Microstructure and Chemical Transformation of Natural Ilmenite during Isothermal Roasting Process in Air Atmosphere
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Methods
3. Results and Discussion
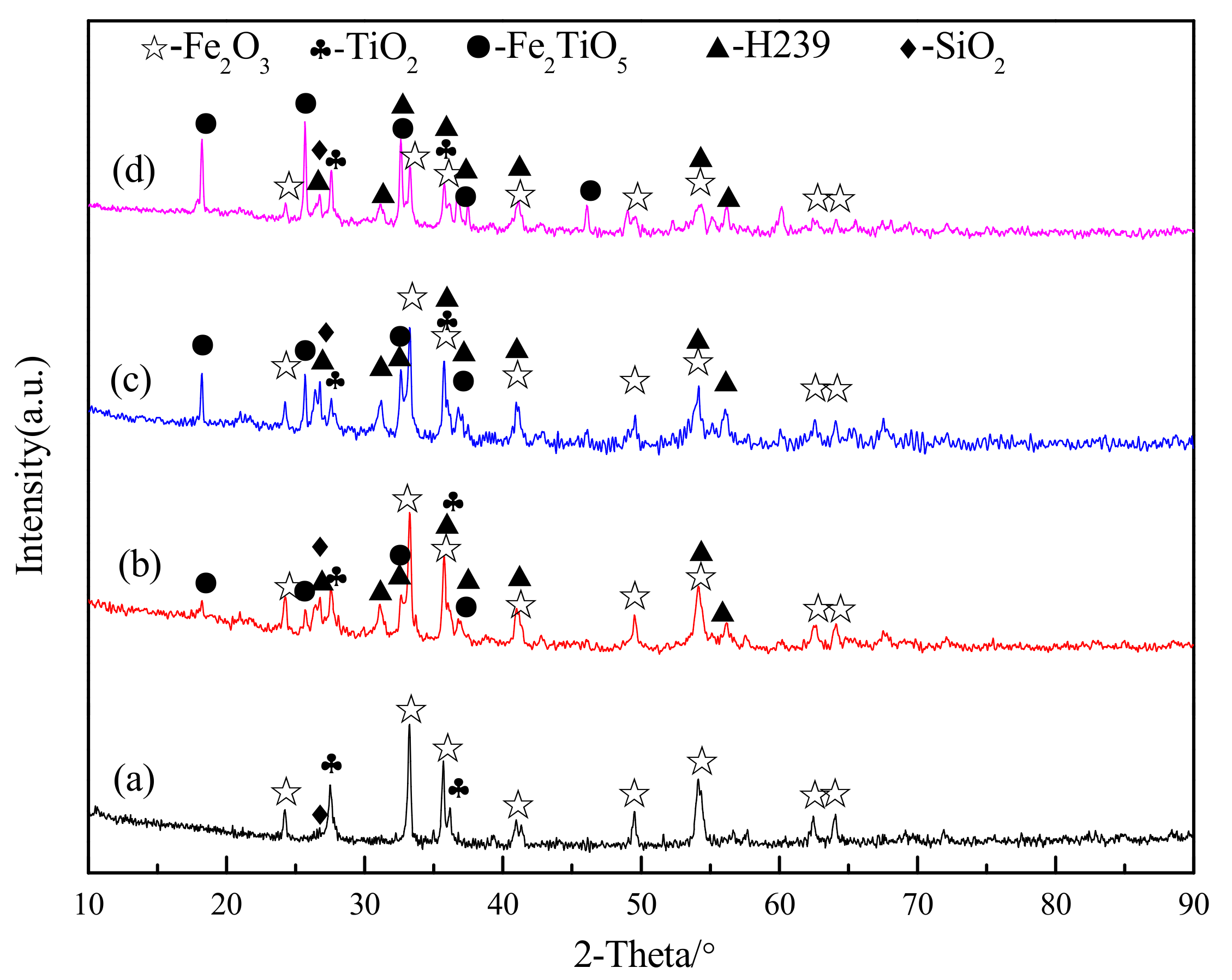
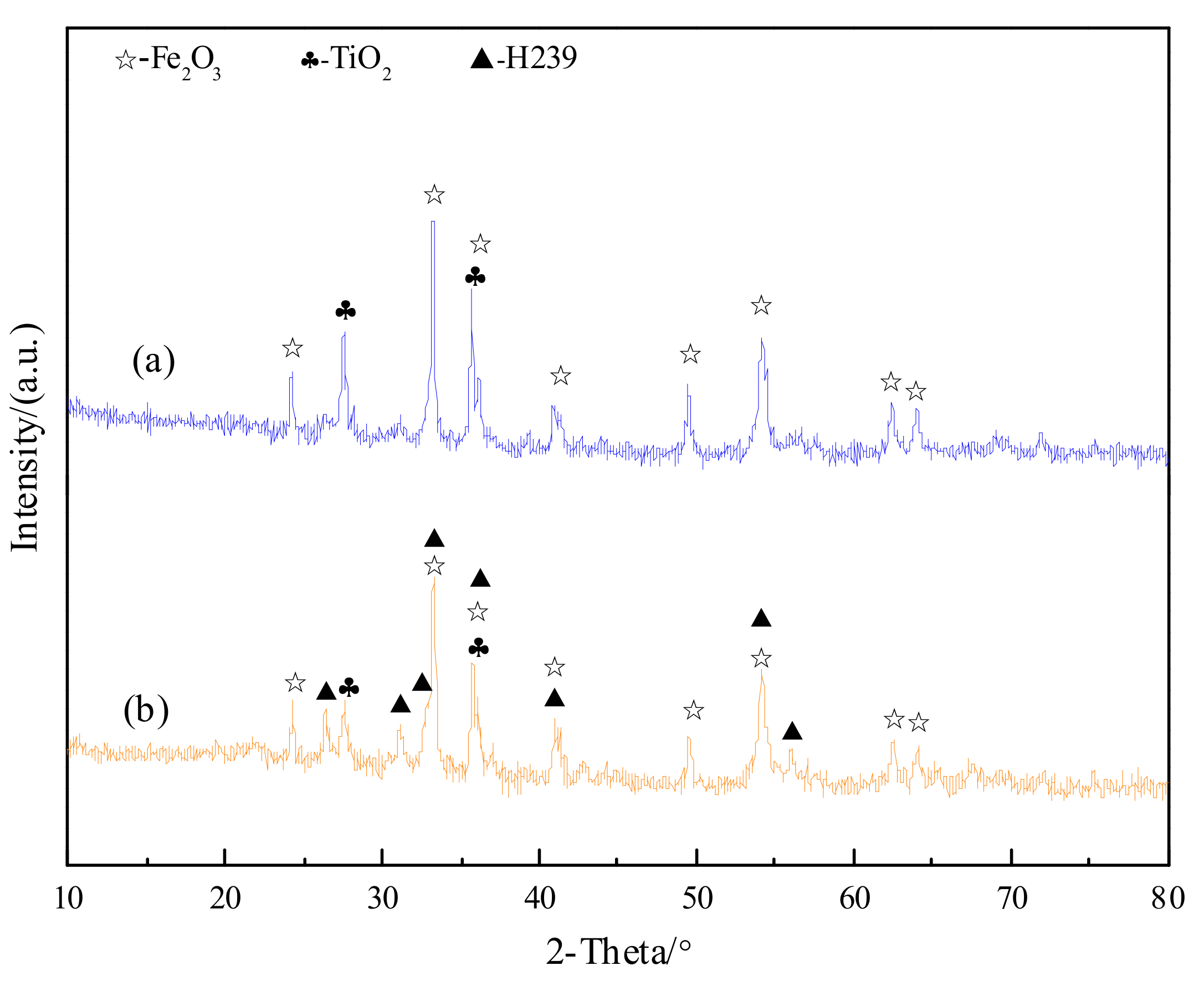
3.1. Confirmation of Oxidation Roasting Products of Natural Ilmenite
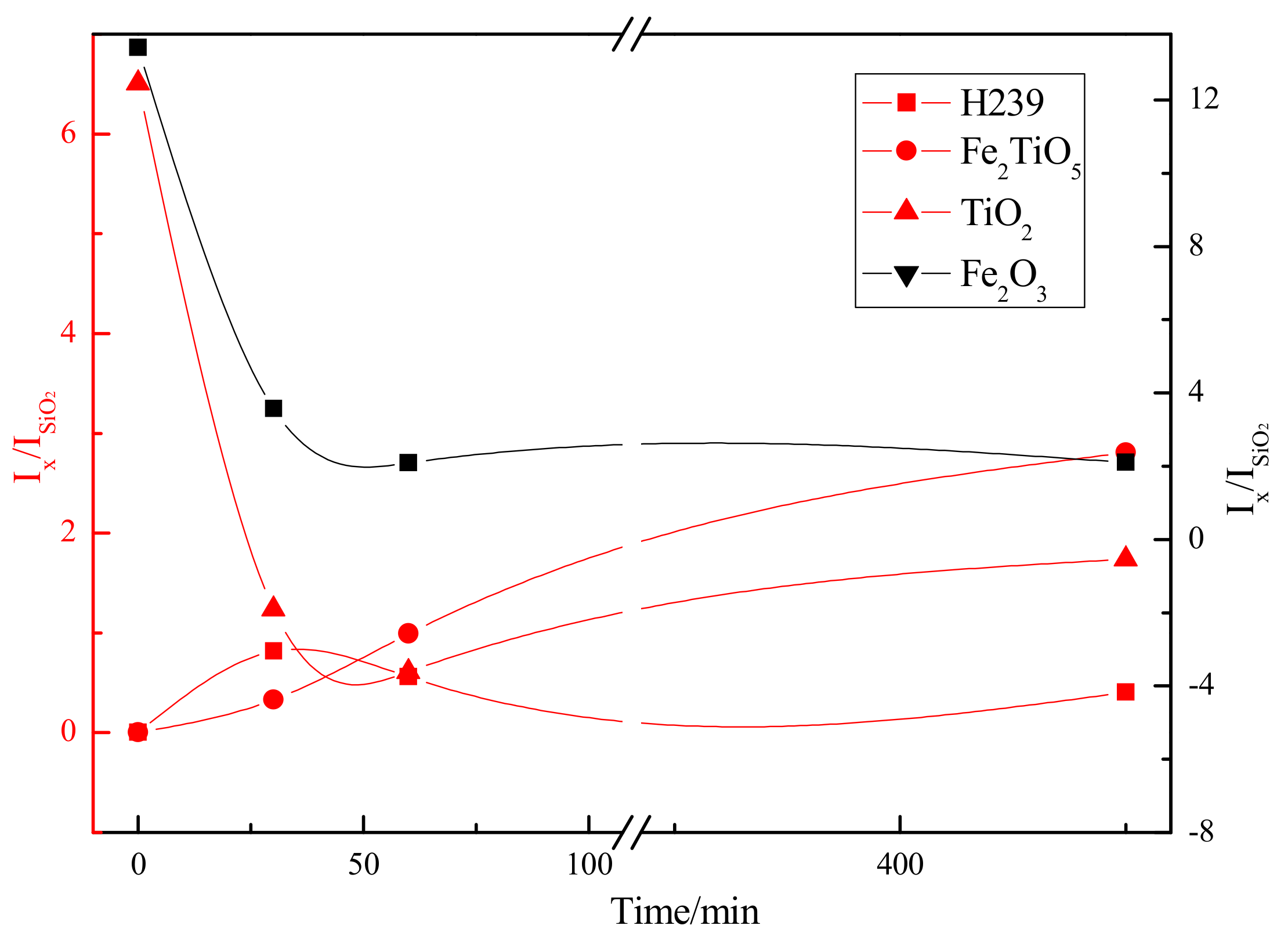
3.2. Chemical Transformation of Natural Ilmenite above 800 °C
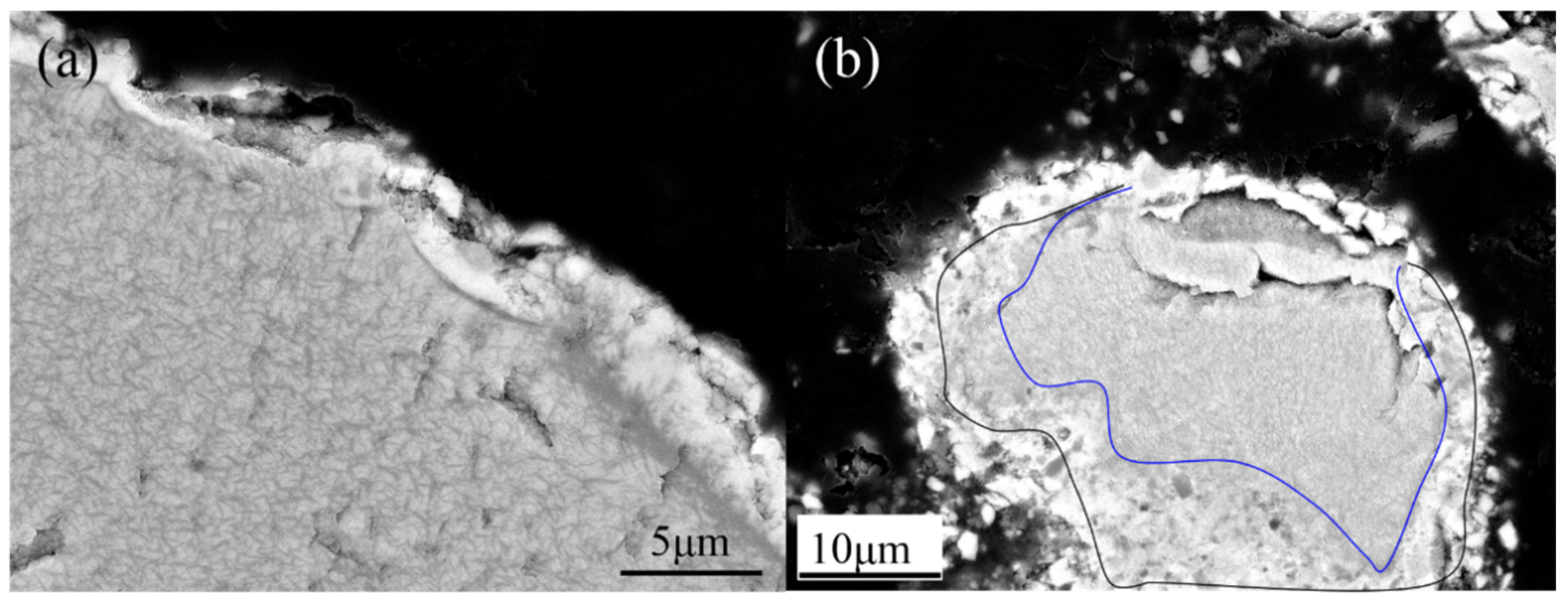
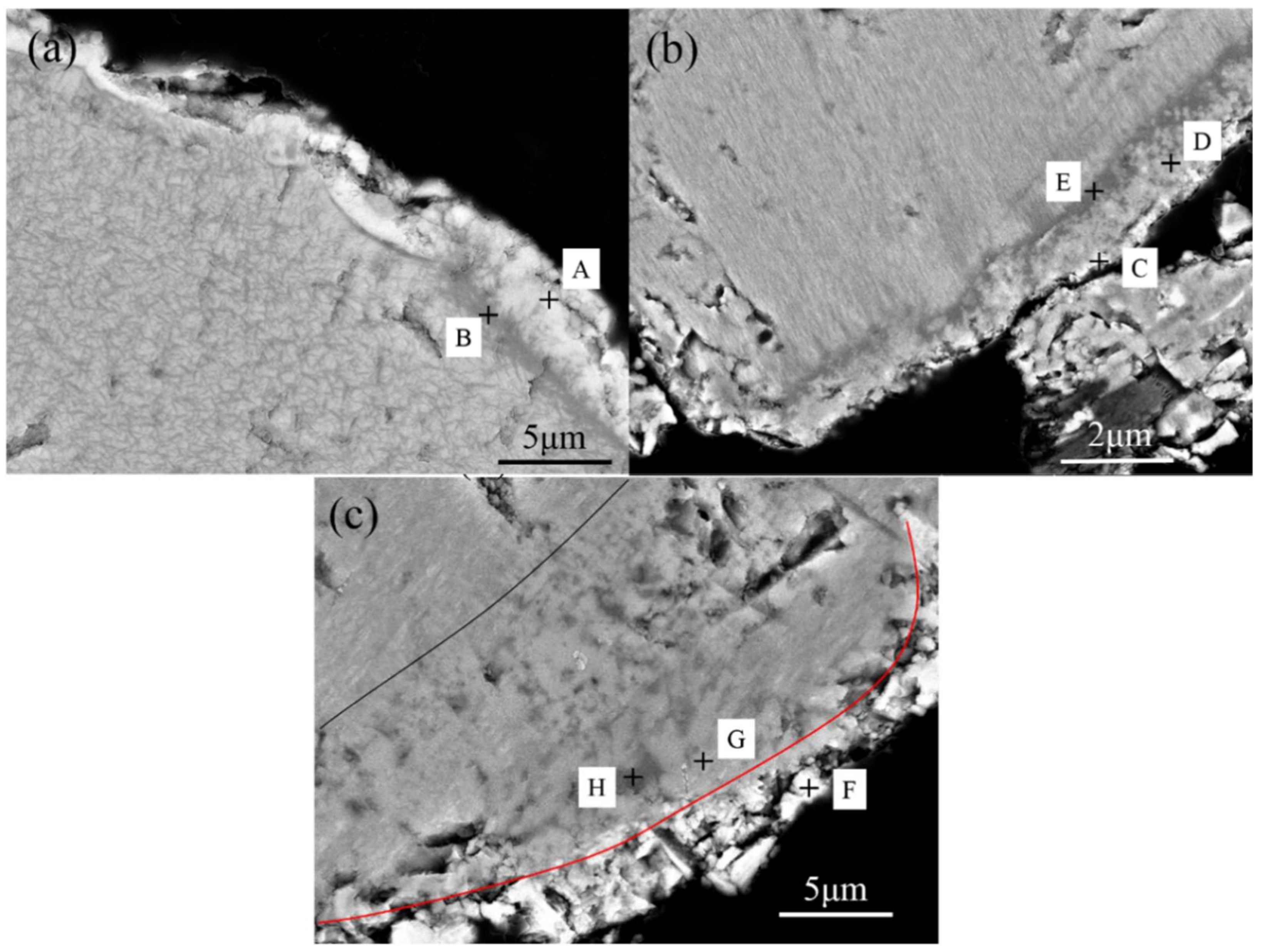
3.3. Microstructure Transition of Natural Ilmenite above 800 °C
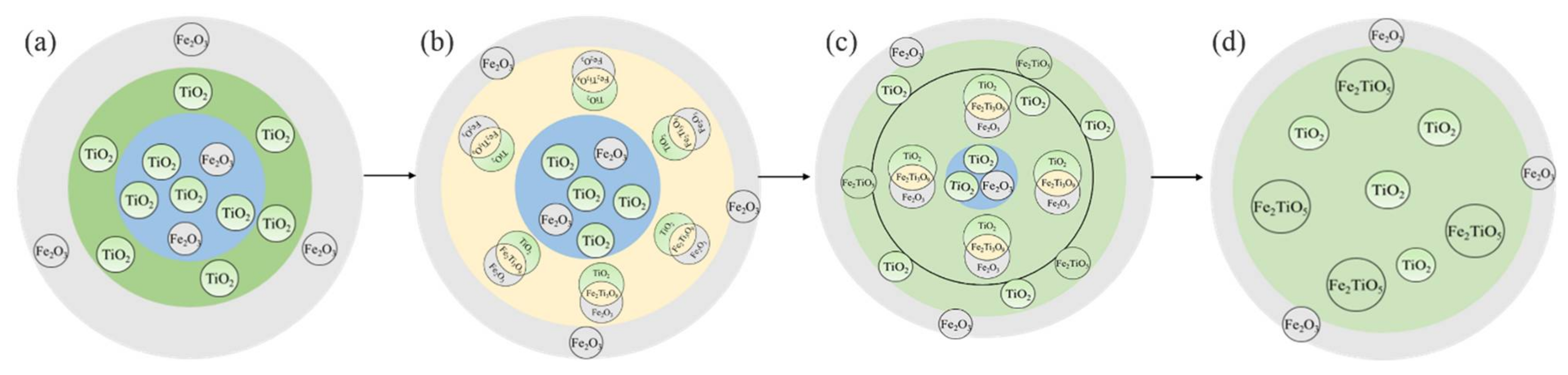
3.4. Chemical Transformation Mechanisms of Pseudorutile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Tang, A.; Xiao, X. Effect of milling time on carbothermic reduction of ilmenite. Trans. Nonferrous Met. Soc. China 2015, 25, 4201–4206. [Google Scholar] [CrossRef]
- Panigrahi, M.; Shibata, E.; Iizuka, A.; Nakamura, T. Production of Fe-Ti alloy from mixed ilmenite and titanium dioxide by direct electrochemical reduction in molten calcium chloride. Electrochim. Acta 2013, 93, 143–151. [Google Scholar] [CrossRef]
