Organic Petrographic and Geochemical Evaluation of the Black Shale of the Duwi Formation, El Sebaiya, Nile Valley, Egypt
Abstract
:1. Introduction
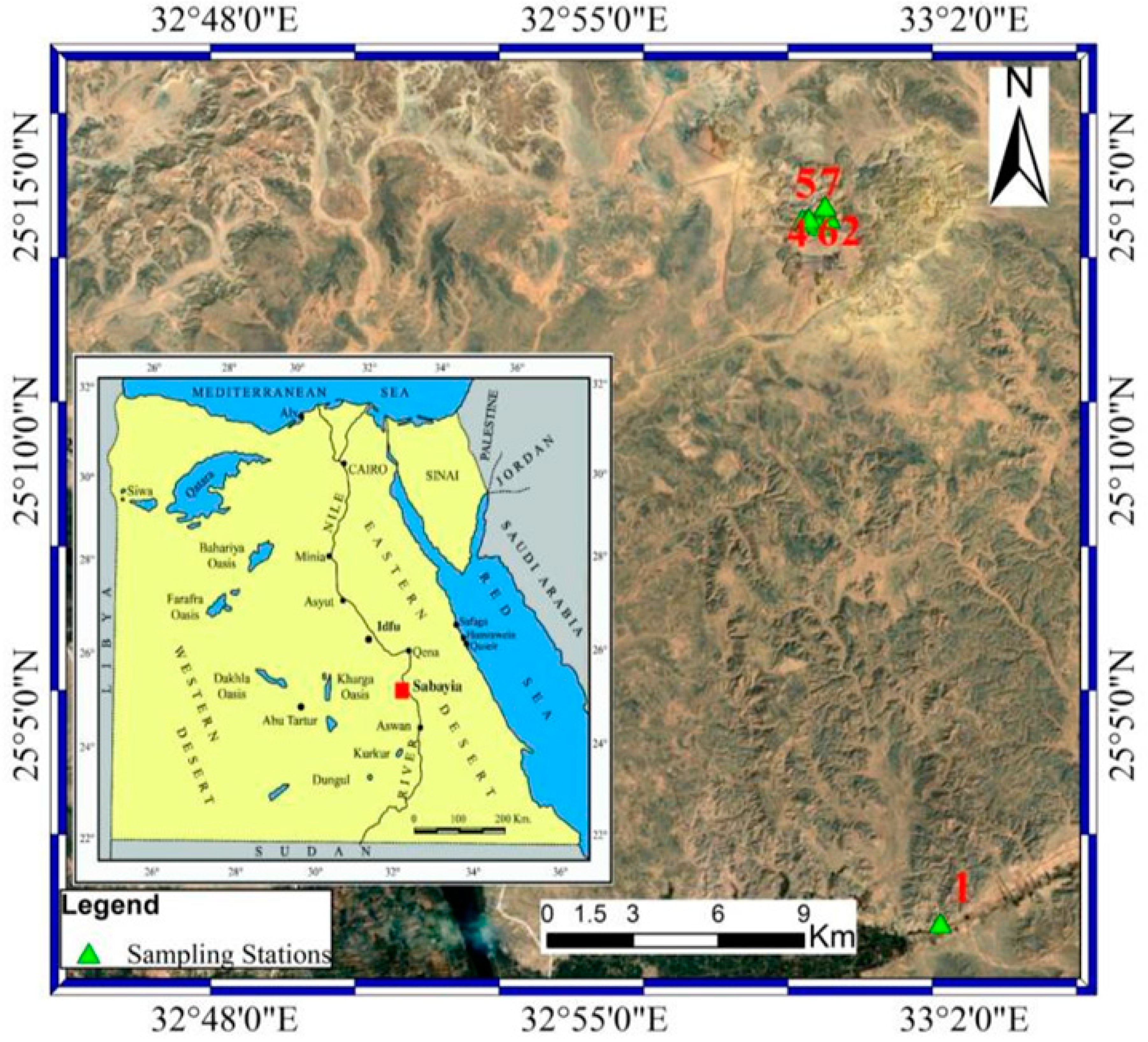
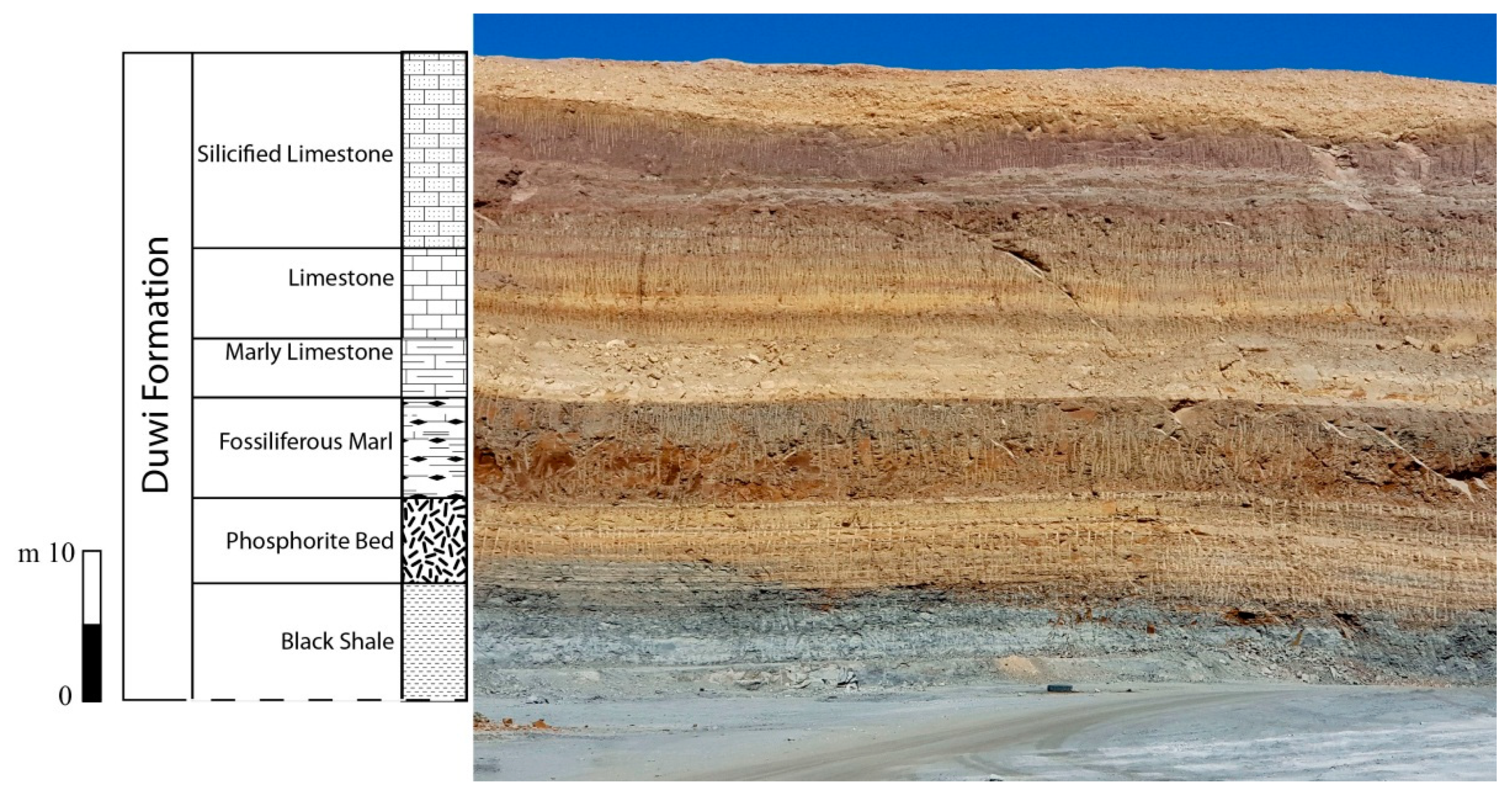
2. Geologic Setting
3. Materials and Methods
3.1. Sampling, Chemical and Mineralogy Techniques
3.2. TOC Analysis and Open-System Pyrolysis
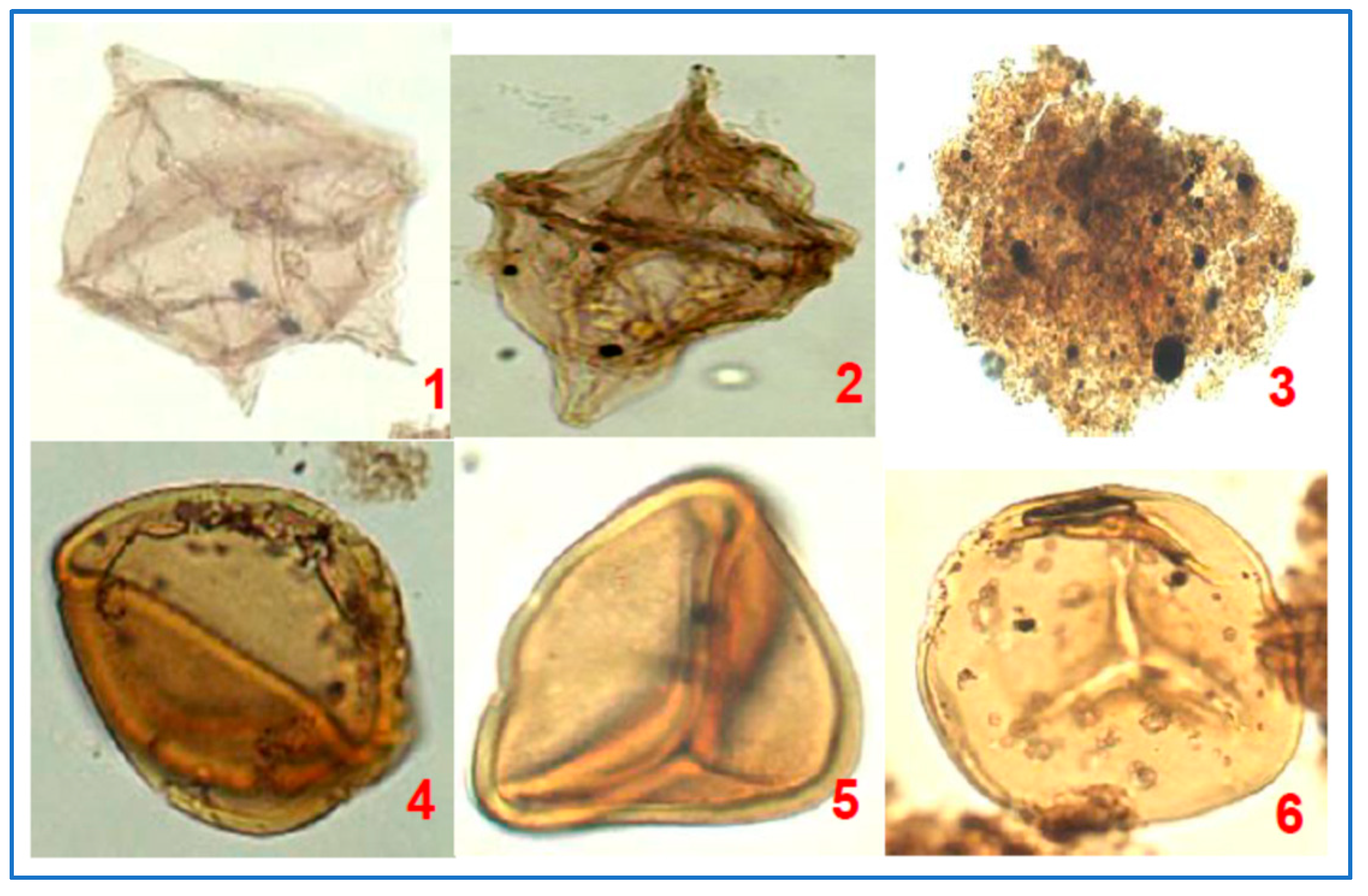
3.3. Palynology
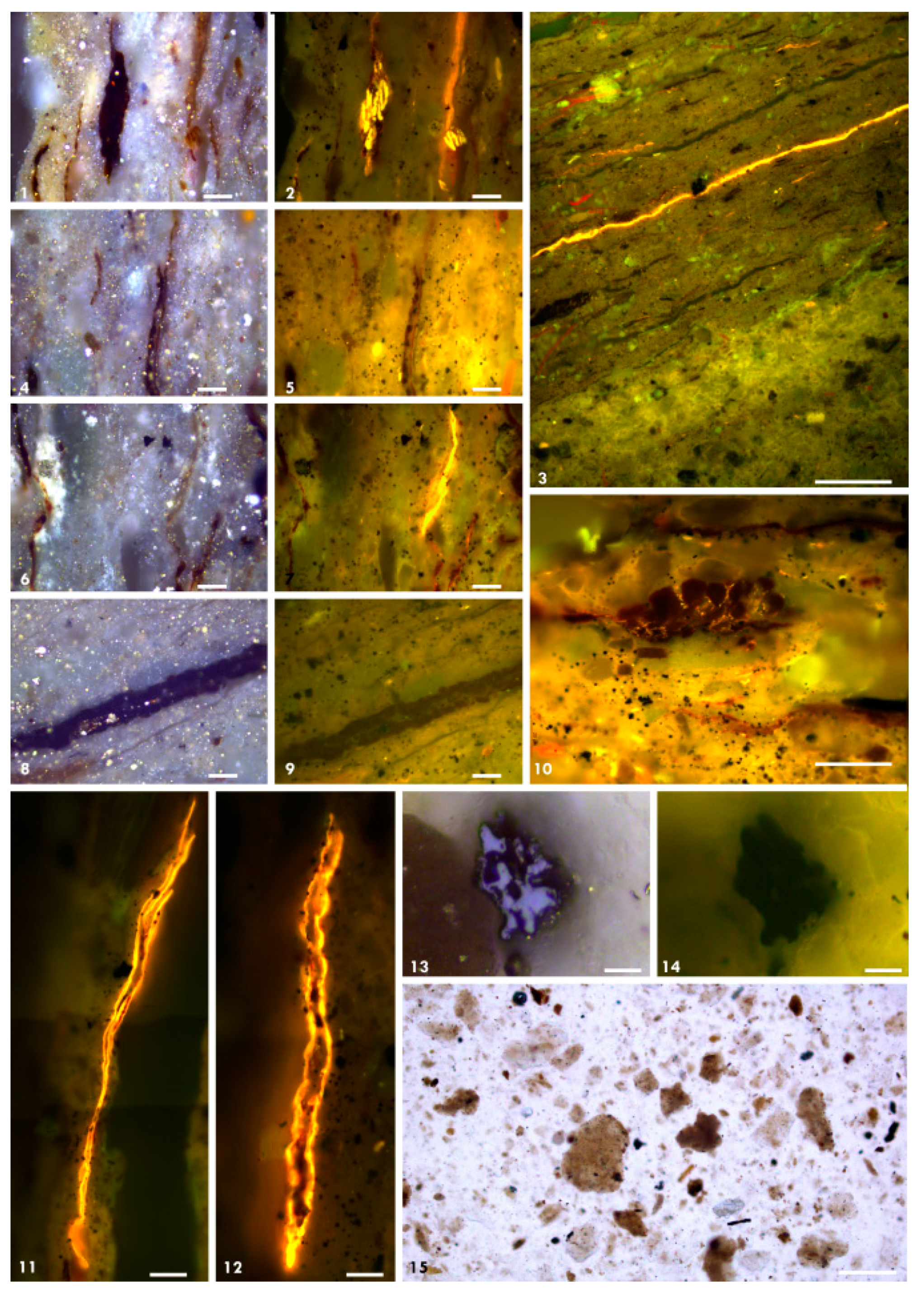
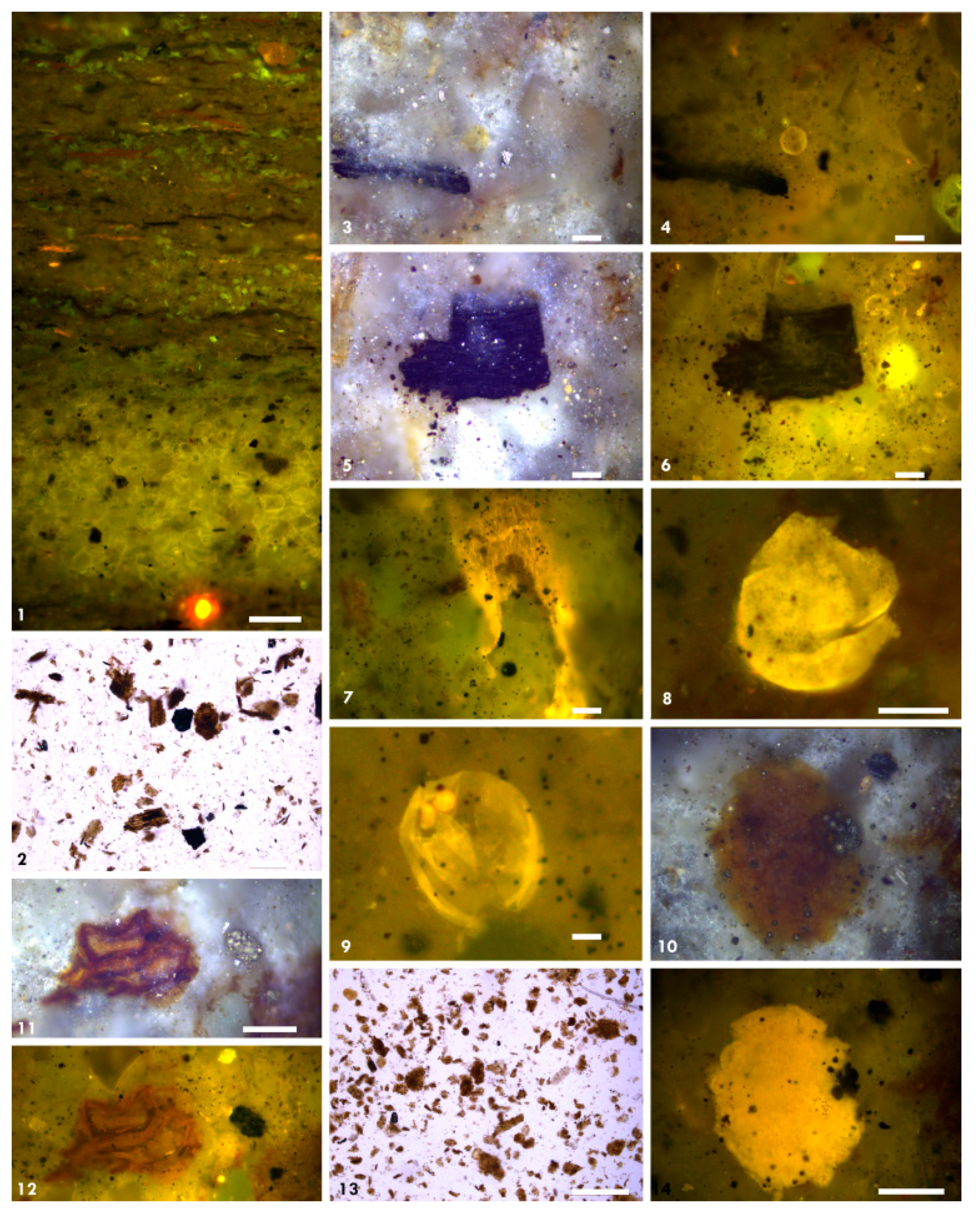
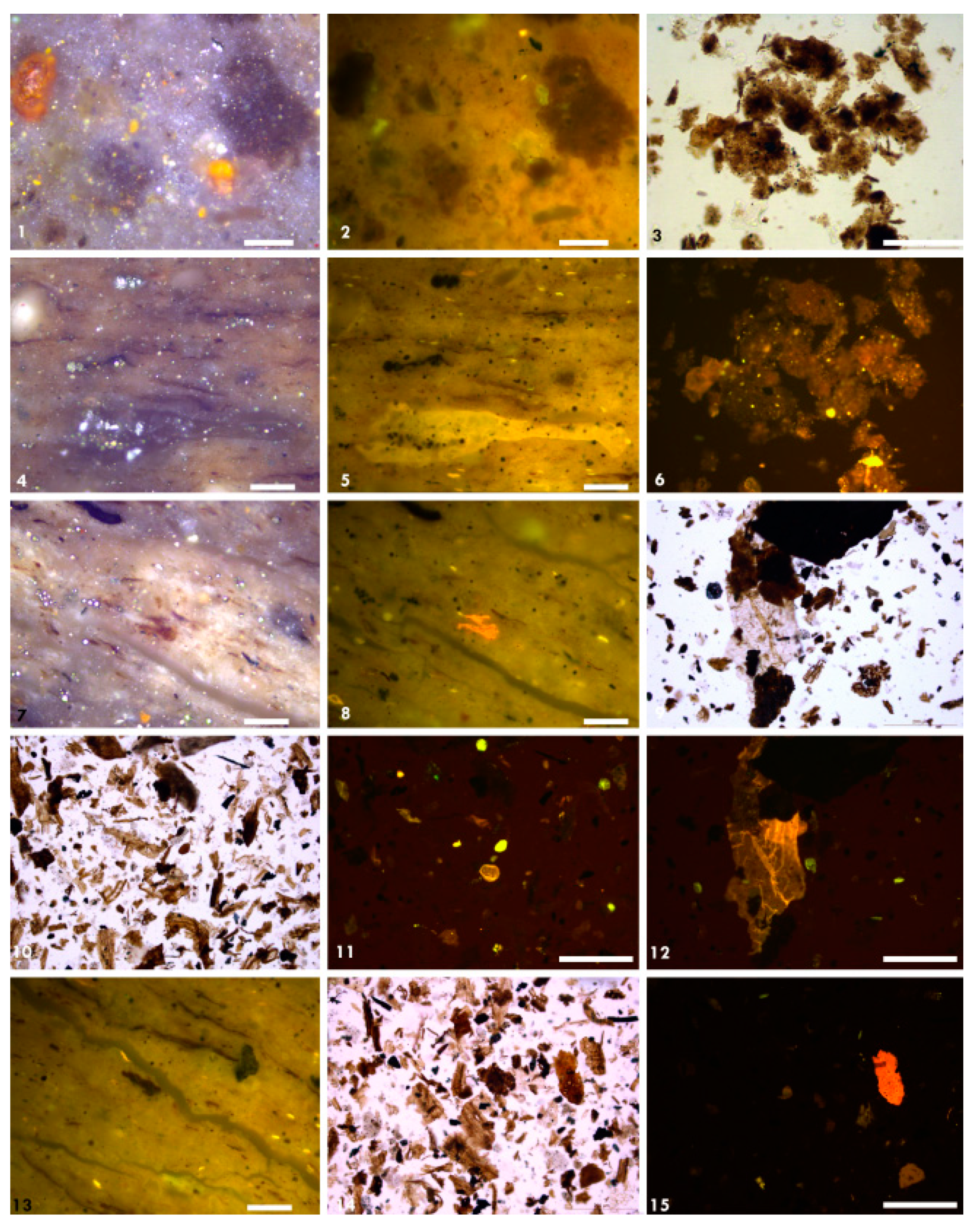
3.4. Organic Petrography
4. Results and Discussion
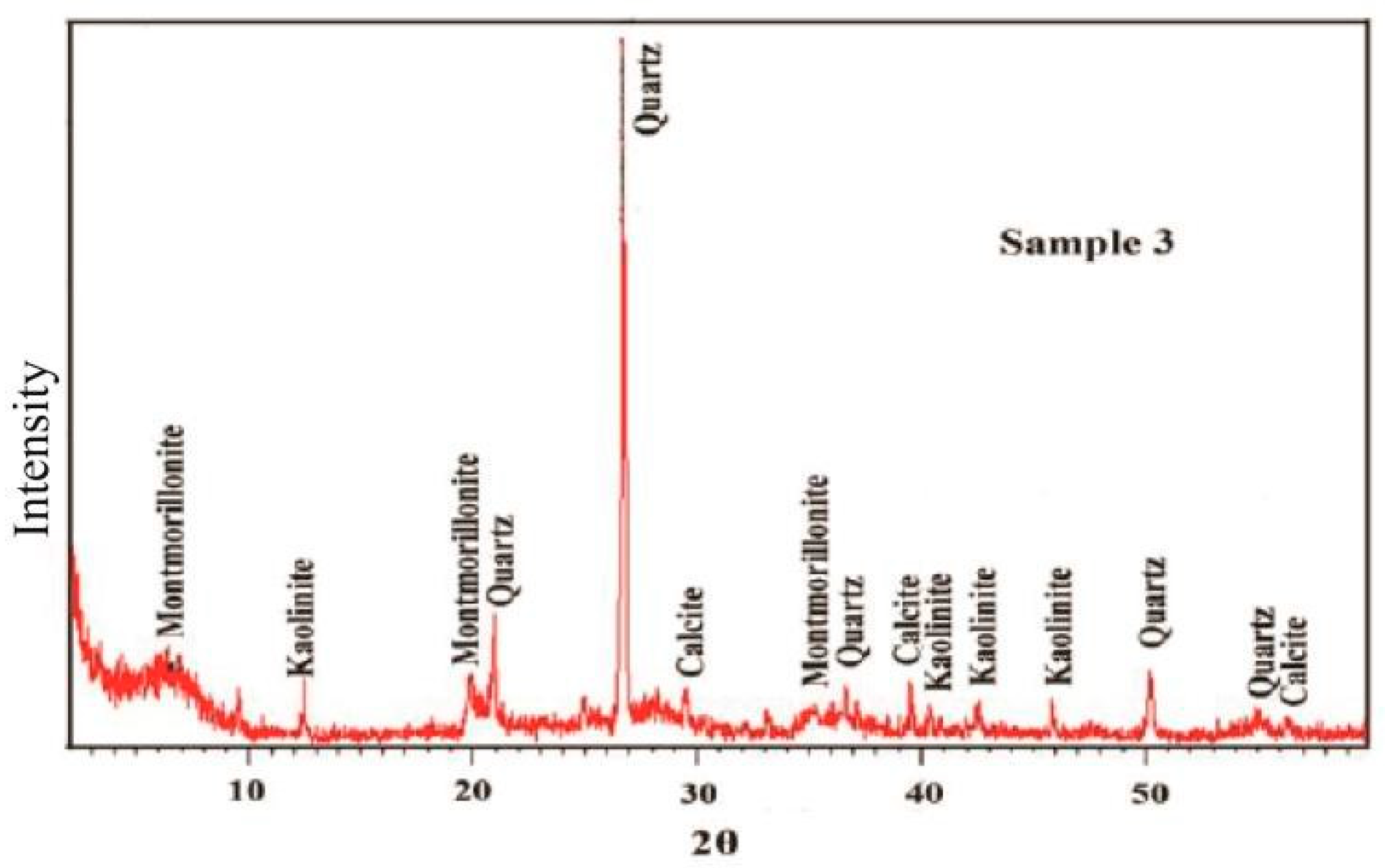
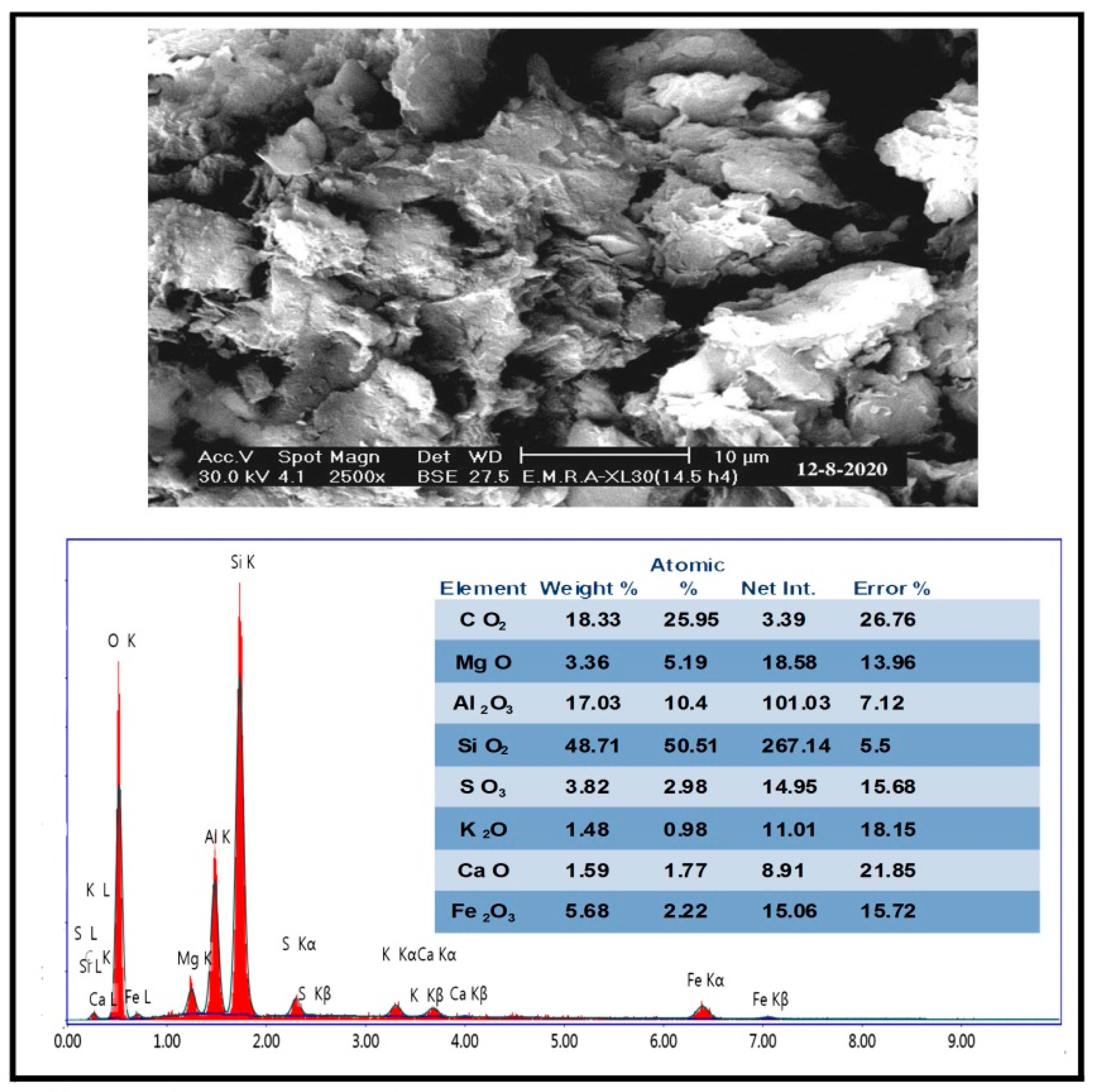
4.1. Mineralogy and SEM
4.2. Inorganic Geochemistry
4.2.1. Major and Trace Elements
4.2.2. Distribution of Trace Elements and Redox Conditions
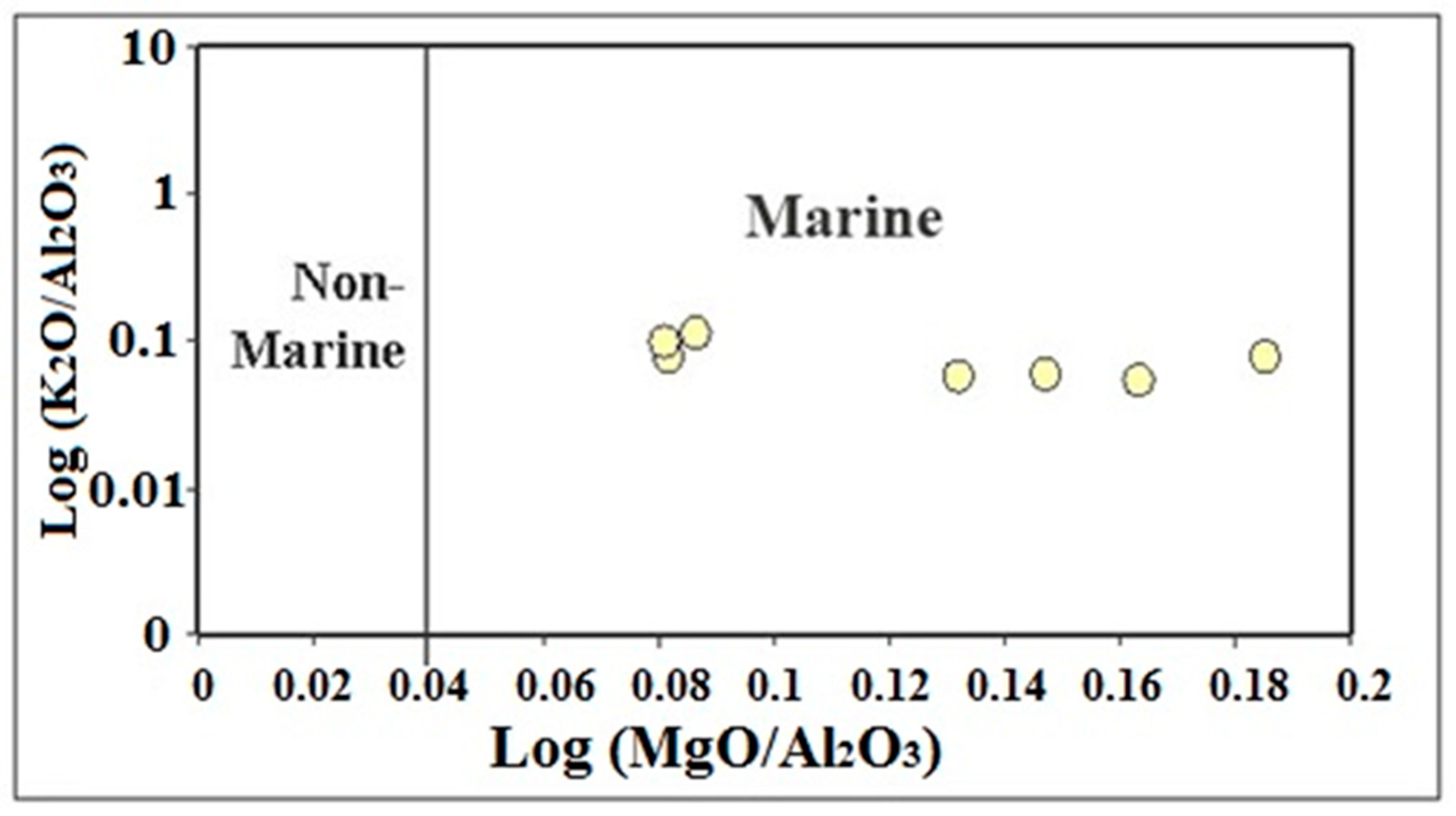
4.2.3. Paleoclimate
4.3. Organic Geochemistry
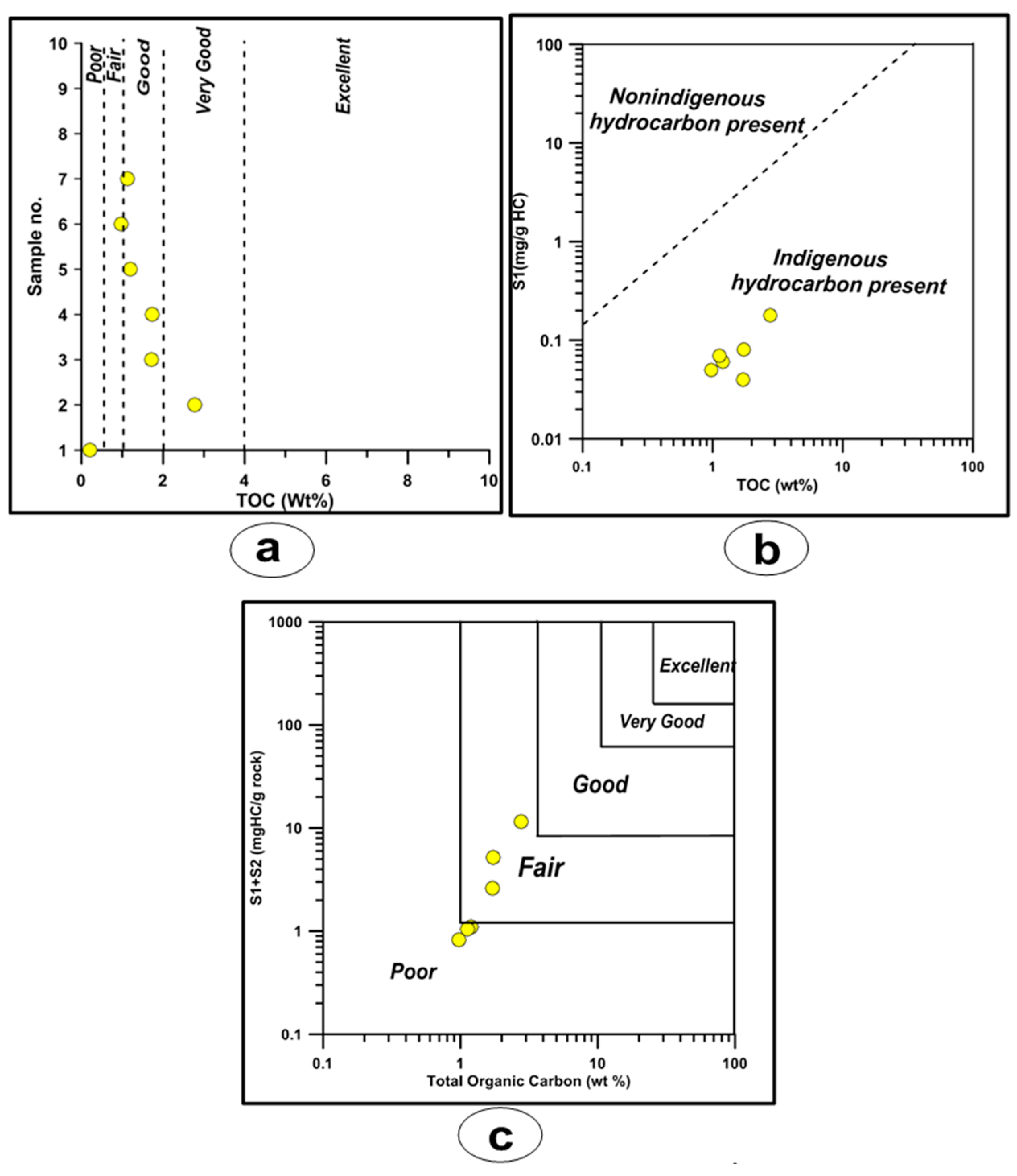
4.3.1. Organic Richness
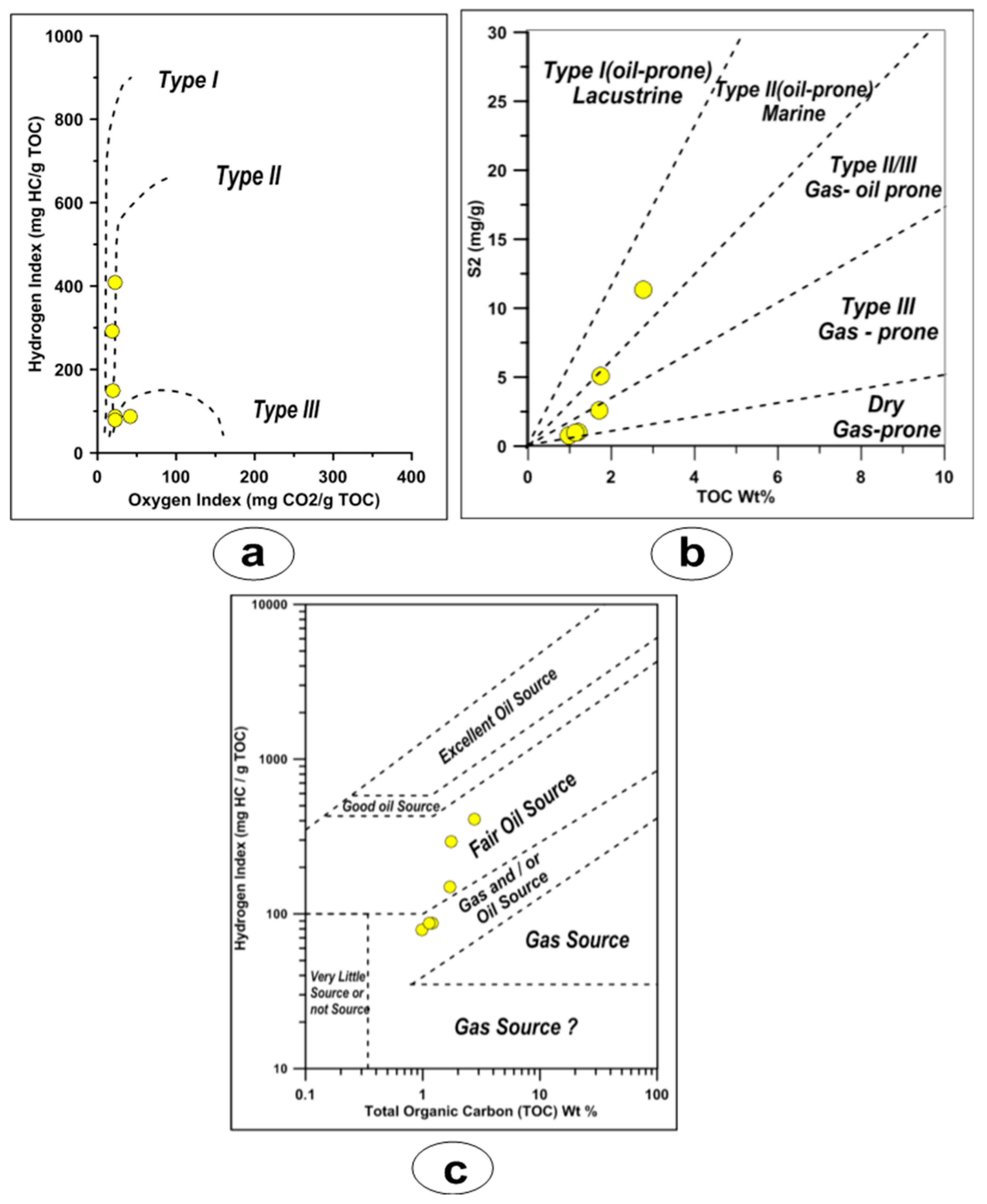
4.3.2. Kerogen Type and Generation Capability
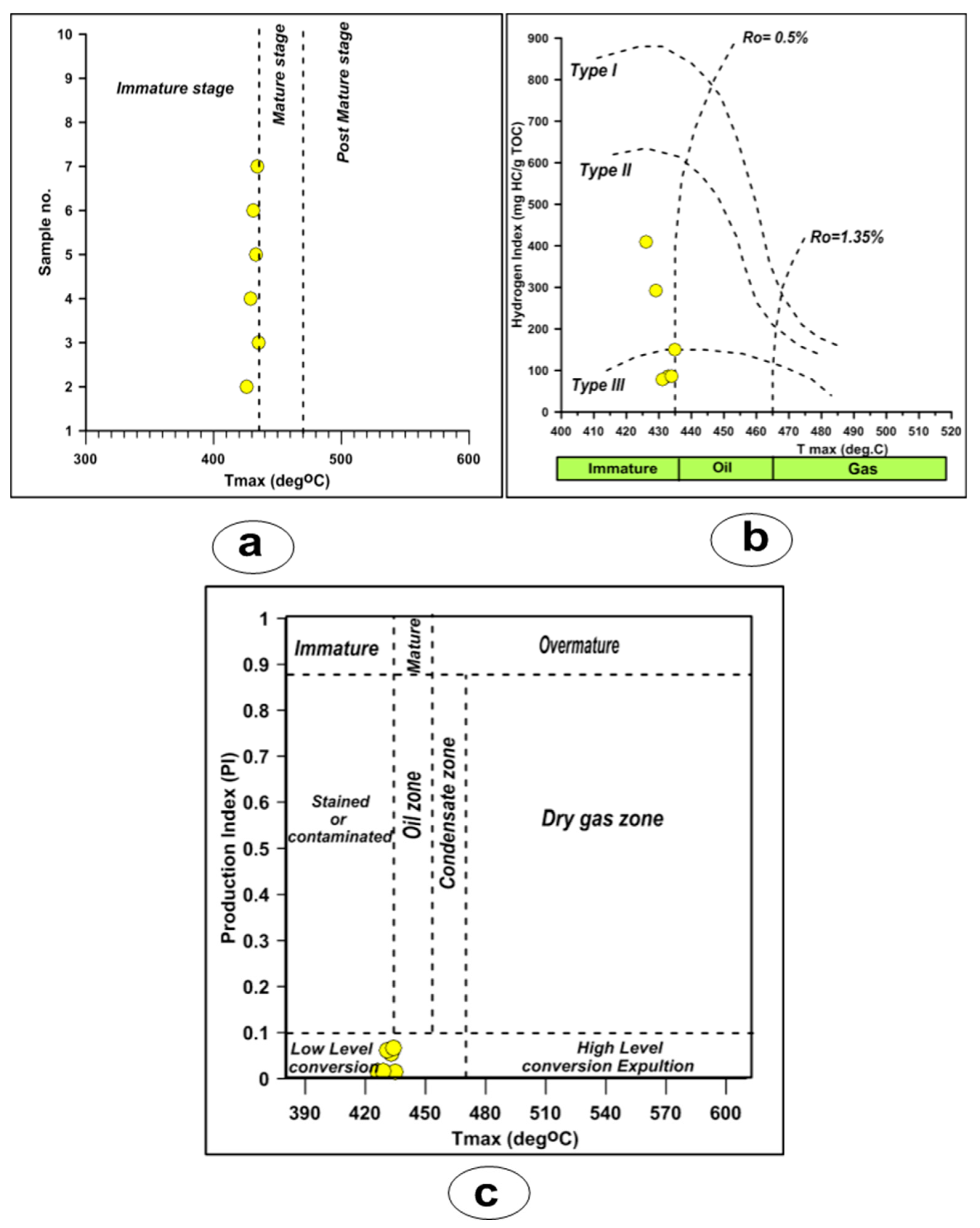
4.3.3. Thermal Maturity
4.3.4. Organic Petrography and Microscopic Composition of Organic Matter
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Ali, R.; El-Kammar, A.; Kussb, J. Chemostratigraphy of the Upper Cretaceous-Paleogene organic-rich succession on the southern Tethys margin in Egypt. J. Afr. Earth Sci. 2020, 164, 103779. [Google Scholar] [CrossRef]
- Awan, R.S.; Liu, C.; Aadil, N.; Yasin, Q.; Salaam, A.; Hussain, A.; Yang, S.; Jadoon, A.K.; Wu, Y.; Gul, M.A. Organic geochemical evaluation of Cretaceous Talhar Shale for shale oil and gas potential from Lower Indus Basin, Pakistan. J. Petrol. Sci. Eng. 2021, 200, 108404. [Google Scholar] [CrossRef]
- Leckie, R.M.; Bralower, T.J.; Cashman, R. Oceanic anoxic events and plankton evolution: Biotic response to tectonic forcing during the mid-Cretaceous. Paleoceanography 2002, 17, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Makled, W.A.; Mustafa, T.F.; Maky, A.F. Mechanism of Late Campanian–Early Maastrichtian oil shale deposition and its sequence stratigraphy implications inferred from the palynological and geochemical analysis. Egypt J. Petrol. 2014, 23, 427–444. [Google Scholar] [CrossRef] [Green Version]
- Baioumy, H.; Tada, R. Origin of Late Cretaceous phosphorites in Egypt. Cretac. Res. 2005, 26, 261–275. [Google Scholar] [CrossRef]
- Glenn, C.R.; Mansour, S.E.A. Reconstruction of the depositional and diagenetic history of phosphorites and associated rocks of the Duwi Formation (late Cretaceous) Eastern Desert, Egypt. Ann. Geol. Surv. Egypt 1979, IX, 388–407. [Google Scholar]
- Tröger, U. The oil shale potential of Egypt. Berl. Gewissen Schaftliche Abh. A 1984, 50, 375–380. [Google Scholar]
- Hassaan, M.M.; Sakr, S.M.; Abd El-Gawad, E.A.; El Naggar, I.M. Reconnaissance Lithological-Geochemical Exploration for Organic Matter and Total Organic Carbon in the Late Campanian-Paleocene black shale Belt, Upper Egypt. Int. J. Innov. Sci. Eng. Technol. 2016, 3, 699–709. [Google Scholar]
