The Influence of Zeolitic By-Product Containing Ammonium Ions on Properties of Hardened Cement Paste
Abstract
:1. Introduction
2. Methods and Materials
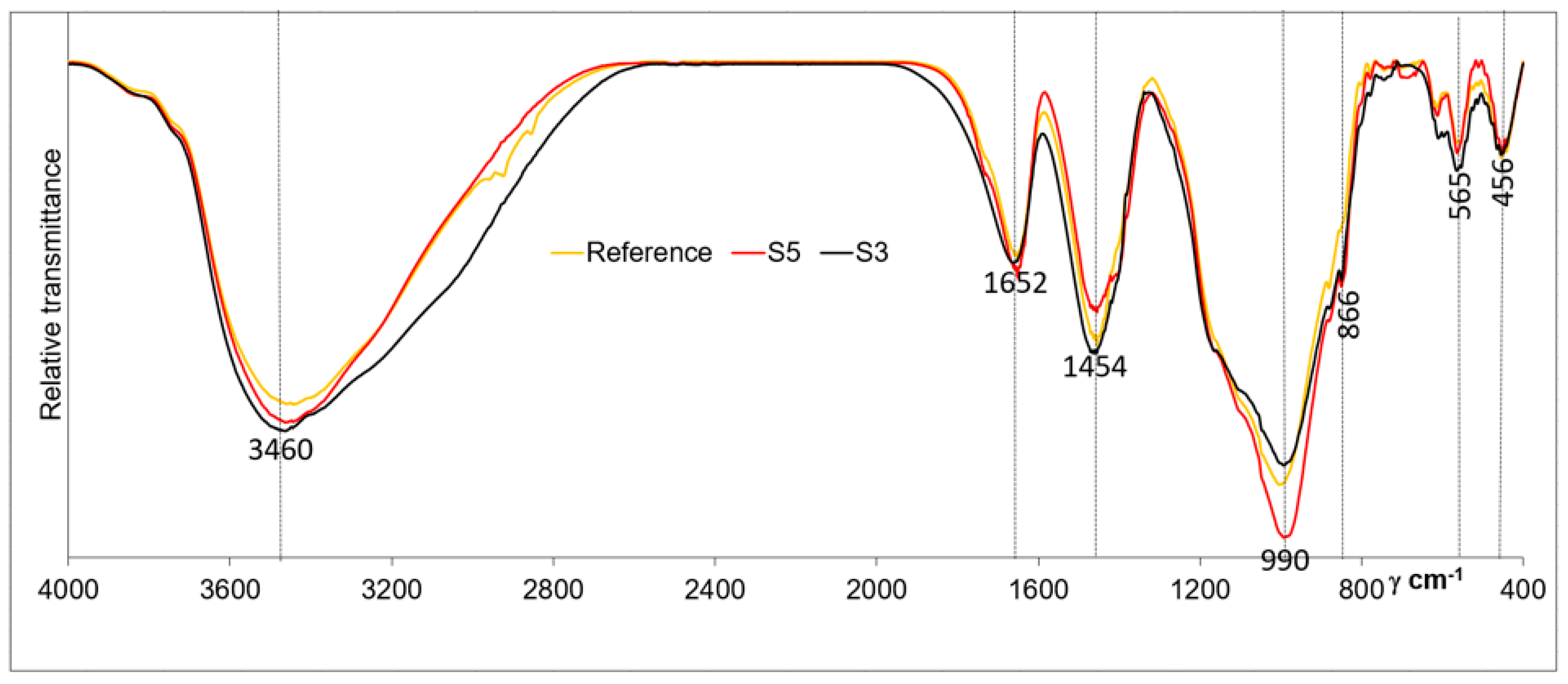
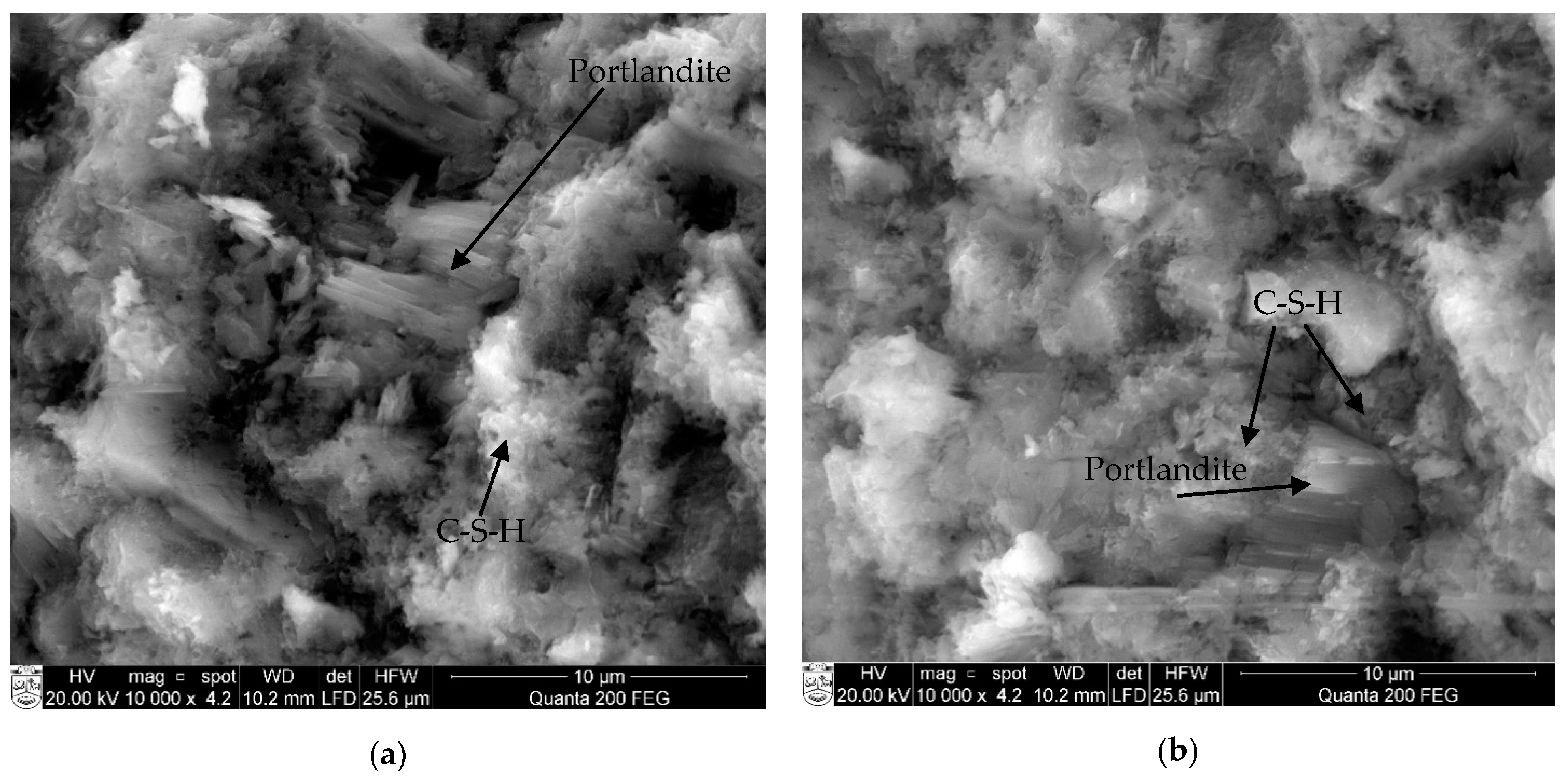
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Panesar, D.K. Supplementary cementing materials. In Developments in the Formulation and Reinforcement of Concrete, 2nd ed.; Mindess, S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 55–85. [Google Scholar]
- Rahla, K.M.; Mateus, R.; Bragança, L. Comparative sustainability assessment of binary blended concretes using Supplementary Cementitious Materials (SCMs) and Ordinary Portland Cement (OPC). J. Clean. Prod. 2019, 220, 445–459. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Recycling of quarry dust for supplementary cementitious materials in low carbon cement. Constr. Build. Mater. 2020, 237, 117608. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Huang, H. Effect of supplementary materials on the autogenous shrinkage of cement paste. Materials 2020, 13, 3367. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ye, G.; De Schutter, G. Investigation on the potential utilization of zeolite as an internal curing agent for autogenous shrinkage mitigation and the effect of modification. Constr. Build. Mater. 2019, 198, 669–676. [Google Scholar] [CrossRef]