- Xiong, L.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Zhang, Q.; Zhou, Z.; Zhang, Y.; Ru, J. Effect of CaO addition on preparation of ferrotitanium from ilmenite by electrochemical reduction in CaCl2NaCl molten salt. J. Alloy. Compd. 2016, 676, 383–389. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, Y.; Xu, C.; Li, J.; Li, Y.; Gong, K.; Ru, J.; Xiong, L. Preparation of Ferrotitanium from Ilmenite by Electrolysis-Assisted Calciothermic Reduction in CaCl2-NaCl Molten Salt. Jom 2015, 68, 532–539. [Google Scholar] [CrossRef]
- Gao, Z.; Cheng, G.; Yang, H.; Xue, X.; Ri, J. Preparation of Ferrotitanium Using Ilmenite with Different Reduction Degrees. Metals 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Dou, Z.; Wang, C.; Fan, S.; Shi, G.; Zhang, T.-A. Al Control in High Titanium Ferro with Low Oxygen Prepared by Thermite Reaction. In The 6th International Symposium on High-Temperature Metallurgical Processing; Jiang, T., Hwang, J.-Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–17. [Google Scholar]
- Akhgar, B.; Pazouki, M.; Ranjbar, M.; Hosseinnia, A.; Salarian, R. Application of Taguchi method for optimization of synthetic rutile nano powder preparation from ilmenite concentrate. Chem. Eng. Res. Des. 2012, 90, 220–228. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Wang, Z.; Guo, H.; Wang, X.; Wu, F.; Fang, J.; Wang, Z.; Li, L. A novel process for producing synthetic rutile and LiFePO4 cathode material from ilmenite. J. Alloy. Compd. 2010, 506, 271–278. [Google Scholar] [CrossRef]
- Hiraki, T.; Maruyama, Y.; Suzuki, Y.; Itoh, S.; Nagasaka, T. Up-grading of natural ilmenite ore by combining oxidation and acid leaching. Int. J. Miner. Metall. Mater. 2018, 25, 729–736. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Li, G. Preparation of nano-titanium dioxide from ilmenite using sulfuric acid-decomposition by liquid phase method. Powder Technol. 2016, 287, 256–263. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Wang, Z.; Guo, H.; Wu, L.; Xiong, X.; Wang, X. Preparation of TiO2 nanosheets and Li4Ti5O12 anode material from natural ilmenite. Powder Technol. 2011, 213, 192–198. [Google Scholar] [CrossRef]
