Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore
Abstract
1. Introduction
2. Materials and Methods
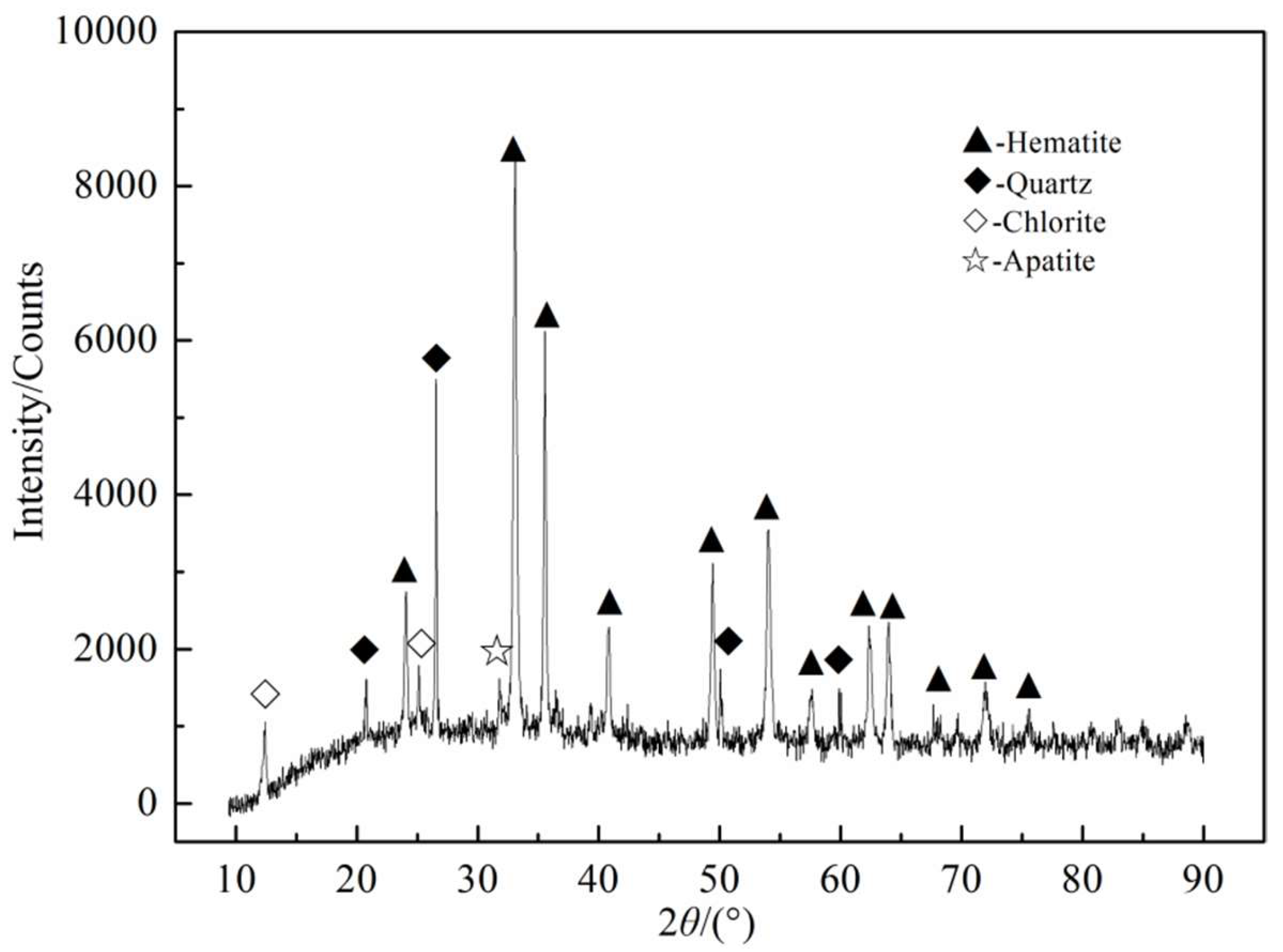
2.1. Materials
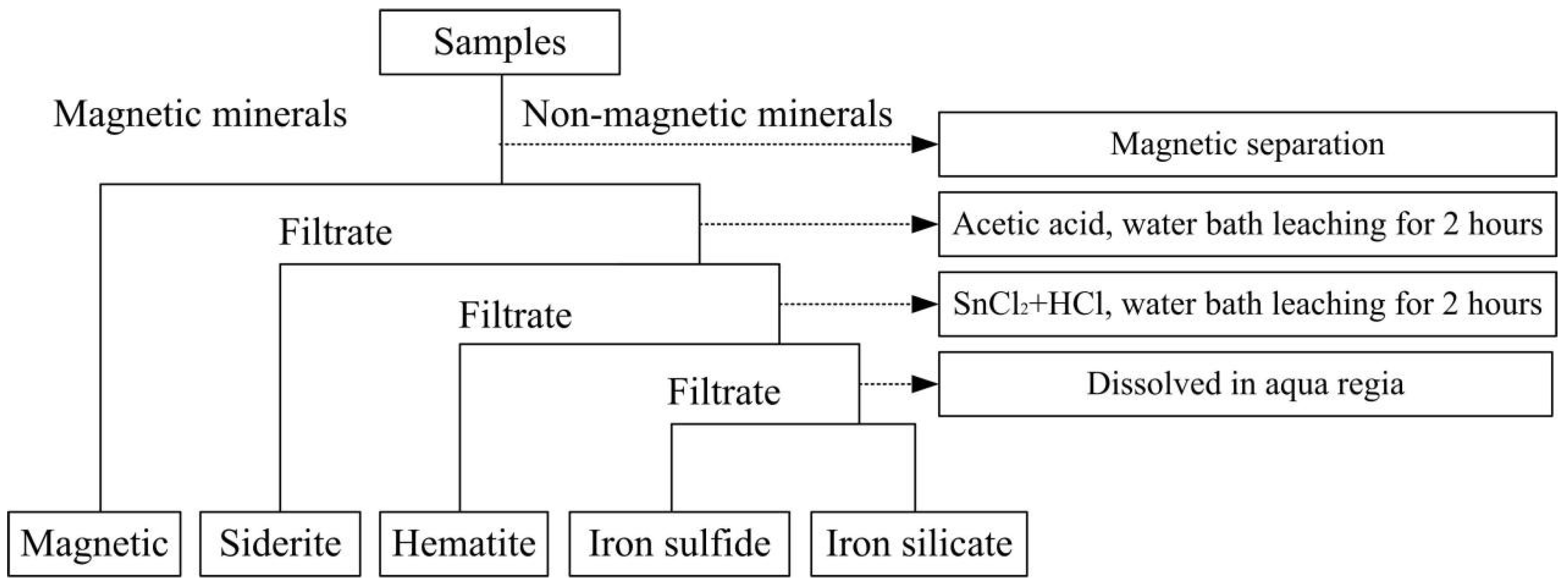
2.2. Methods and Equipment
3. Results and Discussion
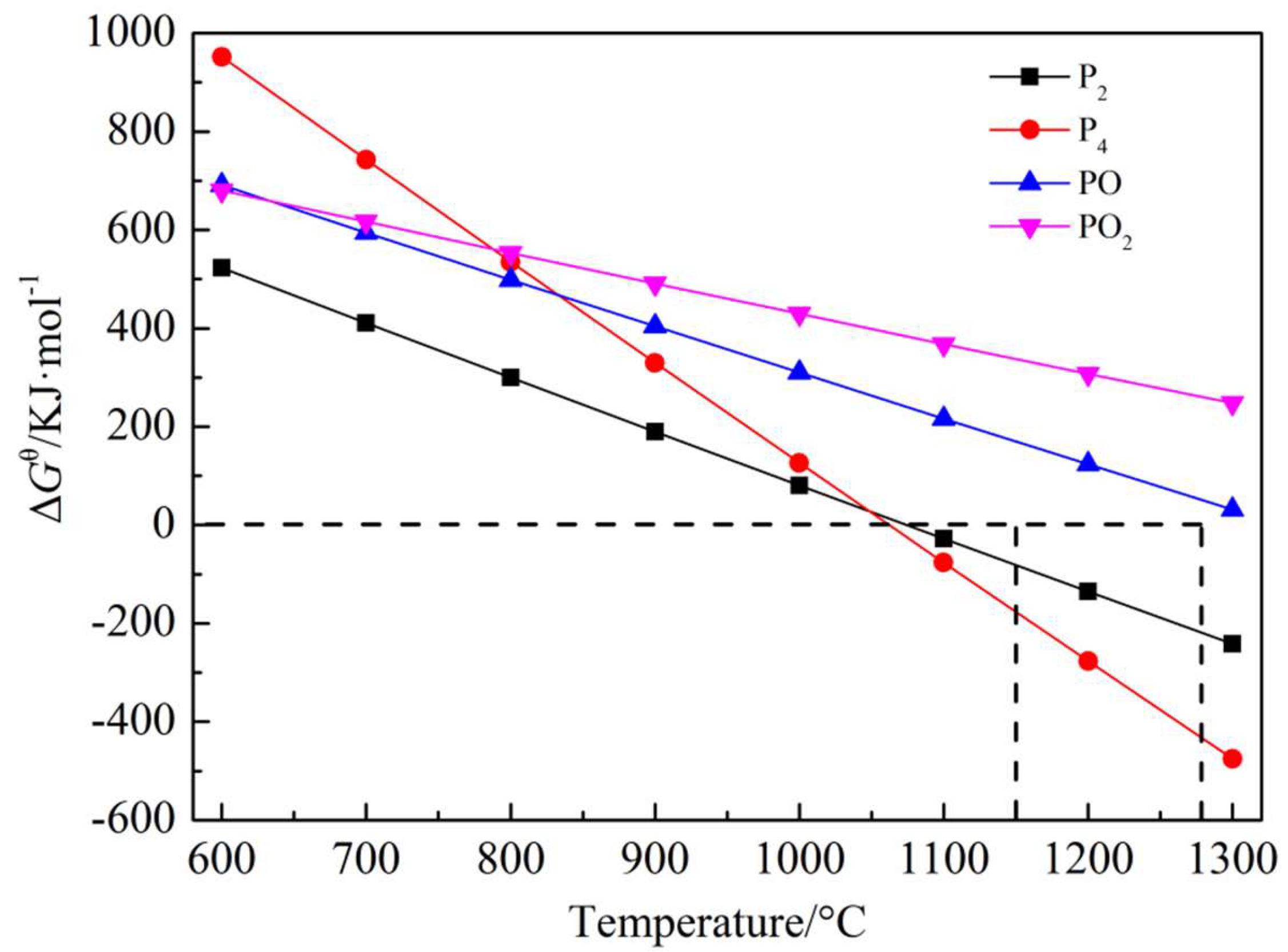
3.1. Analysis of Volatile Phase of Phosphorus
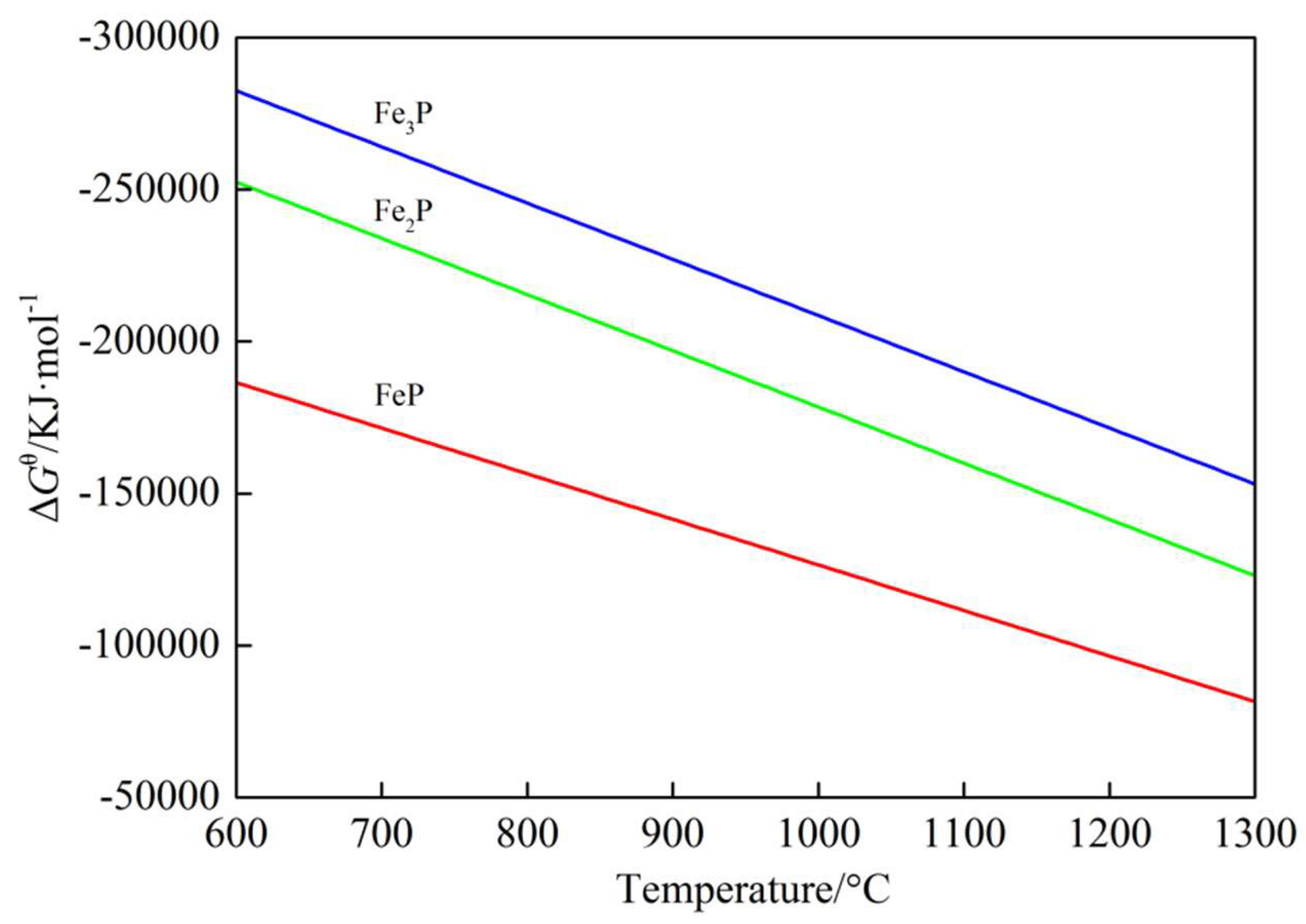
3.2. Reduction Behavior of Phosphorus Minerals
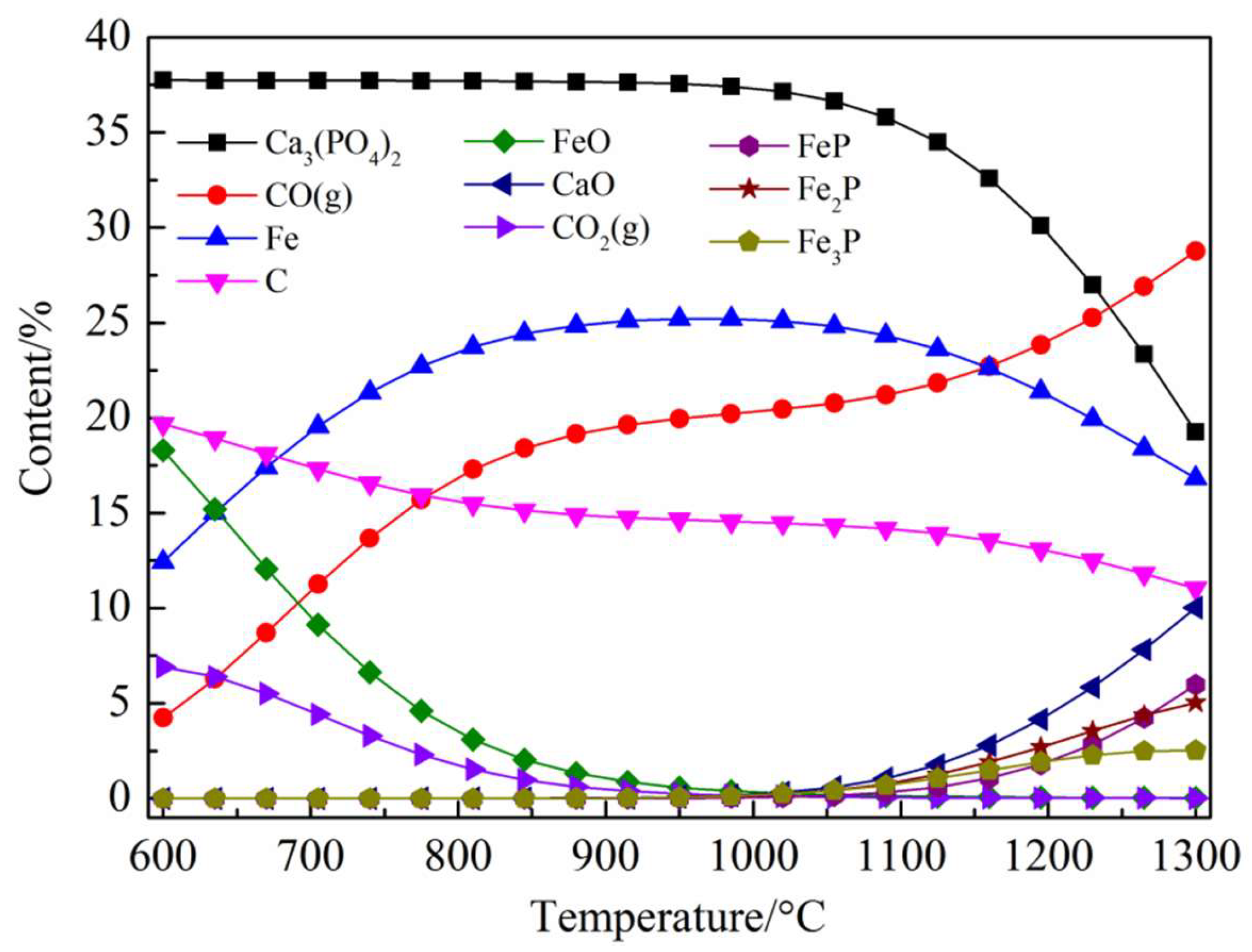
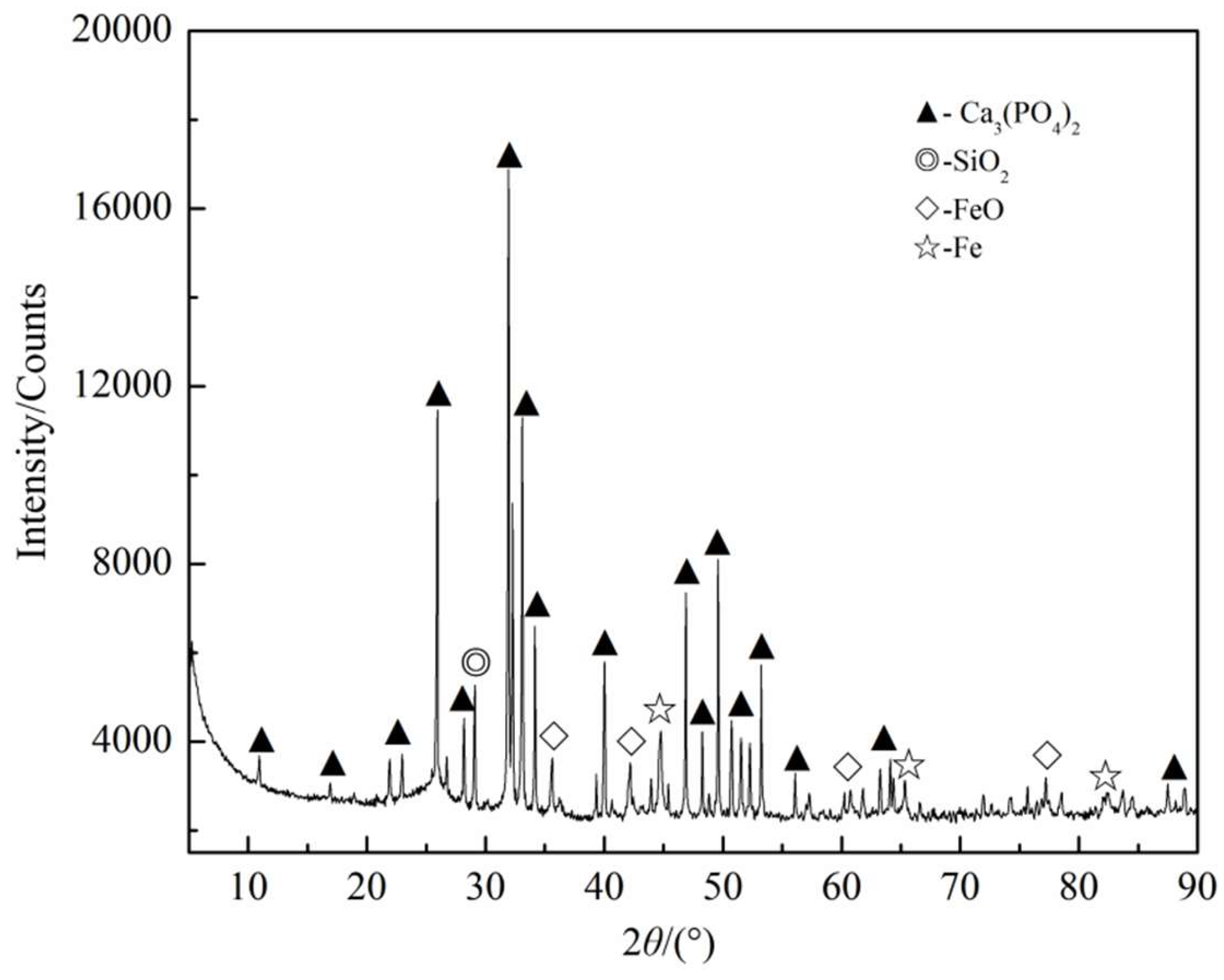
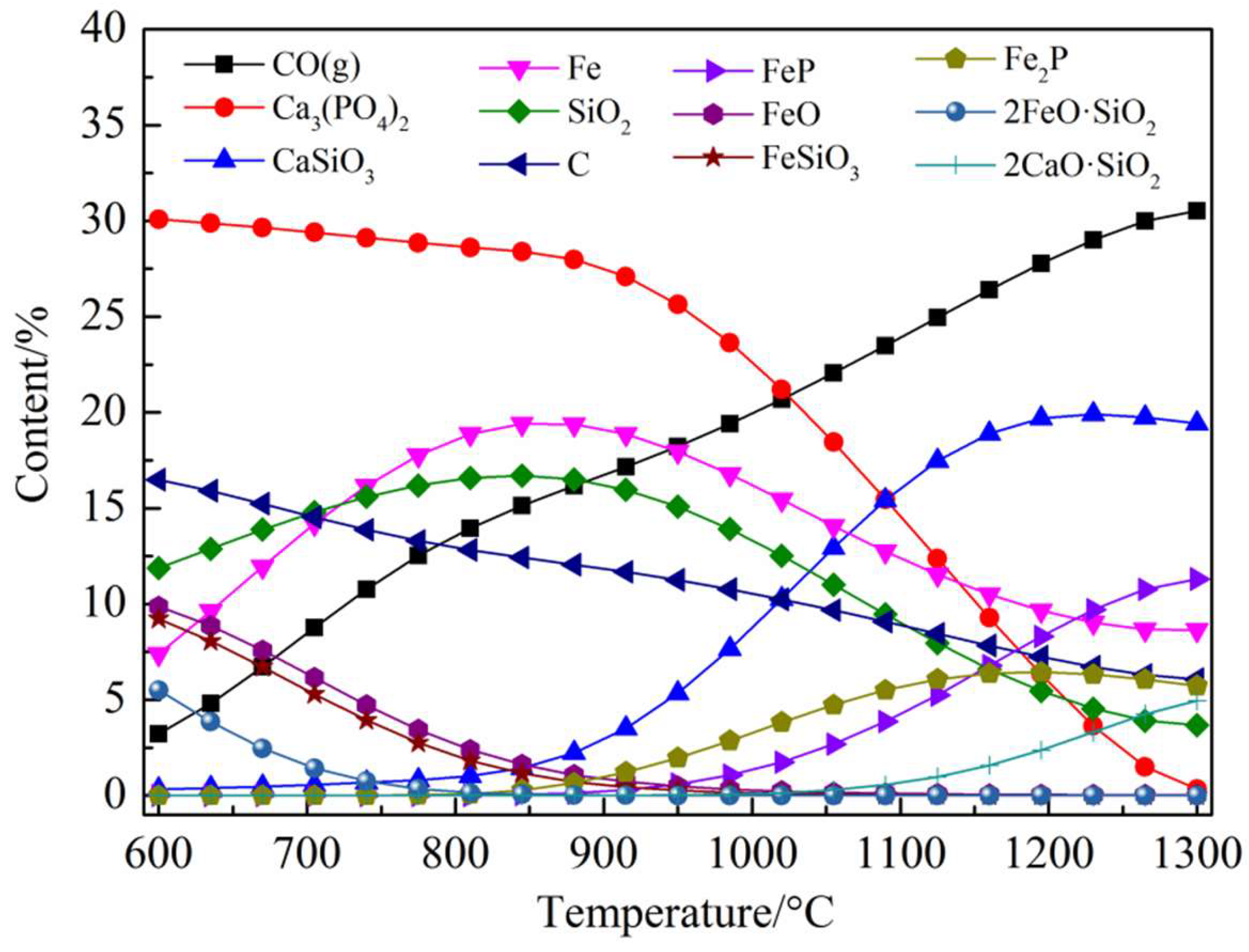
3.2.1. Ca3(PO4)2–C–Fe2O3 System
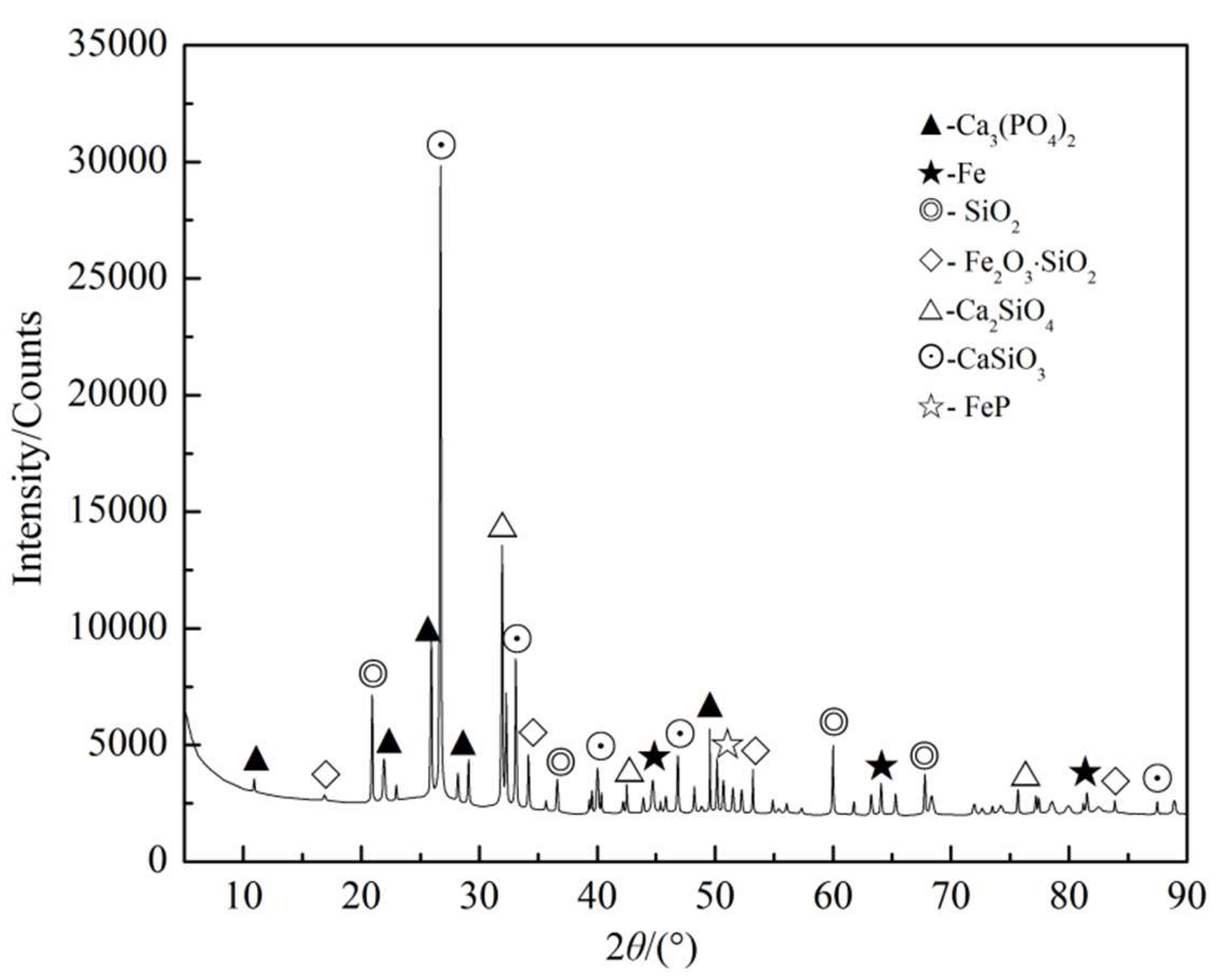
3.2.2. Ca3(PO4)2–C–Fe2O3–SiO2 System
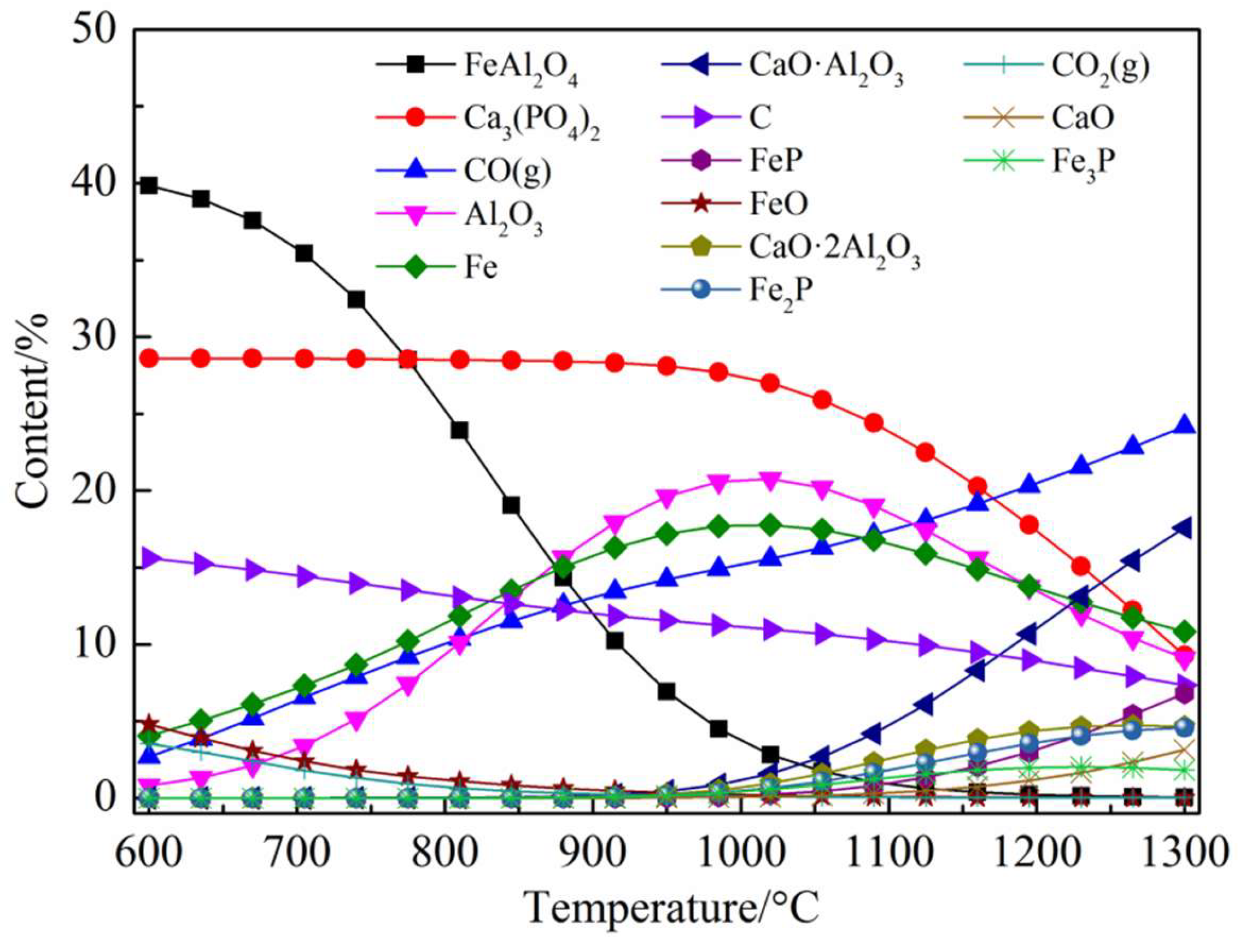
3.2.3. Ca3(PO4)2–C–Fe2O3–Al2O3 System
3.3. Existing Form of Phosphorus
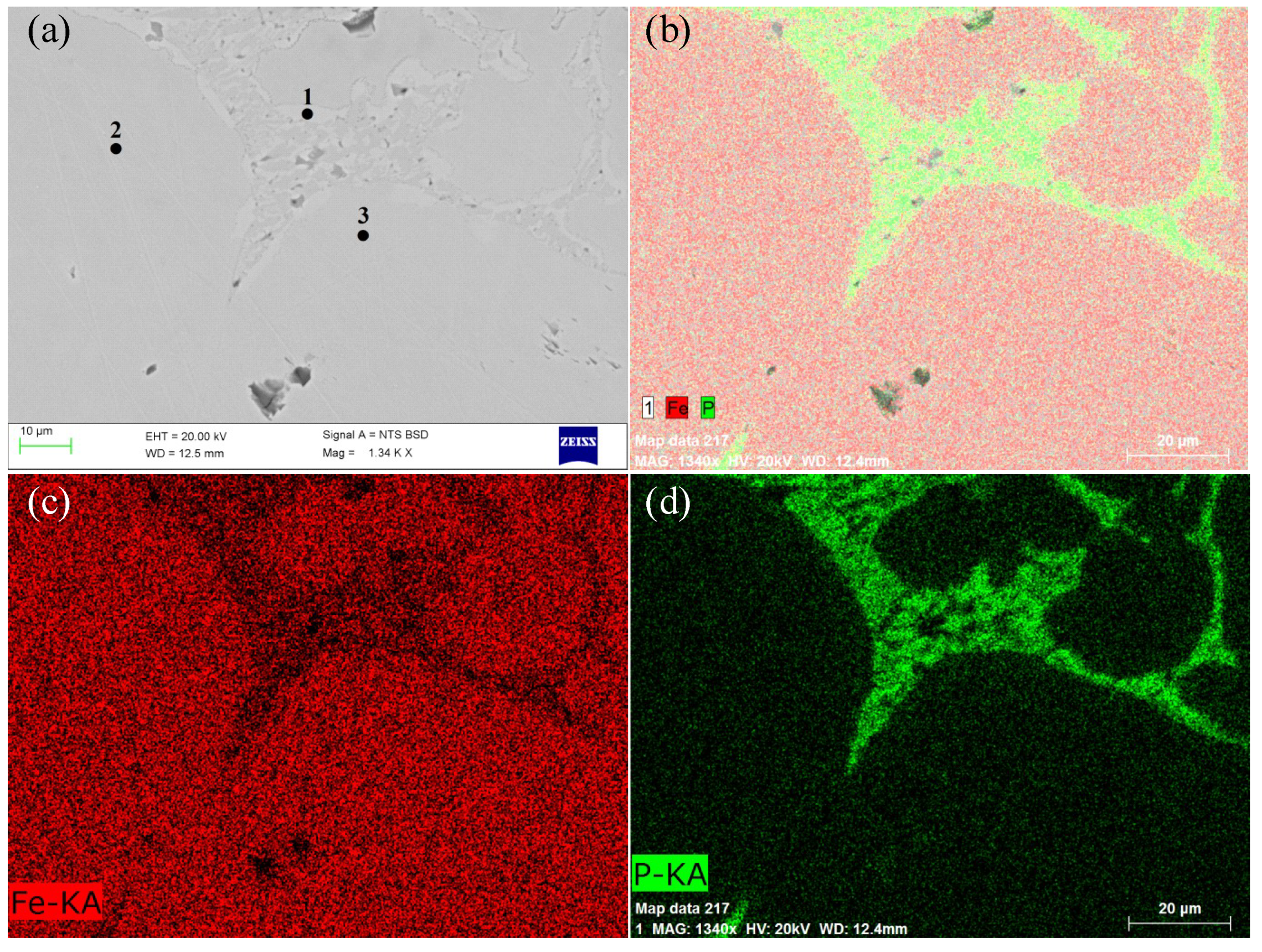
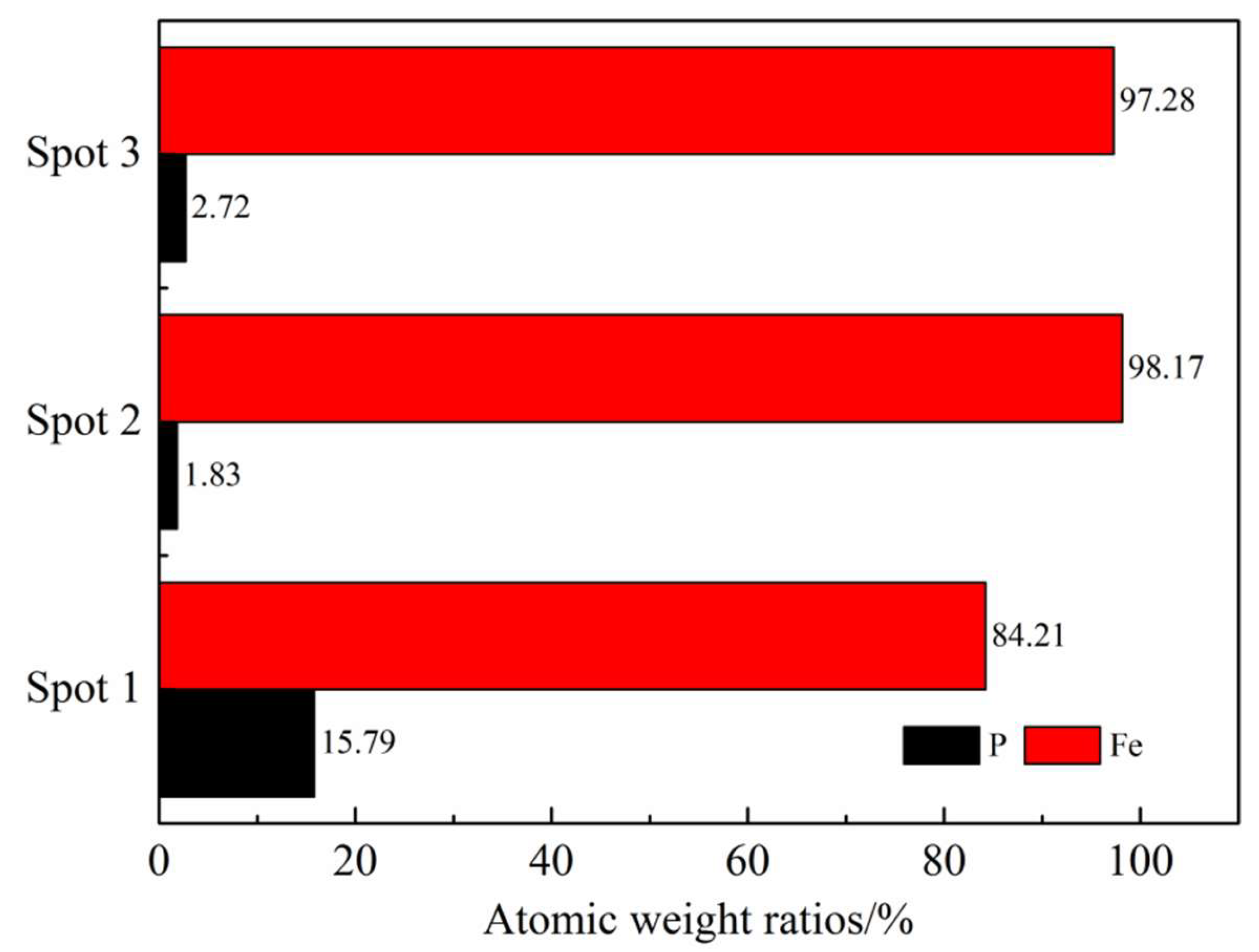
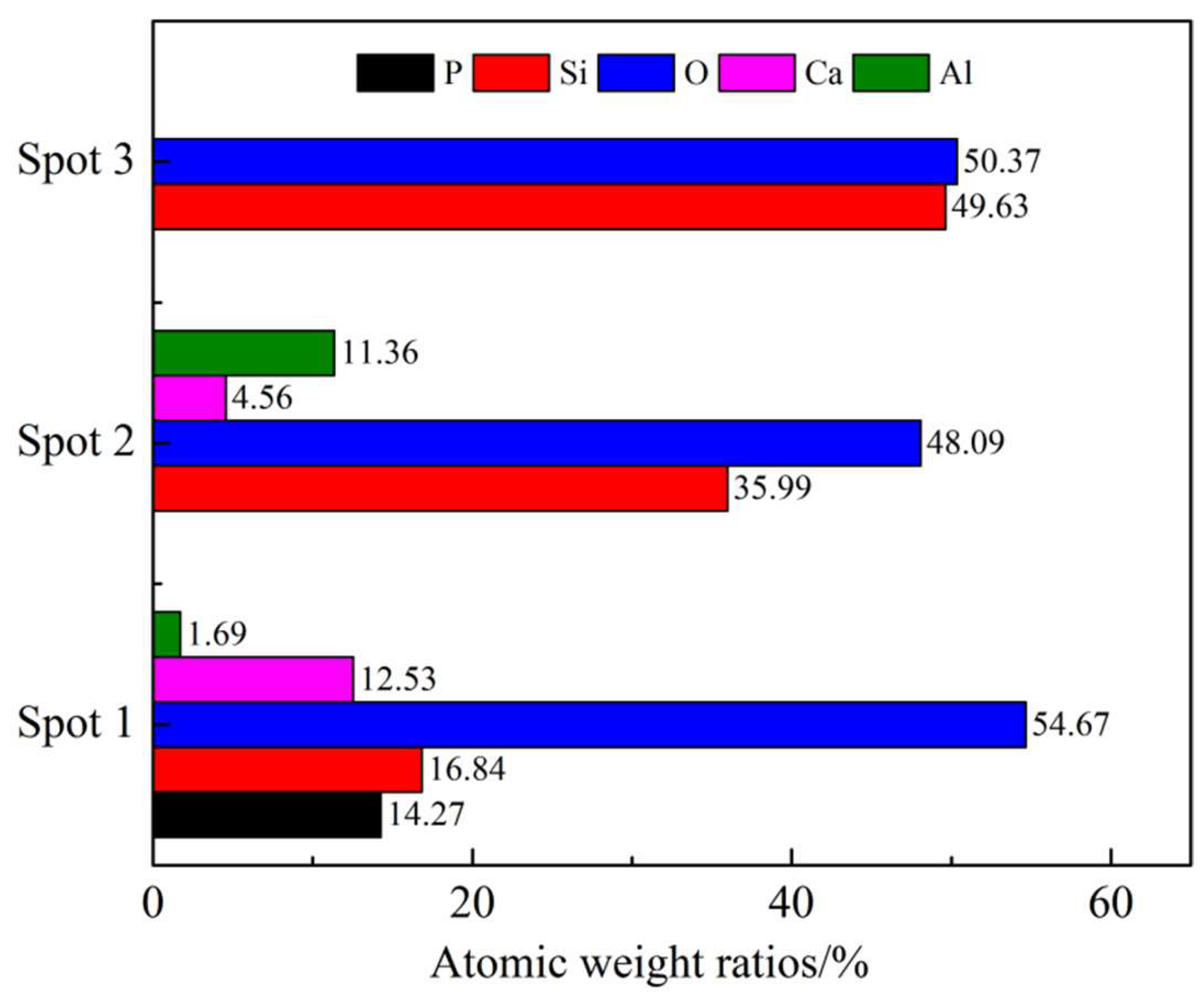
3.3.1. Phosphorus in the Metal Phase
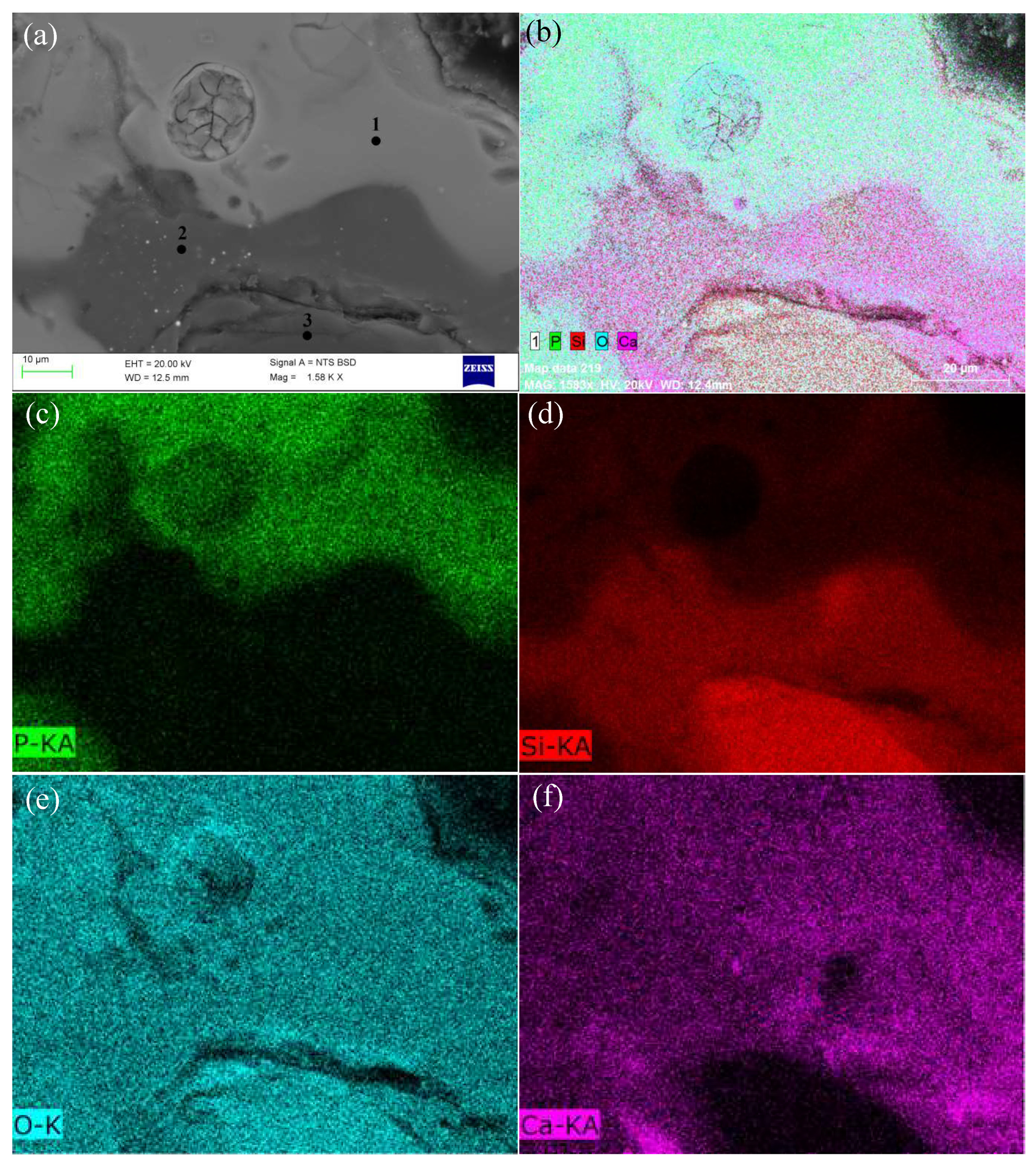
3.3.2. Phosphorus in the Slag Phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.L.; Gu, X.T.; Han, Y.X.; Parra-Álvarez, N.; Claremboux, V.; Kawatra, S.K. Flotation of iron ores: A review. Min. Proc. Ext. Met. Rev. 2019, 1–29. [Google Scholar] [CrossRef]