- Thang, N.C.; Tuan, N.V.; Yang, K.H.; Phung, Q.T. Effect of zeolite on shrinkage and crack resistance of high-performance cement-based concrete. Materials 2020, 13, 3773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Wang, X.Y. Effect of pre-wetted zeolite sands on the autogenous shrinkage and strength of ultra-high-performance concrete. Materials 2020, 13, 2356. [Google Scholar] [CrossRef] [PubMed]
- Nagrockiene, D.; Girskas, G. Research into the properties of concrete modified with natural zeolite addition. Constr. Build. Mater. 2016, 113, 964–969. [Google Scholar] [CrossRef]
- Rahhal, V.F.; Pavlík, Z.; Tironi, A.; Castellano, C.C.; Trezza, M.A.; Černý, R.; Irassar, E.F. Effect of cement composition on the early hydration of blended cements with natural zeolite. J. Therm. Analys. Calorim. 2017, 128, 721–733. [Google Scholar] [CrossRef]
- Lilkov, V.; Rostovsky, I.; Petrov, O. Physical and mechanical characteristics of cement mortars and concretes with addition of clinoptilolite from Beli Plast deposit (Bulgaria), silica fume and fly ash. Clay Miner. 2011, 46, 213–223. [Google Scholar] [CrossRef]
- Lilkov, V.; Petrov, O.; Petkova, V.; Petrova, N.; Tzvetanova, Y. Study of the pozzolanic activity and hydration products of cement pastes with addition of natural zeolites. Clay Miner. 2011, 46, 241–250. [Google Scholar] [CrossRef]
- Girskas, G.; Skripkiūnas, G.; Šahmenko, G.; Korjakins, A. Durability of concrete containing synthetic zeolite from aluminum fluoride production waste as a supplementary cementitious material. Constr. Build. Mater. 2016, 117, 99–106. [Google Scholar] [CrossRef]
- Da, Y.; He, T.; Wang, M.; Shi, C.; Xu, R.; Yang, R. The effect of spent petroleum catalyst powders on the multiple properties in blended cement. Constr. Build. Mater. 2020, 231, 117203. [Google Scholar] [CrossRef]