- Palliyaguru, L.; Kulathunga, U.S.; Jayarathna, L.I.; Jayaweera, C.D.; Jayaweera, P.M. A simple and novel synthetic route to prepare anatase TiO2 nanopowders from natural ilmenite via the H3PO4/NH3 process. Int. J. Miner. Metall. Mater. 2020, 27, 846–855. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, Z.; Wu, L.; Yue, P.; Guo, H.; Wu, F.; Ma, T. Preparation and characterization of Li4Ti5O12 from ilmenite. Powder Technol. 2010, 204, 198–202. [Google Scholar] [CrossRef]
- Deng, Y.; Xing, M.; Zhang, J. An advanced TiO2 /Fe2 TiO5 /Fe 2O3 triple-heterojunction with enhanced and stable visible-light-driven fenton reaction for the removal of organic pollutants. Appl. Catal. B Environ. 2017, 211, 157–166. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, C. Electronic structures and optic properties of Fe2TiO5 using LSDA+U approach. Prog. Nat. Sci. Mater. Int. 2013, 23, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Zeinali Moghaddam, H.; Sharifitabar, M.; Roudini, G. Microstructure and wear properties of Fe–TiC composite coatings produced by submerged arc cladding process using ferroalloy powder mixtures. Surf. Coat. Technol. 2019, 361, 91–101. [Google Scholar] [CrossRef]
