A Novel Open-System Method for Synthesizing Muscovite from a Biotite-Rich Coal Tailing
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Its Preparation
2.2. Synthesizing Approach
2.3. Characterization
3. Results and Discussion
3.1. XRD and SEM Analyzes
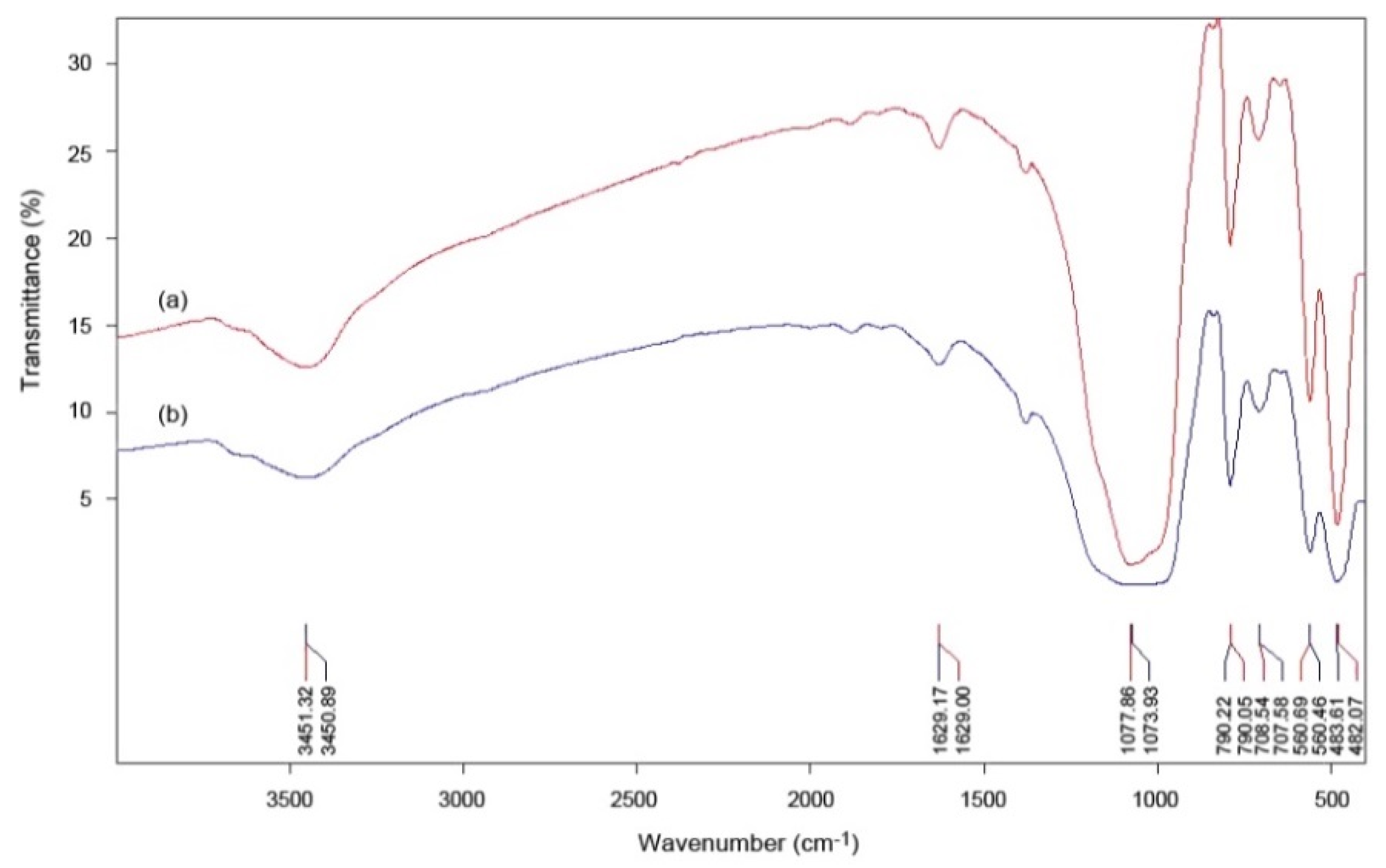
3.2. Infrared Spectroscopy Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
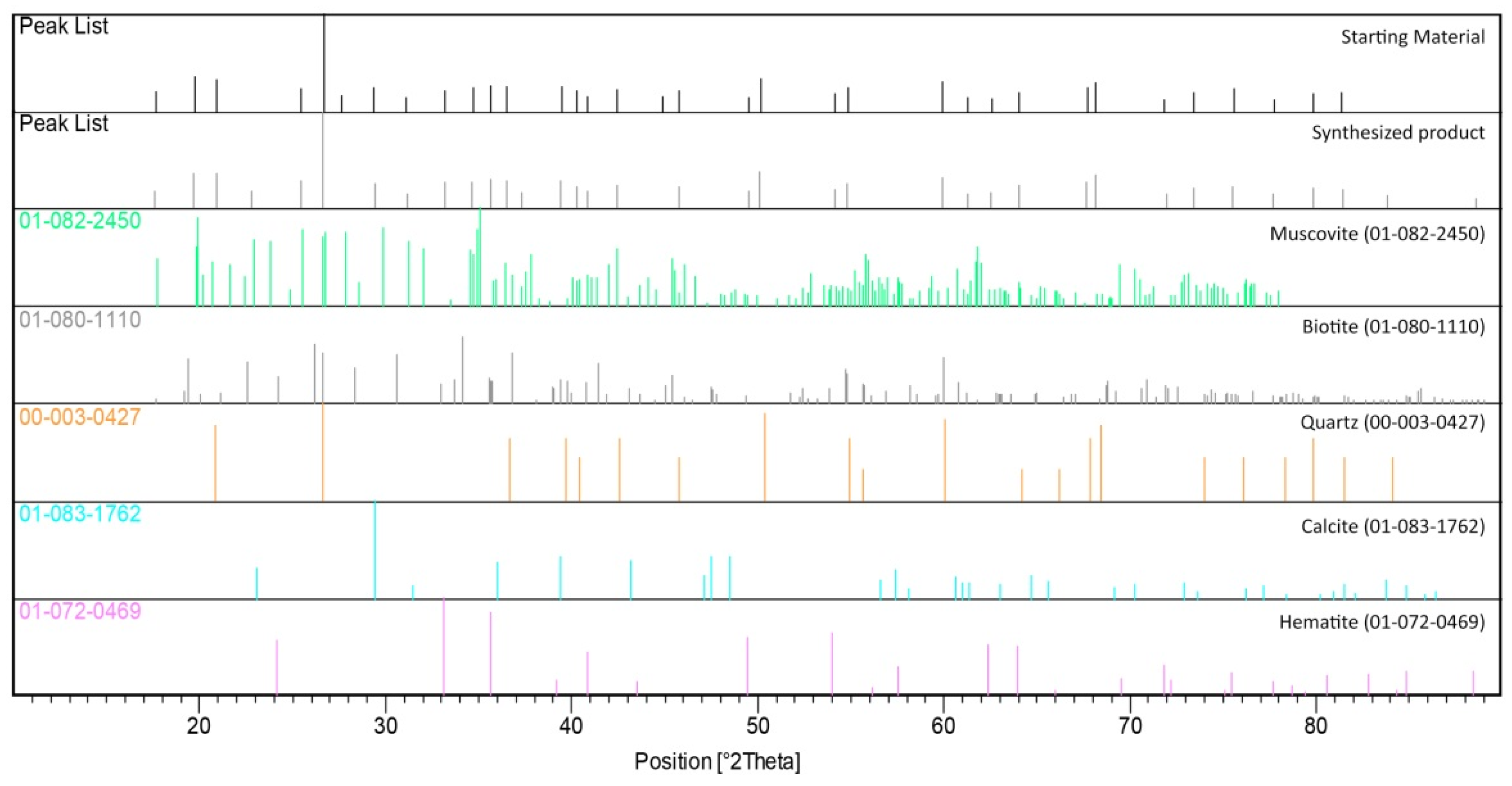
| Phase | Major Phases (wt%) | Minor Phases (wt%) | |||
|---|---|---|---|---|---|
| Sample | Biotite | Muscovite | Quartz | Calcite | Hematite |
| Starting material | 64.4 | 28.7 | 3.9 | 2.0 | 1.0 |
| Product | 27.6 | 67.7 | 2.7 | 1.0 | 1.0 |
| 2θ (Degree) | d (Å) | hkl | Kα1 (Degree) | Kα2 (Degree) |
|---|---|---|---|---|
| 39.4824 | 2.282 | 220 | 39.448 | 39.55 |
| 44.8831 | 2.01953 | 005 | 44.844 | 44.961 |
| 71.8665 | 1.31372 | 064 | 71.797 | 72.003 |
| 75.5809 | 1.25811 | 262 | 75.507 | 75.728 |
| 77.7888 | 1.22783 | 172 | 77.712 | 77.942 |
| 79.8642 | 1.20106 | −405 | 79.785 | 80.023 |
| 81.4022 | 1.18124 | −228 | 81.402 | 81.647 |
| 2θ (Degree) | d (Å) | hkl | Kα1 (Degree) | Kα2 (Degree) |
|---|---|---|---|---|
| 34.6530 | 2.58863 | 200 | 34.623 | 34.712 |
| 40.2683 | 2.23967 | 220 | 40.233 | 40.338 |
| 50.1131 | 1.82034 | 0210 | 50.069 | 50.202 |
| 63.9994 | 1.45483 | 0213 | 63.940 | 64.118 |
| 68.1187 | 1.37655 | −337 | 68.054 | 68.247 |
| 71.9970 | 1.31164 | −1115 | 71.928 | 72.135 |
| 73.4356 | 1.28946 | 068 | 73.365 | 73.577 |
| 2θ | θ (Radian) | Λ | Sinθ | Sin2θ | λ α12/λ α22 | Sin2θ | h | k | l | 4(Sin2θ)/λ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 39.448 | 0.344249 | kα1 | 0.33749 | 0.1138992 | 0.995048962 | 0.1138992 | 2 | 2 | 0 | 0.191965649 |
| 39.55 | 0.345139 | kα2 | 0.338327 | 0.1144654 | 0.995048962 | 0.1138987 | 2 | 2 | 0 | 0.191964722 |
| 44.844 | 0.391338 | kα1 | 0.381425 | 0.1454853 | 0.995048962 | 0.1454853 | 0 | 0 | 5 | 0.245200787 |
| 44.961 | 0.392359 | kα2 | 0.382369 | 0.146206 | 0.995048962 | 0.1454822 | 0 | 0 | 5 | 0.245195507 |
| 71.797 | 0.626547 | kα1 | 0.586351 | 0.3438077 | 0.995048962 | 0.3438077 | 0 | 6 | 4 | 0.579453137 |
| 72.003 | 0.628345 | kα2 | 0.587806 | 0.3455164 | 0.995048962 | 0.3438057 | 0 | 6 | 4 | 0.579449878 |
| 75.507 | 0.658923 | kα1 | 0.612266 | 0.3748691 | 0.995048962 | 0.3748691 | 2 | 6 | 2 | 0.631804109 |
| 75.728 | 0.660851 | kα2 | 0.613789 | 0.3767373 | 0.995048962 | 0.374872 | 2 | 6 | 2 | 0.631809002 |
| 77.712 | 0.678165 | kα1 | 0.627365 | 0.3935871 | 0.995048962 | 0.3935871 | 1 | 7 | 2 | 0.663351385 |