- Allahverdi, A.; Shahrbabaki, M.N.; Ghezelasheghi, M.; Mahinroosta, M. Sulfate resistance of RFCC spent catalyst-blended Portland cement. Boletín Soc. Española Cerámica Vidr. 2019, 58, 103–114. [Google Scholar] [CrossRef]
- Su, N.; Fang, H.Y.; Chen, Z.H.; Liu, F.S. Reuse of waste catalysts from petrochemical industries for cement substitution. Cem. Concr. Res. 2000, 30, 1773–1783. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. J. Haz. Mater. 2020, 393, 122481. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Mei, L.; Hao, S.; Li, D.; Li, X.; Zhang, Q.; Jin, X.; Li, C. Na@La modified zeolite particles for simultaneous removal of ammonia nitrogen and phosphate from rejected water: Performance and mechanism. Water Sci. Technol. 2020, 82, 2975–2989. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.Z.; Chen, D. Adsorbent materials for ammonium and ammonia removal: A review. J. Clean. Product. 2020, 283, 124611. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a potential medium for ammonium recovery and second cheese whey treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Gao, X.; Li, Q. Influences of coal fly ash containing ammonium salts on properties of cement paste. J. Environ. Manag. 2019, 249, 109374. [Google Scholar] [CrossRef]
- Lea, F.M. The action of ammonium salts on concrete. Magaz. Concr. Res. 1965, 17, 115–116. [Google Scholar] [CrossRef]
- Myrdal, R. Accelerating Admixtures for Concrete. State of the Art; COIN—Concrete Innovation Centre: Trondheim, Norway, 2007.