- El-Kammar, A.M. Oil shale resources in Egypt: The present status and future vision, Arab. Geo-Front 2014, 1, 1–34. [Google Scholar] [CrossRef]
- Philobbos, E.R. The phosphatic sediments of the Nile Valley and Eastern Desert of Egypt in view of the Upper Cretaceous-Lower Tertiary sedimentation tectonics. Geol. Soc. Egypt Spec. Publ. 1996, 2, 313–352. [Google Scholar]
- El Kammar, M.M. Organic and Inorganic Components of the Upper Cretaceous-Lower Tertiary Black Shales from Egypt and Their Hydrocarbon Potentialities. Ph.D. Thesis, Cairo University, Giza, Egypt, 1993. [Google Scholar]
- El-Azabi, M.H.; Farouk, S. High resolution sequence stratigraphy of the Maasstrichtian—Ypresian succession along the eastern scarp face of Kharga Oasis, Southern Western Desert, Egypt. Sedimentology 2010, 58, 579–617. [Google Scholar] [CrossRef]
- Issawi, B.; Maher, F.; El-Sayed, A.A.; Youssef; Rifaat, Y.; Osman, A. The Phanerozoic geology of Egypt A geodynamic approach. Egypt. Geol. Surv. 2009, 76, 166–200. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. Treatise Geochem. 2014, 3, 1–64. [Google Scholar]
- Sutherland, R.A. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control: A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Blaser, P.; Zimmermann, S.; Luster, J.; Shotyk, W. Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb and Zn in Swiss forest soils. Sci. Total Environ. 2000, 249, 257–280. [Google Scholar] [CrossRef]
- Bam, E.K.P.; Akiti, T.T.; Osea, S.D.; Ganyaglo, S.Y.; Gibrilla, A. Multivariate cluster analysis of some major andtrace elements distribution in an un- saturated zone profile, Densu river Basin, Ghana. Afr. J. Environ. Sci. Technol. 2011, 5, 155–167. [Google Scholar]
- Espitalié, J.; La Porte, J.L.; Madec, M.; Marquis, F.; Le Plat, P.; Paulet, J.; Bautefeu, A. Methodé rapide de caractérisation des roches mères de leur potential potential pétrolier et de leur degre d‘évolution. Rev. de L’institut Français du Pétrole 1977, 2, 23–42. [Google Scholar] [CrossRef]
- Peters, K.E.; Cassa, M.R. Applied source rock geochemistry, The Petroleum System-From Source to Trap: AAPG Memoir. Tulsa Okla. USA 1994, 60, 93–120. [Google Scholar]
- Behar, F.; Beaumont, V.; Penteado, H.D. Rock-Eval 6 Technology Performances and Developments. Oil Gas Sci. Technol. 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Carvajal-Ortiz, H.; Gentzis, T. Critical considerations when assessing hydrocarbon plays using Rock-Eval pyrolysis and organic petrology data: Data quality revisited. Int. J. Coal Geol. 2015, 152, 113–122. [Google Scholar] [CrossRef]
- Riding, J.B. A guide to preparation protocols in Palynology. Palynology 2021, 45, 1–110. [Google Scholar] [CrossRef]
- ISO (International Organisation for Standardisation). Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples; ISO 7404-2; The International Organization for Standardization: London, UK, 2009. [Google Scholar]
- ISO (International Organisation for Standardisation). Methods for the Petrographic Analysis of Coals; ISO 7404-5; The International Organization for Standardization: London, UK, 2009. [Google Scholar]
