Experimental Study of Pt Solubility in the CO-CO2 Fluid at Low fO2 and Subsolidus Conditions of the Ultramafic-Mafic Intrusions
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- Albite glass trap (AGT). It is a powder at the beginning of experiment when it is filled with fluid. It is sintered rapidly into the dense material with isolated fluid bubbles. In the loose state, the trap can be mechanically contaminated with platinum particles of the walls. These particles have larger size (up to 10–15 µm) than particles (usually of submicron size) deposited from the fluid. Mechanically produced particles contain less iron than deposited ones. Using of the bulk analysis of albite trap leads to the severely overestimated solubility. After the initial trials, albite traps were used only for probing experimental fluid conserved in the bubbles.
- (2)
- Industrial corundum filter for high temperature gasses (CRT). The advantages of this filter are the high mechanical stability and chemical resistance of the main corundum component. The main disadvantage is the use of alkaline aluminosilicate glass as a binder in the production of ceramics. This glass can react with the fluid and thus affect the platinum solubility. Another drawback of the corundum traps is the impossibility of their analysis by the Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) method.
- (3)
- Trap from the optic silica glass (CGT). These traps were prepared by the high-temperature sintering in the vacuum furnace at T = 1370 °C during an hour. Porosity of this trap measured after experiments at P = 200 MPa is about 40 ± 5%. Traps are not strong enough to clean their surface with mechanical abrasion after experiment, therefore some surface mechanical contamination with platinum is possible. To avoid this effect, trap was split into three pieces analyzed separately with Electrothermal Atomic Absorption (ET-AAS) method.
3. Results
3.1. Qualitative Observations of the Reduced Carbonic Fluid Activity
3.2. Fluid Composition
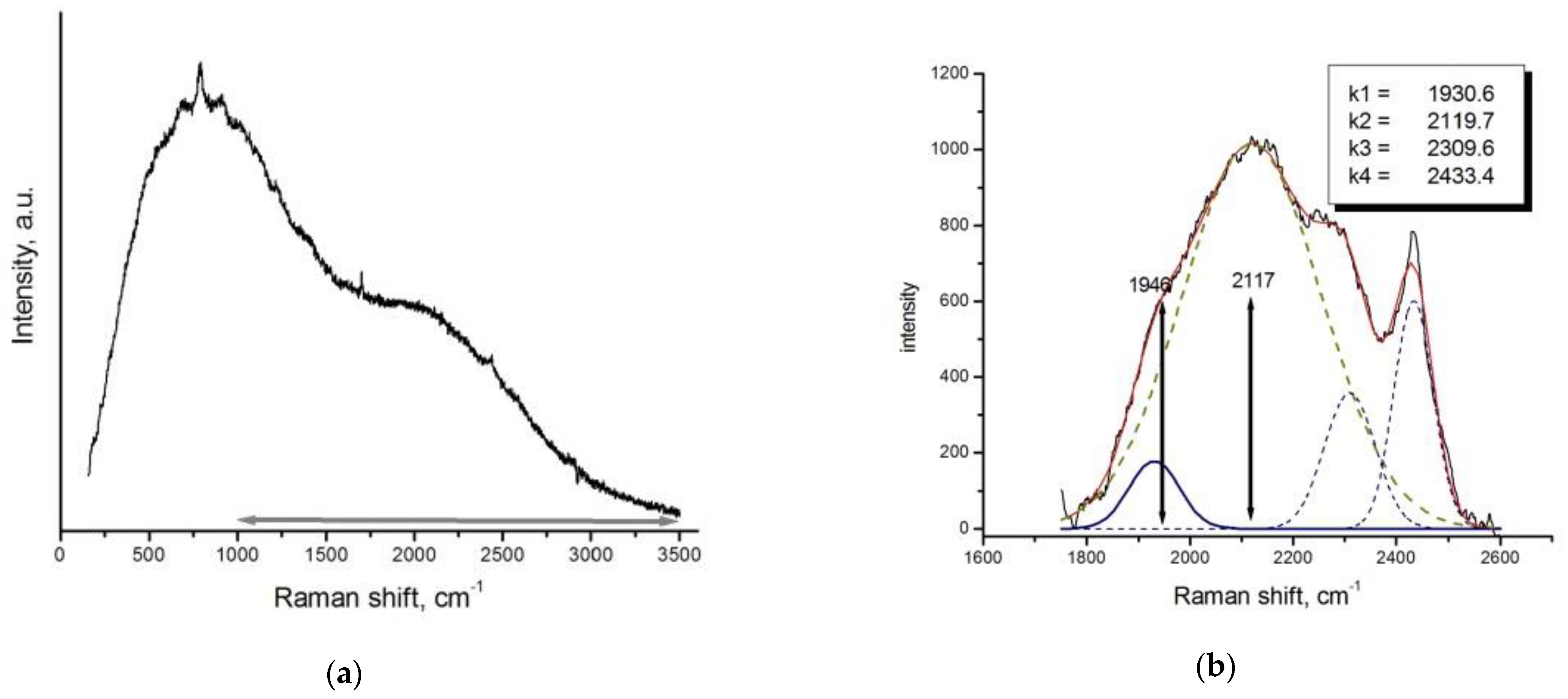
3.2.1. Raman Data Interpretation
Results of Micro-Raman Study
3.3. Platinum Dissolution in the Fluid
3.3.1. Pt Content in the Traps
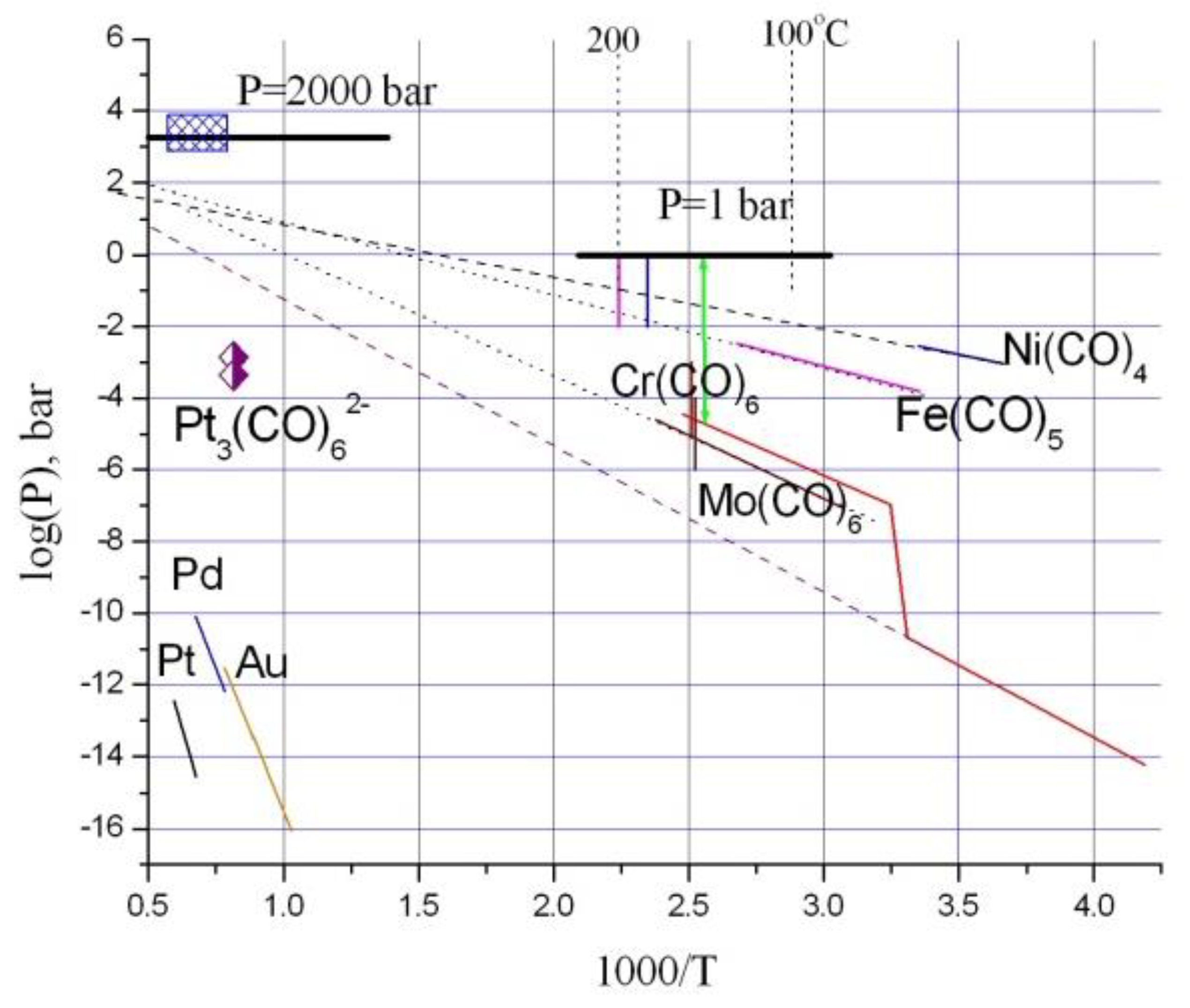
3.3.2. Influence of Pressure and CO Concentration on Pt Solubility