- Frybort, S.; Mauritz, R.; Teischinger, A.; Müller, U. Determination of the bond strength of treated wood strands embedded in a cement matrix by means of a pull-out test. Eur. J. Wood Wood Prod. 2009, 68, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Frybort, S.; Mauritz, R.; Teischinger, A.; Müller, U. Cement bonded composites—A mechanical review. BioResources. 2008, 3, 602–626. [Google Scholar]
- Bruker. X-ray S8 Tiger WD Series 2 Technical Details. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/x-ray-fluorescence/s8-tiger.html (accessed on 14 December 2020).
- Bruker. D8 Advance Diffractometer (Bruker AXS) Technical Details. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/x-ray-diffraction/d8-advance.html (accessed on 14 December 2020).
- CILAS. 1090 Particle Size Analyzer. Available online: https://www.pharmaceuticalonline.com/doc/cilas-1090-particle-size-analyzer-0002 (accessed on 14 December 2020).
- Zeiss. EVO MA and LS Series Scanning Electron Microscopes for Materials Analysis and Life Science; Carl Zeiss SMT: Oberkochen, Germany, 2008; Available online: https://www.scribd.com/document/391914988/EVO-Series-UserGuide (accessed on 1 January 2008).
- Vaičiukynienė, D.; Mikelionienė, A.; Baltušnikas, A.; Kantautas, A.; Radzevičius, A. Removal of ammonium ion from aqueous solutions by using unmodified and H2O2-modified zeolitic waste. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Velázquez, S.; Monzó, J.; Borrachero, M.V.; Soriano, L.; Payá, J. Evaluation of the pozzolanic activity of spent FCC catalyst/fly ash mixtures in Portland cement pastes. Thermochim. Acta. 2016, 632, 29–36. [Google Scholar] [CrossRef]
- García de Lomas, M.; de Rojas, M.S.; Frías, M. Pozzolanic reaction of a spent fluid catalytic cracking catalyst in FCC-cement mortars. J. Therm. Analys. Calorimetr. 2007, 90, 443–447. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.J.; Tay, J.H. Adsorption mechanisms of high levels of ammonium onto natural and NaCl-modifed zeolites. Separ. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Sadiku, N.A.; Sanusi, A. Wood pre-treatment influence on the hydration of Portland cement in combination with some tropical wood species. Pro Ligno 2014, 10, 3–10. [Google Scholar]
