Microbial Diversity and P Content Changes after the Application of Sewage Sludge and Glyphosate to Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimantal Design
2.1.1. Soil Characteristics
2.1.2. Sewage Sludge Characteristics
2.1.3. Pot Experiment
- C—control
- SS—soil with sewage sludge
- GL—soil with glyphosate
- SS+GL—soil with sewage sludge and glyphosate (as Roundup 360 SL)
2.2. Soil Sampling
2.3. Soil Analysis
2.3.1. Soil Total Phosphorus Content Analysis during Experiment
2.3.2. Analysis of Soil Microbial Quantity and Soil Enzymes Activities
2.3.3. Soil Microbial Diversity by Terminal Restriction Fragments Lengths Polymorphism (t-RFLP) Analysis
2.4. Data Analysis
3. Results
3.1. Total Phosphorus Content in Soil
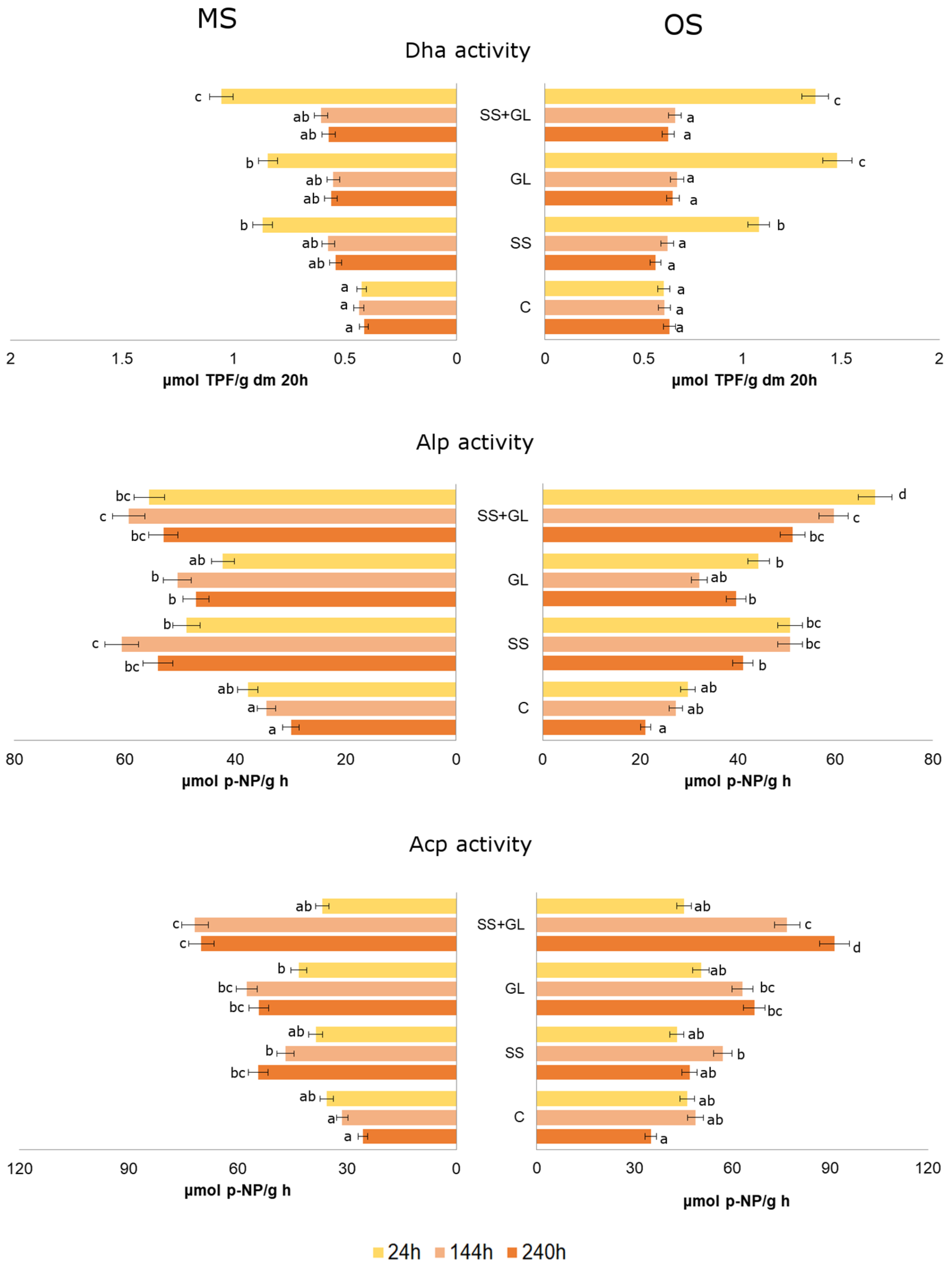
3.2. Soil Microbial Quantity and Soil Enzymes Activities
3.3. Soil Microbial Diversity by t-RFLP Analysis
4. Discussion
4.1. TP Content in MS and OS Soils after Sewage Sludge and Glyphosate Application
4.2. Effect of Sewage Sludge and Glyphosate Application on Soil Microbial Quantity, Enzymes Activities and Microbial Diversity
4.3. Relation of TP Content in MS and OS with Biological Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TP | total phosphorus |
| Alp | alkaline phosphatase |
| Acp | acid phosphatase |
| Dha | dehydrogenases |
| EPSP | 5-endolpyruvate shikimoyl-3-phosphate synthase |
| SS | sewage sludge |
| GL | glyphosate |
| DT50 | half-life persistent |
| t-RFLP | terminal restriction fragment length polymorphism |
| TOC | total organic carbon |
| OM | organic matter |
| ATP | adenosine triphosphate |
| CTP | cytidine triphosphate |
| GTP | guanosine triphosphate |
| USDA | The U.S. Department of Agriculture |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| p-NP | p-nitrophenol |