- ASTM. Standard Test Method for Microscopical Determination of the Reflectance of Vitrinite Dispersed in Sedimentary Rocks, Petroleum Products, Lubricants, and Fossil Fuels; Gaseous Fuels; Coal and Coke, sec. 5, v. 05.06 ASTM D7708; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Abou El-Anwar, E.A.; Samy, Y. Clay Mineralogy and Geochemical characterization of Some Quaternary sediments on Giza-Fayium District, Western side of the Nile Valley, Egypt: Relationships to weathering and provenance. Aust. J. Appl. Sci. Res. 2013, 9, 4765–4780. [Google Scholar]
- Algeo, T.J.; Ingall, E.D. Sedimentary Corg:P ratios, paleocean ventilation, and Phanerozoic atmospheric pO2. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 256, 130–155. [Google Scholar] [CrossRef]
- Marynowski, L.; Pisarzowska, A.; Derkowski, A.; Rakociński, M.; Szaniawski, R.; Środoń, J.; Cohen, A.S. Influence of palaeoweathering on trace element concentrations and environmental proxies in black shales. Palaeogeo. Palaeoclim. Palaeoecol. 2017, 472, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; p. 312. [Google Scholar]
- Fedo, C.M.; Eriksson, K.; Krogstad, E.J. Geochemistry of shale from the Archean (~3.0 Ga) Buhwa greenstone belt, Zimbabwe: Implications for provenance and source area weathering. Geochem. Cosmoch. Acta 1996, 60, 1751–1763. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A. Mineralogical, petrographical, geochemical, diageneses and provenance of the Cretaceous black shale Duwi Formation at Quseir-Safaga, Red Sea, Egypt. Egypt J. Petrol. 2017, 26, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Abou El-Anwar, E.A.; Mekky, H.S.; Abdel Wahab, W. Characterization and Depositional Environment of P2O5–F-U of Phosphatic Rocks for the Duwi Formation, Qussier-Safaga Region, Red Sea Coast, Egypt. Egypt J. Chem. 2019, 62, 2213–2228. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A.; Mekky, H.S.; Abdel Wahab, W. Geochemistry, mineralogy and depositional environment of black shales of the Duwi Formation, Qusseir area, Red Sea coast, Egypt. Carbonates Evaporites 2018, 34, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Swanson, V. A Review Geology and Geochemistry of Uranium in Marine Black Shales; United States Government Printing Office: Washington, DC, USA, 1961; p. 112.
- Arning, E.T.; Lückge, A.; Breuer, C.; Gussone, N.; Birgel, D.; Peckmann, J. Genesis of phosphorite crusts off Peru. Mar. Geol. 2009, 262, 68–81. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A.; Mekky, H.S.; Abd El Rahim, S.H. Discovery of spherulitic dahllite associated with carbonates at Hamadat phosphorite mine, Qusseir, Central Eastern Desert, Egypt. Carbonates Evaporites 2020, 35, 106. [Google Scholar] [CrossRef]
- Venter, R.; Boylett, M. The evaluation of various oxidants used in acid leaching of uranium. In Hydrometallurgy Conference; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2009; pp. 445–456. [Google Scholar]
- Bots, P.; Behrends, T. Uranium mobility in subsurface aqueous systems: The influence of redox conditions. Miner. Mag. 2008, 72, 381–384. [Google Scholar] [CrossRef]