3.4. Solubility of the Pt Quenching Phases in Organic Solvents
3.4.1. Pt in Carbon-Periclase Matrix
3.4.2. Pt in Pure Periclase Matrix
3.5. Surface-Enhanced Raman Scattering (SERS) Spectra of the Quenching Phase Solution
3.5.1. Excitation of Raman Spectrum with Infrared Laser
3.5.2. Excitation of Raman Spectrum with Green Laser
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mochalov, A.G. A genetic model of PGM in cumulative gabbro-pyroxenite-dunite complexes of Koryak Highland, Russia. Geol. Ore Depos. 2013, 66, 145–161. [Google Scholar] [CrossRef]
- Kanitpanyacharoen, W.; Boudreau, A.E. Sulfide-associated mineral assemblages in the Bushveld Complex, South Africa: Platinum-group element enrichment by vapor refining by chloride–carbonate fluids. Miner Depos. 2013, 48, 193–210. [Google Scholar] [CrossRef]
- Yudovskaya, M.; Kinnaird, J.; Naldrett, A.J.; Rodionov, N.; Antonov, A.; Simakin, S.; Kuzmin, D. Trace-element study and age dating of zircon from chromitites of the Bushveld Complex (South Africa). Miner. Petrol. 2013, 107, 915–942. [Google Scholar] [CrossRef]
- Mathez, E.A.; Dietrich, V.J.; Holloway, J.R.; Boudreau, E. Carbon Distribution in the Stillwater Complex and Evolution of Vapor during Crystallization of Stillwater and Bushveld Magmas. J. Petrol. 1989, 30, 153–173. [Google Scholar] [CrossRef] [Green Version]
- Ballhaus, C.G.; Stumpfl, E.F. Occurrence and petrological significance of graphite in the Upper Critical Zone, Western Bushveld Complex, South Africa. Earth Planet. Sci. Lett. 1985, 74, 58–68. [Google Scholar] [CrossRef]
- Hanley, J.J.; Mungall, J.E.; Pettke, T.; Spooner, E.T.C.; Bray, C.J. Fluid and Halide Melt Inclusions of Magmatic Origin in the Ultramafic and Lower Banded Series, Stillwater Complex, Montana, USA. J. Petrol. 2008, 49, 1133–1160. [Google Scholar] [CrossRef] [Green Version]
- Konnikov, E.G.; Vasyukova, O.N. Composition of Fluid Inclusions from Intrusive Rocks of the Norite–Cortlandite Complex, Kamchatka. Geol. Ore Depos. 2007, 49, 227–237. [Google Scholar] [CrossRef]
- Konnikov, E.G.; Meuer, W.P.; Neruchev, S.S.; Prasolov, E.M.; Kislov, E.V.; Orsoev, D.A. Fluid regime of platinum group elemens (PGE) and gold-bearing reef formation in the Dovyren mafic-ultramafic layered complex, eastern Siberia, Russia. Miner. Depos. 2000, 35, 526–532. [Google Scholar] [CrossRef]