| 77.942 | 0.680172 | kα2 | 0.628927 | 0.3955491 | 0.995048962 | 0.3935907 | 1 | 7 | 2 | 0.663357484 |
| 79.785 | 0.696255 | kα1 | 0.641349 | 0.4113288 | 0.995048962 | 0.4113288 | −4 | 0 | 5 | 0.693253191 |
| 80.023 | 0.698332 | kα2 | 0.642941 | 0.4133736 | 0.995048962 | 0.411327 | −4 | 0 | 5 | 0.693250075 |
| 81.402 | 0.710366 | kα1 | 0.652112 | 0.4252496 | 0.995048962 | 0.4252496 | −2 | 2 | 8 | 0.716715267 |
| 81.647 | 0.712504 | kα2 | 0.653731 | 0.4273643 | 0.995048962 | 0.4252484 | −2 | 2 | 8 | 0.716713211 |
| 2θ | θ (Radian) | λ | Sinθ | Sin2θ | λ α12/λ α22 | Sin2θ | h | k | l | 4(Sin2θ)/λ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 34.623 | 0.302143 | kα1 | 0.297566501 | 0.0885458 | 0.995048962 | 0.0885458 | 2 | 2 | 0 | 0.149227687 |
| 34.712 | 0.302919 | kα2 | 0.2983079 | 0.0889876 | 0.995048962 | 0.088547 | 2 | 2 | 0 | 0.149229709 |
| 40.233 | 0.351099 | kα1 | 0.34393012 | 0.1182879 | 0.995048962 | 0.1182879 | 0 | 0 | 5 | 0.199352531 |
| 40.338 | 0.352015 | kα2 | 0.344790375 | 0.1188804 | 0.995048962 | 0.1182918 | 0 | 0 | 5 | 0.199359093 |
| 50.069 | 0.436934 | kα1 | 0.423163908 | 0.1790677 | 0.995048962 | 0.1790677 | 0 | 2 | 10 | 0.30178564 |
| 50.202 | 0.438095 | kα2 | 0.424215228 | 0.1799586 | 0.995048962 | 0.1790676 | 0 | 2 | 10 | 0.301785446 |
| 63.940 | 0.557982 | kα1 | 0.529475155 | 0.2803439 | 0.995048962 | 0.2803439 | 0 | 2 | 13 | 0.472468114 |
| 64.118 | 0.559567 | kα2 | 0.530792256 | 0.2817404 | 0.995048962 | 0.2803455 | 0 | 2 | 13 | 0.472470763 |
| 68.054 | 0.593883 | kα1 | 0.559583516 | 0.3131337 | 0.995048962 | 0.3131337 | −3 | 3 | 7 | 0.527772924 |
| 68.247 | 0.595567 | kα2 | 0.560978578 | 0.314697 | 0.995048962 | 0.3131389 | −3 | 3 | 7 | 0.5277337965 |
| 71.928 | 0.62769 | kα1 | 0.587276816 | 0.3448941 | 0.995048962 | 0.3448941 | −1 | 1 | 15 | 0.581255459 |
| 72.135 | 0.629497 | kα2 | 0.588737945 | 0.3466124 | 0.995048962 | 0.3448963 | −1 | 1 | 15 | 0.581259197 |
| 73.365 | 0.64023 | kα1 | 0.597380231 | 0.35686367 | 0.995048962 | 0.3568631 | 0 | 6 | 8 | 0.601427143 |
| 73.577 | 0.64208 | kα2 | 0.598862869 | 0.3586367 | 0.995048962 | 0.3568611 | 0 | 6 | 8 | 0.601423725 |
| 77.633 | 0.677476 | kα1 | 0.626828219 | 0.3929136 | 0.995048962 | 0.3929136 | −1 | 1 | 16 | 0.662183585 |
| 77.862 | 0.679474 | kα2 | 0.628384036 | 0.3948665 | 0.995048962 | 0.3929115 | −1 | 1 | 16 | 0.662180015 |
References
- Yuan, J.; Yang, J.; Ma, H.; Su, S.; Chang, Q.; Komarneni, S. Green synthesis of nano-muscovite and niter from feldspar through accelerated geomimicking process. Appl. Clay Sci. 2018, 165, 71–76. [Google Scholar] [CrossRef]
