Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Mix Design
2.2.1. Sample Nomenclature
2.2.2. Preparation of Geopolymer Mortar Samples
2.3. Testing Methods
2.3.1. Mechanical Testing
2.3.2. Setting Time
2.4. Sample Characterisation
2.4.1. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometry (EDS)
2.4.2. X-ray Diffraction (XRD)
2.4.3. Nuclear Magnetic Resonance (NMR)
3. Results and Discussion
4. Summary and Conclusions
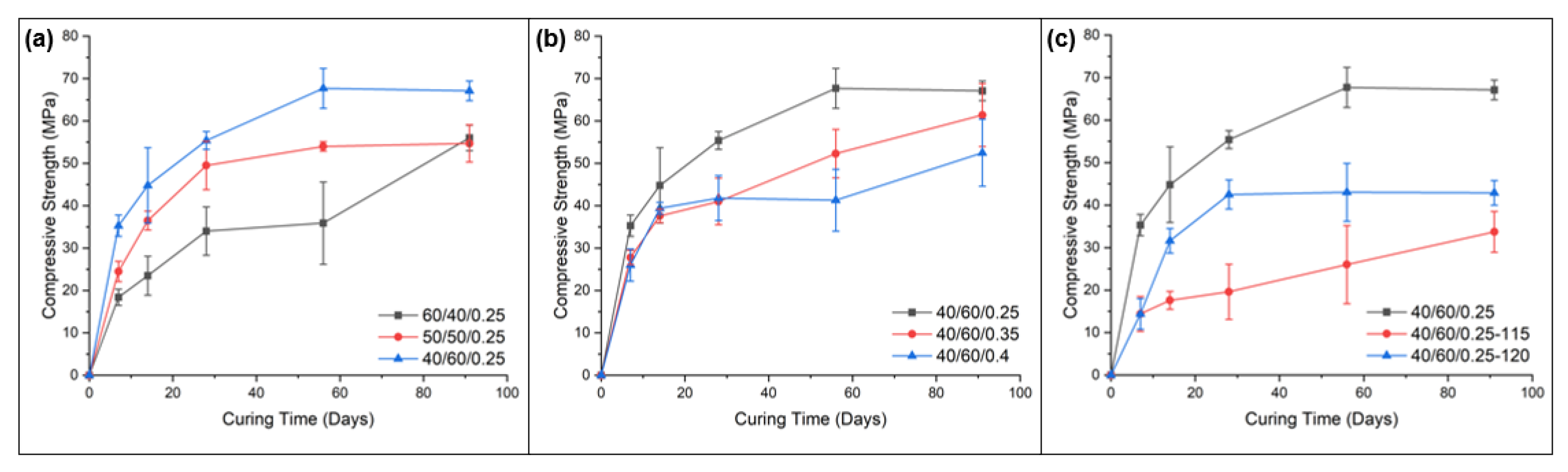
- Owing to the higher reactivity of the GGBFS to the FA, the initial and final setting times were shorter for compositions with higher GGBFS contents compared to those with higher FA content.
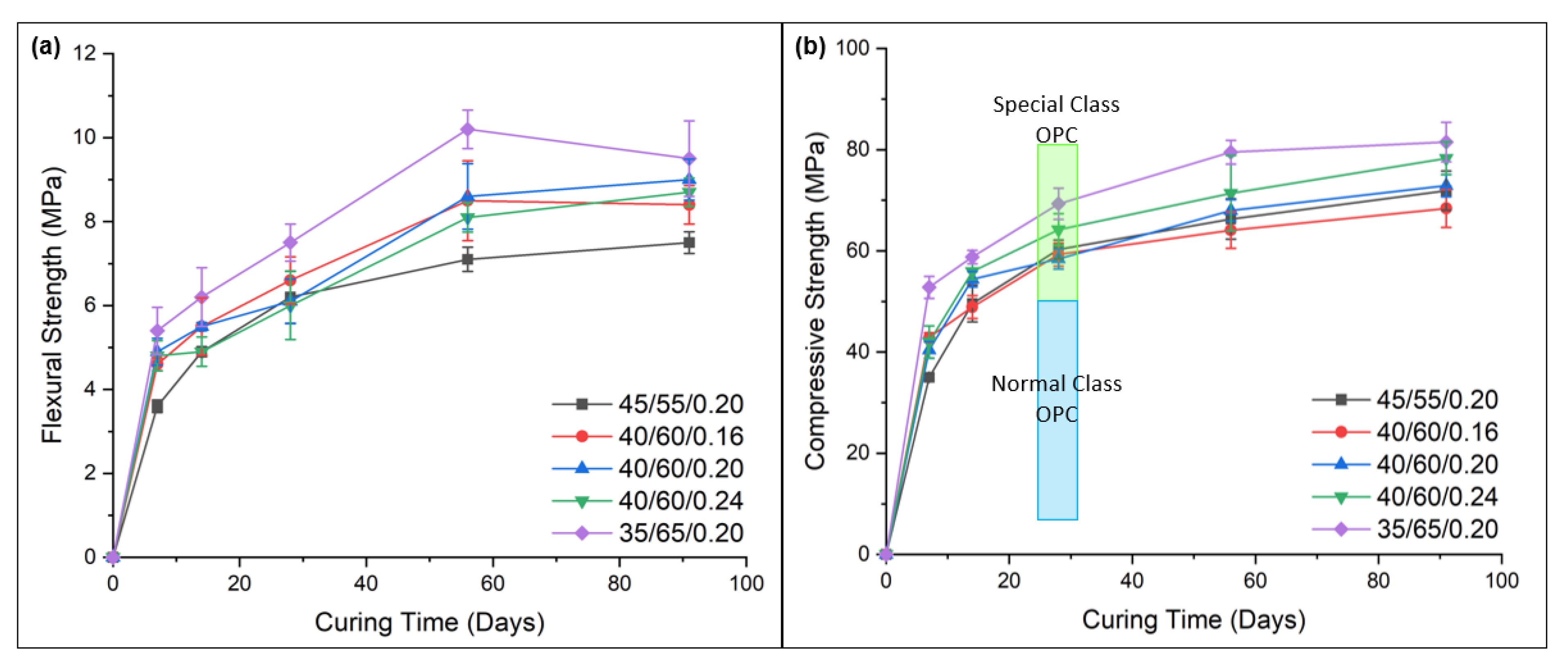
- Powder activators in pre-dry mixed geopolymer cements helped to produce mortars with flexural and compressive strengths of up to ~9 MPa and ~80 MPa at 91-days, respectively. The compressive strength values were superior to the OPC control sample, which showed ~55 MPa at 91-days. Thus, these geopolymers activated using solid sodium metasilicate were observed to be a viable alternative to conventional OPC.
- Mixes with a higher ratio of GGBFS had a greater bulk density, with reduced porosity due to inferior particle packing, which in turn increased its compressive and flexural strength.