- Zhang, X.L.; Han, Y.X.; Sun, Y.S.; Lv, Y.; Li, Y.J.; Tang, Z.D. An novel method for iron recovery from iron ore tailings with pre-concentration followed by magnetization roasting and magnetic separation. Min. Proc. Ext. Met. Rev. 2020, 2, 117–129. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Tian, J.; Lu, Z.; Sun, W.; Hu, Y. Adsorption of Pb (II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhu, X.R.; Han, Y.X.; Li, Y.J. Green magnetization roasting technology for refractory iron ore using siderite as a reductant. J. Clean Prod. 2019, 206, 40–50. [Google Scholar] [CrossRef]
- Gao, P.; Li, G.F.; Han, Y.X.; Sun, Y.S. Reaction behavior of phosphorus in coal-based reduction of an oolitic hematite ore and pre-dephosphorization of reduced iron. Metals 2016, 6, 82. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhang, Q.; Han, Y.X.; Gao, P.; Li, G.F. Comprehensive utilization of iron and phosphorus from high-phosphorus refractory iron ore. JOM 2018, 70, 144–149. [Google Scholar] [CrossRef]
- Kang, Y. Desiliconisation and dephosphorisation behaviours of various oxygen sources in hot metal pre-treatment. Metals 2019, 2, 251. [Google Scholar] [CrossRef]