- Perraki, T.; Kakali, G.; Kontoleon, F. The effect of natural zeolites on the early hydration of Portland cement. Micropor. Mesopor. Mater. 2003, 61, 205–212. [Google Scholar] [CrossRef]
- Perraki, T.; Kontori, E.; Tsivilis, S.; Kakali, G. The effect of zeolite on the properties and hydration of blended cements. Cem. Concr. Comp. 2010, 32, 128–133. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Pundienė, I.; Kantautas, A.; Augonis, A.; Janavičius, E.; Vaičiukynas, V.; Alobeid, J. Synergistic effect of dry sludge from waste wash water of concrete plants and zeolitic by-product on the properties of ternary blended ordinary Portland cements. J. Clean. Prod. 2020, 244, 118493. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Grinys, A.; Vaitkevičius, V.; Kantautas, A. Purified waste fcc catalyst as a cement replacement material. Ceram. Silikáty. 2015, 59, 103–108. [Google Scholar]
- Cao, Y.; Guo, L.; Chen, B.; Fei, X. Modeling early age hydration kinetics and the hydrated phase of cement paste blended with chloride and sulfate. Constr. Build. Mater. 2020, 261, 120537. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Díaz, J.E.; de Gutierrez, R.M.; Torres, J. Blended cement containing fluid catalytic cracking catalyst residue (FCC): Hydration and paste microstructure. Rev. Ing. Constr. 2013, 28, 141–154. [Google Scholar]