| DNA | deoxyribonucleic acid |
| 6FAM | 6-carboxyfluorescein |
| ITS | the internal transcribed spacer |
| ANOVA | analysis of variance |
| PCA | Principal component analysis |
| tRFs | terminal restriction fragments |
| PERMANOVA | Permutational multivariate analysis of variance |
| CFU | colony forming unit |
| bp | base of pairs |
| H’ | Shannon diversity index |
| AMPA | aminomethylphosphonic acid |
| MS | loamy sand soil |
| OS | loamy sand soil with a higher amount of TOC |
References
- Gianessi, L.P. The Increasing Importance of Herbicides in Worldwide Crop Production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef]
- Vázquez, M.; Moreno, M.; Amodeo, M.; Bianchinotti, M. Effects of Glyphosate on Soil Fungal Communities: A Field Study. Rev. Argent. Microbiol. 2021, 1–10, in press. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental Contamination, Toxicity and Potential Risks to Human Health via Food Contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Kaczynski, P.; Lozowicka, B.; Wolejko, E.; Iwaniuk, P.; Konecki, R.; Dragowski, W.; Lozowicki, J.; Amanbek, N.; Rusilowska, J.; Pietraszko, A. Complex Study of Glyphosate and Metabolites Influence on Enzymatic Activity and Microorganisms Association in Soil Enriched with Pseudomonas Fluorescens and Sewage Sludge. J. Hazard. Mater. 2020, 393, 122443. [Google Scholar] [CrossRef]
- Sang, Y.; Mejuto, J.-C.; Xiao, J.; Simal-Gandara, J. Assessment of Glyphosate Impact on the Agrofood Ecosystem. Plants 2021, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Kalnenieks, U.; Muter, O. Metabolic Response of Bacteria to Elevated Concentrations of Glyphosate-Based Herbicide. Ecotoxicol. Environ. Saf. 2019, 173, 373–380. [Google Scholar] [CrossRef] [PubMed]
- EFSA Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015, 13, 4302.