- Mamdouh, O.; Timo, F.; Riku, M. Effect of microwave pre-treatment on the magnetic properties of iron ore and its implications on magnetic separation. Sep. Purif. Technol. 2014, 136, 223–232. [Google Scholar]
- Delvasto, P.; Ballester, A.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Igual, J.M.; Valverde, A.; García-Balboa, C. Mobilization of phosphorus from iron ore by the bacterium Burkholderia caribensis FeGL03. Min. Eng. 2009, 22, 1–9. [Google Scholar] [CrossRef]
- Mamdouh, O.; Timo, F.; Riku, M. Influence of microwave radiation on the magnetic properties of molybdenite and arsenopyrite. Powder Technol. 2017, 15, 276–281. [Google Scholar]
- Ionkov, K.; Gaydardzhiev, S. Amenability for processing of oolitic iron ore concentrate for phosphorus removal. Min. Eng. 2013, 46–47, 119–127. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Zhang, Y.R.; Sun, T.C. Dephosphorization behavior of high-phosphorus oolitic hematite-solid waste containing carbon briquettes during the process of direct reduction-magnetic separation. Metals 2018, 11, 897. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xue, Q.G.; Wang, G.; Wang, J.S. Phosphorus containing mineral evolution and thermodynamics of phosphorus vaporization during carbothermal reduction of high-phosphorus iron ore. Metals 2018, 6, 451. [Google Scholar] [CrossRef]
- Mamdouh, O.; Timo, F.; Riku, M. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore. Appl. Surf. Sci. 2015, 45, 127–140. [Google Scholar]
- Xia, W.T.; Ren, Z.D.; Gao, Y.F. Removal of phosphorus from high phosphorus iron ores by selective HCl leaching method. J. Iron Steel Res. Int. 2011, 18, 1–4. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, W.T.; Han, Y.X.; Li, Y.J. Efficient enrichment of low grade refractory rhodochrosite by preconcentration-neutral suspension roasting-magnetic separation process. Powder Technol. 2020, 361, 529–539. [Google Scholar] [CrossRef]
- Tang, Z.D.; Gao, P.; Sun, Y.S.; Han, Y.X.; Li, E.L.; Chen, J.; Zhang, Y.H. Studies on the fluidization performance of a novel fluidized bed reactor for iron ore suspension roasting. Powder Technol. 2020, 360, 649–657. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Zhang, Z.Q.; Luo, L.Q.; Yu, H. Behavior of Fe and P during reduction magnetic roasting-separation of phosphorus-rich oolitic hematite. Energ. Source. Part. A 2019, 1, 47–64. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhou, W.T.; Han, Y.X.; Li, Y.J. Effect of different additives on reaction characteristics of fluorapatite during coal-based reduction of iron ore. Metals 2019, 9, 923. [Google Scholar] [CrossRef]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Yu, J.W. Size distribution behavior of metallic iron particles in coal-based reduction products of an oolitic iron ore. Min. Proc. Ext. Met. Rev. 2015, 36, 249–257. [Google Scholar] [CrossRef]
- Sun, Y.S.; Li, Y.F.; Han, Y.X.; Li, Y.J. Migration behaviors and kinetics of phosphorus during coal-based reduction of high-phosphorus oolitic iron ore. Int. J. Min. Met. Mater. 2019, 8, 938–945. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.H.; Rao, M.J.; Mi, H.P.; Liang, B.J.; You, J.X.; Peng, Z.W.; Jiang, T. Growth of metallic iron particles during reductive roasting of boron-bearing magnetite concentrate. J. Cent. South. Univ. 2020, 5, 1484–1494. [Google Scholar] [CrossRef]
- Xie, R.Q.; Zhu, Y.M.; Liu, J.; Wang, X.; Li, Y.J. Differential collecting performance of a new complex of decyloxy-propyl-amine and α-bromododecanoic acid on flotation of spodumene and feldspar. Min. Eng. 2020, 153, 106377. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.L.; Sun, H.R.; Yin, W.Z.; Hong, J.S.; Cao, S.H.; Tang, Y.; Zhao, C.; Yao, J. Improving flotation separation of apatite from dolomite using PAMS as a novel eco-friendly depressant. Min. Eng. 2020, 156, 106492. [Google Scholar] [CrossRef]
- Liu, J.; Xie, R.Q.; Zhu, Y.M.; Li, Y.J.; Liu, C. Flotation behavior and mechanism of styrene phosphonic acid as collector on the flotation separation of fluorite from calcite. J. Mol. Liq. 2021, 115261. [Google Scholar] [CrossRef]
- Xie, R.Q.; Zhu, Y.M.; Liu, J.; Li, Y.J. The flotation behavior and adsorption mechanism of a new cationic collector on the separation of spodumene from feldspar and quartz. Sep. Purif. Technol. 2021, 264, 118445. [Google Scholar] [CrossRef]
- Zhou, W.T.; Sun, Y.S.; Han, Y.X.; Gao, P.; Li, Y.J. Recycling iron from oolitic hematite via microwave fluidization roasting and magnetic separation. Min. Eng. 2021, 106851. [Google Scholar]
- Wang, N.; Liang, Z.G.; Chen, M.; Zou, Z.S. Phosphorous enrichment in molten adjusted converter slag: Part I Effect of adjusting technological conditions. J. Iron Steel. Res. Int. 2011, 18, 17–19, 39. [Google Scholar] [CrossRef]