- Bata, T. Evidences of widespread Cretaceous deep weathering and its consequences: A review. Earth Sci. Res. 2016, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.D.; Taylor, C.D. Environmental geochemistry of shale- hosted Ag-Pb-Zn massive sulfide deposits in northwest Alaska: Natural background concentration of metals in water from mineralized areas. Appl. Geochem. 1997, 12, 397–409. [Google Scholar] [CrossRef]
- Goodfellow, W.D. Geology, genesis and exploration of SEDEX deposits with emphasis on the Selwyn Basin, Canada. In Sediment-Hosted Lead-Zinc Sulphide Deposits: Attributes and Models of Some Major Deposits of India, Australia and Canada; Deb, M., Goodfellow, W.D., Eds.; Narosa Publishing House: Delhi, India, 2004; pp. 24–99. [Google Scholar]
- Nicholson, K. Contrasting mineralogical-geochemical signatures of manganese oxides: Guides to metallogenesis. Econ. Geol. 1992, 87, 1253–1264. [Google Scholar] [CrossRef]
- Eric, B.E.; Fralick, P.; Emile, E.; Konfor, N.I.; Betrant, B.S.; Florent, A.D.; Zacharie, E.B.A. Geochemical characteristics of shales in the Mamfe Basin, South West Cameroon: Implication for depositional environments and oxidation conditions. J. Afr. Earth Sci. 2019, 149, 131–142. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Galarraga, F.; Reategui, K.; Martïnez, A.; Martínez, M.; Llamas, J.F.; Márquez, G. V/Ni ratio as a parameter in palaeo-environmental characterization of non-mature medium-crude oils from several Latin American basins. J. Petrol. Sci. Eng. 2008, 61, 9–14. [Google Scholar] [CrossRef]
- Shi, C.; Cao, J.; Bao, J.; Zhu, C.; Jiang, X.; Wu, M. Source characterization of highly mature pyrobitumens using trace and rare earth element geochemistry: Sinian-Paleozoic paleo-oil reservoirs in south China. Org. Geochem. 2015, 83, 77–93. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A. Lithologic characterization of the phosphorite-bearing Duwi Formation (Campanian), South Esna West Nile Valley, Egypt. Carbonates Evaporites 2019, 34, 793–805. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A. Petrography, geochemistry and genesis of the Upper Eocene carbonate terraces (II and III), Qasr El-Sagha Formation, El-Faiyum Egypt. Sedimentol. Egypt 2005, 13, 243–260. [Google Scholar]
- Abou El-Anwar, E.A. Petrography, geochemistry and genesis of some Middle Eocene rocks at Qattamia area, Cairo-Suez Road Egypt. NRC Egypt 2006, 3, 519–543. [Google Scholar]
- Abou El-Anwar, E.A. Petrographical, geochemical and diagenetic studies of the middle Eocene carbonates, Mokattam Formation of Darb el-Fayium area. Int. Conf. Geol. Sci. Eng. Fr. Paris August 2011, 80, 1315–1325. [Google Scholar]
- Abou El-Anwar, E.A. Contribution to the composition and origin of the reef terraces in Ras Mohamed, Sharm El-Sheikh Coast, Southern Sinai, Egypt. Egypt J. Geol. 2012, 56, 33–48. [Google Scholar]
- Abou El-Anwar, E.A. Composition and origin of the dolostones of Um Bogma Formation, Lower Carboniferous, west central Sinai, Egypt. Carbonates Evaporates 2014, 29, 129–205. [Google Scholar] [CrossRef]
- Abou El-Anwar, E.A.; EL-Wekeil, S.S.; Gaafar, F.S. Contribution to the mineralogy, geochemistry, and provenance of the Lower Eocene Esna shale in the Farafra Oasis, Western Desert, Egypt. J. Appl. Sci. Res. 2013, 9, 5344–5369. [Google Scholar]