- Hosseini, E. Crystals and Minerals, 1st ed.; Ruykard-e Novin Publishing Cooperation: Tehran, Iran, 2000. [Google Scholar]
- Habashi, F. A Textbook of Hydrometallurgy, 2nd ed.; Métallurgie Extractive Québec: Québec, QC, Canada, 1999. [Google Scholar]
- Yoder, H.S. Experimental studies on micas: A synthesis. In Proceedings of the 6th National Conference on Clays and Clay Minerals, Berkley, CA, USA, 19–23 August 1957. [Google Scholar]
- Zhang, H.; Tangparitkul, S.; Hendry, B.; Harper, J.; Kim, Y.K.; Hunter, T.N.; Lee, J.W.; Harbottle, D. Selective separation of cesium contaminated clays from pristine clays by flotation. Chem. Eng. J. 2019, 355, 797–804. [Google Scholar] [CrossRef]
- Ben Said, A.; Frances, F.; Grandjean, A.; Latrille, C.; Faure, S. Study of a foam flotation process assisted by cationic surfactant for the separation of soil clay particles: Processing parameters and scaling-up sensitivity. Chem. Eng. Process. Process Intensif. 2019, 142, 107547. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, W.; Hu, S.; Yang, Z.; Liu, X.; Fan, Z. Comprehensive utilization of foundry dust: Coal powder and clay minerals separation by ultrasonic-assisted flotation. J. Hazard. Mater. 2021, 402, 124124. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, W.; Xu, J.; Ding, J.; Wang, Q.; Wang, A. Solid-phase oxalic acid leaching of natural red palygorskite-rich clay: A solvent-free way to change color and properties. Appl. Clay Sci. 2020, 198, 105848. [Google Scholar] [CrossRef]
- Wang, L.; Xin, J.; Nai, H.; Zheng, T.; Tian, F.; Zheng, X. Sorption of DONs onto clay minerals in single-solute and multi-solute systems: Implications for DONs mobility in the vadose zone and leachability into groundwater. Sci. Total Environ. 2020, 712, 135502. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; Fulton, L.; Ulkem, J.; Aiken, S.; Blackwell, A.; Walsh, J.; Walker, P.; Rezanezhad, F. Lepidolite extraction solid by-product: Mitigation of thallium leaching and utilization of radiogenic strontium isotopes as a tracer. Environ. Adv. 2021, 3, 100035. [Google Scholar] [CrossRef]
- Bagheri, K.; Razavi, S.M.; Ahmadi, S.J.; Kosari, M.; Abolghasemi, H. Thermal resistance, tensile properties, and gamma radiation shielding performance of unsaturated polyester/nanoclay/PbO composites. Radiat. Phys. Chem. 2018, 146, 5–10. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, S.; Chanda, J.; Das, A.; Wiessner, S.; Sinha Ray, S.; Bandyopadhyay, A. Distribution of nanoclay in a new TPV/nanoclay composite prepared through dynamic vulcanization. Polym. Test. 2020, 83, 106374. [Google Scholar] [CrossRef]