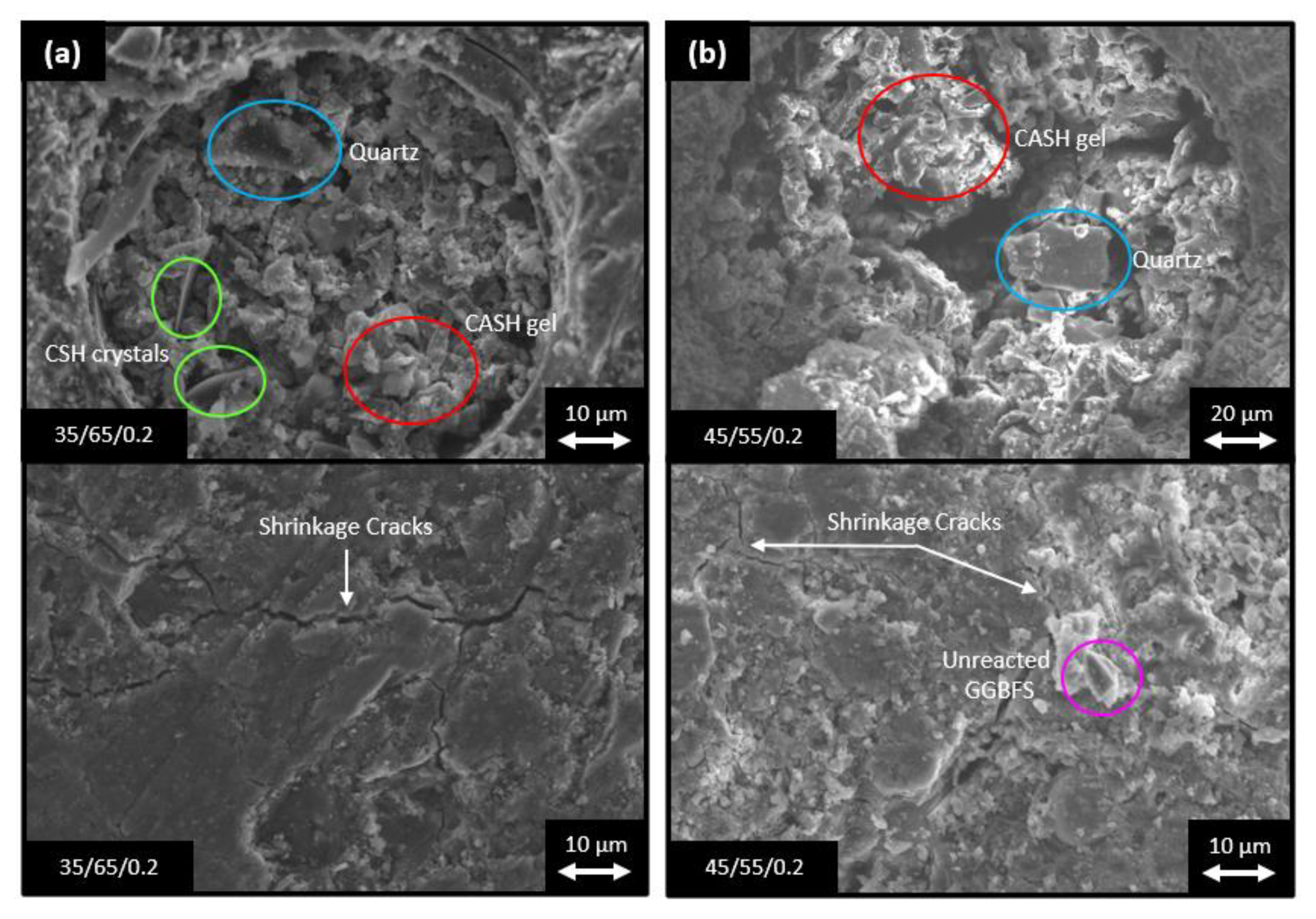
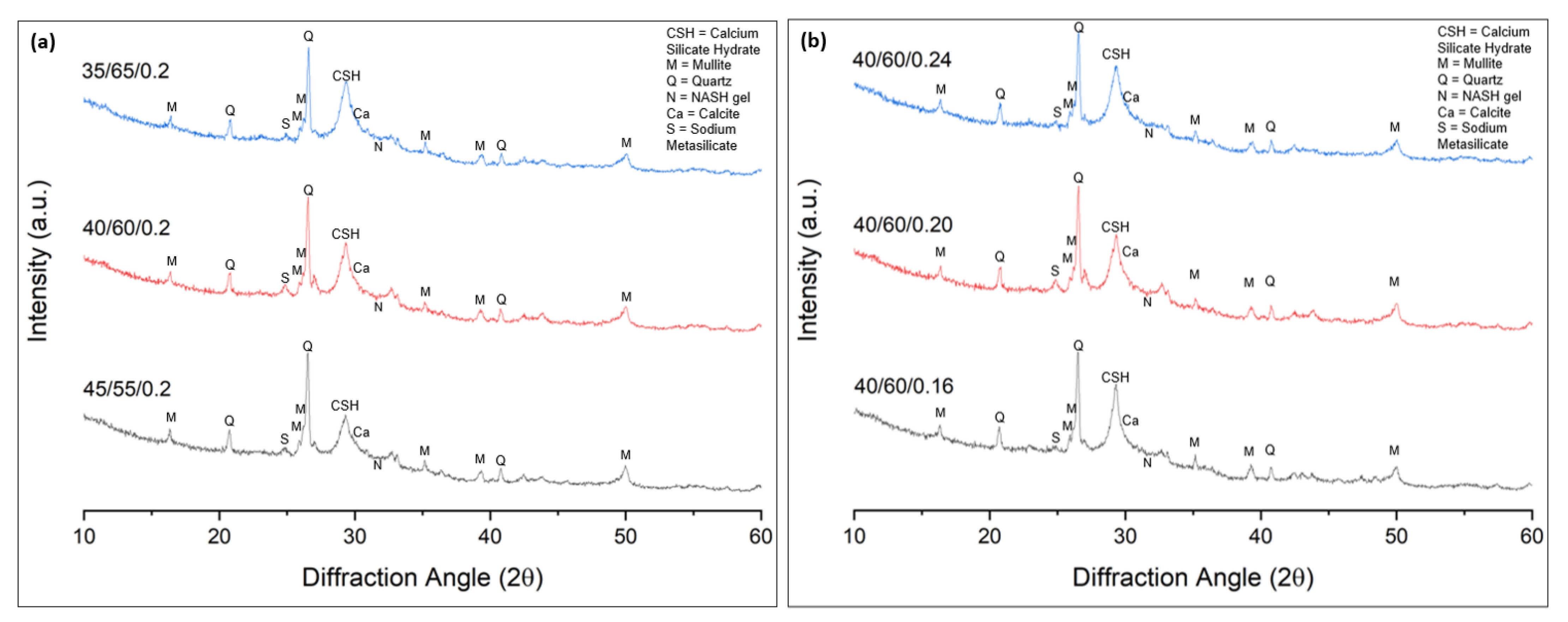
- All samples displayed similar mineralogical constituents at all tested curing points, but with slight differences in their amounts. Higher intensities of the CSH gel were observed with increase in the GGBFS content and Na2SiO3 content in the samples, and a slightly larger hump of the NASH gel was observed with increased FA content and decreased Na2SiO3 content.