- Abou El-Anwar, E.A.; Mekky, H.S.; Samy, Y.M. Contribution to the mineralogical, geochemical and provenance of the Cretaceous black shales, Duwi Formation, Quseir-Safaga, Red Sea Coast Egypt. Egypt J. Geol. 2014, 58, 303–322. [Google Scholar]
- Abou El-Anwar, E.A.; Mekky, H.S.; Abd El Rahim, S.H.; Aita, S.K. Mineralogical, geochemical characteristics and origin of Late Cretaceous phosphorite in Duwi Formation (Geble Duwi Mine) Red Sea region, Egypt. Egypt J. Pet. 2017, 26, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Roaldset, E. Mineralogical and Chemical Changes during Weathering, Transportation and Sedimentation in Different Environments with Particular References to the Distribution of Yttrium and Lanthanide Elements. Ph.D. Thesis, University of Oslo, Oslo, Norway, 1978. [Google Scholar]
- Hayashi, K.I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of ~1.9 Ga sedimentary rocks from ortheastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Cox, R.; Lower, D.R.; Cullers, R.L. The influence of sediment recycling and basement composition evolution of mudrock chemistry in the southwestern United States. Geochim. Cosmochim. Acta 1995, 59, 2919–2940. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 199, 715–717. [Google Scholar] [CrossRef]
- McLennan, S.M. Weathering and global denudation. J. Geol. 1993, 101, 295–303. [Google Scholar] [CrossRef]
- Hunt, J.M. Petroleum Geochemistry and Geology, 2nd ed.; Freeman and Company: New York, NY, USA, 1996; p. 743. [Google Scholar]
- Dembicki, H. Three common source rock evaluation errors made by geologists during prospect or play appraisals. AAPG Bull. 2009, 93, 341–356. [Google Scholar] [CrossRef]
- Jackson, K.S.; Hawkins, P.J.; Bennett, A.J.R. Regional facies and geochemical evaluation of southern Denison Trough. APEA J. 1985, 20, 143–158. [Google Scholar] [CrossRef]
- Dahl, B.; Bojesen-Koefoed, J.; Holm, A.; Justwan, H.; Rasmussen, E.; Thomsen, E. A new approach to interpreting Rock-Eval S2 and TOC data for kerogen quality assessment. Org. Geochem. 2004, 35, 1461–1477. [Google Scholar] [CrossRef]
- Espitalie, J.; Deroo, G.; Marquis, F. Rock-Eval pyrolysis and its application. Inst. Fr. Prepr. 1985, 33578, 72. [Google Scholar]
- Langford, F.F.; Blanc-Valleron, M.M. Interpreting Rock-Eval Pyrolysis Data Using Graphs of Pyrolizable Hydrocarbons vs. Total Organic Carbon. AAPG Bull. 1990, 74, 799–804. [Google Scholar]
- Taylor, G.H.; Teichmüller, D.A.; Diessel, C.F.K.; Littke, R.; Robert, P. Organic Petrology; Gerbrüder Borntraeger: Berlin, Germany, 1998; p. 704. [Google Scholar]
- Tyson, R.V. Sedimentary Organic Matter-Organic Facies and Palynofacies; Chapman & Hall: London, UK, 1995; p. 615. [Google Scholar]


| Index | Index Value | Soil Quality |
|---|---|---|
| EF | EF < 2 | depletion to minimal enrichment |
| 2 ≤ EF < 5 | moderate enrichment | |
| 5 ≤ EF < 20 | significant enrichment | |
| 20 ≤ EF < 40 | very high enrichment | |
| EF ≥ 40 | Extremely high enrichment. | |
| CF | CF < 1 | low CF |
| 1 ≤ CF < 3 | moderate CF | |