- Sen, B.; Fulmali, A.O.; Ganesh Gupta, K.B.N.V.S.; Kumar Prusty, R.; Chandra Ray, B. A study of the effect of carbon nanotube/nanoclay binary nanoparticle reinforcement on glass fibre/epoxy composites. Mater. Today 2020, 26, 2026–2031. [Google Scholar] [CrossRef]
- Shettar, M.; Kowshik, C.S.S.; Manjunath, M.; Hiremath, P. Experimental investigation on mechanical and wear properties of nanoclay–epoxy composites. J. Mater. Res. Technol. 2020, 9, 9108–9116. [Google Scholar] [CrossRef]
- Tullio, S.C.M.C.; Chalcraft, D.R. Converting natural nanoclay into modified nanoclay augments the toxic effect of natural nanoclay on aquatic invertebrates. Ecotox. Environ. Safe. 2020, 197, 110602. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, T.; Kim, Y.-H.; Yang, Z.; Li, Y.; Yu, T. Different-structured nanoclays incorporated composites: Computational and experimental analysis on mechanical properties. Compos. Sci. Technol. 2021, 203, 108612. [Google Scholar] [CrossRef]
- Kundu, K.; Afshar, A.; Katti, D.R.; Edirisinghe, M.; Katti, K.S. Composite nanoclay-hydroxyapatite-polymer fiber scaffolds for bone tissue engineering manufactured using pressurized gyration. Compos. Sci. Technol. 2021, 202, 108598. [Google Scholar] [CrossRef]
- Hamilton, D.L.; Henderson, C.M.B. The preparation of silicate compositions by a gelling method. Mineral. Mag. J. Mineral. Soc. 1968, 36, 832–838. [Google Scholar] [CrossRef]
- Chatterjee, N.D. Synthesis and upper thermal stability limit of 2M margatite, CaAl2[Al2Si2O10(OH)2]. Schweiz. Miner. Petrog. 1974, 54, 753–767. [Google Scholar]
- Gruner, J.W. Formation and stability of muscovite in acid solution at elevated temperatures. Am. Mineral. 1939, 24, 624–628. [Google Scholar]
- Gruner, J.W. Conditions for the formation of paragonite. Am. Mineral. 1942, 27, 131–144. [Google Scholar]
- Gruner, J.W. The hydrothermal alteration of feldspars in acid solutions between 300 and 400°. Econ. Geol. 1944, 39, 578–589. [Google Scholar] [CrossRef]
- Kiyoura, R.; Ito, Y. Hydrothermal reactions of silicates: III. Hydrothermal reactions and synthesis of sericite. J. Ceram. Assoc. Jpn. 1953, 61, 415–419. [Google Scholar] [CrossRef][Green Version]
- Yoder, H.S.; Eugster, H.P. Synthetic and natural muscovites. Geochim. Cosmochim. Ac. 1955, 8, 225–280. [Google Scholar] [CrossRef]
- Velde, B. Experimental determination of muscovite polymorph stabilities. Am. Mineral. 1965, 50, 436–449. [Google Scholar]
