rich wastewater. Chem. Eng. Sci. 2017, 158, 606–620. [Google Scholar] [CrossRef]
- Silva, P.M.O.; Raulino, G.S.C.; Vidal, C.B.; do Nascimento, R.F. Selective precipitation of Cu2+, Zn2+ and Ni2+ ions using H2S(g) produced by hydrolysis of thioacetamide as the precipitating agent. Desalination Water Treat. 2017, 95, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Rostamnezhad, N.; Kahforoushan, D.; Sahraei, E.; Ghanbarian, S.; Shabani, M. A method for the removal of Cu(II) from aqueous solutions by sulfide precipitation employing heavy oil fly ash. Desalination Water Treat. 2016, 57, 17593–17602. [Google Scholar] [CrossRef]
- Zvimba, J.N.; Mulopo, J.; de Beer, M.; Bologo, L.; Mashego, M. The dissolution characteristics of calcium sulfide and utilization as a precipitation agent in acidic wastewater effluent treatment. Water Sci. Techonol. 2011, 63, 2860–2866. [Google Scholar] [CrossRef]
- Hong, T.; Wei, Y.; Li, L.; Mumford, K.; Stevens, G. An investigation into the precipitation of copper sulfide from acidic sulfate solutions. Hydrometallurgy 2020, 192, 105288. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Bo, A.; Tana, T.; Liu, X.; Zhao, F.; Sarina, S.; Jia, M.; Yang, C.; Gu, Y.; et al. Simultaneous removal of cationic and anionic heavy metal contaminants from electroplating effluent by hydrotalcite adsorbent with disulfide (S2−) intercalation. J. Hazard. Mater. 2020, 382, 121111. [Google Scholar] [CrossRef]
- Zhu, F.; Hu, X.; Kong, L.; Peng, X. Calcium sulfide-organosilicon complex for sustained release of H2S in strongly acidic wastewater: Synthesis, mechanism and efficiency. J. Hazard. Mater. 2021, 421, 126745. [Google Scholar] [CrossRef]