- Wang, N.; Liang, Z.G.; Chen, M.; Zou, Z.S. Phosphorous enrichment in molten adjusted converter slag: Part II Enrichment behavior of phosphorus in CaO-SiO2-FeOx-P2O5 molten slag. J. Iron Steel. Res. Int. 2011, 18, 22–26. [Google Scholar] [CrossRef]
- Inoue, R.; Suito, H. Phosphorous partition between 2CaO·SiO2 particles and 2CaO-SiO2-FetO slags. ISIJ Int. 2006, 46, 174–179. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Behavior of phosphorous transfer from CaO-FetO-P2O5(-SiO2) slag to CaO particles. ISIJ Int. 2006, 46, 180–187. [Google Scholar] [CrossRef]
- Fukagai, S.; Hamamo, T.; Tsukihashi, F. Formation reaction of phosphate compound in multi phase flux at 1573 K. ISIJ Int. 2007, 47, 187–189. [Google Scholar] [CrossRef]
- Yang, X.; Matsuura, H.; Tsukihashi, F. Condensation of P2O5 at the interface between 2CaO·SiO2 and CaO-SiO2-FeOx-P2O5 slag. ISIJ Int. 2009, 49, 1298–1307. [Google Scholar] [CrossRef]



| Component | TFe | SiO2 | Al2O3 | CaO | MgO | P | FeO | Fe2O3 | S |
|---|---|---|---|---|---|---|---|---|---|
| Content | 42.21 | 21.80 | 5.47 | 4.33 | 0.59 | 1.31 | 4.31 | 55.51 | 0.13 |