- Voncken, J.H.L.; Eerden, A.M.J.; Jansen, J.B.H. Synthesis of a Rb analogue of 2M1 muscovite. Am. Mineral. 1987, 72, 551–554. [Google Scholar]
- Jung, I.; Schreyer, W. Synthesis, properties and stability of end member boromuscovite, KAl2[BSi3O10] (OH)2. Contrib. Mineral. Petr. 2003, 144, 507. [Google Scholar] [CrossRef]
- Roy, R. Decomposition and synthesis of the micas. J. Am. Ceram. Soc. 1949, 32, 204–209. [Google Scholar]
- Caillere, S.; Henin, S. Transformation of minerals of the montmorillonite family into 10 Å micas. Mineral. Mag. 1949, 28, 606–611. [Google Scholar] [CrossRef]
- Gillingham, T.E. The solubility and transfer of silica and other non-volatile in steam. Econ. Geol. 1948, 43, 241–272. [Google Scholar] [CrossRef]
- Norton, F.H. Hydrothermal formation of clay minerals in the laboratory. Am. Mineral. 1939, 24, 1–17. [Google Scholar]
- O’Neill, T.F. The hydrothermal alteration of feldspars at 250° to 400°. Econ. Geol. 1948, 43, 167–180. [Google Scholar] [CrossRef]
- Haselton, H.T.; Cygan, G.L.; Jenkins, D.M. Experimental study of muscovite stability in pure H2O and 1 molal KCI-HCl solutions. Geochim. Cosmochim. Acta 1995, 59, 429–442. [Google Scholar] [CrossRef]
- Hoisch, T.D. A muscovite-biotite geothermometer. Am. Mineral. 1989, 74, 565–572. [Google Scholar]
- Munoz, J.L.; Ludington, S. Flourine-hydroxyl exchange in synthetic muscovite and its application to muscovite-biotite assemblages. Am. Mineral. 1977, 62, 304–308. [Google Scholar]
- Lin, H.; Li, G.; Dong, Y.; Li, J. Effect of pH on the release of heavy metals from stone coal waste rocks. Int. J. Miner. Process. 2017, 165, 1–7. [Google Scholar] [CrossRef]
- Civeira, M.S.; Pinheiro, R.N.; Gredilla, A.; Vallejuelo, S.F.O.; Oliveira, M.L.S.; Ramos, C.G.; Taffarel, S.R.; Kautzmann, R.M.; Madariaga, J.M.; Silva, L.F.O. The properties of the nano-minerals and hazardous elements: Potential environmental impacts of Brazilian coal waste fire. Sci. Total Environ. 2016, 544, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, B. Coal Science and Engineering, 1st ed.; Woodhead Publishing India: New Delhi, India, 2012. [Google Scholar]
- Chudy, K.; Marszałek, H.; Kierczak, J. Impact of hard-coal waste dump on water quality—A case study of LudwikowiceKłodzkie (NowaRuda Coalfield, SW Poland). J. Geochem. Explor. 2014, 146, 127–135. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinusradiata. Water Air Soil Poll. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Opara, A.; Adams, D.J.; Free, M.L.; McLennan, J.; Hamilton, J. Microbial production of methane and carbon dioxide from lignite, bituminous coal, and coal waste materials. Int. J. Coal Geol. 2012, 96, 1–8. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, T.; Rudolph, V.; Golding, S.D. Biogenic methane production from Bowen Basin coal waste materials. Int. J. Coal Geol. 2017, 169, 22–27. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Strizhak, P.A. Environmentally and economically efficient utilization of coal processing waste. Sci. Total Environ. 2017, 598, 21–27. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Lapin, D.A.; Lyrschikov, S.Y.; Shevyrev, S.A. Ignition of coal–water fuels made of coal processing wastes and different oils. Appl. Therm. Eng. 2018, 128, 235–243. [Google Scholar] [CrossRef]
- Frías, M.; Sanchez de Rojas, M.I.; García, R.; Juan Valdés, A.; Medina, C. Effect of activated coal mining wastes on the properties of blended cement. Cem. Concr. Compos. 2012, 34, 678–683. [Google Scholar] [CrossRef]
- Modarres, A.; Ayar, P. Coal waste application in recycled asphalt mixtures with bitumen emulsion. J. Clean. Prod. 2014, 83, 263–272. [Google Scholar] [CrossRef]
- Modarres, A.; Rahmanzadeh, A. Application of coal waste powder as filler in hot mix asphalt. Constr. Build. Mater. 2014, 66, 476–483. [Google Scholar] [CrossRef]
- Modarres, A.; Rahmanzadeh, A.; Ayar, P. Effect of coal waste powder in hot mix asphalt compared to conventional fillers: Mix mechanical properties and environmental impacts. J. Clean. Prod. 2015, 91, 262–268. [Google Scholar] [CrossRef]
- Stolboushkin, A.Y.; Ivanov, A.I.; Fomina, O.A. Use of coal-mining and processing wastes in production of bricks and fuel for their burning. Procedia Eng. 2016, 150, 1496–1502. [Google Scholar] [CrossRef][Green Version]
- Taha, Y.; Benzaazoua, M.; Hakkou, R.; Mansori, M. Coal mine wastes recycling for coal recovery and eco-friendly bricks production. Miner. Eng. 2017, 107, 123–138. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Q.; Hu, S.; Wu, K. A concrete material with waste coal gangue and fly ash used for farmland drainage in high groundwater level areas. J. Clean. Prod. 2016, 112, 631–638. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Bin Haider, J.; Xu, B.; Johir Aneek, M.A.H.; Karmakar, K.; Zhou, J.L.; Boshir Ahmed, M.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A critical review of waste resources, synthesis, and applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- He, X.; Yao, B.; Xia, Y.; Huang, H.; Gan, Y.; Zhang, W. Coal fly ash derived zeolite for highly efficient removal of Ni2+ in waste water. Powder Technol. 2020, 367, 40–46. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Motak, M.; Gálvez, M.E.; Da Costa, P. Ni/zeolite X derived from fly ash as catalysts for CO2 methanation. Fuel 2020, 267, 117139. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- DeAquino, T.F.; Estevam, S.T.; Viola, V.O.; Marques, C.R.M.; Zancan, F.L.; Vasconcelos, L.B.; Riella, H.G.; Pires, M.J.R.; Morales-Ospino, R.; Torres, A.E.B.; et al. CO2 adsorption capacity of zeolites synthesized from coal fly ashes. Fuel 2020, 276, 118143. [Google Scholar]
- Khoshdast, H. Alternative Methods for Recycling of Zarand Coal Washing Plant’s Fine and Coarse Tailings. Technical Report; Ministry of Industry, Mine and Trade: Kerman, Iran, 2010. [Google Scholar]
- Darezereshki, E. Synthesis of maghemite (γ-Fe2O3) nanoparticles by wet chemical method at room temperature. Mater. Let. 2010, 64, 1471–1472. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Warren, B.E. X-ray Diffraction; Dover Publications: New York, NY, USA, 1990. [Google Scholar]
- Rickwood, P.C. The largest crystals. Am. Mineral. 1981, 66, 885–907. [Google Scholar]
- Busigny, V.; Cartigny, P.; Philippot, P.; Javoy, M. Quantitative analysis of ammonium in biotite using infrared spectroscopy. Am. Mineral. 2004, 89, 1625–1630. [Google Scholar] [CrossRef]
- Chaussidon, J. The IR spectrum of structural hydroxyls of K-depleted biotites. Clays Clay Miner. 1972, 20, 59–67. [Google Scholar] [CrossRef]
- Liese, H.C. Tetrahedrally coordinated aluminum in some natural biotites: An infrared absorption analysis. Am. Mineral. 1963, 48, 980–990. [Google Scholar]
- Nayak, P.S.; Singh, B.K. Instrumental characterization of clay by XRF, XRD and FTIR. Bull. Mater. Sci. 2007, 30, 235–238. [Google Scholar] [CrossRef]
- Shih, Y.J.; Shen, Y.H. Swelling of sericite by LiNO3-hydrothermal treatment. Appl. Clay Sci. 2009, 43, 282–288. [Google Scholar] [CrossRef]
- Van der Marel, H.M.; Bentelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and Their Admixtures; Elsevier Science Publishers: New York, NY, USA, 1976. [Google Scholar]
- Gadsen, J.A. Infrared Spectra of Minerals and Related Inorganic Compounds; Butterworths: London, UK, 1975. [Google Scholar]
- Matteson, A.; Herron, M.M. Quantitative Mineral Analysis by Fourier Transform Infrared Spectroscopy. In Proceedings of the Society of Core Analysts Conference: Advances in Core Technology, Houston, TX, USA, 3–6 October 1993. [Google Scholar]
- Hofmeister, A.M.; Keppel, E.; Speck, A.K. Absorption and reflection infrared spectra of MgO and other diatomic compounds. Mon. Not. R. Astron. Soc. 2003, 345, 16–38. [Google Scholar] [CrossRef]