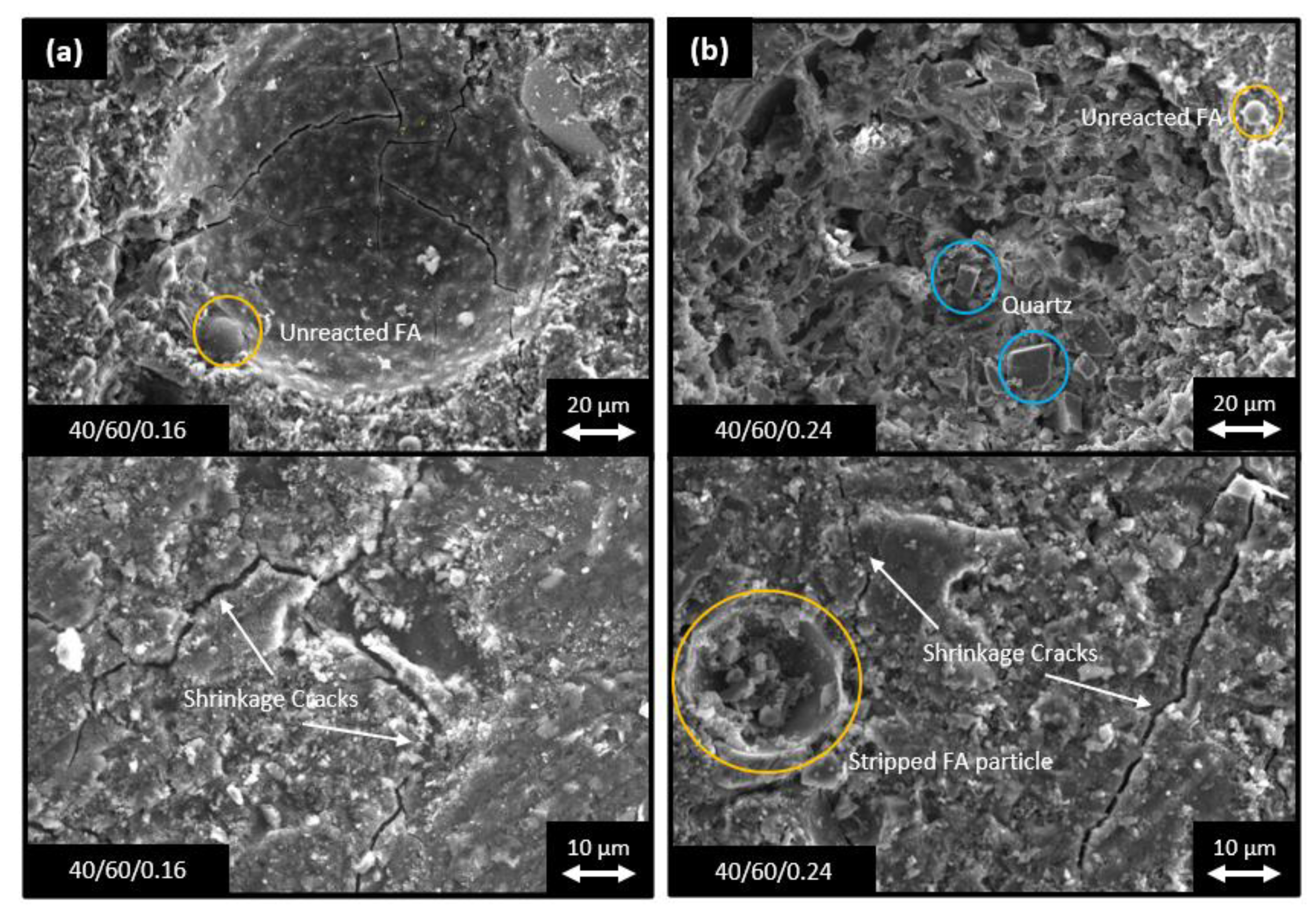
- High GGBFS-containing compositions produced higher mechanical strengths than those with higher FA contents. Samples with high GGBFS proportions in the binders exhibited significantly greater early age compressive strengths, i.e., 52.8 MPa for 35/65/0.2 at 7-days curing vs. 35.0 MPa for 45/55/0.2 for the same curing time. This was attributed to the smaller particle sizes and higher CaO content of the GGBFS, which contributed to higher reactivity and greater density and homogeneity of the matrix. However, higher amounts of slag resulted in increased numbers of fine cracks being present from rapid gel formation.
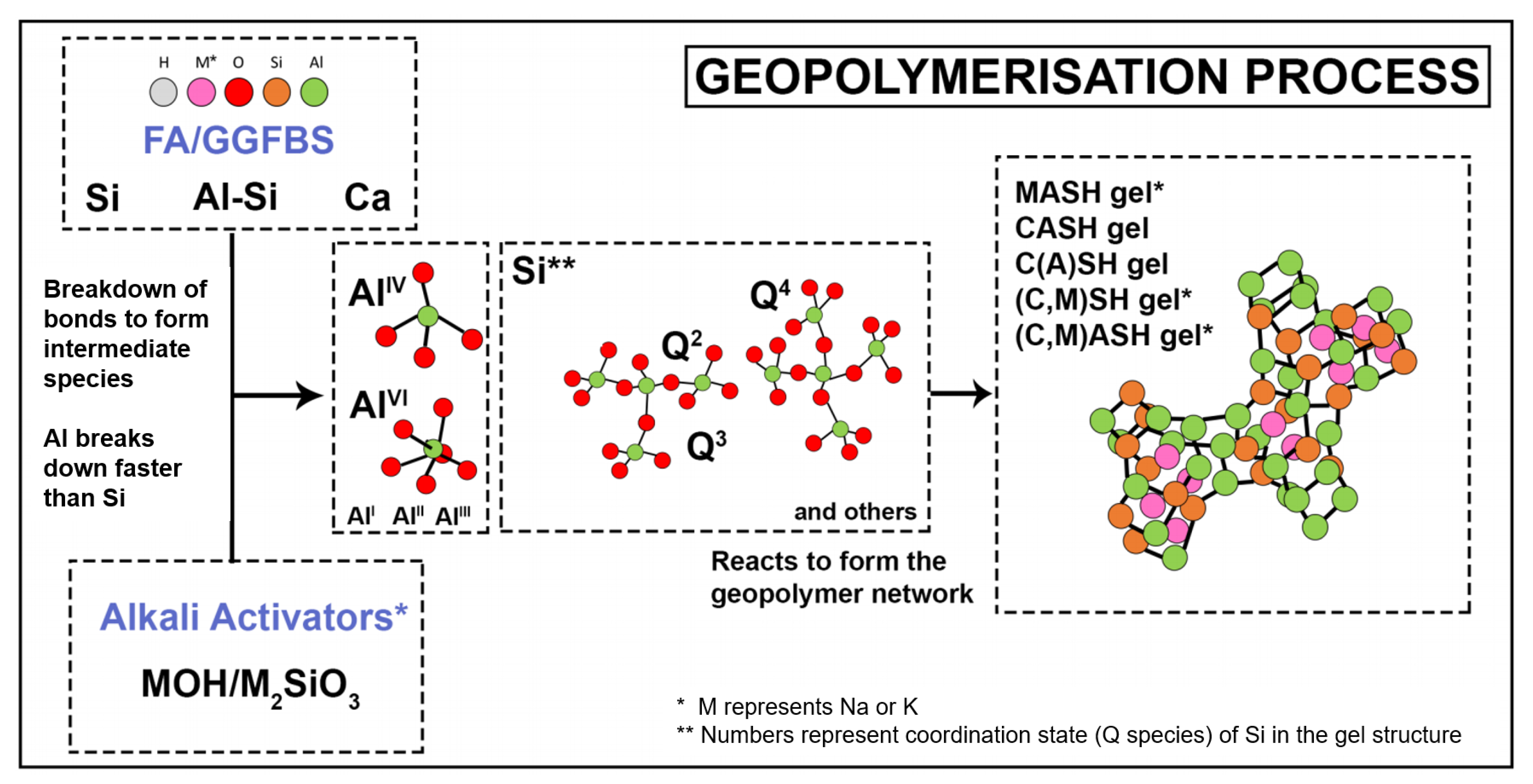
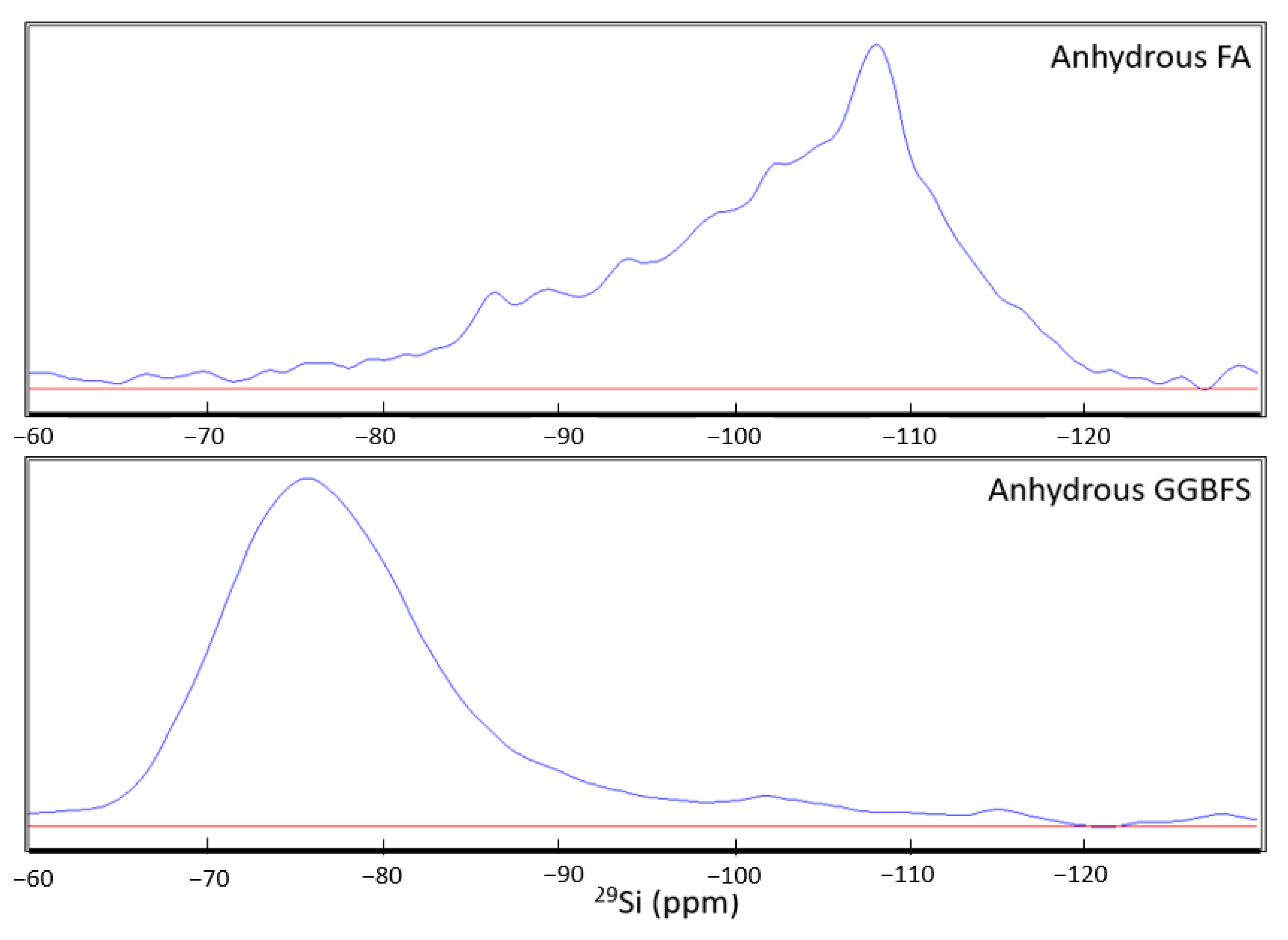
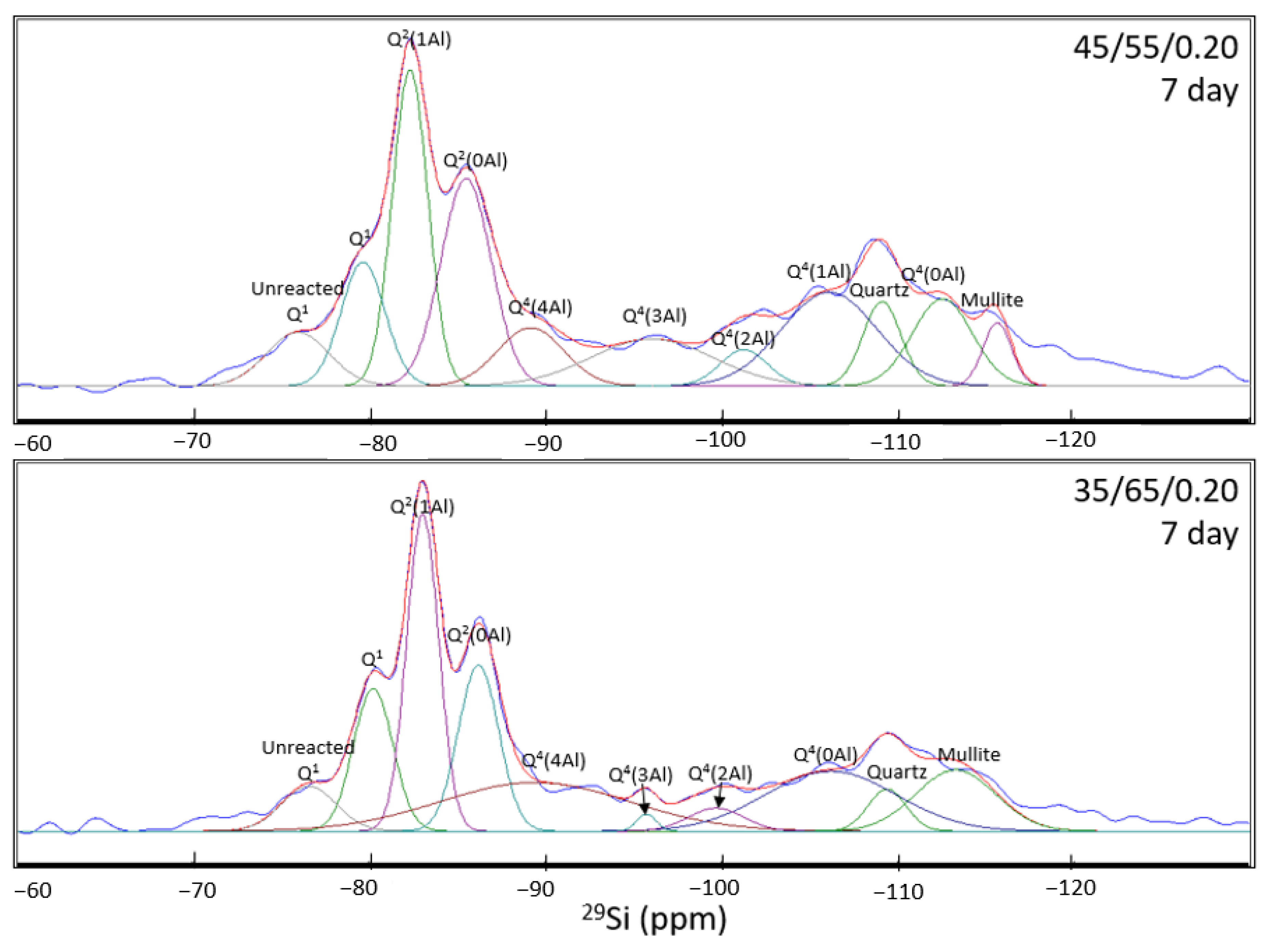
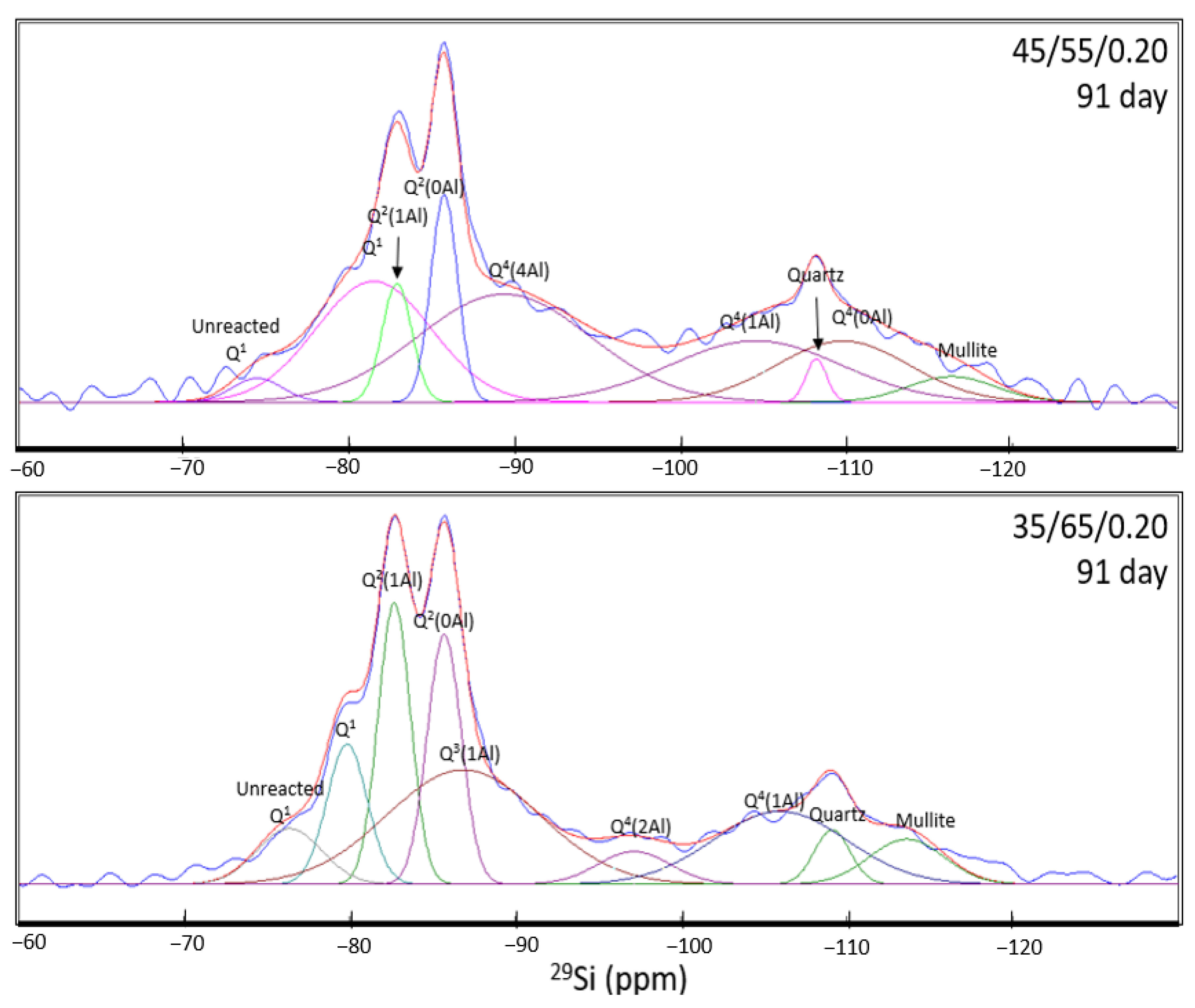
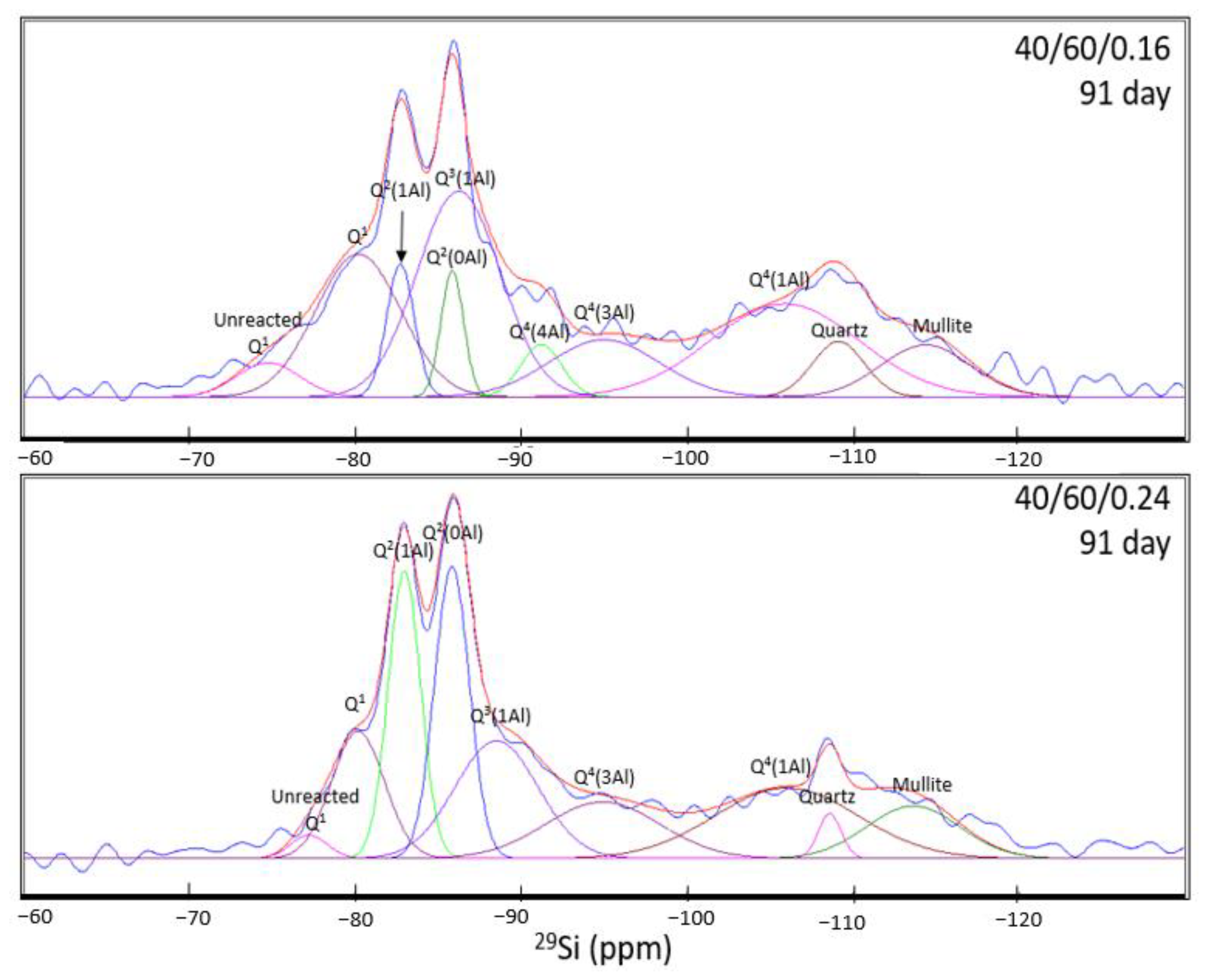
- NMR analysis revealed that the anhydrous GGBFS consists mainly of low-Q silicate species, whilst anhydrous FA consists mainly of high-Q silicate species. As a result, the FA is expected to have a much slower activation rate than the GGBFS. Therefore, through the deconvolution of the spectra for the 7-day and 91-day cured samples, higher