Metallogenic Characteristics and Formation Mechanism of Naomugeng Clay-Type Lithium Deposit in Central Inner Mongolia, China
Abstract
:1. Introduction
2. Regional Geological Setting and Ore Geology in Studied Area
3. Methodology
3.1. Sampling and Analytic Methods
3.2. Mass Balance Calculations
4. Results
4.1. Petrography and Altered Minerals
4.2. Whole-Rock Geochemistry
4.3. Mass Changes Related to Hydrothermal Alteration
5. Discussion
5.1. Occurrence of Lithium
5.2. Metallogenic Characteristics
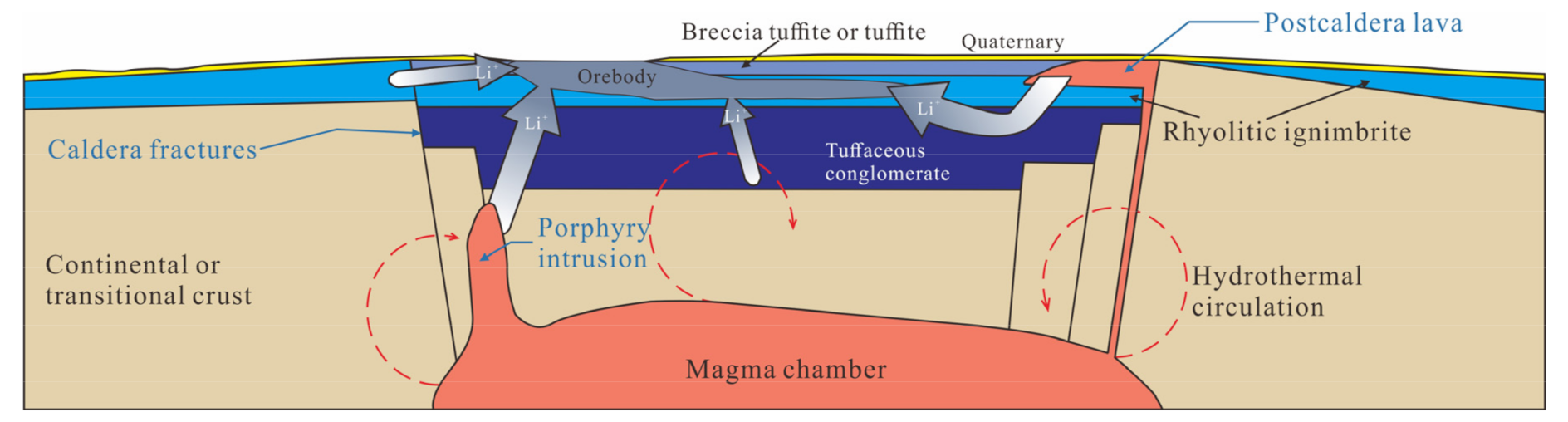
5.3. Formation Mechanism and Prospective Potential of Lithium Deposits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, T.R.; Coble, M.A.; Rytuba, J.J.; Mahood, G.A. Lithium enrichment in intracontinental rhyolite magmas leads to Li deposits in caldera basins. Nat. Commun. 2017, 8, 270. [Google Scholar] [CrossRef]
- Castor, S.B.; Henry, C.D. Lithium-rich claystone in the McDermitt Caldera, Nevada, USA: Geologic, mineralogical, and geochemical characteristics and possible origin. Minerals 2020, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Munk, L.A.; Hynek, S.A.; Bradley, D.; Boutt, D.; Labay, K.; Jochens, H. Lithium brines: A global perspective. In Reviews in Economic Geology; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2016; Volume 18, pp. 339–365. [Google Scholar]
- Partington, G.A.; Mcnaughton, N.J.; Williams, I.S. A review of the geology, mineralization, and geochronology of the Greenbushes pegmatite, Western Australia. Econ. Geol. 1995, 90, 616–635. [Google Scholar] [CrossRef]
- Dai, H.; Wang, D.H.; Liu, L.J.; Yu, Y.; Dai, J.J. Geochronology and Geochemistry of Li(Be)-bearing granitic pegmatites from the Jiajika superlarge Li-polymetallic deposit in western Sichuan, China. J. Earth Sci. 2019, 707–727. [Google Scholar] [CrossRef]
- Swanson, S.E. Mineralogy of spodumene pegmatites and related rocks in the rin-spodumene belt of north Carolina and south Carolina, USA. Can. Mineral. 2012, 50, 1589–1608. [Google Scholar] [CrossRef]
- Ma, S.C.; Wang, D.H.; Sun, Y.; Li, C.; Zhong, H.R. Geochronology and geochemical characteristics of lower-middle Triassic clay rock and their significances for prospecting clay-type lithium deposit. Earth Sci. 2019, 44, 427–440. [Google Scholar] [CrossRef]
- Wen, H.J.; Luo, C.G.; Du, S.J.; Yu, W.X.; Gu, H.N.; Ling, K.Y.; Cui, Y.; Li, Y.; Yang, J.H. Carbonate-hosted clay-type lithium deposit and its prospecting significance. Chin. Sci. Bull. 2020, 65, 53–59. [Google Scholar] [CrossRef]