| Phase Analysis of Iron | Iron in Hematite | Iron in Magnetic | Iron in Siderite | Iron in Sulfide | Iron in Silicate | Total |
|---|---|---|---|---|---|---|
| Content | 40.97 | 0.54 | 0.37 | 0.13 | 0.19 | 42.21 |
| Distribution rate | 97.09 | 1.28 | 0.88 | 0.31 | 0.45 | 100.00 |
| P | TFe | SiO2 | CaO | Al2O3 | MgO | F | Cl |
|---|---|---|---|---|---|---|---|
| 17.56 | 0.012 | 3.44 | 53.49 | <0.01 | <0.01 | 4.73 | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Zhou, W.; Sun, Y.; Han, Y.; Li, Y. Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore. Minerals 2021, 11, 247. https://doi.org/10.3390/min11030247
Jin J, Zhou W, Sun Y, Han Y, Li Y. Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore. Minerals. 2021; 11(3):247. https://doi.org/10.3390/min11030247
Chicago/Turabian StyleJin, Jianping, Wentao Zhou, Yongsheng Sun, Yuexin Han, and Yanjun Li. 2021. "Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore" Minerals 11, no. 3: 247. https://doi.org/10.3390/min11030247
APA StyleJin, J., Zhou, W., Sun, Y., Han, Y., & Li, Y. (2021). Reaction Characteristics and Existing Form of Phosphorus during Coal-Based Reduction of Oolitic Iron Ore. Minerals, 11(3), 247. https://doi.org/10.3390/min11030247