- Mokone, T.P.; Lewis, A.E.; van Hille, R.P. Effect of post-precipitation conditions on surface properties of colloidal metal sulphide precipitates. Hydrometallurgy 2012, 119–120, 55–66. [Google Scholar] [CrossRef]
- Nduna, M.; Rodriguez-Pascual, M.; Lewis, A.E. Effect of dissolved precipitating ions on the settling characteristics of copper sulphide. J. South. Afr. Inst. Min. Metall. 2013, 113, 435–439. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S2225-62532013000500009&lng=en&nrm=iso (accessed on 20 August 2021).
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Nduna, M.K.; Lewis, A.E.; Nortier, P. A model for the zeta potential of copper sulphide. Colloids Surface A 2014, 441, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Villa-Gomez, D.K.; van Hullebusch, E.D.; Maestro, R.; Farges, F.; Nikitenko, S.; Kramer, H.; Gonzalez-Gil, G.; Lens, P.N.L. Morphology, Mineralogy, and Solid-Liquid Phase Separation Characteristics of Cu and Zn Precipitates Produced with Biogenic Sulfide. Environ. Sci. Technol. 2014, 48, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wrighton-Araneda, K.; Ruby-Figueroa, R.; Estay, H.; Cortés-Arriagada, D. Interaction of H2O with (CuS)n, (Cu2S)n, and (ZnS)n small clusters (n = 1–4, 6): Relation to the aggregation characteristics of metal sulfides at aqueous solutions. J. Mol. Model. 2019, 25, 291. [Google Scholar] [CrossRef]
- Xia, Z.; Peng, X.; Kong, L.; Hu, X. Hydrophilicity/hydrophobicity of metal sulfide particles as a determinator of aggregation performance in wastewater. J. Water Process Eng. 2021, 40, 101900. [Google Scholar] [CrossRef]
- Mirazimi, M.; Liu, W. Aqueous arsenic and sulfur speciation analysis and solid phase characterization in the dissolution of arsenic trisulfide. Hydrometallurgy 2021, 199, 105545. [Google Scholar] [CrossRef]