- Baoyinwuliji; Zhao, W.T.; Wang, X.K.; Cui, L.W.; Li, X.R.; Bao, F.Q. Geochemical anomaly and metallogenic potential of the Naomugengsumu lithium mineralization area in Inner Mongolia. Geol. Resour. 2014, 23, 531–534. [Google Scholar] [CrossRef]
- Hong, D.W.; Wang, S.G.; Xie, X.L.; Zhang, J.S.; Wang, T. Metallogenic province derived from mantle sources: A case study of Central Asian Orogenic Belt. Miner. Depos. 2003, 22, 41–55. [Google Scholar] [CrossRef]
- Xiao, W.J.; Shu, L.S.; Gao, J.; Xiong, X.L.; Wang, J.B.; Guo, Z.J.; Li, J.Y.; Sun, M. Continental dynamics of the central asian orogenic belt and its metallogeny. Xinjiang Geol. 2008, 26, 4–8. [Google Scholar]
- Cogn, J.P.; Kravchinsky, V.A.; Halim, N.; Hankard, F. Late Jurassic-Early Cretaceous closure of the Mongol-Okhotsk Ocean demonstrated by new Mesozoic palaeomagnetic results from the Trans-Bakal area (SE Siberia). Geophys. J. R. Astron. Soc. 2010, 163, 813–832. [Google Scholar] [CrossRef] [Green Version]
- Windley, B.F.; Alexeiev, D.; Xiao, W.; KröNer, A.; Badarch, G. Tectonic models for accretion of the Central Asian Orogenic Belt. J. Geol. Soc. 2007, 164, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Chen, Y.; Xu, B.; Faure, M.; Shi, G.; Choulet, F. Did the Paleo-Asian Ocean between North China Block and Mongolia Block exist during the late Paleozoic? First paleomagnetic evidence from central-eastern Inner Mongolia, China. J. Geophys. Res. Solid Earth 2013, 118, 1873–1894. [Google Scholar] [CrossRef] [Green Version]
- Li, P.C.; Li, S.C.; Liu, Z.H.; Li, G.; Bai, X.H.; Wan, L. Formation age and tectonic environment of volcanic rocks from Manketouebo Formation in Linxi area, Inner Mongolia. Glob. Geol. 2016, 35, 77–88. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Y.J.; Li, H.Y.; Dong, P.P. Post- orogeny of the Hegenshan suture zone: Zircon U-Pb age and geochemical constraints from volcanic rocks of the Manketouebo Formation. Geol. Bull. China 2019, 38, 1443–1454. [Google Scholar]
- Yin, Z.G.; Hao, K.; Liu, C.L.; Wang, Y.; Li, H.N.; Li, M.; Gong, Z.M.; Zhang, S.T. Geochronological and geochemical characteristics of rhyolite in the Manketou’ebo Formation of Dong Ujimqin Banner, Inner Mongolia, and its geological implications. Geol. Bull. China 2019, 38, 1825–1836. [Google Scholar]