- Hanley, J.J.; Mungall, J.E.; Pettke, T.; Spooner, E.T.C.; Bray, C.J. Ore metal redistribution by hydrocarbon–brine and hydrocarbon–halide melt phases, North Range footwall of the Sudbury Igneous Complex, Ontario, Canada. Miner. Depos. 2005, 40, 237–256. [Google Scholar] [CrossRef] [Green Version]
- Shcheka, G.G.; Lehmann, B.; Gierth, E.; Gömann, K.; Wallianos, A. Macrocrystals of Pt–Fe alloy from the Kondyor PGE placer deposit, Khabarovskiy Kray, Russia: Trace-element content, mineral inclusions and reaction assemblages. Can. Miner. 2004, 42, 601–617. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Borisova, A.Y.; Harrichoury, J.C. The effect of sulfur on vapor-liquid fractionation of metals in hydrothermal systems. Earth Planet Sci. Lett. 2008, 266, 345–362. [Google Scholar] [CrossRef]
- Uel’skiy, A.A.; Grebennikov, A.V.; Storozhenko, P.A. Carbonyl Materials: Preparation, Properties, and Application. Polym. Sci. Ser. D Glues Seal. Mater. 2011, 4, 228–235. [Google Scholar] [CrossRef]
- Simakin, A.G.; Kislov, E.V.; Salova, T.P.; Shaposhnikova, O.Y.; Nekrasov, A.N. Reduced CO2 Fluid as an Agent of Ore-Forming Processes: A Case Study of Dolomite-Replacement Skarns at the Yoko-Dovyren Massif. Petrology 2019, 27, 1–16. [Google Scholar] [CrossRef]
- Torok, K.; Degi, J.; Szep, A.; Marosi, G. Reduced carbonic fluids in mafic granulite xenoliths from the Bakony–Balaton Highland Volcanic Field, W-Hungary. Chem. Geol. 2005, 223, 93–108. [Google Scholar] [CrossRef]
- Bergman, S.C.; Dubessy, J. CO2–CO fluid inclusions in a composite peridotite xenolith: Implications for upper mantle oxygen fugacity. Contrib. Miner. Petrol. 1984, 85, 1–13. [Google Scholar] [CrossRef]
- Simakin, A.; Salova, T.; Devyatova, V.; Zelensky, M. Reduced carbonic fluid and possible nature of high K magmas of Tolbachik. J. Volcanol. Geotherm. Res. 2015, 307, 210–221. [Google Scholar] [CrossRef]
- Gamer, M. Vapor Pressures and Thermodynamic Properties of Tungsten, Chromium, Cobalt and Rhodium Carbonyls. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 1994; p. 80. [Google Scholar]
- Moskvin, A.B. (Ed.) New Handbook of Chemist and Technologist. v.12. General Information: Structure of Matter; World and Family: Saint Petersburg, Russia, 2006; p. 1464. (In Russian) [Google Scholar]