- Kong, L.; Xia, Z.; Hu, X.; Peng, X. Chemical solidification/stabilization of arsenic sulfide and oxide mixed wastes using elemental sulfur: Efficiencies, mechanisms and long-term stabilization enhancement by dicyclopentadiene. J. Hazard. Mater. 2021, 419, 126390. [Google Scholar] [CrossRef]
- Breuer, P. Dealing with copper in gold ores; implemented and future approaches. In Proceedings of the Gold-PM Conference, 20th Anniversary Event, ALTA 2015, Perth, Australia, 23–30 May 2015. [Google Scholar]
- Estay, H.; Carvajal, P.; Hedjazi, F.; Van Zeller, T. The SART process experience in the Gedabek plant. In Proceedings of the Hydroprocess 2012, 4th International Workshop on Process Hydrometallurgy, Santiago, Chile, 11–13 July 2012. [Google Scholar]
- Gqebe, S.; Rodriguez-Pascual, M.; Lewis, A. A modification of the zeta potential of copper sulphide by the application of a magnetic field in order to improve particle settling. J. South. Afr. Inst. Min. Metall. 2016, 116, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Chai, L.; Li, Q.; Ye, L.; Yang, B.; Wang, Q. Abiological Granular Sludge Formation Benefit for Heavy Metal Wastewater Treatment Using Sulfide Precipitation. Clean-Soil Air Water 2017, 45, 1500730. [Google Scholar] [CrossRef]
- Peng, X.; Xia, Z.; Kong, L.; Hu, X.; Wang, X. UV light irradiation improves the aggregation and settling performance of metal sulfide particles in strongly acidic wastewater. Water Res. 2019, 163, 114860. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.K.; Wang, L.K. Sulfide Precipitation for Treatment of Metal Wastes. In Handbook of Advanced Industrial and Hazardous Waste Management; Wang, L.K., Wang, M.H.S., Hung, Y.T., Shammas, N.K., Chen, J.P., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 185–238. [Google Scholar]
