How Relevant Is It to Use Mineral Proxies to Mimic the Atmospheric Reactivity of Natural Dust Samples? A Reactivity Study Using SO2 as Probe Molecule
Abstract
:1. Introduction
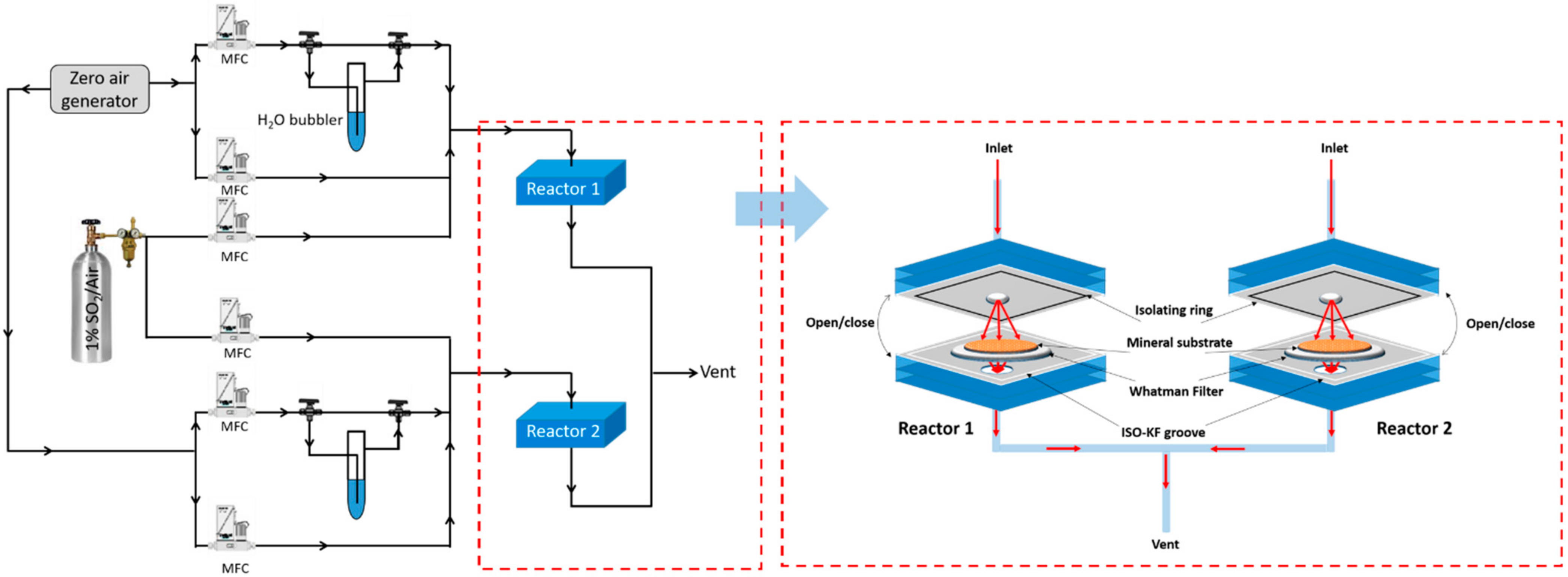
2. Materials and Methods
2.1. Volcanic Samples, Desert Dusts and Metal Oxides
2.2. Characterization of the Samples
2.3. Chemicals and Reagents
2.4. Preparation of Solutions for HPLC Analysis
2.5. Dust and Mineral Surrogate Ageing. Extraction of Sulfites and Sulfates
2.6. Instrumentation and Chromatographic Conditions
3. Results and Discussion
3.1. First Insights on SO2 Uptake on Mineral Samples
3.2. Classification of Mineral Samples Based on Their Efficiency to Form Sulfites and/or Sulfates. Can a Natural Dust be Represented by One Chosen Individual Component?
3.3. Can a Natural Dust be Characterized by a Sum of “Representative” Simple Mineral Proxies?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usher, C.R.; Michel, A.E.; Grassian, V.H. Reactions on mineral dust. Chem. Rev. 2003, 103, 4883–4940. [Google Scholar] [CrossRef]
- Bristow, C.S.; Hudson-Edwards, K.A.; Chappell, A. Fertilizing the Amazon and equatorial Atlantic with West African dust. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Andreae, M.O. Climatic effects of changing atmospheric aerosol levels. In World Survey of Climatology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 16, pp. 347–398. [Google Scholar]
- Rose, W.I.; Wunderman, R.L.; Hoffman, M.F.; Gale, L. A volcanologist’s review of atmospheric hazards of volcanic activity: Fuego and Mount St. Helens. J. Volcanol. Geotherm. Res. 1983, 17, 133–157. [Google Scholar] [CrossRef]
- McCormick, M.P.; Thomason, L.W.; Trepte, C.R. Atmospheric effects of the Mt Pinatubo eruption. Nat. Cell Biol. 1995, 373, 399–404. [Google Scholar] [CrossRef]
- Langmann, B. Volcanic Ash versus Mineral Dust: Atmospheric Processing and Environmental and Climate Impacts. Available online: https://www.hindawi.com/journals/isrn/2013/245076/ (accessed on 11 April 2018).