- Guo, J.G.; Zhang, Y.J.; Zhang, C.; Li, W.; Wang, Y.; Wang, Q.Z.; Guo, J. Formation age and geochemical characteristics of volcanic rocks in Manketou’ebo Formation in Kundu area of Ar Horqin Banner, Inner Mongolia. Geol. Bull. China 2018, 37, 1652–1661. [Google Scholar] [CrossRef]
- Bor-Ming, J.; Wu, F.; Chen, B. Granitoids of the Central Asian Orogenic Belt and continental growth in the Phanerozoic. Trans R Soc Edinburgh Earth Sci. Earth Environ. Sci. Trans. R. Soc. Edinb. 1999, 91, 181–193. [Google Scholar] [CrossRef]
- Li, X.H.; Li, Z.X.; Wingate, M.T.D.; Chung, S.L.; Liu, Y.; Lin, G.C. Geochemistry of the 755 ma mundine well dyke swarm, northwestern australia: Part of a neoproterozoic mantle superplume beneath rodinia? Precambrian Res. 2006, 146, 1–15. [Google Scholar] [CrossRef]
- Bézos, A.; Escrig, S.; Langmuir, C.H.; Michael, P.J.; Asimow, P.D. Origins of chemical diversity of back-arc basin basalts: A segment-scale study of the eastern lau spreading center. J. Geophys. Res. Solid Earth 2009, 114, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Xia, M. Modification of mechanical and color properties of polypropylene with colored nano SiO2 prepared from diatomite. J. Appl. Polym. Sci. 2020, 137, 48328. [Google Scholar] [CrossRef]
- Ague, J.J. Mass transfer during Barrovian metamorphism of Pelites, South-Central Connecticut. I: Evidence for changes in composition and volume. Am. J. Sci. 1994, 294, 989–1057. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, X.F.; Xiao, R.; Bai, Y.P.; Yang, F.; Mao, W.; Jiang, S.K. Elements mobilization of mineralized porphyry rocks during hydrothermal alteration at Zhushahong porphyry copper deposit, Dexing district, South China. Acta Petrol. Sin. 2012, 28, 3869–3886. [Google Scholar]
- Ague, J.J. Extreme channelization of fluid and the problem of element mobility during barrovian metamorphism. Am. Mineral. 2011, 96, 333–352. [Google Scholar] [CrossRef]
- Dong, K.; Chen, S.; Graham, I.; Zhao, J.; Fu, P.; Xu, Y.; Tian, G.; Qin, W.; Chen, J. Geochemical behavior during mineralization and alteration events in the Baiyinchang volcanic-hosted massive sulfide deposits, Gansu Province, China. Ore Geol. Rev. 2017, 91, 559–572. [Google Scholar] [CrossRef]