- Zeng, W.; Guo, W.; Li, B.; Xiao, R.; Hu, H.; Yan, Y.; Wu, L.; Wei, Z.; Chai, L. Experimental and simulation studies of metal sulfide precipitates separation in copper smelting waste acid using a gravitation field-flow fractionation method. J. Water Process Eng. 2020, 36, 101330. [Google Scholar] [CrossRef]
- Mirazimi, M.; Fan, J.; Liu, W. Kinetics of arsenic and sulfur release from amorphous arsenic trisulfide. Hydrometallurgy 2021, 200, 105555. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Girão, A.V.; Trindade, T.; Costa, M.C.; Duarte, A.; Rocha-Santos, T. Biological synthesis of nanosized sulfide semiconductors: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 8283–8302. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Groeneveld, J.D.; Pokhrel, S.; Mädler, L. Metal Sulfide Nanoparticles: Precusor Chemistry. Chem. Eur. J. 2021, 27, 6390–6406. [Google Scholar] [CrossRef] [PubMed]
- Balischewski, C.; Choi, H.S.; Behrens, K.; Beqiraj, A.; Körzdörfer, T.; Gebner, A.; Wendel, A. Metal Sulfide Nanoparticle Synthesis with Ionic Liquids-State of the Art and Future Perspectives. ChemistryOpen 2021, 10, 272–295. [Google Scholar] [CrossRef]
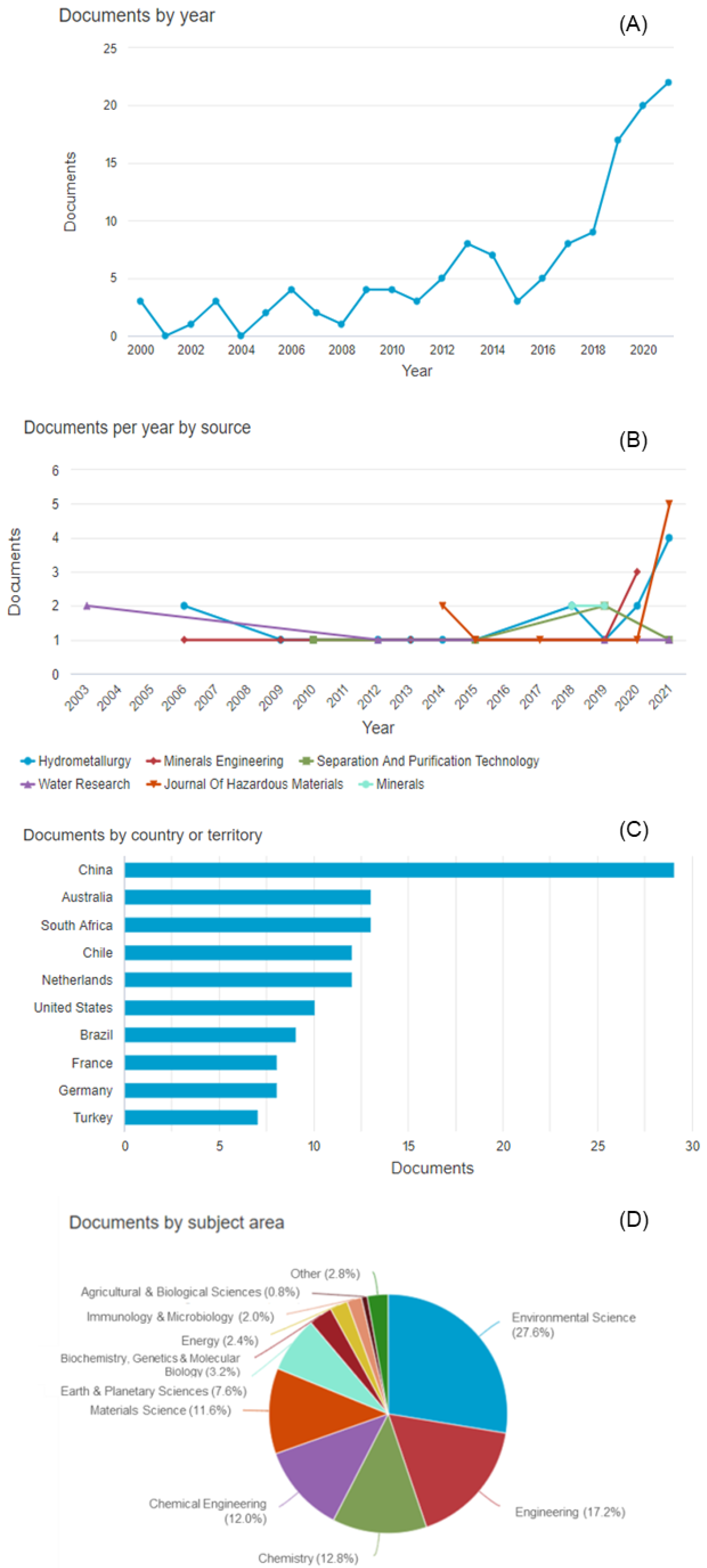
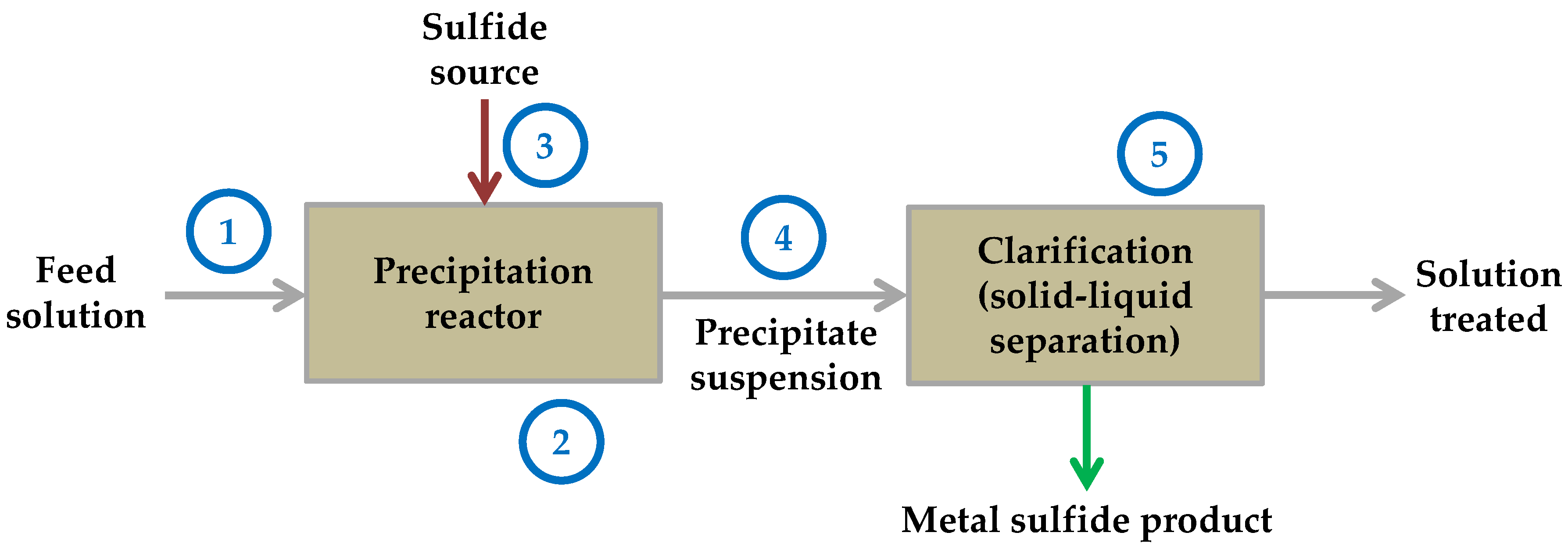
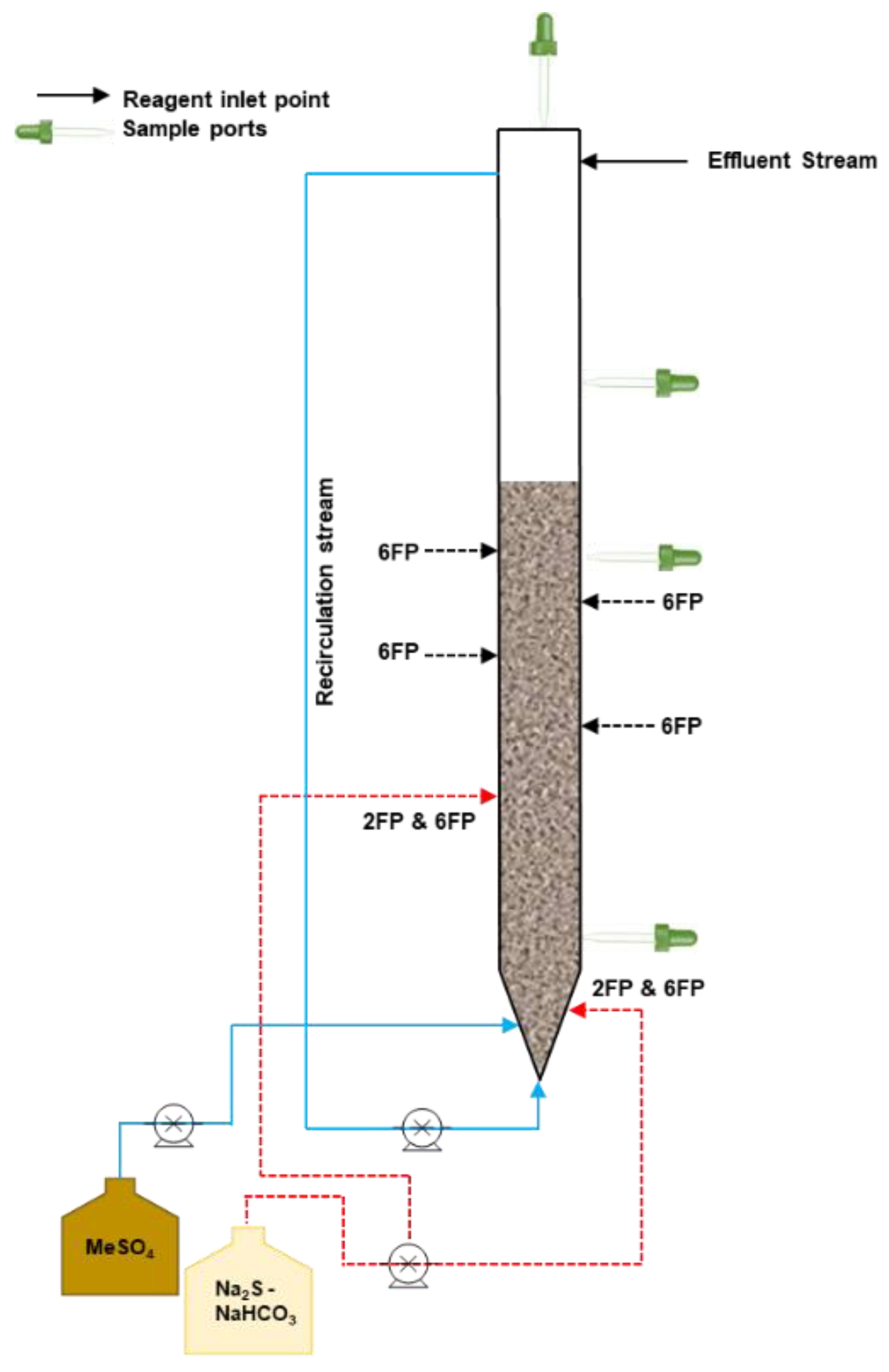
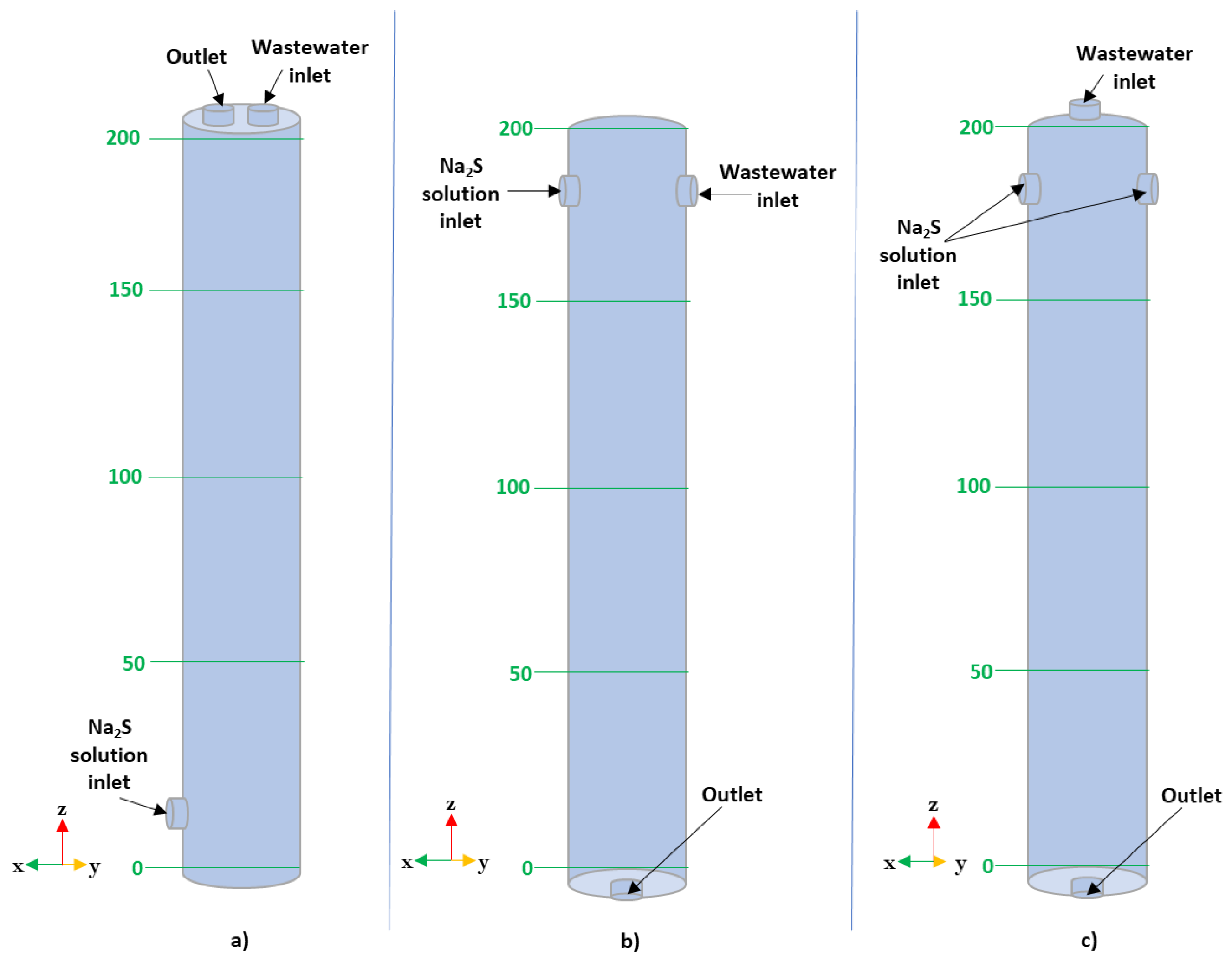
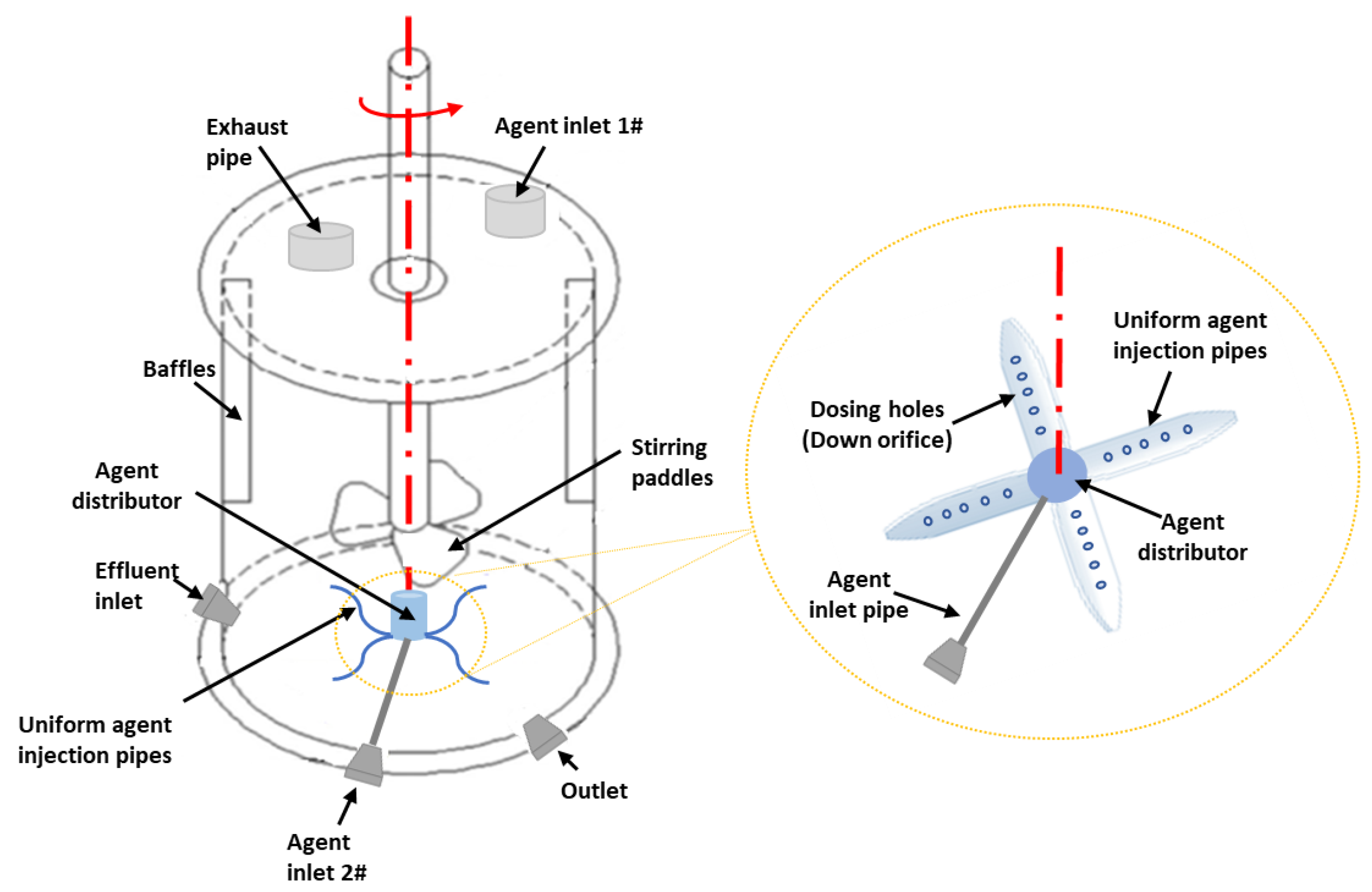

| Element | pKsp |
|---|---|
| Bi3+ | 98.8 |
| Hg2+ | 52.2 |
| Ag+ | 49.2 |
| Cu+ | 47.7 |
| Cu2+ | 35.9 |
| Cd2+ | 28.9 |
| Pb2+ | 28.1 |
| Sn2+ | 27.5 |
| Zn2+ | 24.5 |
| Co2+ | 22.1 |
| Ni2+ | 21.0 |
| Fe2+ | 18.8 |
| Mn2+ | 13.3 |
| Feed Solution Type | Metals/Metalloids | Operational Conditions | Conversion, % | Sulfide Reagent Source | Reference | |
|---|---|---|---|---|---|---|
| pH | Sulfide Dosage, Molar Ratio (S2−/M) | |||||