- Prata, A.J.; Carn, S.A.; Stohl, A.; Kerkmann, J. Long range transport and fate of a stratospheric volcanic cloud from Soufrière Hills volcano, Montserrat. Atmos. Chem. Phys. Discuss. 2007, 7, 5093–5103. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, G.; Rossi, E.; Biass, S.; Bonadonna, C. Timing and nature of volcanic particle clusters based on field and numerical investigations. J. Volcanol. Geotherm. Res. 2016, 327, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Schepanski, K. Transport of mineral dust and its impact on climate. Geosciences 2018, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Maters, E.C.; Cimarelli, C.; Casas, A.S.; Dingwell, D.B.; Murray, B.J. Volcanic ash ice-nucleating activity can be enhanced or depressed by ash-gas interaction in the eruption plume. Earth Planet. Sci. Lett. 2020, 551, 116587. [Google Scholar] [CrossRef]
- Maters, E.C.; Dingwell, D.B.; Cimarelli, C.; Müller, D.; Whale, T.F.; Murray, B.J. The importance of crystalline phases in ice nucleation by volcanic ash. Atmospheric Chem. Phys. Discuss. 2019, 19, 5451–5465. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, Z.M.; Zhang, Y.H.; Zhu, T.; Li, J.L.; Ding, J. Kinetics and mechanism of heterogeneous oxidation of sulfur dioxide by ozone on surface of calcium carbonate. Atmospheric Chem. Phys. Discuss. 2006, 6, 2453–2464. [Google Scholar] [CrossRef] [Green Version]
- Ayris, P.M.; Delmelle, P.; Cimarelli, C.; Maters, E.C.; Suzuki, Y.J.; Dingwell, D.B. HCl uptake by volcanic ash in the high temperature eruption plume: Mechanistic insights. Geochim. et Cosmochim. Acta 2014, 144, 188–201. [Google Scholar] [CrossRef]
- Casas, A.S.; Wadsworth, F.B.; Ayris, P.M.; Delmelle, P.; Vasseur, J.; Cimarelli, C.; Dingwell, D.B. SO2 scrubbing during percolation through rhyolitic volcanic domes. Geochim. Cosmochim. Acta 2019, 257, 150–162. [Google Scholar] [CrossRef]
- Van Eaton, A.R.; Harper, M.A.; Wilson, C.J.N. High-flying diatoms: Widespread dispersal of microorganisms in an explosive volcanic eruption. Geology 2013, 41, 1187–1190. [Google Scholar] [CrossRef]
- Durant, A.J.; Bonadonna, C.; Horwell, C.J. Atmospheric and environmental impacts of volcanic particulates. Elements 2010, 6, 235–240. [Google Scholar] [CrossRef]
- Witt, V.; Ayris, P.M.; Damby, D.E.; Cimarelli, C.; Kueppers, U.; Dingwell, D.B.; Wörheide, G. Volcanic ash supports a diverse bacterial community in a marine mesocosm. Geobiology 2017, 15, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Vodyanitskii, Y.N. Elements oxides as a source of errors in the gross chemical composition of soil and ways to eliminate the errors. Ann. Agrar. Sci. 2018, 16, 90–93. [Google Scholar] [CrossRef]
- Delmelle, P.; Wadsworth, F.B.; Maters, E.C.; Ayris, P.M. High temperature reactions between gases and ash particles in volcanic eruption plumes. Rev. Miner. Geochem. 2018, 84, 285–308. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Arnalds, O.; Olafsson, H.; Hladil, J.; Skala, R.; Navratil, T.; Chadimova, L.; Meinander, O. Snow–Dust Storm: Unique case study from Iceland, March 6–7, 2013. Aeolian Res. 2015, 16, 69–74. [Google Scholar] [CrossRef]