- Md. Meftaul, I.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Asaduzzaman, M.; Parven, A.; Megharaj, M. Controversies over Human Health and Ecological Impacts of Glyphosate: Is It to Be Banned in Modern Agriculture? Environ. Pollut. 2020, 263, 114372. [Google Scholar] [CrossRef]
- Wolmarans, K.; Swart, W.J. Influence of Glyphosate, Other Herbicides and Genetically Modified Herbicide-Resistant Crops on Soil Microbiota: A Review. S. Afr. J. Plant Soil 2014, 31, 177–186. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S.; Sahni, D.; Sehgal, M.; Srivastava, D.S.; Singh, A. Pesticides Use and Its Effect on Soil Bacteria and Fungal Populations, Microbial Biomass Carbon and Enzymatic Activity. Curr. Sci. 2019, 116, 643. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Asif Naeem, M.; Niazi, N.K. A Critical Review of Different Factors Governing the Fate of Pesticides in Soil under Biochar Application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Simpson, G.; Haslett, J.R.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between Biodiversity Attributes and Ecosystem Services: A Systematic Review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Cornea, C.P.; Voaideş, C.; Ciuca, M.; Stan, V.; Gament, E.; Razec, I.; Duşa, M. Molecular Methods for Assessement the Bacterial Communities from Different Type of Soils in Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 64. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Zhu, Y.; Zhu, L.; Zhang, J.; Li, B.; Wang, J.; Wang, J.; Zhang, C.; Cheng, C. Effects of the Herbicide Mesotrione on Soil Enzyme Activity and Microbial Communities. Ecotoxicol. Environ. Saf. 2018, 164, 571–578. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of β-Glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Łozowicki, J.; Tujtebajeva, G.; Wydro, U.; Jablońska-Trypuć, A. Effect of Microorganism on Behaviour of Two Commonly Used Herbicides in Wheat/Soil System. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Arora, S.; Sahni, D. Pesticides Effect on Soil Microbial Ecology and Enzyme Activity—An Overview. J. Appl. Nat. Sci. 2016, 8, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Goll, D.S.; Ciais, P.; Peng, S.; Margalef, O.; Asensio, D.; Sardans, J.; Peñuelas, J. Spatial Pattern and Environmental Drivers of Acid Phosphatase Activity in Europe. Front. Big Data 2020, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Maroušek, J.; Maroušková, A.; Zoubek, T.; Bartoš, P. Economic Impacts of Soil Fertility Degradation by Traces of Iron from Drinking Water Treatment. Environ. Dev. Sustain. 2021, 1–10. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Sas-Paszt, L.; Frąc, M. The Status of Soil Microbiome as Affected by the Application of Phosphorus Biofertilizer: Fertilizer Enriched with Beneficial Bacterial Strains. Int. J. Mol. Sci. 2020, 21, 8003. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. ISBN 9789811090448. [Google Scholar]
- Taliman, N.A.; Dong, Q.; Echigo, K.; Raboy, V.; Saneoka, H. Effect of Phosphorus Fertilization on the Growth, Photosynthesis, Nitrogen Fixation, Mineral Accumulation, Seed Yield, and Seed Quality of a Soybean Low-Phytate Line. Plants 2019, 8, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wydro, U.; Jabłońska-Trypuć, A.; Hawrylik, E.; Butarewicz, A.; Rodziewicz, J.; Janczukowicz, W.; Wołejko, E. Heavy Metals Behavior in Soil/Plant System after Sewage Sludge Application. Energies 2021, 14, 1584. [Google Scholar] [CrossRef]
- Ondreičková, K.; Gubišová, M.; Piliarová, M.; Horník, M.; Matušinský, P.; Gubiš, J.; Klčová, L.; Hudcovicová, M.; Kraic, J. Responses of Rhizosphere Fungal Communities to the Sewage Sludge Application into the Soil. Microorganisms 2019, 7, 505. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Agrawal, M. Potential Benefits and Risks of Land Application of Sewage Sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Jezierska-Tys, S.; Frąc, M.; Tys, J. Microbiological Hazards Resulting from Application of Dairy Sewage Sludge: Effects on Occurrence of Pathogenic Microorganisms in Soil. J. Toxicol. Environ. Health Part A 2010, 73, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Miguel, N.; Sarasa, J.; López, A.; Gómez, J.; Mosteo, R.; Ormad, M.P. Study of Evolution of Microbiological Properties in Sewage Sludge-Amended Soils: A Pilot Experience. Int. J. Environ. Res. Public Health 2020, 17, 6696. [Google Scholar] [CrossRef] [PubMed]
- Suhadolc, M.; Schroll, R.; Hagn, A.; Dörfler, U.; Schloter, M.; Lobnik, F. Single Application of Sewage Sludge–Impact on the Quality of an Alluvial Agricultural Soil. Chemosphere 2010, 81, 1536–1543. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Jaisi, D.P. Degradation of Glyphosate and Bioavailability of Phosphorus Derived from Glyphosate in a Soil-Water System. Water Res. 2019, 163, 114840. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Botttomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994. [Google Scholar]
- Margesin, R. Acid and Alkaline Phosphomonoesterase Activity with the Substrate p-Nitrophenyl Phosphate. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Mattana, S.; Chelinho, S.; Sousa, J.P.; Alcañiz, J.M.; Domene, X. Nonylphenol Causes Shifts in Microbial Communities and Nitrogen Mineralization in Soil Microcosms. Ecotoxicol. Environ. Saf. 2019, 181, 395–403. [Google Scholar] [CrossRef]
- Available online: http://inismor.ucd.ie/talign/ (accessed on 10 November 2021).