| 3 ≤ CF < 6 | considerable CF | |
| CF ≥ 6 | very high CF |
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | SO3 | Cl | LOI | V | Cr | Mn | Co | Ni | Cu | Zn | As | Zr | Cd | Pb |
| % | ppm | ||||||||||||||||||||||
| 1 | 49.4 | 1.0 | 15.8 | 9.0 | 2.6 | 0.7 | 1.5 | 0.9 | 0.1 | 0.1 | 2.1 | 16.6 | 104.0 | 81.2 | 321.4 | 17.3 | 42.0 | 13.7 | 52.5 | 0.4 | 249.6 | 2.2 | 10.9 |
| 2 | 59.7 | 0.5 | 9.6 | 4.4 | 1.8 | 4.0 | 0.6 | 0.7 | 2.2 | 2.3 | 0.1 | 13.8 | 81.5 | 94.1 | 96.6 | 9.2 | 46.3 | 9.7 | 149.7 | 2.9 | 76.7 | 2.6 | 12.6 |
| 3 | 58.9 | 0.8 | 14.6 | 4.8 | 1.9 | 1.8 | 0.9 | 0.8 | 0.5 | 1.7 | 0.1 | 13.0 | 106.8 | 85.3 | 63.0 | 14.5 | 38.6 | 10.8 | 81.9 | 2.1 | 151.6 | 2.6 | 14.4 |
| 4 | 61.0 | 0.6 | 11.3 | 5.1 | 1.7 | 2.2 | 0.7 | 0.7 | 1.0 | 2.4 | 0.1 | 13.2 | 120.1 | 90.7 | 61.9 | 7.3 | 37.6 | 0.5 | 67.7 | 2.7 | 63.9 | 2.5 | 11.4 |
| 5 | 53.4 | 1.1 | 17.0 | 6.1 | 1.4 | 1.3 | 1.1 | 1.3 | 0.7 | 4.4 | 0.1 | 11.7 | 77.1 | 77.7 | 298.1 | 14.1 | 32.9 | 9.7 | 105.2 | 6.0 | 354.8 | 2.3 | 14.6 |
| 6 | 48.6 | 1.3 | 15.3 | 7.5 | 1.2 | 2.1 | 0.9 | 1.5 | 1.1 | 5.7 | 0.1 | 13.8 | 91.7 | 82.0 | 322.0 | 16.2 | 35.7 | 10.4 | 48.6 | 0.8 | 397.9 | 3.4 | 13.2 |
| 7 | 48.1 | 1.5 | 14.1 | 7.6 | 1.2 | 2.6 | 0.9 | 1.6 | 0.5 | 5.5 | 0.1 | 16.0 | 80.1 | 78.7 | 222.1 | 17.1 | 33.9 | 11.4 | 61.8 | 0.0 | 348.0 | 2.3 | 14.0 |
| Min | 48.1 | 0.5 | 9.6 | 4.4 | 1.2 | 0.7 | 0.6 | 0.7 | 0.1 | 0.1 | 0.1 | 11.7 | 77.1 | 77.7 | 61.9 | 7.3 | 32.9 | 0.5 | 48.6 | 0.0 | 63.9 | 2.2 | 10.9 |
| Max | 61.0 | 1.5 | 17.0 | 9.0 | 2.6 | 4.0 | 1.5 | 1.6 | 2.2 | 5.7 | 2.1 | 16.6 | 120.1 | 94.1 | 322.0 | 17.3 | 46.3 | 13.7 | 149.7 | 6.0 | 397.9 | 3.4 | 14.6 |
| Mean | 54.2 | 1.0 | 14.0 | 6.3 | 1.7 | 2.1 | 0.9 | 1.1 | 0.9 | 3.2 | 0.4 | 14.0 | 94.5 | 84.2 | 197.9 | 13.7 | 38.1 | 9.5 | 81.1 | 2.1 | 234.6 | 2.6 | 13.0 |
| Sample | Rb | Sr | Th | Mo | La | U | CIA | U/Th | V/Cr | TOC/P | V/(V + Ni) | Cr/Ni | Ni/Co | U/Mo | Al/Si | V/Ni | K2O/Al2O3 | Al2O3/TiO2 | |||||
| ppm | |||||||||||||||||||||||
| 1 | 24.6 | 216.2 | 10.4 | 1.3 | 26.2 | 1.7 | 80.78 | 0.16 | 1.28 | 4.8 | 0.56 | 1.93 | 2.43 | 1.31 | 0.32 | 2.48 | 0.05 | 15.84 | |||||
| 2 | 21.5 | 194.8 | 7.6 | 24.5 | 30.7 | 4.8 | 83.99 | 0.63 | 0.87 | 2.9 | 0.46 | 2.03 | 5.03 | 0.20 | 0.16 | 1.76 | 0.08 | 19.59 | |||||
| 3 | 32.6 | 165.3 | 13.3 | 6.1 | 22.1 | 2.6 | 84.61 | 0.20 | 1.25 | 7.8 | 0.56 | 2.21 | 2.66 | 0.43 | 0.25 | 2.77 | 0.06 | 17.83 | |||||
| 4 | 25.1 | 134.7 | 7.9 | 31.5 | 18.3 | 4.5 | 84.30 | 0.57 | 1.32 | 4.0 | 0.57 | 2.41 | 5.15 | 0.14 | 0.18 | 3.19 | 0.06 | 19.45 | |||||
| 5 | 36.1 | 153 | 11.2 | 1.7 | 39.8 | 1.8 | 82.52 | 0.16 | 0.99 | 3.9 | 0.50 | 2.36 | 2.33 | 1.06 | 0.32 | 2.34 | 0.08 | 15.27 | |||||
| 6 | 38.1 | 174.7 | 14.3 | 2.3 | 21.3 | 2.3 | 82.69 | 0.16 | 1.12 | 2.0 | 0.53 | 2.30 | 2.20 | 1.00 | 0.31 | 2.57 | 0.10 | 11.76 | |||||
| 7 | 40.6 | 178.9 | 11.8 | 2.2 | 45.8 | 1.5 | 81.16 | 0.13 | 1.02 | 5.2 | 0.50 | 2.32 | 1.98 | 0.68 | 0.29 | 2.36 | 0.11 | 9.59 | |||||
| Min | 21.5 | 134.7 | 7.6 | 1.3 | 18.3 | 1.5 | 80.78 | 0.13 | 0.87 | 2.0 | 0.46 | 1.93 | 4.51 | 1.15 | 0.20 | 2.34 | 0.07 | 9.59 | |||||