- Crowley, J.N.; Ammann, M.; Cox, R.A.; Hynes, R.G.; Jenkin, M.E.; Mellouki, A.; Rossi, M.J.; Troe, J.; Wallington, T.J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume V–heterogeneous reactions on solid substrates. Atmospheric Chem. Phys. Discuss. 2010, 10, 9059–9223. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.L.; Li, P.; Usher, C.R.; Grassian, V.H. Heterogeneous uptake of sulfur dioxide on aluminum and magnesium oxide particles. J. Phys. Chem. A 2001, 105, 6109–6120. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, G.; Chen, J.; Wang, Y.; Wang, X.; An, Z.; Zhang, P. Heterogeneous reactions of sulfur dioxide on typical mineral particles. J. Phys. Chem. B 2006, 110, 12588–12596. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Deng, Y.; Fu, H.; Zhang, L.; Chen, J. Emerging investigator series: Heterogeneous reactions of sulfur dioxide on mineral dust nanoparticles: From single component to mixed components. Environ. Sci. Nano 2018, 5, 1821–1833. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Deng, Y.; Fu, H.; Zhang, L.; Chen, J. Adsorption of SO2 on mineral dust particles influenced by atmospheric moisture. Atmos. Environ. 2018, 191, 153–161. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Pettibone, J.; Grassian, V.H. Sulfur dioxide adsorption and photooxidation on isotopically-labeled titanium dioxide nanoparticle surfaces: Roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed sulfite and sulfate. Phys. Chem. Chem. Phys. 2012, 14, 6957–6966. [Google Scholar] [CrossRef]
- Wu, L.Y.; Tong, S.R.; Wang, W.G.; Ge, M.F. Effects of temperature on the heterogeneous oxidation of sulfur dioxide by ozone on calcium carbonate. Atmospheric Chem. Phys. Discuss. 2011, 11, 6593–6605. [Google Scholar] [CrossRef] [Green Version]
- Usher, C.R.; Al-Hosney, H.; Carlos-Cuellar, S.; Grassian, V.H. A laboratory study of the heterogeneous uptake and oxidation of sulfur dioxide on mineral dust particles. J. Geophys. Res. Space Phys. 2002, 107, ACH 16-1–ACH 16-9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tong, S.; Ge, M.; Jing, B.; Hou, S.; Tan, F.; Chen, Y.; Guo, Y.; Wu, L. The influence of relative humidity on the heterogeneous oxidation of sulfur dioxide by ozone on calcium carbonate particles. Sci. Total. Environ. 2018, 633, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, Y.; Li, H.; Chen, Z. Hydrogen peroxide maintains the heterogeneous reaction of sulfur dioxide on mineral dust proxy particles. Atmos. Environ. 2016, 141, 552–559. [Google Scholar] [CrossRef]
- Bluth, G.J.S.; Schnetzler, C.C.; Krueger, A.J.; Walter, L.S. The contribution of explosive volcanism to global atmospheric sulphur dioxide concentrations. Nat. Cell Biol. 1993, 366, 327–329. [Google Scholar] [CrossRef]
- Highwood, E.-J.; Stevenson, D.S. Atmospheric impact of the 1783–1784 Laki Eruption: Part II Climatic effect of sulphate aerosol. Atmos. Chem. Phys. Discuss. 2003, 3, 1177–1189. [Google Scholar] [CrossRef] [Green Version]
- Daniel, J.J. Introduction to Atmospheric Chemistry; Princeton University Press: Princeton, NJ, USA, 1999; ISBN 978-1-4008-4154-7. [Google Scholar]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 978-0-08-052907-3. [Google Scholar]
- Schmidt, A.; Ostro, B.; Carslaw, K.S.; Wilson, M.; Thordarson, T.; Mann, G.W.; Simmons, A.J. Excess mortality in Europe following a future Laki-style Icelandic eruption. Proc. Natl. Acad. Sci. USA 2011, 108, 15710–15715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US EPA. O. Sulfur Dioxide Basics. Available online: https://www.epa.gov/so2-pollution/sulfur-dioxide-basics (accessed on 14 August 2020).