- Gou, H.-P.; Zhang, G.-H.; Chou, K.-C. Influence of Pre-oxidation on Carbothermic Reduction Process of Ilmenite Concentrate. Isij Int. 2015, 55, 928–933. [Google Scholar]
- Zhang, J.; Zhu, Q.; Xie, Z.; Lei, C.; Li, H. Morphological changes of Panzhihua ilmenite during oxidation treatment. Metall. Mater. Trans. B 2013, 44, 897–905. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Wei, F. Phase Transitions and Reaction Mechanism of Ilmenite Oxidation. Metall. Mater. Trans. A 2010, 41, 1338–1348. [Google Scholar] [CrossRef]
- Teufer, G.; Temple, A. Pseudorutile—a new mineral intermediate between ilmenite and rutile in the N alteration of ilmenite. Nature 1966, 211, 179. [Google Scholar] [CrossRef]
- Lv, W.; Lv, X.; Zhang, Y.; Li, S.; Tang, K.; Song, B. Isothermal oxidation kinetics of ilmenite concentrate powder from Panzhihua in air. Powder Technol. 2017, 320, 239–248. [Google Scholar]
- Zhang, G.; Ostrovski, O. Effect of preoxidation and sintering on properties of ilmenite concentrates. Int. J. Miner. Process. 2002, 64, 201–218. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajakumar, V.; Grieveson, P. Phase transformations during heating of llmenite concentrates. Metall. Trans. B 1991, 22, 711–716. [Google Scholar] [CrossRef]
- Rao, D.B.; Rigaud, M. Kinetics of the oxidation of ilmenite. Oxid. Met. 1975, 9, 99–116. [Google Scholar]
- Xiao, W.; Lu, X.-G.; Zou, X.-L.; Wei, X.-M.; Ding, W.-Z. Phase transitions, micro-morphology and its oxidation mechanism in oxidation of ilmenite (FeTiO3) powder. Trans. Nonferrous Met. Soc. China 2013, 23, 2439–2445. [Google Scholar] [CrossRef]
- Grey, I.; Reid, A. Shear structure compounds (Cr, Fe)2Tin−2O2n−1 derived from the α-PbO2 structural type. J. Solid State Chem. 1972, 4, 186–194. [Google Scholar]
- Lin, F.; Song, H.; Tian, S.; Chen, X.; Zhou, J.; Wang, F. Fe1.5Ti0.5O3 nanoparticles as an anode material for lithium-ion batteries. Electrochim. Acta 2012, 83, 305–310. [Google Scholar] [CrossRef]