slag content and higher amounts of metasilicate activator increased the proportion of CASH gel formed owing to the faster reactions of the slag and the effect of the sodium silicate in opening the CSH structure to allow for Al incorporation. NASH gel formation occurred at a slower rate and contributed to late-age strength development. Furthermore, the NASH gel was observed to be Al-deficient owing to the competing Al incorporation in the CASH gel network.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemmings, R.T.; Berry, E.E. On the Glass in Coal Fly Ashes: Recent Advances; McCarthy, G.J., Glasser, F.P., Roy, D.M., Hemmings, R.T., Eds.; Cambridge University Press: Cambridge, UK, 1987; p. 113. [Google Scholar]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM C618-19. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Kosmatka, S.; Kerkhoff, B.; Panarese, W. Chapter 3: Fly ash, slag, silica fume, and natural pozzolans. In Design and Control of Concrete Mixtures; Portland Cement Association: Skokie, IL, USA, 2002; pp. 57–72. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Stutzman, P.E.; Centeno, L. Compositional Analysis of Beneficiated Fly Ashes; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1995.
- Yeoh, M.L.Y.; Ukritnukun, S.; Rawal, A.; Davies, J.; Kang, B.J.; Burrough, K.; Aly, Z.; Dayal, P.; Vance, E.R.; Gregg, D.J.; et al. Mechanistic Impacts of long-term gamma irradiation on physicochemical, structural, and mechanical stabilities of radiation-responsive geopolymer pastes. J. Hazard. Mater. 2002, 407, 124805. [Google Scholar] [CrossRef] [PubMed]
- Ukritnukun, S.; Koshy, P.; Rawal, A.; Castel, A.; Sorrell, C.C. Predictive model of setting times and compressive strengths for low-alkali, ambient-cured, fly ash/slag-based geopolymers. Minerals 2020, 10, 920. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Improvement of early strength of fly ash-slag based one-part alkali activated mortar. Constr. Build. Mater. 2020, 246, 118533. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Kovalchuk, G.U.; Ordoñez, L.M.; Naranjo, M.C. OPC-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 2007, 42, 2958–2966. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes, 1st ed.; CRC Press: London, UK, 2003. [Google Scholar]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of one-part geopolymer binders made from fly ash. Waste Biomass Valorization 2017, 8, 225–233. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K. Workability loss and compressive strength development of cementless mortars activated by combination of sodium silicate and sodium hydroxide. J. Mater. Civil Eng. 2009, 21, 119–127. [Google Scholar] [CrossRef]
- Yahya, Z.; Abdullah, M.M.A.B.; Kamarudin, H.; Nizar, K.; Razak, R.A.; Sandu, A.V. Effect of solids to liquids, Na2SiO3 to NaOH and curing temperature on the palm oil boiler ash (Si + Ca) geopolymerisation system. Materials 2015, 8, 2227–2242. [Google Scholar] [CrossRef]
- Provis, J. Chapter 4: Activating solution chemistry for geopolymers. In Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing Series in Civil and Structural Engineering; Elsevier Science B.V.: Amsterdam, The Netherlands, 2009; pp. 50–71. [Google Scholar]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels: Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels: FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernandez-Jimeneza, A. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef] [Green Version]
- VUTChempoint. Geopolymerisation Mechanism. 2011. Available online: https://www.youtube.com/watch?v=_BX8E0IEkVI (accessed on 20 June 2020).