- Mori, Y.; Nishiyama, T.; Yanagi, T. Mass transfer and reaction paths in alteration zones around carbonate veins in the Nishisonogi Metamorphic Rocks, southwest Japan. Am. Mineral. 2003, 88, 611–623. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon analysis: A brief review of the method and applications. Phys. Chem. Earth 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Villaplaza, B.R.B.; Buena, A.E.; Pacle, N.A.D.; Payot, B.D.; Gabo-Ratio, J.A.S.; Ramos, N.T.; Dimalanta, C.B.; Faustino-Eslava, D.V.; Queaño, K.L.; Yumul, G.P., Jr. Alteration and lithogeochemistry in the Masara gold District, Eastern Mindanao, Philippines, as tools for exploration targeting. Ore Geol. Rev. 2017, 91, 530–540. [Google Scholar] [CrossRef]
- Grant, J.A. The isocon diagram: A simple solution to Gresens’ equation for metasomatic alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- Maclean, W.; Kranidiotis, P. Immobile elements as monitors of mass transfer in hydrothermal alteration; Phelps Dodge massive sulfide deposit, Matagami, Quebec. Econ. Geol. 1987, 82, 951–962. [Google Scholar] [CrossRef]
- Kessel, R.; Schmidt, M.W.; Ulmer, P.; Pettke, T. Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature 2005, 437, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Kuwatani, T.; Yoshida, K.; Ueki, K.; Oyanagi, R.; Uno, M.; Akaho, S. Sparse isocon analysis: A data-driven approach for material transfer estimation. Chem. Geol. 2020, 532, 119345. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Hendricks, S.B. Lattice structure of clay minerals and some properties of clays. J. Geol. 1942, 50, 276–290. [Google Scholar] [CrossRef]
- Rakov, L.T. Mechanisms of isomorphic substitution in quartz. Geochem. Int. 2006, 40, 1004–1014. [Google Scholar] [CrossRef]
- Liu, S.K.; Li, J.J.; Liu, J.M. An updated model of Rietveld structure refinement of Na/K-feldspar. Period. Mineral. 2017, 86, 75–85. [Google Scholar] [CrossRef]
- Müller, G. Preparation of hydrogen and lithium feldspars by ion exchange. Nature 1988, 332, 435–436. [Google Scholar] [CrossRef]
- Ribbe, P.H. Feldspar Minerals. Vol. 1: Crystal structure and physical properties; Vol. 2: Chemical and textural properties. Geosci. Can. 1975, 83, 537–538. [Google Scholar] [CrossRef]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An introduction to properties and utilization. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; Zoveidavianpoor, M., Ed.; IntechOpen: London, UK, 2018; pp. 3–22. [Google Scholar]
- Madejová, J.; Barlog, M.; Jankovi, U.; Slan, M.; Pálková, H. Comparative study of alkylammonium- and alkylphosphonium-based analogues of organo-montmorillonites. Appl. Clay Sci. 2021, 200, 105894. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Shu, S.K.; Peng, C.L.; Shi, W.G.; Qin, L.; Luo, S.H. Adsorption characteristics and mechanism of ammonium ions on montmorillonite surface. Chin. Rare Earths 2020, 41, 68–77. [Google Scholar]