- Kundig, E.P.; McIntosh, D.; Moskovits, M.; Ozin, G.A. Binary Carbonyls of Platinum, Pt(CO)n, (Where n = 1–4). A Comparative Study of the Chemical and Physical Properties of M(CO)n, (Where M = Ni, Pd, or Pt; n = 1–4). J. Am. Chem. Soc. 1973, 95, 7234–7241. [Google Scholar] [CrossRef]
- Longoni, G.; Chini, P. Synthesis and Chemical Characterization of Platinum Carbonyl Dianions [Pt3(C0)6]n2- (n = −10, 6, 5, 4, 3, 2, 1). A New Series of Inorganic Oligomers. J. Am. Chem. Soc. 1976, 98, 7225–7231. [Google Scholar] [CrossRef]
- Torigoe, K.; Remita, H.; Picq, G.; Belloni, J.; Bazin, D. Structural Characterization of Supported Platinum Carbonyl Clusters by X-ray Absorption Spectroscopy. J. Phys. Chem. B 2000, 104, 7050–7056. [Google Scholar] [CrossRef] [Green Version]
- Li, G.J.; Fujimoto, T.; Fukuoka, A.; Ichikawa, M. Ship-in-Bottle synthesis of Pt9-Pt15 carbonyl clusters inside NaY and NaX zeolites, in-situ FTIR and EXAFS characterization and the catalytic behaviors in 13CO exchange reaction and NO reduction by CO. Catal. Lett. 1992, 12, 171–186. [Google Scholar] [CrossRef]
- Bradford, C.W. The Carbonyls of the Platinum Group Metals. Platin. Metals Rev. 1972, 16, 50–55. [Google Scholar]
- Kessel, R.; Schmidt, M.W.; Ulmer, P.; Pettke, T. Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 2005, 437, 724–727. [Google Scholar] [CrossRef]
- Simakin, A.G.; Salova, T.P.; Gabitov, R.I.; Isaenko, S.I. Dry CO2–CO fluid as an important potential deep Earth solvent. Geofluids 2016, 16, 1043–1057. [Google Scholar] [CrossRef]
- Kerrick, D.M.; Jacobs, G.K. A modified redlich-kwong equation of state for H2O, CO2, and H2O–CO2 mixtures at elevated pressures and temperatures. Am. J. Sci. 1981, 281, 735–767. [Google Scholar]
- Shi, P.; Saxena, S.K. Thermodynamic modeling of the C–H–O–S fluid system. Am. Miner. 1992, 77, 1038–1049. [Google Scholar]
- Borisova, A.Y.; Toutain, J.-P.; Stefansson, A.; Gouy, S.; de Parseval, P. Processes controlling the 2010 Eyjafjallajökull explosive eruption. J. Geophys. Res. 2012, 117, B05202. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Nabiullina, S.N.; Tyutyunnik, O.A. Au and PGE Determination in Geochemical Materials: Experience in Applying Spectrometric Techniques. Geochem. Int. 2020, 58, 377–390. [Google Scholar] [CrossRef]
- Burke, E.A.J. Raman microspectrometry of fluid inclusions. Lithos 2001, 55, 139–158. [Google Scholar] [CrossRef]