- Kocak, Y.; Tascı, E.; Kaya, U. The effect of using natural zeolite on the properties and hydration characteristics of blended cements. Constr. Build. Mater. 2013, 47, 720–727. [Google Scholar] [CrossRef]
- Jose, A.; Nivitha, M.R.; Krishnan, J.M.; Robinson, R.G. Characterization of cement stabilized pond ash using FTIR spectroscopy. Constr. Build. Mater. 2020, 263, 120136. [Google Scholar] [CrossRef]
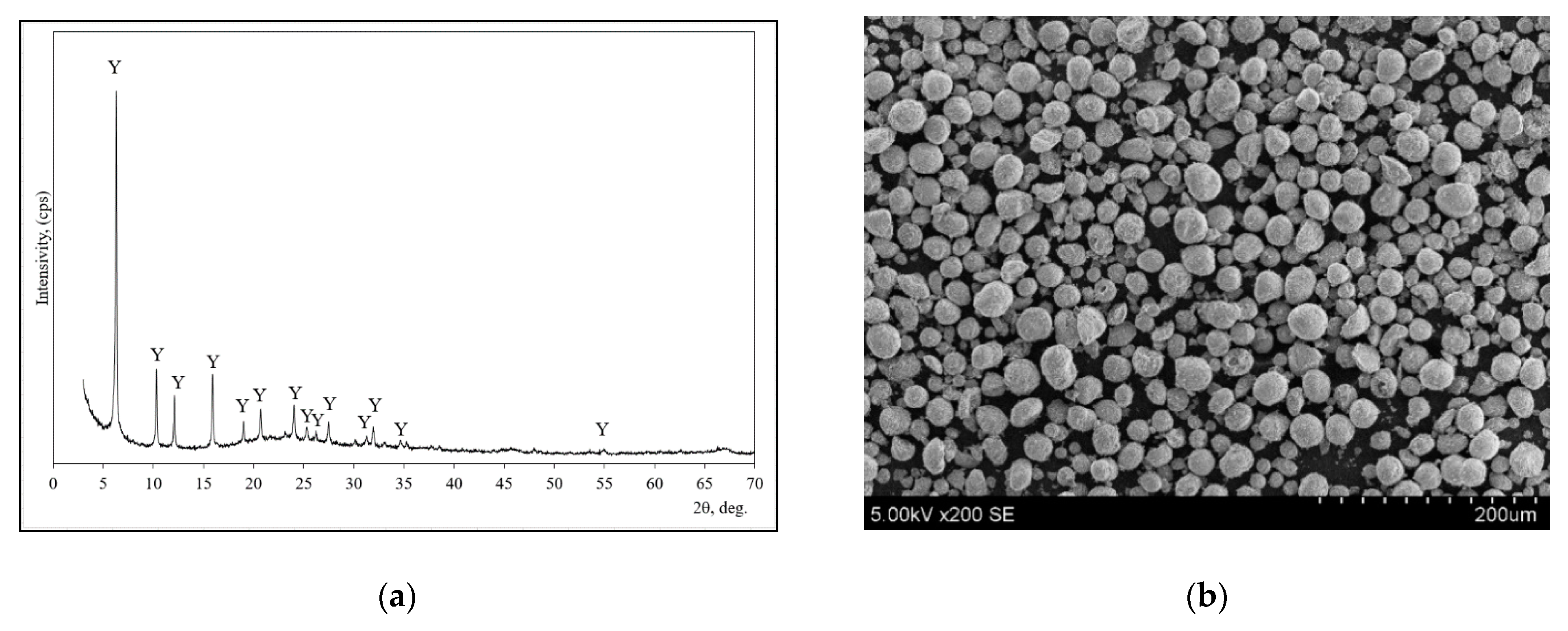
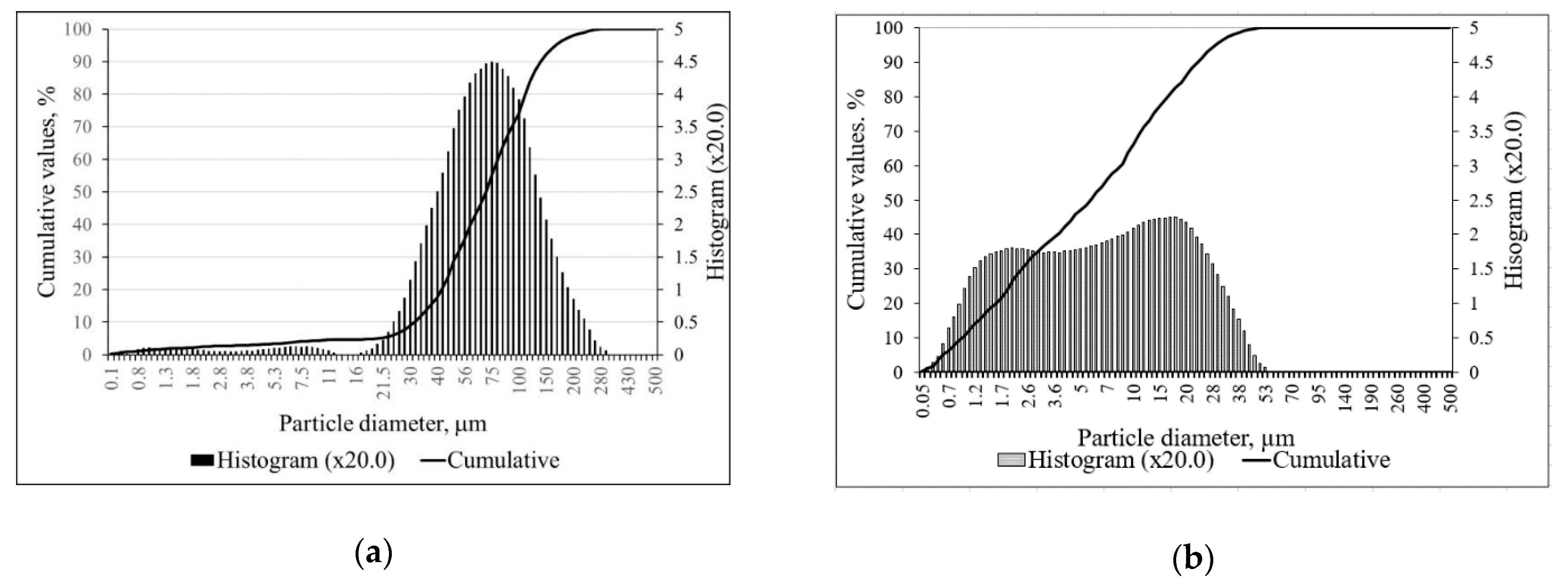
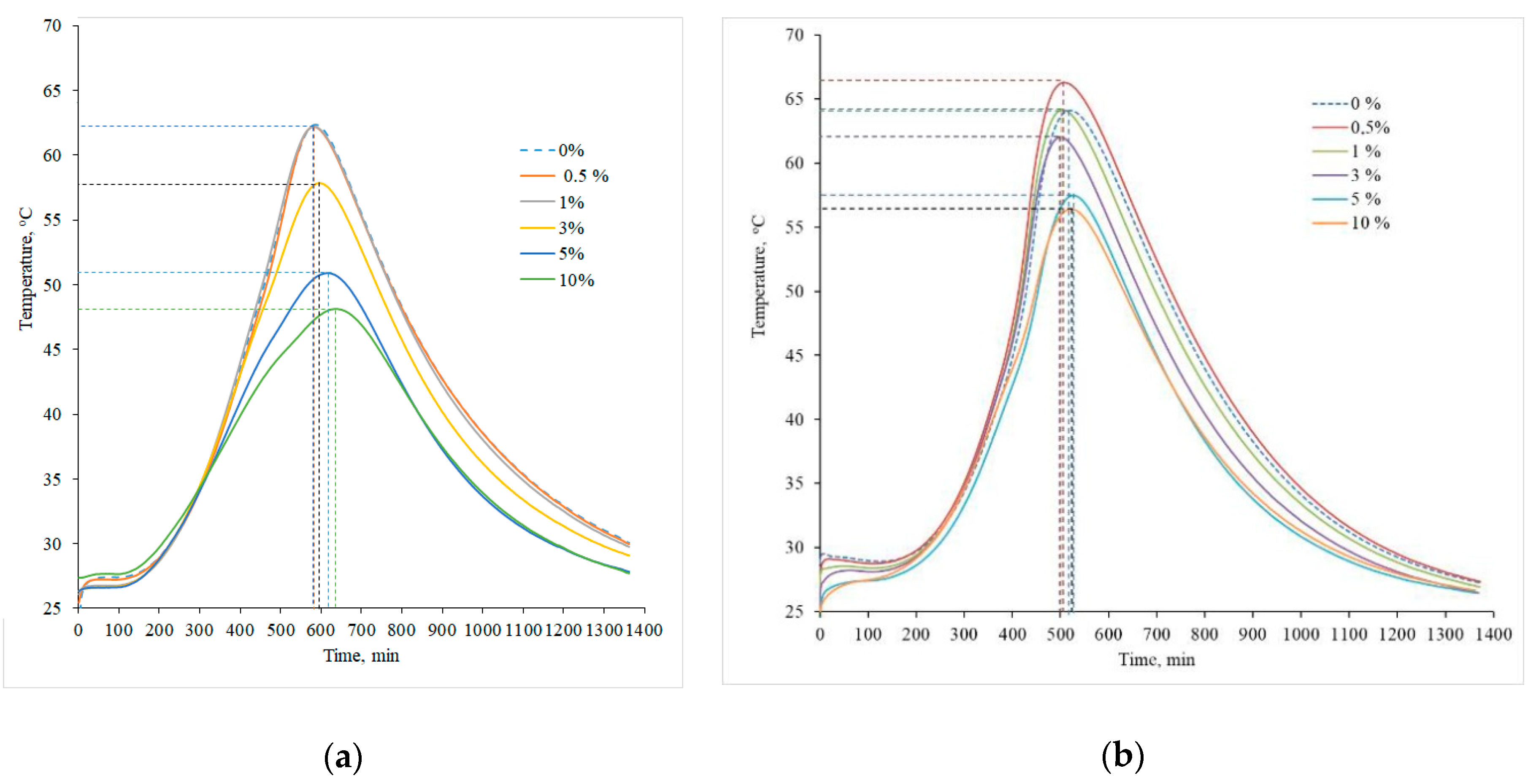

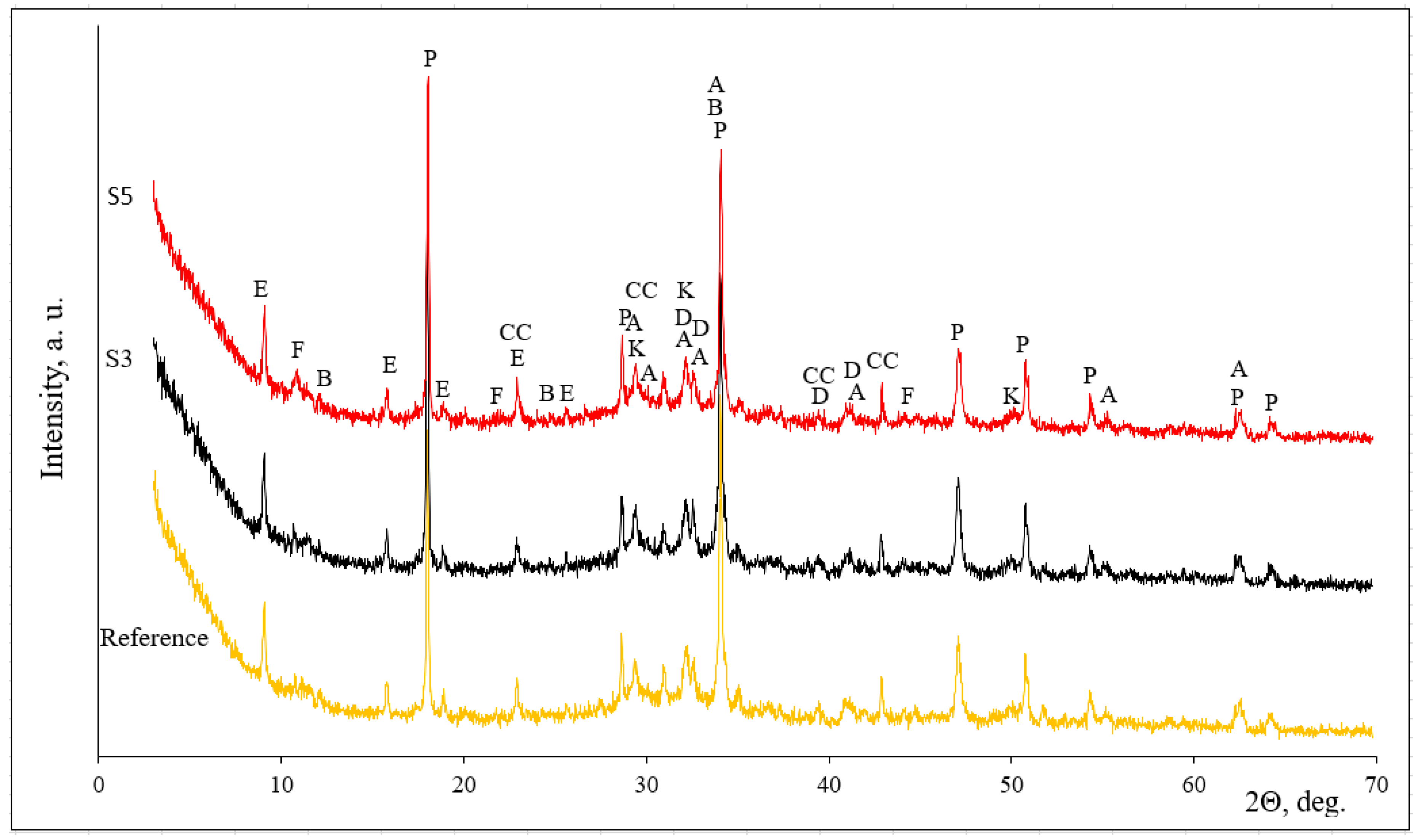
| Parameter | Portland Cement CEM I 52.5R | Zeolitic By-Product |
|---|---|---|
| SiO2 | 21.00 | 35.4 |
| Al2O3 | 3.90 | 48.77 |
| Fe2O3 | 2.90 | 1.02 |
| La2O3 | - | 1.63 |
| TiO2 | - | 3.57 |
| MgO | 2.70 | 0.44 |
| CaO | 66.00 | 0.37 |
| Na2O | - | 0.312 |
| SO3 | 3.40 | 0.07 |
| P2O5 | - | 0.08 |
| K2O | - | - |
| Cl | 0.06 | 2.57 |
| Other | - | 5.77 |
| Bulk density, kg/m3 | 1236 | 864 |
| Specific density, kg/m3 | 3122 | 2679 |
| Surface area (by Blaine), m2/g | 350.0 | 142.1 |