| AMD | As | 4.0 | Not reported | 8–95.4 | Biogenic H2S | [5] |
| AMD | As | ~0 | 3–5 | ~100 | FeS | [6] |
| AMD | Sb | 7 | 15 | ~100 | Biogenic H2S | [7] |
| AMD | Cu | 2.34–2.56 | Not reported | 10–80 | Biogenic H2S | [8] |
| AMD | Cu | 3.7 | Not reported | 99.8 | Biogenic H2S | [9] |
| Zn | 5.0 | 99.9 | ||||
| AMD | Cu | 2.0 | 2.91 | >95 | Na2S 1M | [10] |
| Zn | 3.0 | 3.84 | >95 | |||
| AMD | Cu | 6.7 | Not reported | 99.99 | Biogenic H2S | [11] |
| Fe | 87.64 | |||||
| Zn | 99.88 | |||||
| AMD | Cu | 3.2 | Not reported | >99.9 | Biogenic H2S | [12] |
| Pb | >99.9 | |||||
| Zn | >99.9 | |||||
| AMD | Cu | 3.0–7.0 | Not reported | >90 | Biogenic H2S | [13] |
| Cd | >90 | |||||
| Pb | >90 | |||||
| Zn | >90 | |||||
| Fe | >88 | |||||
| Ni | >82 | |||||
| AMD | Cu | 4.0 | Not reported | 93.3 | Biogenic H2S | [14] |
| Fe | 99.2 | |||||
| Zn | ~100 | |||||
| AMD | Cu | 2.2 | Not reported | 99 | Biogenic H2S | [15] |
| AMD | Cu | 2.0–6.0 | 0.5–1.5 | 80.0–97.9 | Na2S solution | [16] |
| Zn | 3.0–8.0 | 0.5–1.5 | 57.5–99.95 | |||
| AMD | Cu | 4.0 | Not reported | >99 | Biogenic H2S | [17] |
| Fe | >99 | |||||
| Zn | >99 | |||||
| AMD | Cu | 7.0–8.0 | Not reported | >99 | Biogenic H2S | [18] |
| Zn | >99 | |||||
| AMD | Cu | 2.2 | Not reported | 70 | Biogenic H2S | [19] |
| Pb | 37 | |||||
| Zn | 79 | |||||
| Fe | 65 | |||||
| AMD | Cu | 1.7–3.3 | 1.0–1.5 | 75–100 | 1 M NaHS solution | [20] |
| Industrial wastewater | Tl | 12.0 | >250 | >99 | 1 g/L Na2S solution | [21] |
| Cd | >87 | |||||
| Pb | >40 | |||||
| Cu | >94 | |||||
| Zn | >67 | |||||
| Industrial wastewater | Cu | 4.0 | 2.5 | ~100 | NaHS | [22] |
| Zn | 2.3 | 60 | ||||
| Industrial wastewater | Cu | 7.0 | Not reported | >99 | Biogenic H2S | [23] |
| Zn | >95 | |||||