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K.; Ashour, A.F.; Le, E.T. Properties of cementless mortars activated by sodium silicate. Constr. Build. Mater. 2008, 22, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.H.; Song, J.K.; Lee, K.S.; Ashour, A.F. Flow and compressive strength of alkali-activated mortars. ACI Mater. J. 2009, 106, 50–58. [Google Scholar]
- Yang, K.H.; Song, J.K.; Lee, J.S. Properties of alkali-activated mortar and concrete using lightweight aggregates. Mater. Struct. 2010, 43, 403–416. [Google Scholar] [CrossRef]
- ASTM C348-20. Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C109/109M-20a. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C191-19. Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; le Calvé, S.; Alonso, B.; Durand, J.O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- AS 3972. General Purpose and Blended Cements; Standards Australia: Sydney, Australia, 2010. [Google Scholar]
- Datasheet—Specifying Concrete; Cement, Concrete and Aggregates Australia (CCAA): Mascot, Australia, 2017; pp. 1–4.
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]


| Binder | Aggregate Type | FA:BFS Ratio | (Na2O)x•(SiO2)y /Binder Ratio | NaOH/Binder Ratio | Silicate Modulus Ms | H2O/Binder Ratio | Curing Temp. (°C) | 7 d UCS [MPa] | 28 d UCS [MPa] | 56 d UCS [MPa] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | None | 100:0 | 0.377 | 0.066 | N/A | 0.490 | 40 | 42.0 | 57.0 | -- | [14] |
| FA | Sand | 100:0 | 0.155 | 0.002 | 0.90 | 0.603 | 23 | 1.5 | 3.5 | 5.0 | [15] |
| BFS | Sand | 0:100 | 0.155 | 0.002 | 0.90 | 0.586 | 23 | 38.5 | 49.6 | 58.4 | |
| FA | Sand | 100:0 | 0.485 | -- | 0.90 | 0.742 | 23 | 3.2 | 9.5 | 14.0 | [23] |
| BFS | Sand | 0:100 | 0.485 | -- | 0.90 | 0.742 | 23 | 47.1 | 51.3 | 58.4 | |
| FA | Sand | 100:0 | N/A | N/A | 0.75 | 0.500 | 23 | 0.5 | 2.20 | 2.5 | [24] |
| BFS | Sand | 0:100 | N/A | N/A | 0.75 | 0.500 | 23 | 42.1 | 48.6 | 51.5 | |
| BFS | Sand | 0:100 | N/A | -- | 0.90 | 0.500 | 23 | 48.1 | 61.1 | 62.8 | |
| BFS | Clay Granule | 0:100 | 0.215 | -- | 0.90 | 0.487 | 23 | 49.3 | 53.8 | 62.1 | [25] |
| FA/BFS | Sand | 50:50 | 0.100 | -- | 0.92 | 0.300 | 30 | ~62.0 | ~83 | -- | [9] |
| FA/BFS | Sand | 60:40 | 0.100 | -- | 0.92 | 0.300 | 20 | ~55.0 | ~80 | -- |
| Elemental Oxide (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | CaO | TiO2 | Na2O | MgO | P2O5 | L.O.I |
| 69.8 | 19.78 | 2.68 | 1.54 | 1.44 | 0.79 | 0.55 | 0.47 | 0.2 | 2.32 |
| Elemental Oxide (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | TiO2 | SO3 | Fe2O3 | Mn3O4 | K2O | Na2O | BaO | Wt. Gain |
| 41.49 | 35.28 | 14.69 | 5.76 | 1.25 | 1.01 | 0.5 | 0.42 | 0.28 | 0.24 | 0.1 | 0.12 |
| Material | Avg. Particle Size (D[3,2], µm) | Avg. Particle Size (D[4,3], µm) | D10 (µm) | D50 (µm) | D90 (µm) |
|---|---|---|---|---|---|
| FA | 7.8 | 37.0 | 4.5 | 22.8 | 83.7 |
| GGBFS | 4.4 | 14.9 | 1.7 | 11.7 | 30.9 |
| Series A | FA (g) | GGBFS (g) | Na2SiO3 (g) | H2O (g) | Sand (g) |
|---|---|---|---|---|---|
| 60/40/0.25 | 150 | 100 | 62.5 | 150 | 500 |
| 60/40/0.35 | 150 | 100 | 87.5 | 150 | 500 |
| 60/40/0.4 | 150 | 100 | 100 | 150 | 500 |
| 50/50/0.25 | 125 | 125 | 62.5 | 150 | 500 |
| 50/50/0.35 | 125 | 125 | 87.5 | 150 | 500 |
| 50/50/0.4 | 125 | 125 | 100 | 150 | 500 |
| 40/60/0.25 | 100 | 150 | 62.5 | 150 | 500 |
| 40/60/0.35 | 100 | 150 | 87.5 | 150 | 500 |
| 40/60/0.4 | 100 | 150 | 100 | 150 | 500 |
| 40/60/0.25–115 | 100 | 150 | 62.5 | 165 | 500 |
| 40/60/0.25–120 | 100 | 150 | 62.5 | 170 | 500 |
| 40/60/0.35–115 | 100 | 150 | 87.5 | 165 | 500 |
| 40/60/0.35–120 | 100 | 150 | 87.5 | 170 | 500 |
| 40/60/0.4–115 | 100 | 150 | 100 | 165 | 500 |
| 40/60/0.4–120 | 100 | 150 | 100 | 170 | 500 |
| Series B | FA (g) | GGBFS (g) | Na2SiO3 (g) | H2O (g) | Sand (g) |
|---|---|---|---|---|---|
| 45/55/0.2 | 112.5 | 137.5 | 50 | 126 | 500 |
| 40/60/0.16 | 100 | 150 | 40 | 126 | 500 |
| 40/60/0.2 | 100 | 150 | 50 | 126 | 500 |
| 40/60/0.24 | 100 | 150 | 60 | 126 | 500 |
| 35/65/0.2 | 87.5 | 162.5 | 50 | 126 | 500 |
| ID (7 day) | Raw Slag | CASH | NASH | Quartz | Mullite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45/55/0.2 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −75.96 | −79.66 | −82.33 | −85.51 | - | −89.18 | −96.05 | −101.27 | −106.12 | −112.55 | −109.12 | −115.66 | |||
| FWHM | 4.16 | 2.92 | 2.37 | 3.37 | - | 4.39 | 8.12 | 3.17 | 6.39 | 3.98 | 2.54 | 1.89 | |||
| Intensity | 14.33 | 33.21 | 84.80 | 55.63 | - | 57.88 | 15.59 | 12.55 | 9.66 | 25.09 | 24.34 | 17.25 | 22.57 | 16.94 | |
| Integral (%) | 5.48 | 8.90 | 18.46 | 17.23 | - | 44.59 | 6.29 | 9.36 | 2.81 | 14.73 | 8.54 | 41.73 | 5.26 | 2.94 | |