- Available online: https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 10 November 2021).
- Fredriksson, N.J.; Hermansson, M.; Wilén, B.-M. Tools for T-RFLP Data Analysis Using Excel. BMC Bioinform. 2014, 15, 361. [Google Scholar] [CrossRef] [Green Version]
- Wierzbowska, J.; Sienkiewicz, S.; Zalewska, M.; Żarczyński, P.; Krzebietke, S. Phosphorus Fractions in Soil Fertilised with Organic Waste. Environ. Monit. Assess. 2020, 192, 315. [Google Scholar] [CrossRef]
- Stávková, J.; Maroušek, J. Novel Sorbent Shows Promising Financial Results on P Recovery from Sludge Water. Chemosphere 2021, 276, 130097. [Google Scholar] [CrossRef] [PubMed]
- Kahiluoto, H.; Kuisma, M.; Ketoja, E.; Salo, T.; Heikkinen, J. Phosphorus in Manure and Sewage Sludge More Recyclable than in Soluble Inorganic Fertilizer. Environ. Sci. Technol. 2015, 49, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Zuo, W.; Gu, C.; Zhang, W.; Xu, K.; Wang, Y.; Bai, Y.; Shan, Y.; Dai, Q. Sewage Sludge Amendment Improved Soil Properties and Sweet Sorghum Yield and Quality in a Newly Reclaimed Mudflat Land. Sci. Total Environ. 2019, 654, 541–549. [Google Scholar] [CrossRef]
- Nobile, C.; Houben, D.; Michel, E.; Firmin, S.; Lambers, H.; Kandeler, E.; Faucon, M.-P. Phosphorus-Acquisition Strategies of Canola, Wheat and Barley in Soil Amended with Sewage Sludges. Sci. Rep. 2019, 9, 14878. [Google Scholar]
- Houben, D.; Michel, E.; Nobile, C.; Lambers, H.; Kandeler, E.; Faucon, M.-P. Response of Phosphorus Dynamics to Sewage Sludge Application in an Agroecosystem in Northern France. Appl. Soil Ecol. 2019, 137, 178–186. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P. Application of Biochar to Sewage Sludge Reduces Toxicity and Improve Organisms Growth in Sewage Sludge-Amended Soil in Long Term Field Experiment. Sci. Total Environ. 2018, 625, 8–15. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Athamneh, B.M. Changes in Soil Fertility and Plant Uptake of Nutrients and Heavy Metals in Response to Sewage Sludge Application to Calcareous Soils. J. Agron. 2004, 3, 229–236. [Google Scholar] [CrossRef]
- de Jonge, H.; de Jonge, L.W.; Jacobsen, O.H.; Yamaguchi, T.; Moldrup, P. Glyphosate Sorption in Soils of Different pH and Phosphorus Content. Soil Sci. 2001, 166, 230–238. [Google Scholar] [CrossRef]
- Haney, R.L.; Senseman, S.A.; Hons, F.M.; Zuberer, D.A. Effect of Glyphosate on Soil Microbial Activity and Biomass. Weed Sci. 2000, 48, 89–93. [Google Scholar] [CrossRef]
- Kacprzak, M.; Grobelak, A.; Grosser, A.; Napora, A. The Potential of Biosolid Application for the Phytostabilisation of Metals. Desalin. Water Treat. 2014, 52, 3955–3964. [Google Scholar] [CrossRef]
- Gryta, A.; Frąc, M.; Oszust, K. Community Shift in Structure and Functions across Soil Profile in Response to Organic Waste and Mineral Fertilization Strategies. Appl. Soil Ecol. 2019, 143, 55–60. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent Advances in Glyphosate Biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef]