- Hosseini-Zori, M. Synthesis and characterization of red pearlescent pigments based on muscovite and zirconia-nanoencapsulated hematite. Prog. Color Colorants Coat. J. 2012, 5, 7–13. [Google Scholar]
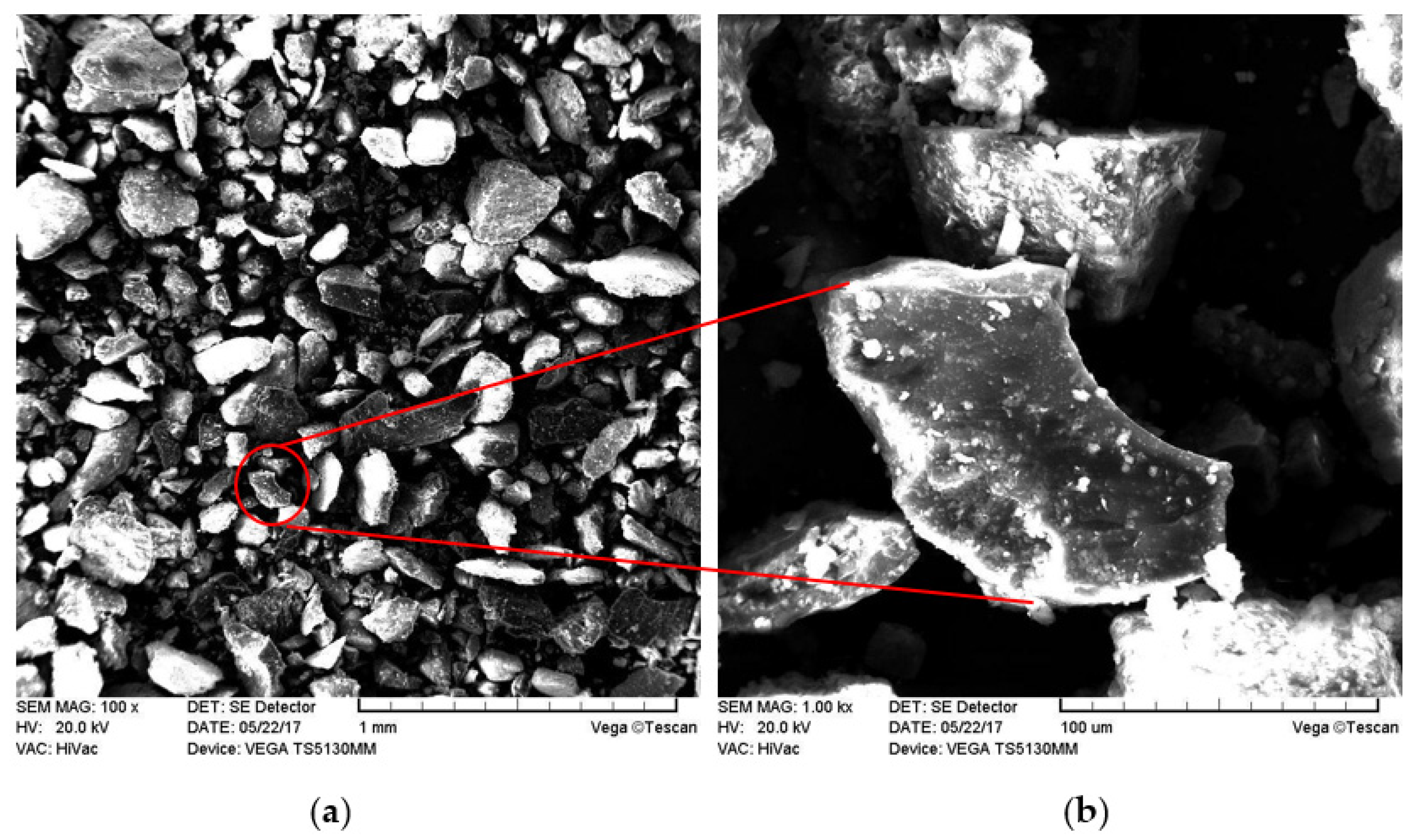
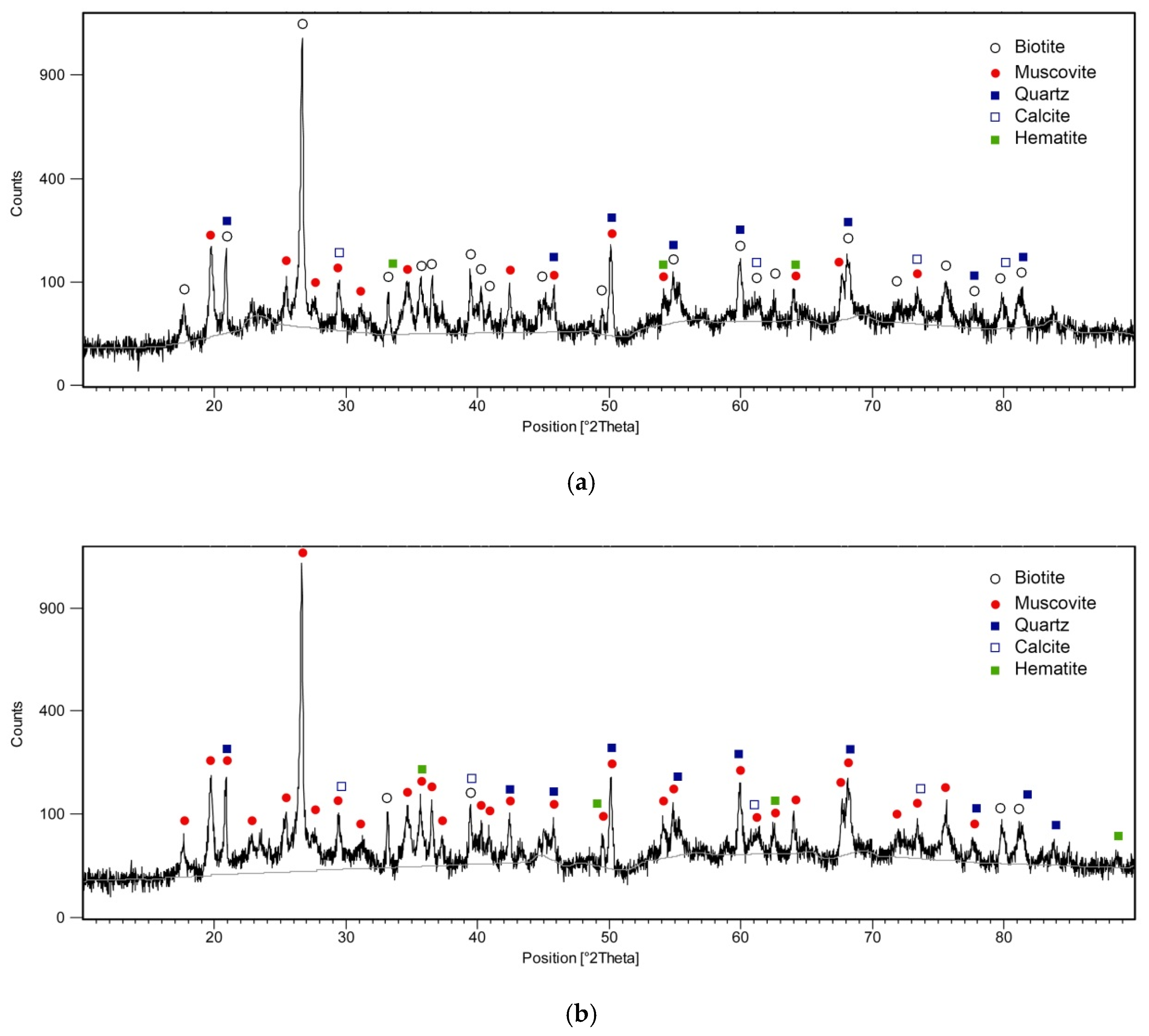

| Component | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | L.O.I * |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 49.1 | 25.8 | 5.9 | 5.5 | 4.7 | 2.0 | 4.6 | 1.9 | 0.5 |
| Chemical Name | Lattice Parameters | |||
|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | β (Degree) | |
| Biotite (standard) | 5.3370 | 9.2420 | 10.2110 | 100.1500 |
| Biotite (starting material) | 5.3343 | 9.2267 | 10.2560 | 100.0800 |
| Muscovite (standard) | 5.1940 | 9.0130 | 20.0640 | 95.8000 |
| Muscovite (synthesized) | 5.1767 | 8.9327 | 20.0786 | 96.8771 |
| Reference Code | Lattice Parameters | |||
|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | β (Degree) | |
| 01-082-2450 | 5.1940 | 9.0130 | 20.0640 | 95.8000 |
| 01-082-2451 | 5.1510 | 8.9310 | 19.3990 | 95.8000 |
| 00-025-0649 | 9.1120 | 5.2600 | 20.0330 | 97.8700 |
| 01-085-2147 | 5.1740 | 8.9750 | 19.7700 | 95.2000 |
| Band (cm−1) | Assignment(s) | |
|---|---|---|
| Starting Material | Synthesized Product | |
| 3451.32 | 3450.89 | ‒O‒H |
| 1629.00 | 1629.17 | H‒O‒H |
| 1077.86 | 1073.93 | Si‒O |
| 790.05 | 790.22 | Al‒O‒Al |
| 708.54 | 707.58 | Si‒O‒Al |
| 560.69 | 560.46 | Si‒O; Si‒O‒Al |
| 482.07 | 483.61 | Si‒O; Si‒O‒Fe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoshdast, H.; Shojaei, V.; Hassanzadeh, A.; Niedoba, T.; Surowiak, A. A Novel Open-System Method for Synthesizing Muscovite from a Biotite-Rich Coal Tailing. Minerals 2021, 11, 269. https://doi.org/10.3390/min11030269
Khoshdast H, Shojaei V, Hassanzadeh A, Niedoba T, Surowiak A. A Novel Open-System Method for Synthesizing Muscovite from a Biotite-Rich Coal Tailing. Minerals. 2021; 11(3):269. https://doi.org/10.3390/min11030269
Chicago/Turabian StyleKhoshdast, Hamid, Vahideh Shojaei, Ahmad Hassanzadeh, Tomasz Niedoba, and Agnieszka Surowiak. 2021. "A Novel Open-System Method for Synthesizing Muscovite from a Biotite-Rich Coal Tailing" Minerals 11, no. 3: 269. https://doi.org/10.3390/min11030269
APA StyleKhoshdast, H., Shojaei, V., Hassanzadeh, A., Niedoba, T., & Surowiak, A. (2021). A Novel Open-System Method for Synthesizing Muscovite from a Biotite-Rich Coal Tailing. Minerals, 11(3), 269. https://doi.org/10.3390/min11030269