| 35/65/0.2 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −76.60 | −80.24 | −83.05 | −86.22 | - | −89.41 | −95.74 | −99.69 | - | −106.20 | −109.40 | −113.30 | |||
| FWHM | 3.65 | 2.64 | 2.14 | 2.70 | - | 12.69 | 1.30 | 3.68 | - | 8.76 | 2.54 | 5.34 | |||
| Intensity | 15.81 | 50.23 | 111.51 | 58.43 | - | 73.39 | 17.12 | 6.01 | 8.14 | - | 21.12 | 13.10 | 14.78 | 21.49 | |
| Integral (%) | 4.9 | 11.22 | 20.27 | 13.39 | - | 44.88 | 18.43 | 0.66 | 2.54 | - | 15.68 | 37.31 | 3.18 | 9.73 | |
| 40/60/0.16 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −76.30 | −80.30 | −83.14 | −86.26 | - | −89.97 | - | −99.69 | - | −107.45 | −109.64 | −114.40 | |||
| FWHM | 3.75 | 3.31 | 2.11 | 2.73 | - | 12.49 | - | 5.43 | - | 9.37 | 2.54 | 4.93 | |||
| Intensity | 15.58 | 42.70 | 98.12 | 59.96 | - | 65.93 | 15.66 | - | 8.38 | - | 25.80 | 16.61 | 18.00 | 15.75 | |
| Integral (%) | 5.00 | 12.11 | 17.69 | 13.30 | - | 43.10 | 16.75 | - | 3.90 | - | 20.69 | 41.34 | 3.91 | 6.65 | |
| 40/60/0.24 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −76.85 | −80.16 | −82.96 | −86.04 | −88.48 | −90.25 | - | −103.06 | - | −111.19 | −109.00 | −116.43 | |||
| FWHM | 4.36 | 2.55 | 2.22 | 2.62 | 1.31 | 8.23 | - | 11.01 | - | 6.39 | 2.54 | 2.84 | |||
| Intensity | 13.23 | 47.72 | 106.41 | 65.66 | 9.65 | 57.36 | 17.39 | - | 17.08 | - | 19.95 | 18.14 | 7.49 | 11.33 | |
| Integral (%) | 5.20 | 10.97 | 21.26 | 15.49 | 1.14 | 48.86 | 12.90 | - | 16.94 | - | 11.49 | 41.33 | 1.72 | 2.90 | |
| ID (91 day) | Raw Slag | CASH | NASH | Quartz | Mullite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45/55/0.2 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −74.42 | −81.44 | −82.85 | −85.70 | - | −89.31 | - | - | −104.51 | −109.77 | −108.18 | −116.34 | |||
| FWHM | 3.53 | 8.22 | 2.11 | 1.92 | - | 12.26 | - | - | 12.14 | 9.43 | 1.47 | 6.15 | |||
| Intensity | 8.05 | 41.05 | 40.31 | 70.42 | - | 50.59 | 36.73 | - | - | 20.8 | 20.75 | 26.09 | 14.64 | 8.68 | |
| Integral (%) | 1.83 | 21.63 | 5.45 | 8.65 | - | 35.73 | 28.89 | - | - | 16.2 | 12.54 | 57.63 | 1.38 | 3.42 | |
| 35/65/0.2 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −76.36 | −79.84 | −82.69 | −85.71 | −86.86 | - | - | −97.16 | −105.97 | - | −109.15 | −113.63 | |||
| FWHM | 4.60 | 2.88 | 2.31 | 2.42 | 10.82 | - | - | 5.17 | 9.48 | - | 2.54 | 5.3 | |||
| Intensity | 17.92 | 45.00 | 90.44 | 80.28 | 36.85 | 63.14 | - | - | 10.52 | 23.46 | - | 16.99 | 17.48 | 14.51 | |
| Integral (%) | 5.84 | 9.17 | 14.79 | 13.78 | 28.23 | 65.97 | - | - | 3.85 | 15.75 | - | 19.60 | 3.14 | 5.45 | |
| 40/60/0.16 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −74.74 | −80.19 | −82.73 | −85.85 | −86.24 | −91.22 | −94.99 | - | −105.88 | - | −109.13 | −114.38 | |||
| FWHM | 4.42 | 6.06 | 1.95 | 1.60 | 5.92 | 2.91 | 7.81 | - | 10.56 | - | 3.68 | 6.38 | |||
| Intensity | 10.72 | 44.63 | 41.49 | 39.51 | 64.36 | 47.50 | 16.3 | 17.81 | - | 29.07 | - | 21.06 | 17.29 | 16.19 | |
| Integral (%) | 3.15 | 17.98 | 5.38 | 4.2 | 25.36 | 52.92 | 3.15 | 9.26 | - | 20.42 | - | 32.83 | 4.23 | 6.9 | |
| 40/60/0.24 | Site Type | Q1 | Q1 | Q2(1Al) | Q2(0Al) | Q3(1Al) | Total CASH | Q4(4Al) | Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Total NASH | Q4(0Al) | Q4(0Al) |
| Pos. (ppm) | −77.20 | −80.17 | −82.96 | −85.83 | −88.52 | - | −94.92 | - | −106.00 | - | −108.61 | −113.63 | |||
| FWHM | 2.59 | 3.76 | 2.30 | 2.47 | 5.92 | - | 8.11 | - | 10.13 | - | 1.62 | 6.62 | |||
| Intensity | 8.46 | 46.08 | 104.23 | 106.04 | 42.65 | 74.75 | - | 20.52 | - | 25.68 | - | 23.10 | 16.27 | 19.00 | |
| Integral (%) | 1.43 | 11.34 | 15.70 | 17.17 | 16.51 | 60.72 | - | 10.89 | - | 17.01 | - | 27.90 | 1.73 | 8.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, V.; Jervis, W.; Fishburn, B.; Numata, T.; Joe, W.; Rawal, A.; Sorrell, C.C.; Koshy, P. Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions. Minerals 2021, 11, 143. https://doi.org/10.3390/min11020143
Wong V, Jervis W, Fishburn B, Numata T, Joe W, Rawal A, Sorrell CC, Koshy P. Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions. Minerals. 2021; 11(2):143. https://doi.org/10.3390/min11020143
Chicago/Turabian StyleWong, Vincent, William Jervis, Benjamin Fishburn, Takafumi Numata, William Joe, Aditya Rawal, Charles Christopher Sorrell, and Pramod Koshy. 2021. "Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions" Minerals 11, no. 2: 143. https://doi.org/10.3390/min11020143
APA StyleWong, V., Jervis, W., Fishburn, B., Numata, T., Joe, W., Rawal, A., Sorrell, C. C., & Koshy, P. (2021). Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions. Minerals, 11(2), 143. https://doi.org/10.3390/min11020143