| Ni | >95 | |||||
| Industrial wastewater | Cu | 7.5 | Not reported | ~100 | Biogenic H2S | [24] |
| Industrial wastewater | Cu | 7.0–8.3 | Not reported | >99.5 | Biogenic H2S | [25] |
| Zn | >99 | |||||
| Ni | >99 | |||||
| Industrial wastewater | As | <1.0 | 1.65 | ~100 | Na2S wt. 10% solution | [26] |
| Industrial wastewater | Pd | 1.7 | 0.5–2.0 | 87.9–99.8 | Biogenic H2S | [27] |
| Fe | 2.3 | 0.6–3.75 | 53.8–98.3 | |||
| Plating industrial effluent | Cu | 10.0 | Not reported | 93.9 | Na2S | [28] |
| Zn | 99.4 | |||||
| Cr | 99.99 | |||||
| Plating industrial effluent | Cu | 1.7 | Not reported | >99 | Biogenic H2S | [29] |
| Zn | 1.76 | 85–97 | ||||
| Ni | 25–92 | |||||
| Fe | 2–99 | |||||
| Plating industrial effluent | Ni | 8–12 | Not reported | 65.8–95.3 | Na2S solution | [30] |
| Zn | 93.8 | |||||
| Cu | 100 | |||||
| Cu-laden electroplating effluent | Cu | 6.5–8.0 | Not reported | 99.9 | Biogenic H2S | [31] |
| Co-Mo Catalyst leachate | Mo | alkaline | >1000 | 98.2 | Na2S wt. 40% solution | [32] |
| Co | 1.0 | 98.0 | ||||
| Recycled mineral sludge leachate | Mo | 1.0 | 10 | 40.0–95.0 | 1 M Na2S solution | [33] |
| Co | 52.0–98.0 | |||||
| Ni | 48.0–98.0 | |||||
| Ni-Cd battery leachate | Cd | 0.2–1.4 | 0.5–2.0 | ~100 | Na2S–(NH4)2S–FeS | [34] |
| Waste printed circuit boards leachate | Cu | 10.6 | 1.0–1.2 | 88–99.5 | 5.2 M NaHS solution | [35] |
| Cathode ray tube powder leachate | Zn | 2.0–2.5 | ~8.8 | ~100 | Na2S 10% w/v solution | [36] |
| Spent refinery catalyst leachate | Mo | 2.0 | Not reported | 36–72 | Biogenic H2S | [37] |
| Co | 3.5 | 16.0 | ||||
| Ni | 3.5 | 23.0 | ||||
| Lithium ion batteries (LIBs) leachate | Co | 2.9–3.1 | 2.0 | 99.2 | (NH4)2S 10% v/v solution | [38] |