- Ren, Z.-S.; Hu, X.-J.; Li, S.-Y.; Xue, X.-X.; Chou, K.-C. Interdiffusion in the Fe2O3-TiO2 system. Int. J. Miner. Metall. Mater. 2013, 20, 273–278. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, D.; Song, S.; Cheng, G.; Xue, X. Selective leaching of vanadium over iron from vanadium slag. J. Hazard. Mater. 2019, 368, 300–307. [Google Scholar]
- Zhang, H.-Q.; Fu, J.-T. Oxidation behavior of artificial magnetite pellets. Int. J. Miner. Metall. Mater. 2017, 24, 603–610. [Google Scholar] [CrossRef]
- Surzhikov, A.P.; Frangulyan, T.S.; Ghyngazov, S.A.; Lysenko, E.N. Investigation of oxidation processes in non-stoichiometric lithium–titanium ferrites using TG analysis. J. Therm. Anal. Calorim. 2010, 102, 883–887. [Google Scholar]
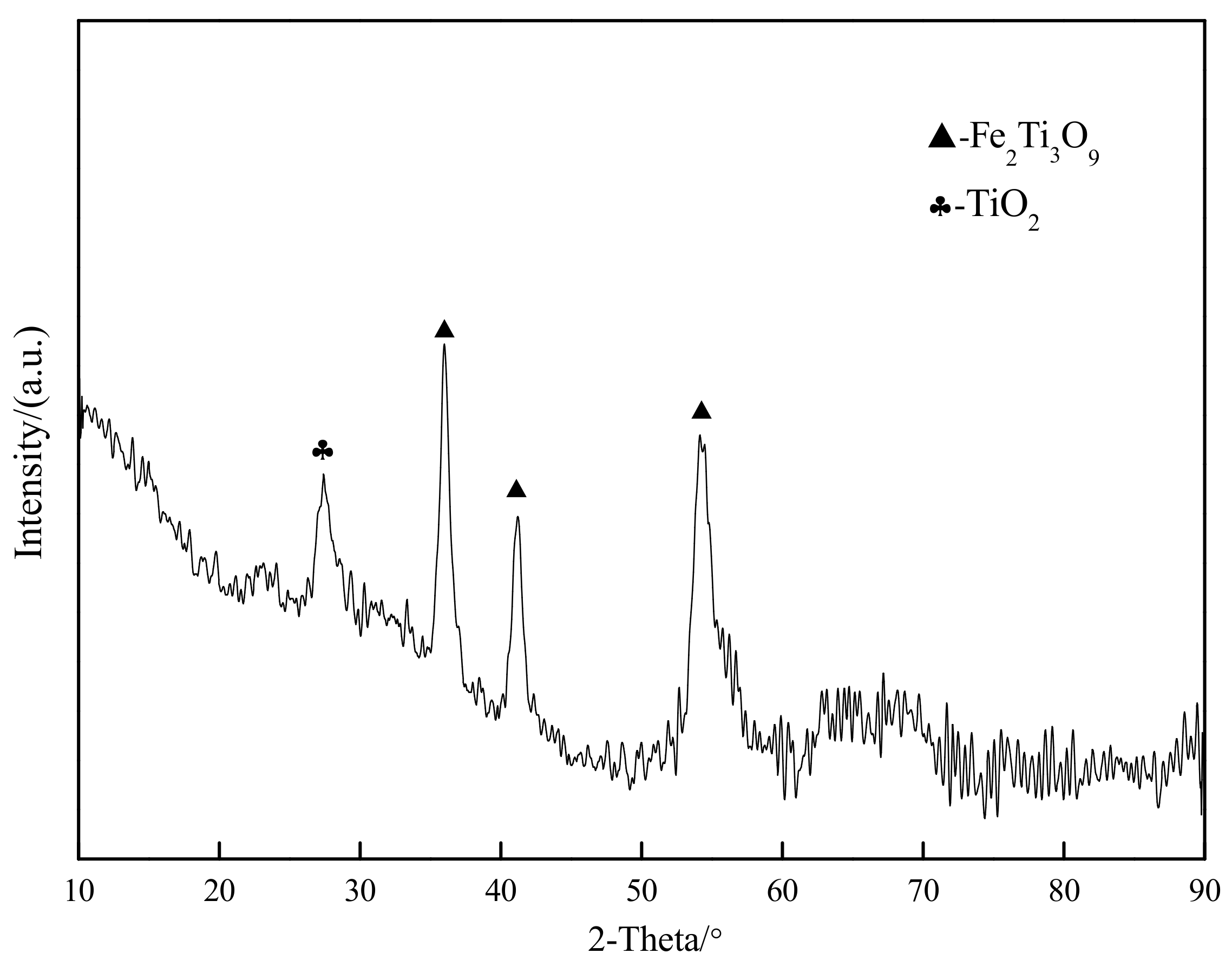
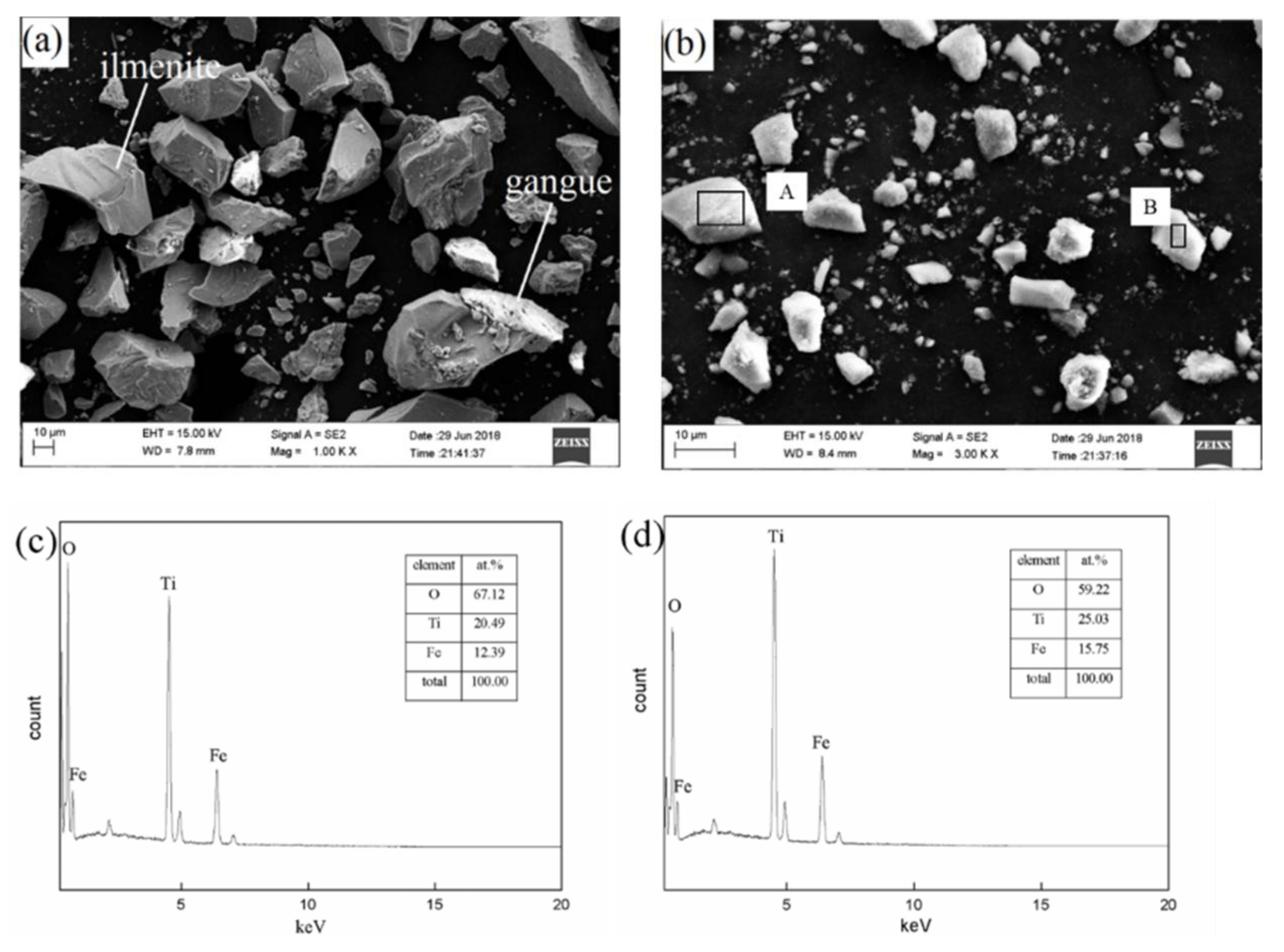
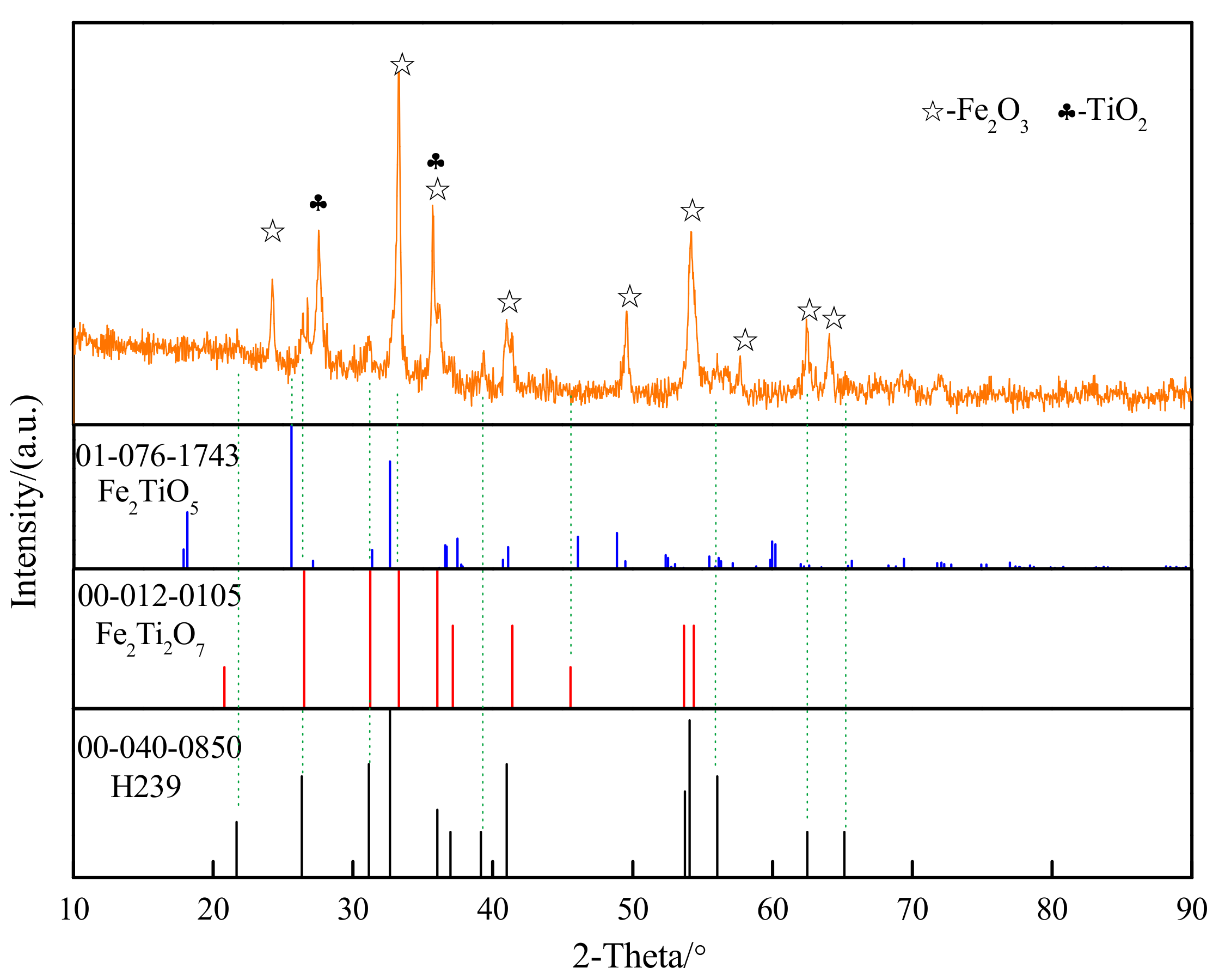
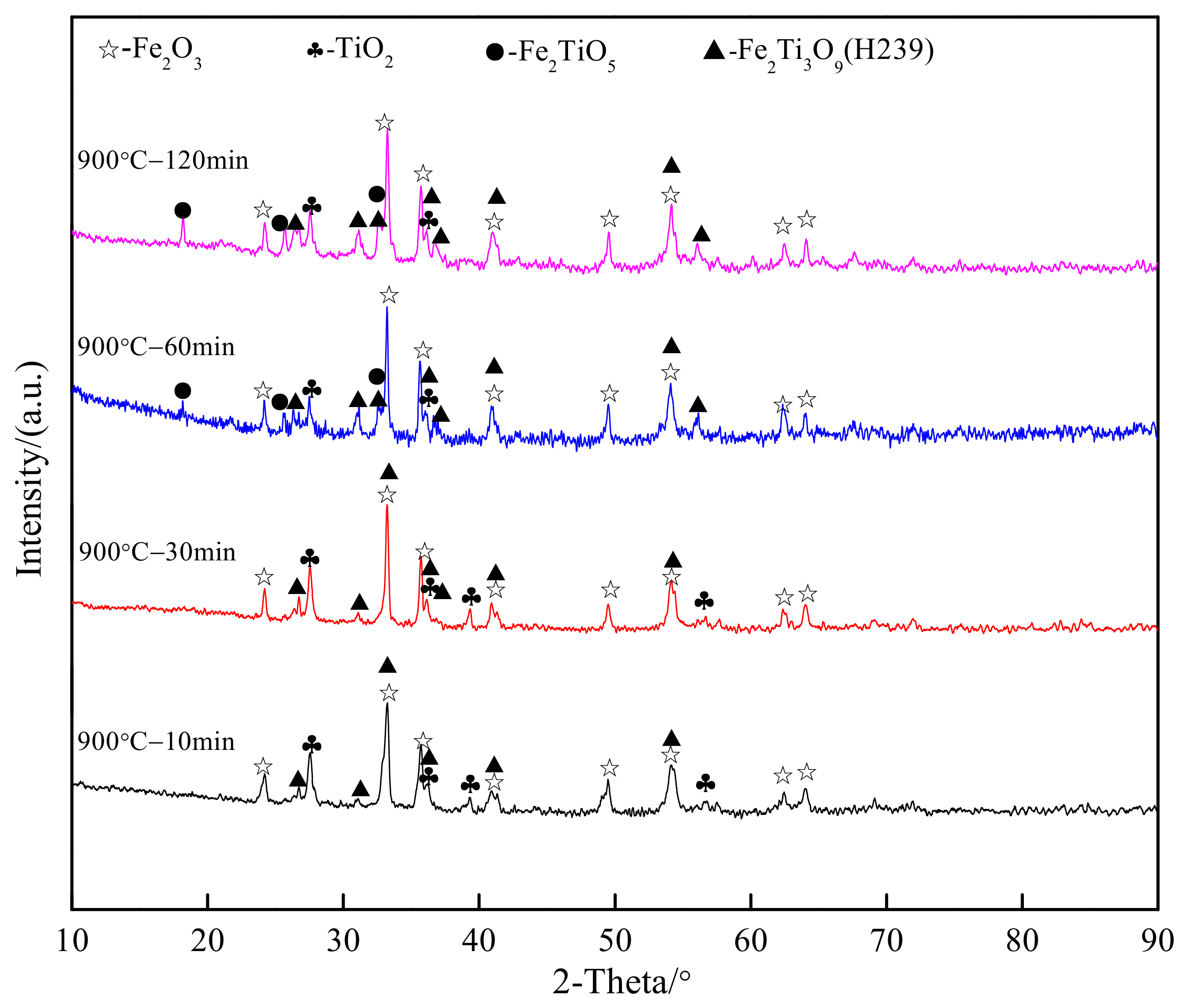

| TFe | FeO | CaO | SiO2 | MgO | Al2O3 | TiO2 | MnO | V2O3 | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| 34.52 | 26.85 | 0.86 | 5.11 | 0.95 | 1.06 | 44.44 | 0.65 | 0.28 | 0.024 | 0.006 |
| Point | Fe | Ti | O |
|---|---|---|---|
| A | 28.67 | 4.01 | 67.32 |
| B | 5.38 | 30.00 | 64.62 |
| C | 33.84 | 6.84 | 59.32 |
| D | 15.15 | 20.89 | 63.96 |