| Max | 40.6 | 216.2 | 14.3 | 31.5 | 45.8 | 4.8 | 84.61 | 0.63 | 1.32 | 7.8 | 0.57 | 2.41 | 2.68 | 0.15 | 0.28 | 2.59 | 0.09 | 19.59 | |||||
| Mean | 31.23 | 173.94 | 10.9 | 9.94 | 29.17 | 2.74 | 82.86 | 0.29 | 1.12 | 4.4 | 0.53 | 2.22 | 2.84 | 0.28 | 0.26 | 2.43 | 0.08 | 15.39 | |||||
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | SO3 | Cl | LOI | V | Cr | Mn | Co | Ni | Cu | Zn | As | Zr | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1.00 | ||||||||||||||||||||||
| TiO2 | −0.91 | 1.00 | |||||||||||||||||||||
| Al2O3 | −0.67 | 0.71 | 1.00 | ||||||||||||||||||||
| Fe2O3 | −0.91 | 0.72 | 0.62 | 1.00 | |||||||||||||||||||
| MgO | 0.17 | −0.45 | 0.00 | 0.17 | 1.00 | ||||||||||||||||||
| CaO | 0.41 | −0.38 | −0.85 | −0.58 | −0.36 | 1.00 | |||||||||||||||||
| Na2O | −0.55 | 0.40 | 0.81 | 0.73 | 0.52 | −0.90 | 1.00 | ||||||||||||||||
| K2O | −0.80 | 0.94 | 0.57 | 0.51 | −0.70 | −0.13 | 0.14 | 1.00 | |||||||||||||||
| P2O5 | 0.48 | −0.52 | −0.76 | −0.62 | −0.30 | 0.88 | −0.81 | −0.22 | 1.00 | ||||||||||||||
| SO3 | −0.45 | 0.67 | 0.20 | 0.11 | −0.95 | 0.21 | −0.31 | 0.87 | 0.16 | 1.00 | |||||||||||||
| Cl | −0.38 | 0.05 | 0.31 | 0.69 | 0.82 | −0.56 | 0.78 | −0.23 | −0.52 | −0.63 | 1.00 | ||||||||||||
| LOI | −0.56 | 0.33 | 0.01 | 0.70 | 0.39 | −0.07 | 0.33 | 0.10 | −0.34 | −0.21 | 0.68 | 1.00 | |||||||||||
| V | 0.42 | −0.45 | −0.17 | −0.11 | 0.49 | −0.31 | 0.07 | −0.64 | −0.30 | −0.58 | 0.25 | 0.04 | 1.00 | ||||||||||
| Cr | 0.81 | −0.89 | −0.92 | −0.70 | 0.21 | 0.68 | −0.69 | −0.78 | 0.73 | −0.42 | −0.22 | −0.18 | 0.37 | 1.00 | |||||||||
| Mn | −0.89 | 0.73 | 0.75 | 0.85 | −0.07 | −0.55 | 0.68 | 0.67 | −0.39 | 0.34 | 0.45 | 0.29 | −0.43 | −0.77 | 1.00 | ||||||||
| Co | −0.88 | 0.85 | 0.80 | 0.79 | 0.03 | −0.54 | 0.67 | 0.67 | −0.65 | 0.23 | 0.41 | 0.48 | −0.32 | −0.85 | 0.73 | 1.00 | |||||||
| Ni | 0.43 | −0.72 | −0.62 | −0.25 | 0.66 | 0.43 | −0.19 | −0.74 | 0.49 | −0.70 | 0.36 | 0.26 | 0.18 | 0.74 | −0.37 | −0.37 | 1.00 | ||||||
| Cu | −0.64 | 0.51 | 0.52 | 0.55 | 0.25 | −0.23 | 0.52 | 0.39 | −0.29 | −0.02 | 0.45 | 0.46 | −0.52 | −0.53 | 0.57 | 0.83 | 0.10 | 1.00 | |||||
| Zn | 0.58 | −0.59 | −0.52 | −0.69 | −0.02 | 0.63 | −0.47 | −0.35 | 0.74 | −0.13 | −0.35 | −0.44 | −0.44 | 0.53 | −0.42 | −0.55 | 0.48 | −0.05 | 1.00 | ||||
| As | 0.48 | −0.36 | 0.04 | −0.55 | −0.18 | −0.02 | −0.08 | −0.16 | 0.26 | 0.03 | −0.39 | −0.85 | −0.23 | 0.08 | −0.13 | −0.46 | −0.15 | −0.33 | 0.62 | 1.00 | |||
| Zr | −0.90 | 0.94 | 0.79 | 0.69 | −0.45 | −0.43 | 0.46 | 0.93 | −0.41 | 0.68 | 0.05 | 0.14 | −0.55 | −0.89 | 0.87 | 0.81 | −0.69 | 0.53 | −0.46 | −0.14 | 1.00 | ||
| Cd | −0.08 | 0.11 | −0.06 | −0.08 | −0.42 | 0.23 | −0.39 | 0.24 | 0.40 | 0.45 | −0.39 | −0.25 | 0.02 | 0.16 | 0.09 | 0.01 | −0.05 | −0.07 | −0.17 | −0.19 | 0.21 | 1.00 | |
| Pb | −0.10 | 0.42 | 0.36 | −0.26 | −0.61 | 0.07 | −0.15 | 0.57 | −0.01 | 0.59 | −0.64 | −0.48 | −0.57 | −0.43 | 0.02 | 0.30 | −0.56 | 0.27 | 0.22 | 0.34 | 0.44 | 0.11 | 1.00 |
| Index | SN | As | Cd | Co | Cr | Cu | Ni | Pb | Zn | V | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF | 1 | 0.02 | 6.86 | 0.28 | 0.25 | 0.14 | 0.25 | 0.18 | 0.22 | 0.30 | 0.28 | 0.18 |