- Zhang, X.K.; Cai, Y.F.; Fan, C.X. Simulation of “chloritization” of montmorillonite to chlorite. In Proceedings of the 17th Annual Academic Meeting of the Chinese Society of Mineral Petrogeochemistry, Hangzhou, China, 19 April 2019; p. 97. [Google Scholar]
- Seyfried, W.E., Jr.; Foustoukos, D.I.; Allen, D.E. Ultramafic-hosted hydrothermal systems at mid-ocean ridges: Chemical and physical controls on pH, redox and carbon reduction reactions. In Proceedings of the American Geophysical Union, Washington, DC, USA, 1 January 2004; p. 267. [Google Scholar]
- Bai, Y.L.; Wang, T.; Wang, Z.Q.; Kang, X.L.; Gu, X.Y.; Qiu, G.D.; Wang, D.X.; Yan, J. Forming age and paleoclimate of Manketouebo Formation in Manzhouli-Xinbarhuyouqi area. Acta Geol. Sin. 2020, 94, 1367–1381. [Google Scholar] [CrossRef]
- He, P.; Guo, S.; Zhang, T.F.; Zhang, Y.L.; Su, H.; Fu, Q.L. Geochronology, geochemistry and tectonic setting of volcanic rocks from Manketouebo Formation in Wulagai area, southern Da Hinggan Mountains. Geol. China 2020, 1–27. Available online: http://kns.cnki.net/kcms/detail/11.1167.P.20200106.1707.007.html (accessed on 20 February 2021).
- Meng, X.F.; Zhang, Z.P.; Xu, C.; Wang, D.Q. Geologic characteristics and lithologic member division in the Late Jurassic Manketou’ebo Formation in Wulanhade area, Dongwuzhumuqinqi, Inner Mongolia, China. Geol. Bull. China 2010, 29, 1649–1654. [Google Scholar] [CrossRef]
- Douville, E.; Bienvenu, P.; Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Appriou, P.; Gamo, T. Yttrium and rare earth elements in fluids from various deep-sea hydrothermal systems. Geochim. Cosmochim. Acta 1999, 63, 627–643. [Google Scholar] [CrossRef]
- Genna, D.; Gaboury, D.; Roy, G. Evolution of a volcanogenic hydrothermal system recorded by the behavior of LREE and Eu: Case study of the Key Tuffite at Bracemac–McLeod deposits, Matagami, Canada. Ore Geol. Rev. 2014, 63, 160–177. [Google Scholar] [CrossRef]
- Ellis, B.S.; Szymanowski, D.; Magna, T.; Neukampf, J.; Dohmen, R.; Bachmann, O.; Ulmer, P.; Guillong, M. Post-eruptive mobility of lithium in volcanic rocks. Nat. Commun. 2018, 9, 3228. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Liang, Q.S. Study on the cause of lithium soil geochemical abnormal in the layer of Angeeryinwula formation in Bayanaobao area, Inner Mongolia. Geol. Chem. Miner. 2019, 41, 82–89. [Google Scholar] [CrossRef]