- Wołejko, E.; Kaczyński, P.; Łozowicka, B.; Wydro, U.; Borusiewicz, A.; Hrynko, I.; Konecki, R.; Snarska, K.; Dec, D.; Malinowski, P. Dissipation of S-Metolachlor in Plant and Soil and Effect on Enzymatic Activities. Environ. Monit. Assess. 2017, 189, 355. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, P.; Farto, M.; Mourinha, C.; Palma, P. Beneficial Use of Dewatered and Composted Sewage Sludge as Soil Amendments: Behaviour of Metals in Soils and Their Uptake by Plants. Waste Biomass Valorization 2016, 7, 1189–1201. [Google Scholar] [CrossRef]
- Picariello, E.; Pucci, L.; Carotenuto, M.; Libralato, G.; Lofrano, G.; Baldantoni, D. Compost and Sewage Sludge for the Improvement of Soil Chemical and Biological Quality of Mediterranean Agroecosystems. Sustainability 2020, 13, 26. [Google Scholar] [CrossRef]
- Frąc, M.; Jezierska-Tys, S.; Oszust, K.; Gryta, A.; Pastor, M. Assessment of Microbiological and Biochemical Properties of Dairy Sewage Sludge. Int. J. Environ. Sci. Technol. 2016, 14, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Marschner, P. Structure and Function of the Soil Microbial Community in a Long-Term Fertilizer Experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Sadet-Bourgeteau, S.; Houot, S.; Karimi, B.; Mathieu, O.; Mercier, V.; Montenach, D.; Morvan, T.; Sappin-Didier, V.; Watteau, F.; Nowak, V.; et al. Microbial Communities from Different Soil Types Respond Differently to Organic Waste Input. Appl. Soil Ecol. 2019, 143, 70–79. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Landi, L.; Smalla, K.; Nannipieri, P.; Brookes, P.C.; Renella, G. Effects of Cd- and Zn-Enriched Sewage Sludge on Soil Bacterial and Fungal Communities. Ecotoxicol. Environ. Saf. 2010, 73, 1255–1263. [Google Scholar] [CrossRef]
- Mossa, A.-W.; Dickinson, M.J.; West, H.M.; Young, S.D.; Crout, N.M.J. The Response of Soil Microbial Diversity and Abundance to Long-Term Application of Biosolids. Environ. Pollut. 2017, 224, 16–25. [Google Scholar] [CrossRef]
- Kanissery, R.G.; Welsh, A.; Gomez, A.; Connor, L.; Sims, G.K. Identification of Metolachlor Mineralizing Bacteria in Aerobic and Anaerobic Soils Using DNA-Stable Isotope Probing. Biodegradation 2017, 29, 117–128. [Google Scholar] [CrossRef]
- Tarrasón, D.; Ojeda, G.; Ortiz, O.; Alcañiz, J.M. Effects of Different Types of Sludge on Soil Microbial Properties: A Field Experiment on Degraded Mediterranean Soils. Pedosphere 2010, 20, 681–691. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Liu, H.; Li, J.; Wang, Y.; Xu, H. Application of Acclimated Sewage Sludge as a Bio-Augmentation/Bio-Stimulation Strategy for Remediating Chlorpyrifos Contamination in Soil With/without Cadmium. Sci. Total Environ. 2017, 579, 657–666. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global Patterns of Phosphatase Activity in Natural Soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Wang, S.; Si, Y.; Yang, W.; Zhu, S.; Ni, W. Variations in Eco-Enzymatic Stoichiometric and Microbial Characteristics in Paddy Soil as Affected by Long-Term Integrated Organic-Inorganic Fertilization. PLoS ONE 2017, 12, e0189908. [Google Scholar] [CrossRef]