| E | 3.63 | 31.19 | 65.19 |
| F | 31.67 | 5.60 | 62.72 |
| G | 22.35 | 16.03 | 61.62 |
| H | 6.59 | 32.23 | 61.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Gao, Z.; Yang, S.; Yang, H.; Xue, X. Microstructure and Chemical Transformation of Natural Ilmenite during Isothermal Roasting Process in Air Atmosphere. Minerals 2021, 11, 137. https://doi.org/10.3390/min11020137
Cheng G, Gao Z, Yang S, Yang H, Xue X. Microstructure and Chemical Transformation of Natural Ilmenite during Isothermal Roasting Process in Air Atmosphere. Minerals. 2021; 11(2):137. https://doi.org/10.3390/min11020137
Chicago/Turabian StyleCheng, Gongjin, Zixian Gao, Songtao Yang, He Yang, and Xiangxin Xue. 2021. "Microstructure and Chemical Transformation of Natural Ilmenite during Isothermal Roasting Process in Air Atmosphere" Minerals 11, no. 2: 137. https://doi.org/10.3390/min11020137
APA StyleCheng, G., Gao, Z., Yang, S., Yang, H., & Xue, X. (2021). Microstructure and Chemical Transformation of Natural Ilmenite during Isothermal Roasting Process in Air Atmosphere. Minerals, 11(2), 137. https://doi.org/10.3390/min11020137