| Lithium ion batteries (LIBs) leachate | Cu | 3.5–5.0 | Not reported | 93.0 | Biogenic H2S | [39] |
| Al | 3.5–5.0 | 98.0 | ||||
| Co | 10 | 99.9 | ||||
| Ni | 10 | 99.9 | ||||
| Zn | 10 | 98.4 | ||||
| Cd | 10 | 98.6 | ||||
| Mn | 10 | 98.9 | ||||
| Fe | 10 | 99.5 | ||||
| Acidic wastewater from smelters | As | 4.0–5.0 | 3.0 | 97.2–99.1 | Na2S 110 g/L solution | [40] |
| Cu smelting ashes leachate | Pb | >12.0 | 2.0–2.5 | >99 | Na2S solution | [41] |
| Zn | >99 | |||||
| Acidic wastewater from smelter | Re | ~0 | Not reported | 98.4–98.9 | Saturated Na2S3O3 solution | [42] |
| Cu | 94.8–98.4 | |||||
| As | 11.6–15.0 | |||||
| Acidic wastewater from smelter | Cu | ~0 | 1.5–3.0 | 70–96 | Synthetic monoclinic FeS | [43] |
| Acidic wastewater from smelter | Re | ~0 | Not reported | 99.0 | H2S with UV irradiation | [44] |
| PLS from ore bioleaching | Cu | 3.2 | Not reported | >99.9 | Biogenic H2S | [45] |
| Zn | 1.5 | Not reported | ||||
| Ni | 2.0 | >99.9 | ||||
| Co | 2.0 | >99.9 | ||||
| PLS from tailing acid leaching | Cu | 3.0 | 1.2 | 93.7 | Na2S wt. 0.5% solution | [46] |
| Zn | 3.6 | 1.1 | 89.7 | |||
| PLS from tailing bioleaching | Cu | 1.24 | 1.1 | >99 | Na2S solution | [47] |
| Pb | >99 | |||||
| Zn | >99 | |||||
| Fe | 2.5 | 75 | ||||
| Chloride PLS | Cu | 1.0 | Not reported | 99.9 | Na2S solution | [48] |
| Zn | 4.0 | 99.9 | ||||
| Cyanide PLS | Cu | 3.5–5.0 | 0.4–0.6 | 81–99.9 | NaHS solution | [49,50] |
| Zn | 3.5–5.5 | 1.0–1.2 | 96.4–99.9 | |||
| Cyanide PLS | Cu | 3.5–5.0 | 0.5–0.6 | 77.5–99.9 | NaHS solution | [51,52,53,54] |
| Alkaline glycine-cyanide PLS | Cu | 10.0 | 1.0–1.6 | 71.2–96.5 | NaHS solution after pre-oxidation | [55,56] |
| Element | Initial Concentration, mg/L | pH | Sulfide Dosage, Molar Ratio (S2−/M) | Temperature, °C | Maximum Conversion, % | Reaction Time to Reach Maximum Conversion, min | Reference |