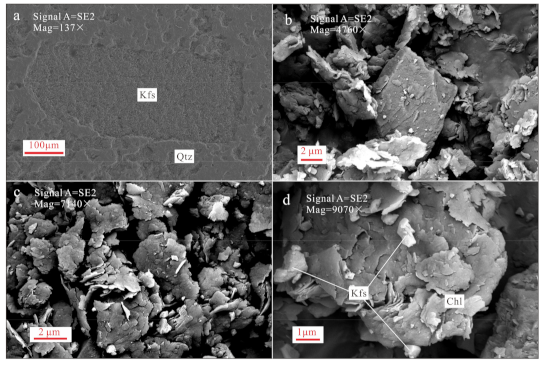
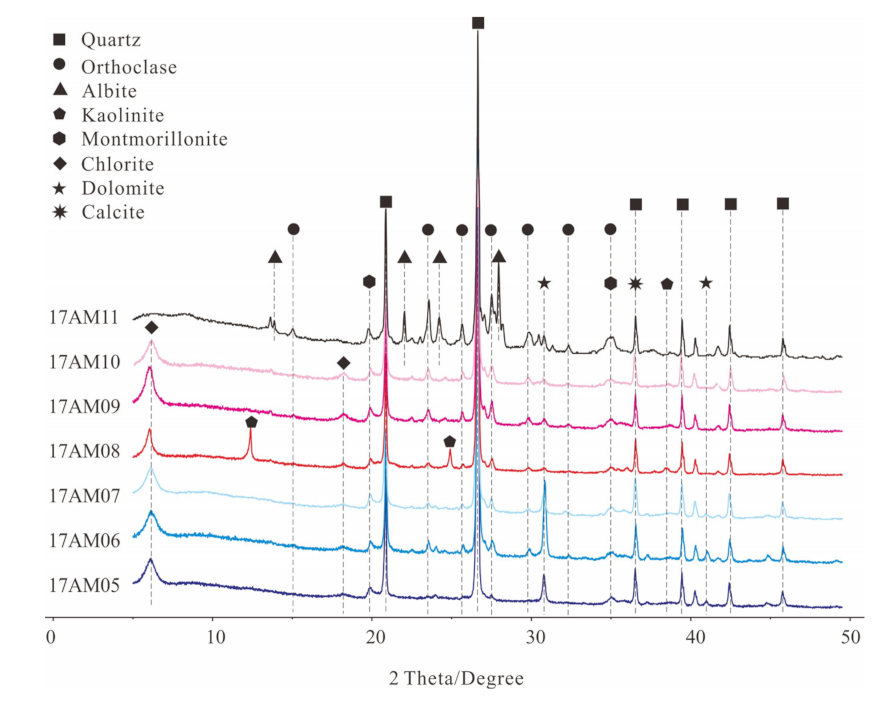


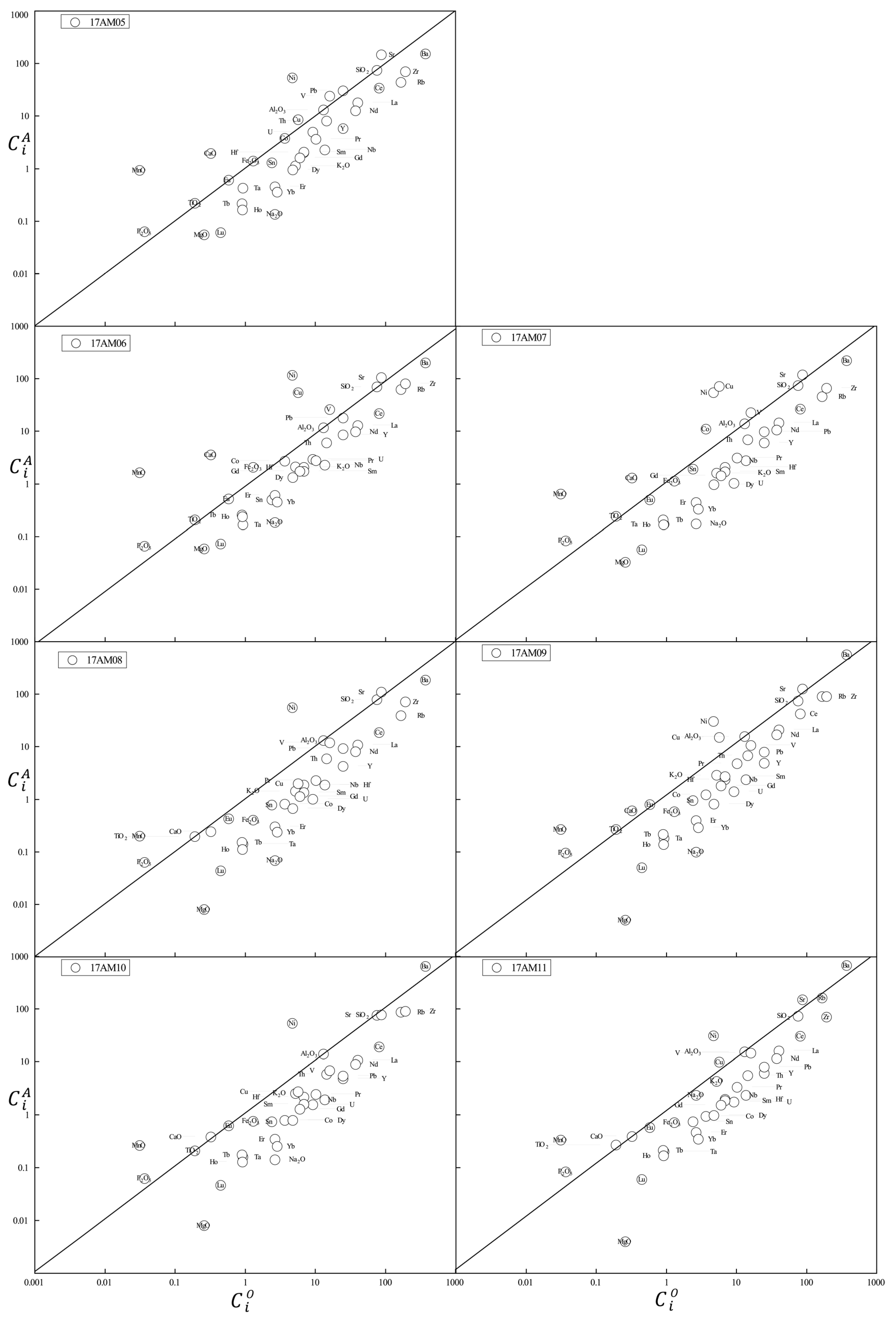

| Sample No. | GPS | Lithology |
|---|---|---|
| 17AM04 (zk04-1, depth: 88 m) | 42°48′14″ N, 111°19′00″ E (storage site of core samples) | Tuffaceous conglomerate |
| 17AM05 (zk04-1, depth: 94 m) | Crystal tuff | |
| 17AM06 (zk04-1, depth: 286 m) | Crystal tuff | |
| 17AM07 (zk04-1, depth: 283 m) | Crystal tuff | |
| 17AM08 | Crystal tuff | |
| 17AM09 | 42°48′11″ N, | Crystal tuff |
| 17AM10 | 111°20′30″ E | Crystal tuff |
| 17AM11 | Crystal tuff |
| Element | 17AM5 | 17AM6 | 17AM7 | 17AM8 | 17AM9 | 17AM10 | 17AM11 |
|---|---|---|---|---|---|---|---|
| SiO2 | 75.0 | 70.7 | 74.8 | 79.5 | 74.4 | 76.8 | 72.7 |
| TiO2 | 0.22 | 0.21 | 0.24 | 0.20 | 0.27 | 0.21 | 0.27 |
| Al2O3 | 13.1 | 11.6 | 13.9 | 13.2 | 15.6 | 13.9 | 15.5 |
| Fe2O3T | 1.39 | 2.09 | 1.13 | 0.40 | 0.58 | 0.75 | 0.71 |
| MnO | 0.06 | 0.06 | 0.03 | 0.01 | 0.01 | 0.01 | 0.00 |
| MgO | 0.94 | 1.63 | 0.65 | 0.20 | 0.27 | 0.26 | 0.33 |
| CaO | 1.96 | 3.59 | 1.29 | 0.24 | 0.61 | 0.38 | 0.39 |
| Na2O | 0.14 | 0.19 | 0.18 | 0.07 | 0.10 | 0.14 | 2.33 |
| K2O | 1.13 | 2.11 | 1.62 | 1.43 | 2.89 | 2.53 | 4.29 |
| P2O5 | 0.06 | 0.07 | 0.08 | 0.06 | 0.10 | 0.06 | 0.08 |
| LOI | 6.42 | 7.91 | 5.68 | 4.13 | 4.75 | 3.98 | 2.96 |
| Total | 100.34 | 100.02 | 99.63 | 99.50 | 99.60 | 99.03 | 99.58 |
| Li | 2229 | 2627 | 3014 | 1421 | 2806 | 2323 | 349 |
| Rb | 43.9 | 62.6 | 46.4 | 39.7 | 90.8 | 88.1 | 163.9 |
| Sr | 149 | 106 | 119 | 111 | 127 | 79 | 152 |
| Ba | 154 | 201 | 223 | 186 | 570 | 657 | 673 |
| Zr | 70.6 | 80.6 | 66.0 | 72.5 | 91.4 | 90.2 | 70.2 |
| Nb | 2.29 | 2.29 | 2.80 | 1.87 | 2.39 | 1.91 | 2.35 |
| Th | 8.07 | 6.10 | 6.98 | 5.97 | 6.85 | 5.86 | 5.55 |
| U | 4.97 | 2.94 | 1.03 | 1.01 | 1.41 | 1.55 | 1.73 |
| Pb | 30.5 | 18.0 | 10.0 | 9.3 | 8.0 | 5.4 | 7.9 |
| V | 24.4 | 26.1 | 22.7 | 12.1 | 10.7 | 6.9 | 14.5 |
| Co | 3.77 | 2.74 | 11.18 | 0.81 | 1.22 | 0.78 | 0.93 |
| Ni | 54 | 117 | 55 | 56 | 31 | 54 | 31 |
| Cu | 8.67 | 55.31 | 72.12 | 1.99 | 15.14 | 2.71 | 9.82 |
| Sn | 1.29 | 0.50 | 1.93 | 0.78 | 0.96 | 0.74 | 0.73 |
| Cs | 4.92 | 8.51 | 5.59 | 3.94 | 7.43 | 6.43 | 7.26 |
| La | 18.0 | 12.9 | 14.4 | 10.8 | 21.2 | 10.7 | 16.1 |
| Ce | 34.4 | 21.9 | 26.6 | 18.8 | 42.8 | 19.0 | 30.8 |
| Pr | 3.68 | 2.76 | 3.11 | 2.30 | 4.84 | 2.42 | 3.32 |