- Lang, M.; Zou, W.; Chen, X.; Zou, C.; Zhang, W.; Deng, Y.; Zhu, F.; Yu, P.; Chen, X. Soil Microbial Composition and PhoD Gene Abundance Are Sensitive to Phosphorus Level in a Long-Term Wheat-Maize Crop System. Front. Microbiol. 2021, 11, 3547. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Wang, F.; Zou, B.; Chen, Y.; Zhao, J.; Mo, Q.; Li, Y.; Li, X.; Xia, H. Effects of Nitrogen and Phosphorus Addition on Soil Microbial Community in a Secondary Tropical Forest of China. Biol. Fertil. Soils 2014, 51, 207–215. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network Analysis Reveals Functional Redundancy and Keystone Taxa amongst Bacterial and Fungal Communities during Organic Matter Decomposition in an Arable Soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Osorio, N.W. Effectiveness of Phosphate Solubilizing Microorganism in Increasing Plant Phosphate Uptake and Growth in Tropical Soils. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–80. ISBN 9783642210617. [Google Scholar]







| Characteristics | MS | OS | SS 1 |
|---|---|---|---|
| pH | 7.4 | 6.2 | 6.9 |
| OM 2 (%) | - | - | 57.8 |
| TOC 3 (%) | 5.84 | 10.78 | - |
| Sand | 77.1 | 71.3 | - |
| Silt | 20.7 | 25.1 | - |
| Clay | 2.2 | 3.6 | - |
| P (% d.m.) | 0.03 | 0.05 | 3.15 |
| Cd, mg/kg d.m. | 0.13 | 0.05 | 0.52 |
| Cr, mg/kg d.m. | 8.2 | 4.0 | 18.4 |
| Cu, mg/kg d.m. | 6.7 | 3.3 | 35 |
| Ni, mg/kg d.m. | 2.7 | 1.8 | 5.1 |
| Pb, mg/kg d.m. | 8.6 | 2.3 | 10 |
| Zn, mg/kg d.m. | 24 | 11 | 310 |
| Primer | Sequences | Length (Bases) | Tm (°C) | GC (%) |
|---|---|---|---|---|
| 8F | AGAGTTTGATCCTGGCTCAG | 20 | 51.8 | 50 |
| 1389R | ACGGGCGGTGTGTACAAG | 18 | 52.6 | 61 |
| ITS1-F | CTTGGTCATTTAGAGGAAGTAA | 22 | 49.2 | 36 |
| ITS4-R | TCCTCCGCTTATTGATATGC | 20 | 49.7 | 45 |
| Treatments | Bacteria | Fungi | ||
|---|---|---|---|---|
| MS | OS | MS | OS | |
| SS | 3.55 * | 6.03 * | 4.84 * | 2.71 |
| GL | 0.59 | 3.97 | 3.25 | 4.71 * |
| SS + GL | 1.96 | 5.86 * | 3.93 | 4.13 |
| Global F | 1.90 | 6.48 * | 2.23 | 4.33 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wydro, U.; Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A. Microbial Diversity and P Content Changes after the Application of Sewage Sludge and Glyphosate to Soil. Minerals 2021, 11, 1423. https://doi.org/10.3390/min11121423
Wydro U, Wołejko E, Łozowicka B, Jabłońska-Trypuć A. Microbial Diversity and P Content Changes after the Application of Sewage Sludge and Glyphosate to Soil. Minerals. 2021; 11(12):1423. https://doi.org/10.3390/min11121423
Chicago/Turabian StyleWydro, Urszula, Elżbieta Wołejko, Bożena Łozowicka, and Agata Jabłońska-Trypuć. 2021. "Microbial Diversity and P Content Changes after the Application of Sewage Sludge and Glyphosate to Soil" Minerals 11, no. 12: 1423. https://doi.org/10.3390/min11121423