- Jégo, S.; Pichavant, M. Gold solubility in arc magmas: Experimental determination of the effect of sulfur at 1000 °C and 0.4 GPa. Geochim. Cosmochim. Acta 2012, 84, 560–592. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Radzol, A.R.M.; Lee, K.Y.; Mansor, W.; Yahaya, S.R. Nano-Scale Characterization of Surface Enhanced Raman Spectroscopic Substrates. Procedia Eng. 2012, 41, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Matricardi, C.; Hanske, C.; Garcia-Pomar, J.L.; Langer, J.; Mihi, A.; Liz-Marzán, L.M. Gold Nanoparticle Plasmonic Superlattices as Surface Enhanced Raman Spectroscopy Substrates. ACS Nano 2018, 12, 8531–8539. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.B.; Sarkar, U. Structure, vibrational, and optical properties of platinum cluster: A density functional theory approach. J. Mol. Model 2014, 20, 2537. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. X-ray photoelectron spectroscopy and Raman microscopy of ferroan platinum crystal from the Kondyor Massif, Russian Far East. Spectrosc. Lett. 2018, 52, 43–48. [Google Scholar] [CrossRef]
- Mountain, B.W.; Wood, S.A. Solubility and Transport of Platinum-Group Elements in Hydrothermal Solutions: Thermodynamic and Physical Chemical Constraints. In Geo-Platinum 87; Prichard, H.M., Potts, P.J., Bowles, J.F.W., Cribb, S.J., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 57–82. [Google Scholar] [CrossRef]
- Sassani, D.C.; Shock, E.L. Solubility and transport of platinum-group elements in supercritical fluids: Summary and estimates of thermodynamic properties for ruthenium, rhodium, palladium, and platinum solids, aqueous ions, and complexes to 1000 °C and 5 kbar. Geochim. Cosmochim. Acta 1998, 62, 2643–2671. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Tyutyunnik, O.A.; Silantyev, S.A. Mobility of Dissolved Palladium and Platinum Species during the Water–Rock Interaction in a Chloride Environment: Modeling of PGE Behavior during Interaction between Oceanic Serpentinites and Seawater Derivatives. Geochem. Int. 2019, 57, 282–289. [Google Scholar] [CrossRef]
- Barnes, S.J.; Liu, W. Pt and Pd mobility in hydrothermal fluids: Evidence from komatiites and from thermodynamic modeling. Ore Geol. Rev. 2012, 44, 49–58. [Google Scholar] [CrossRef]