| Nd | 12.70 | 9.94 | 10.76 | 8.14 | 17.16 | 8.91 | 11.59 |
| Hf | 2.03 | 2.10 | 2.07 | 1.87 | 2.37 | 2.15 | 1.98 |
| Sm | 2.09 | 1.75 | 1.71 | 1.35 | 2.72 | 1.59 | 1.84 |
| Eu | 0.61 | 0.52 | 0.50 | 0.43 | 0.80 | 0.62 | 0.57 |
| Gd | 1.60 | 1.73 | 1.44 | 1.13 | 1.80 | 1.29 | 1.51 |
| Tb | 0.21 | 0.26 | 0.21 | 0.15 | 0.21 | 0.17 | 0.21 |
| Dy | 0.96 | 1.33 | 0.96 | 0.67 | 0.82 | 0.78 | 0.98 |
| Ho | 0.17 | 0.24 | 0.17 | 0.11 | 0.14 | 0.13 | 0.17 |
| Er | 0.46 | 0.61 | 0.45 | 0.30 | 0.39 | 0.35 | 0.46 |
| Yb | 0.36 | 0.45 | 0.33 | 0.24 | 0.29 | 0.25 | 0.35 |
| Lu | 0.06 | 0.07 | 0.06 | 0.04 | 0.05 | 0.05 | 0.06 |
| Y | 5.84 | 8.55 | 6.05 | 4.29 | 4.94 | 4.79 | 6.03 |
| ΣREE | 85.3 | 66.9 | 70.8 | 51.1 | 101.1 | 53.7 | 77.0 |
| LREE/HREE | 5.2 | 2.9 | 4.2 | 4.5 | 7.7 | 4.1 | 5.0 |
| δEu | 1.02 | 0.91 | 0.97 | 1.06 | 1.10 | 1.32 | 1.05 |
| Sub Groups | Core Samples | Earth Surface Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample No. | Composition of “Original Rock” | 17AM5 | 17AM6 | 17AM7 | 17AM8 | 17AM9 | 17AM10 | 17AM11 |
| Reference Frame | 0.99 | 1.11 | 0.93 | 0.98 | 0.83 | 0.93 | 0.84 | |
| SiO2 | 75.2 | −2% | 5% | −7% | 4% | −18% | −5% | −19% |
| TiO2 | 0.19 | 14% | 22% | 19% | 3% | 19% | 3% | 17% |
| Al2O3 | 12.99 | - | - | - | - | - | - | - |
| Fe2O3T | 1.30 | 6% | 79% | −19% | −70% | −63% | −46% | −54% |
| MgO | 0.03 | 2894% | 5803% | 1844% | 539% | 625% | 690% | 802% |
| MnO | 0.26 | −79% | −75% | −88% | −97% | −98% | −97% | −99% |
| CaO | 0.32 | 502% | 1146% | 274% | −25% | 57% | 12% | 2% |
| Na2O | 2.65 | −95% | −92% | −94% | −97% | −97% | −95% | −27% |
| K2O | 5.16 | −78% | −54% | −71% | −73% | −53% | −54% | −31% |
| P2O5 | 0.04 | 72% | 101% | 110% | 69% | 115% | 58% | 89% |
| Rb | 165.7 | −74% | −58% | −74% | −77% | −54% | −50% | −17% |
| Sr | 87.5 | 67% | 35% | 26% | 24% | 20% | −16% | 45% |
| Ba | 372.5 | −59% | −40% | −44% | −51% | 27% | 65% | 51% |
| Y | 24.7 | −77% | −61% | −77% | −83% | −83% | −82% | −80% |
| Zr | 192.9 | −64% | −53% | −68% | −63% | −61% | −56% | −70% |
| Nb | 13.6 | −83% | −81% | −81% | −86% | −85% | −87% | −86% |
| Th | 14.3 | −45% | −53% | −55% | −59% | −60% | −62% | −68% |
| U | 9.2 | −47% | −64% | −90% | −89% | −87% | −84% | −84% |
| Hf | 6.9 | −71% | −66% | −72% | −73% | −71% | −71% | −76% |
| Ta | 0.9 | −54% | −80% | −83% | −85% | −84% | −84% | −82% |
| Pb | 24.7 | 22% | −19% | −63% | −63% | −73% | −80% | −73% |
| V | 15.9 | 51% | 82% | 32% | −26% | −44% | −60% | −24% |