| Samples | Ordinary Portland Cement (wt %) | Zeolitic By-Product with Ammonium Chloride (wt %) | Water-to-Solid Materials Ratio (W/S) |
|---|---|---|---|
| Reference | 100 | 0 | 0.36 |
| S0.5 | 99.5 | 0.5 | 0.36 |
| S1 | 99.0 | 1.0 | 0.36 |
| S3 | 97.0 | 3.0 | 0.36 |
| S5 | 95.0 | 5.0 | 0.36 |
| S10 | 90.0 | 10.0 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaičiukynienė, D.; Mikelionienė, A.; Kantautas, A.; Radzevičius, A.; Bajare, D. The Influence of Zeolitic By-Product Containing Ammonium Ions on Properties of Hardened Cement Paste. Minerals 2021, 11, 123. https://doi.org/10.3390/min11020123
Vaičiukynienė D, Mikelionienė A, Kantautas A, Radzevičius A, Bajare D. The Influence of Zeolitic By-Product Containing Ammonium Ions on Properties of Hardened Cement Paste. Minerals. 2021; 11(2):123. https://doi.org/10.3390/min11020123
Chicago/Turabian StyleVaičiukynienė, Danutė, Agnė Mikelionienė, Aras Kantautas, Algirdas Radzevičius, and Diana Bajare. 2021. "The Influence of Zeolitic By-Product Containing Ammonium Ions on Properties of Hardened Cement Paste" Minerals 11, no. 2: 123. https://doi.org/10.3390/min11020123