- Barnes, S.J.; Campbell, I.H. Role of late magmatic fluids in Merensky-type platinum deposits: A discussion. Geology 1988, 16, 488–491. [Google Scholar] [CrossRef]
- Khanchuk, A.I.; Plyusnina, L.P.; Ruslan, A.V.; Likhoidov, G.G.; Barinov, N.N. Nature of Graphitization and Noble Metal Mineralization in Metamorphic Rocks of the Northern Khanka Terrane, Primorye. Geol. Ore Depos. 2013, 55, 225–244. [Google Scholar] [CrossRef]
- Schouwstra, R.P.; Kinloch, E.D.; Lee, C.A. A Short Geological Review of the Bushveld Complex. Platin. Met. Rev. 2000, 44, 33–39. [Google Scholar]
- Johan, Z.; Ohnenstetter, M.; Slansky, E.; Barron, L.M.; Suppel, D. Platinum mineralization in the Alaskan-type intrusive complex near Fifield, New South Wales, Australia: Part 1. platinum group minerals in clinopyroxenites of the Kelvin Grove Prospect, Owendale Intrusion. Miner. Petrol. 1989, 40, 289–309. [Google Scholar] [CrossRef]
- Johan, Z.; Slansky, E.; Kelly, D.A. Platinum nuggets from the Kompiam area, Enga Province, Papua New Guinea: Evidence for an Alaskan-type complex. Miner. Petrol. 2000, 68, 159–176. [Google Scholar] [CrossRef]
- Ivanov, O.K. Concentrically Zoned Pyroxenite–Dunite Massifs of the Urals: Mineralogy, Petrology, and Genesis; UrGU: Yekaterinburg, Russia, 1997; 32p, ISBN 5-7525-0507-0. (In Russian) [Google Scholar]
- Tolstykh, N.; Krivenko, A.; Sidorov, E.; Laajoki, K.; Podlipsky, M. Ore mineralogy of PGM placers in Siberia and the Russian Far East. Ore Geol. Rev. 2002, 20, 1–25. [Google Scholar] [CrossRef]






| Run | T/P (°C/Kbar) | XCO/XCOeq | LA-ICP-MS (ppm) | ET-AAS (ppm) | Porosity | Cfl/CCO2 2 |
|---|---|---|---|---|---|---|
| ab86 | 950/2 | 0.5/0.14 | 109.5 ± 129.8 | 335.5 | 0.35 1 | mechanical contamination |
| ab86 | 950/2 | 0.5/0.14 | 55.3 ± 53.0 3 | - | 0.35 1 | 489.6/418.5 |
| cor99 | 950/2 | 0.5/0.14 | - | 27.5 | 0.4 | 512.4/437.9 |
| qz98 | 950/2 | 0.5/0.14 | - | 57.7 | 0.35 1 | 490.0/418.4 |
| ab86 | 950/2 | 0.5/0.14 | 32.7 ± 33.7 4 | n.d. | 0.4 1 | 289.5/247.5 |
| qz98 | 950/2 | 0.5/0.14 | 22.1 ± 15.1 | n.d. | 0.30 | 136.3/129.3 |
| cor102 | 950/2 | 0.33/0.14 | - | 11.5 | 0.4 1 | 192.9/183.1 |
| qz118 | 950/2 | 0.15/0.14 | - | 22.9 ± 7.1 | 0.5 | 141.0/133.8 |
| ab108 | 950/1 | 0.5/0.22 | 2.5 ± 2.0 | n.d. | 0.45 | 20.5/18.0 |
| cor105 | 950/1 | 0.5/0.22 | - | 5.1 | 0.5 | 85.5/75.3 |