| Sn | 2.4 | −47% | −77% | −25% | −68% | −67% | −71% | −74% |
| Co | 3.7 | 2% | −17% | 184% | −78% | −72% | −80% | −79% |
| Ni | 4.7 | 1038% | 2686% | 989% | 1068% | 445% | 976% | 463% |
| Cu | 5.7 | 51% | 986% | 1082% | −66% | 122% | −55% | 45% |
| La | 40.3 | −56% | −64% | −67% | −74% | −56% | −75% | −67% |
| Ce | 81.4 | −58% | −70% | −70% | −77% | −56% | −78% | −68% |
| Pr | 10.2 | −64% | −70% | −72% | −78% | −61% | −78% | −73% |
| Nd | 37.5 | −67% | −70% | −73% | −79% | −62% | −78% | −74% |
| Sm | 6.8 | −70% | −71% | −77% | −81% | −67% | −78% | −77% |
| Eu | 0.6 | 4% | 0% | −20% | −28% | 14% | −1% | −18% |
| Gd | 6.0 | −74% | −68% | −78% | −81% | −75% | −80% | −79% |
| Tb | 0.9 | −76% | −67% | −78% | −83% | −80% | −82% | −80% |
| Dy | 4.8 | −80% | −69% | −81% | −86% | −86% | −85% | −83% |
| Ho | 0.9 | −82% | −71% | −83% | −88% | −87% | −87% | −85% |
| Er | 2.7 | −83% | −74% | −84% | −89% | −88% | −88% | −85% |
| Yb | 2.9 | −88% | −82% | −89% | −92% | −92% | −92% | −90% |
| Lu | 0.4 | −86% | −82% | −88% | −90% | −91% | −90% | −89% |
| Alteration zones | Chloritization | Montmorillonitization | Calcitization | Dolomitization |
|---|---|---|---|---|
| Least mineralized sample |  | |||
| Mineralized samples (from earth surface) | ||||
| Mineralized samples (from drilling hole) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, Z.; Wu, T.; Luo, Y.; Zhao, J.; Li, X.; Yang, W.; Chen, X. Metallogenic Characteristics and Formation Mechanism of Naomugeng Clay-Type Lithium Deposit in Central Inner Mongolia, China. Minerals 2021, 11, 238. https://doi.org/10.3390/min11030238
Li C, Li Z, Wu T, Luo Y, Zhao J, Li X, Yang W, Chen X. Metallogenic Characteristics and Formation Mechanism of Naomugeng Clay-Type Lithium Deposit in Central Inner Mongolia, China. Minerals. 2021; 11(3):238. https://doi.org/10.3390/min11030238
Chicago/Turabian StyleLi, Chuan, Zilong Li, Tao Wu, Yaqin Luo, Jun Zhao, Xinren Li, Wencai Yang, and Xuegang Chen. 2021. "Metallogenic Characteristics and Formation Mechanism of Naomugeng Clay-Type Lithium Deposit in Central Inner Mongolia, China" Minerals 11, no. 3: 238. https://doi.org/10.3390/min11030238
APA StyleLi, C., Li, Z., Wu, T., Luo, Y., Zhao, J., Li, X., Yang, W., & Chen, X. (2021). Metallogenic Characteristics and Formation Mechanism of Naomugeng Clay-Type Lithium Deposit in Central Inner Mongolia, China. Minerals, 11(3), 238. https://doi.org/10.3390/min11030238