|---|---|---|---|---|---|---|---|
| Cu | 500–1800 | 3.5–5.0 | 0.5–0.6 | 15 | 83–99 | <1 | [52] |
| Cu | 300 | 10.0 | 1.4 | 25 | 96.5 | 5 | [55] |
| Re | 30 | ~0 | Not reported | 25 | 97.0 1 | 360 | [44] |
| Zn Pb | 2350 4340 | >12.0 | 2.0 | 70 | ~100 ~95 | 75 60 | [41] |
| Re Cu As | 11.5–22.9 16.2–99.9 2170–4381 | ~0 | Not reported | 70 | 98.4 98.4 11.6 | 60 60 120 | [42] |
| Cu | 5420 | 10.6 | 1.0–1.2 | 25 | 88–99.5 | 5 | [35] |
| Mo Co Ni | 1000 1000 1000 | 1.0 | 10 | 20–25 | 95 96 97 | 75 | [33] |
| As | 12,562 | 4.0 | 3.0 | 25 | 99.1 | 60 | [40] |
| Co | 11,900 | 3.0 | 2.0 | 30 | 98.2 | 30 | [38] |
| As | 1000–5000 | ~0 | 2.5 | Room temperature | 99.5 | 60 | [6] |
| Cu Fe | 120 124 | 2.5 7.4 | Not reported | Room temperature | 99.0 99.0 | 2.5 5.0 | [67] |
| As(V) | 800–900 | 1.8 | 10–20 | Room temperature | 80–85 | 120 | [68] |
| Zn Cd Ni Cu | 12,557 6294 1232 635 | 5.5 4.5 7.5 2.5 | Not reported | 45–85 | 40–99.7 35–97 35–98 30–97 | 45 45 45 45 | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estay, H.; Barros, L.; Troncoso, E. Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks. Minerals 2021, 11, 1385. https://doi.org/10.3390/min11121385
Estay H, Barros L, Troncoso E. Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks. Minerals. 2021; 11(12):1385. https://doi.org/10.3390/min11121385
Chicago/Turabian StyleEstay, Humberto, Lorena Barros, and Elizabeth Troncoso. 2021. "Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks" Minerals 11, no. 12: 1385. https://doi.org/10.3390/min11121385
APA StyleEstay, H., Barros, L., & Troncoso, E. (2021). Metal Sulfide Precipitation: Recent Breakthroughs and Future Outlooks. Minerals, 11(12), 1385. https://doi.org/10.3390/min11121385