| qz120 | 950/1 | 0.21/0.22 | - | 10.2 | 0.60 | 80.1/70.5 |
| cor104 | 950/0.5 | 0.5/0.31 | - | 1.4 | 0.60 | 24.1/19.7 |
| ab107 | 950/0.5 | 0.5/0.31 | 0.22 ± 0.17 | n.d. | 0.6 1 | 6.3/5.16 |
| qz119 | 950/0.5 | 0.5/0.31 | - | 11.6/32.4 | 0.21 | 114/93.6 |
| cor106 | 9500/3 | 0.5/0.10 | - | 5.0 | 0.4 1 | 145.8/119.3 |
| qz117 | 950/2;1000/1 | 0.2/0.29 | - | 1.3/6.4 | 0.4 1 | 44.0/40.8 |
| Run | Time, Hours | Dissol. Time, h | XCO/XCOeq | Pt in MgO ppm | Comments | Pt in Flush ppm 1 |
|---|---|---|---|---|---|---|
| qz121 | 21 | 0.5 | 0.5/0.14 | 1469 | MgO + acetone stirred | 8.8 2 |
| qz122 | 2 | 0.5 | 0.5/0.14 | 2393 | stirred | 48.2 2 |
| qz123 | 2 | 0.25 | 0.5/0.14 | 17.4 | not stirred | 4.3 |
| O56 | 21 | 0.25 | 0.16/0.14 | 1.4 | no carbon | 21.5 |
| O57 | 21 | 0.25 | 0.16/0.14 | 3.4 | no carbon | 0.1 |
| qz114 | 2 | 12 | 0.5/- | 21.8 | fluid with XH2O = 0.046 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simakin, A.; Salova, T.; Borisova, A.Y.; Pokrovski, G.S.; Shaposhnikova, O.; Tyutyunnik, O.; Bondarenko, G.; Nekrasov, A.; Isaenko, S.I. Experimental Study of Pt Solubility in the CO-CO2 Fluid at Low fO2 and Subsolidus Conditions of the Ultramafic-Mafic Intrusions. Minerals 2021, 11, 225. https://doi.org/10.3390/min11020225
Simakin A, Salova T, Borisova AY, Pokrovski GS, Shaposhnikova O, Tyutyunnik O, Bondarenko G, Nekrasov A, Isaenko SI. Experimental Study of Pt Solubility in the CO-CO2 Fluid at Low fO2 and Subsolidus Conditions of the Ultramafic-Mafic Intrusions. Minerals. 2021; 11(2):225. https://doi.org/10.3390/min11020225
Chicago/Turabian StyleSimakin, Alexander, Tamara Salova, Anastassia Y. Borisova, Gleb S. Pokrovski, Olga Shaposhnikova, Oksana Tyutyunnik, Galina Bondarenko, Alexey Nekrasov, and Sergey I. Isaenko. 2021. "Experimental Study of Pt Solubility in the CO-CO2 Fluid at Low fO2 and Subsolidus Conditions of the Ultramafic-Mafic Intrusions" Minerals 11, no. 2: 225. https://doi.org/10.3390/min11020225
APA StyleSimakin, A., Salova, T., Borisova, A. Y., Pokrovski, G. S., Shaposhnikova, O., Tyutyunnik, O., Bondarenko, G., Nekrasov, A., & Isaenko, S. I. (2021). Experimental Study of Pt Solubility in the CO-CO2 Fluid at Low fO2 and Subsolidus Conditions of the Ultramafic-Mafic Intrusions. Minerals, 11(2), 225. https://doi.org/10.3390/min11020225