| 2 | 0.55 | 26.37 | 0.49 | 0.93 | 0.32 | 0.90 | 0.68 | 2.04 | 0.77 | 0.66 | 1.62 | |
| 3 | 0.20 | 13.34 | 0.39 | 0.43 | 0.18 | 0.38 | 0.39 | 0.56 | 0.51 | 0.58 | 0.44 | |
| 4 | 0.62 | 30.43 | 0.46 | 1.08 | 0.02 | 0.88 | 0.73 | 1.11 | 1.36 | 0.82 | 1.83 | |
| 5 | 0.25 | 5.04 | 0.16 | 0.17 | 0.07 | 0.14 | 0.17 | 0.31 | 0.16 | 0.21 | 0.13 | |
| 6 | 0.03 | 6.65 | 0.16 | 0.16 | 0.07 | 0.13 | 0.14 | 0.13 | 0.17 | 0.24 | 0.15 | |
| 7 | 0.00 | 5.14 | 0.20 | 0.17 | 0.08 | 0.15 | 0.17 | 0.19 | 0.17 | 0.23 | 0.11 | |
| mean | 0.24 | 12.40 | 0.31 | 0.45 | 0.12 | 0.40 | 0.35 | 0.65 | 0.49 | 0.43 | 0.64 | |
| CF | 1 | 0.08 | 24.44 | 1.00 | 0.88 | 0.49 | 0.89 | 0.64 | 0.78 | 1.07 | 0.99 | 0.63 |
| 2 | 0.60 | 28.89 | 0.53 | 1.02 | 0.35 | 0.99 | 0.74 | 2.23 | 0.84 | 0.72 | 1.78 | |
| 3 | 0.44 | 28.89 | 0.84 | 0.93 | 0.39 | 0.82 | 0.85 | 1.22 | 1.10 | 1.27 | 0.96 | |
| 4 | 0.56 | 27.78 | 0.42 | 0.99 | 0.02 | 0.80 | 0.67 | 1.01 | 1.24 | 0.75 | 1.67 | |
| 5 | 1.25 | 25.56 | 0.82 | 0.84 | 0.35 | 0.70 | 0.86 | 1.57 | 0.79 | 1.07 | 0.67 | |
| 6 | 0.17 | 37.78 | 0.94 | 0.89 | 0.37 | 0.76 | 0.78 | 0.73 | 0.95 | 1.36 | 0.85 | |
| 7 | 0.00 | 25.56 | 0.99 | 0.86 | 0.41 | 0.72 | 0.82 | 0.92 | 0.83 | 1.12 | 0.56 | |
| mean | 0.44 | 28.41 | 0.79 | 0.92 | 0.34 | 0.81 | 0.77 | 1.21 | 0.97 | 1.04 | 1.02 |
| Sample No. | Sulfur wt.% | TOC wt.% | S1 mg/g | S2 mg/g | S3 mg/g | Tmax | HI | OI | PI | S1 + S2 | S2/S3 | Ro |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.14 | 0.21 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2 | 2.48 | 2.77 | 0.18 | 11.34 | 0.61 | 426 | 409 | 22 | 0.02 | 11.52 | 18.59 | |
| 3 | 1.96 | 1.71 | 0.04 | 2.56 | 0.34 | 435 | 150 | 20 | 0.02 | 2.60 | 7.53 | 0.44 |
| 4 | 2.52 | 1.74 | 0.08 | 5.09 | 0.32 | 429 | 293 | 18 | 0.02 | 5.17 | 15.91 | 0.53 |
| 5 | 5.23 | 1.20 | 0.06 | 1.04 | 0.27 | 433 | 87 | 23 | 0.05 | 1.10 | 3.85 | |
| 6 | 5.14 | 0.98 | 0.05 | 0.77 | 0.22 | 431 | 79 | 22 | 0.06 | 0.82 | 3.50 | |
| 7 | 2.95 | 1.13 | 0.07 | 0.98 | 0.47 | 434 | 87 | 42 | 0.07 | 1.05 | 2.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou El-Anwar, E.; Salman, S.; Mousa, D.; Aita, S.; Makled, W.; Gentzis, T. Organic Petrographic and Geochemical Evaluation of the Black Shale of the Duwi Formation, El Sebaiya, Nile Valley, Egypt. Minerals 2021, 11, 1416. https://doi.org/10.3390/min11121416
Abou El-Anwar E, Salman S, Mousa D, Aita S, Makled W, Gentzis T. Organic Petrographic and Geochemical Evaluation of the Black Shale of the Duwi Formation, El Sebaiya, Nile Valley, Egypt. Minerals. 2021; 11(12):1416. https://doi.org/10.3390/min11121416
Chicago/Turabian StyleAbou El-Anwar, Esmat, Salman Salman, Doaa Mousa, Sami Aita, Walid Makled, and Thomas Gentzis. 2021. "Organic Petrographic and Geochemical Evaluation of the Black Shale of the Duwi Formation, El Sebaiya, Nile Valley, Egypt" Minerals 11, no. 12: 1416. https://doi.org/10.3390/min11121416
APA StyleAbou El-Anwar, E., Salman, S., Mousa, D., Aita, S., Makled, W., & Gentzis, T. (2021). Organic Petrographic and Geochemical Evaluation of the Black Shale of the Duwi Formation, El Sebaiya, Nile Valley, Egypt. Minerals, 11(12), 1416. https://doi.org/10.3390/min11121416