- Stevenson, D.S.; Johnson, C.E.; Highwood, E.J.; Gauci, V.; Collins, W.J.; Derwent, R.G. Atmospheric impact of the 1783–1784 Laki eruption: Part I Chemistry modelling. Atmos. Chem. Phys. Discuss. 2003, 3, 487–507. [Google Scholar] [CrossRef] [Green Version]
- Baltrusaitis, J.; Cwiertny, D.M.; Grassian, V.H. Adsorption of sulfur dioxide on hematite and goethite particle surfaces. Phys. Chem. Chem. Phys. 2007, 9, 5542–5554. [Google Scholar] [CrossRef] [PubMed]
- Urupina, D.; Lasne, J.; Romanias, M.; Thiery, V.; Dagsson-Waldhauserova, P.; Thevenet, F. Uptake and surface chemistry of SO2 on natural volcanic dusts. Atmos. Environ. 2019, 217, 116942. [Google Scholar] [CrossRef]
- Urupina, D.; Gaudion, V.; Romanias, M.N.; Verriele, M.; Thevenet, F. Method development and validation for the determination of sulfites and sulfates on the surface of mineral atmospheric samples using reverse-phase liquid chromatography. Talanta 2020, 219, 121318. [Google Scholar] [CrossRef] [PubMed]
- Arnalds, O.; Dagsson-Waldhauserova, P.; Olafsson, H. The Icelandic volcanic aeolian environment: Processes and impacts—A review. Aeolian Res. 2016, 20, 176–195. [Google Scholar] [CrossRef] [Green Version]
- Gislason, S.R.; Hassenkam, T.; Nedel, S.; Bovet, N.; Eiriksdottir, E.S.; Alfredsson, H.A.; Hem, C.P.; Balogh, Z.I.; Dideriksen, K.; Oskarsson, N.; et al. Characterization of Eyjafjallajokull volcanic ash particles and a protocol for rapid risk assessment. Proc. Natl. Acad. Sci.USA 2011, 108, 7307–7312. [Google Scholar] [CrossRef] [Green Version]
- Baldo, C.; Formenti, P.; Nowak, S.; Chevaillier, S.; Cazaunau, M.; Pangui, E.; Di Biagio, C.; Doussin, J.-F.; Ignatyev, K.; Dagsson-Waldhauserova, P.; et al. Distinct chemical and mineralogical composition of Icelandic dust compared to northern African and Asian dust. Atmospheric Chem. Phys. Discuss. 2020, 20, 13521–13539. [Google Scholar] [CrossRef]
- Prospero, J.M.; Ginoux, P.; Torres, O.; Nicholson, S.E.; Gill, T.E. Environmental characterization of global sources of atmospheric soil dust identified with the NIMBUS 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev. Geophys. 2002, 40, 2-1–2-31. [Google Scholar] [CrossRef]
- Marticorena, B.; Chatenet, B.; Rajot, J.L.; Bergametti, G.; Deroubaix, A.; Vincent, J.; Kouoi, A.; Schmechtig, C.; Coulibaly, M.; Diallo, A.; et al. Mineral dust over west and central Sahel: Seasonal patterns of dry and wet deposition fluxes from a pluriannual sampling (2006–2012). J. Geophys. Res. Atmos. 2017, 122, 1338–1364. [Google Scholar] [CrossRef]
- Wang, X.; Romanias, M.N.; Pei, Z.; Rousseau, A.; Thévenet, F. Uptake mechanism of acetic acid onto natural gobi dust. ACS Earth Space Chem. 2020, 4, 1650–1662. [Google Scholar] [CrossRef]
- Tang, M.; Cziczo, D.J.; Grassian, V.H. Interactions of water with mineral dust aerosol: Water adsorption, hygroscopicity, cloud condensation, and ice nucleation. Chem. Rev. 2016, 116, 4205–4259. [Google Scholar] [CrossRef] [Green Version]
- Hatch, C.D.; Wiese, J.S.; Crane, C.C.; Harris, K.J.; Kloss, H.G.; Baltrusaitis, J. Water adsorption on clay minerals as a function of relative humidity: Application of bet and freundlich adsorption models. Langmuir 2012, 28, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Alleman, L.Y.; Lamaison, L.; Perdrix, E.; Robache, A.; Galloo, J.-C. PM10 metal concentrations and source identification using positive matrix factorization and wind sectoring in a French industrial zone. Atmos. Res. 2010, 96, 612–625. [Google Scholar] [CrossRef]
- Maters, E.C.; Delmelle, P.; Rossignol, M.; Ayris, P.M.P.; Bernard, A. Controls on the surface chemical reactivity of volcanic ash investigated with probe gases. Earth Planet. Sci. Lett. 2016, 450, 254–262. [Google Scholar] [CrossRef]
- Ayris, P.; Lee, A.; Wilson, K.D.; Kueppers, U.; Dingwell, D.B.; Delmelle, P. SO2 sequestration in large volcanic eruptions: High-temperature scavenging by tephra. Geochim. Cosmochim. Acta 2013, 110, 58–69. [Google Scholar] [CrossRef]
- Joshi, N.; Romanias, M.N.; Riffault, V.; Thevenet, F. Investigating water adsorption onto natural mineral dust particles: Linking DRIFTS experiments and BET theory. Aeolian Res. 2017, 27, 35–45. [Google Scholar] [CrossRef]
- Ibrahim, S.; Romanias, M.N.; Alleman, L.Y.; Zeineddine, M.N.; Angeli, G.K.; Trikalitis, P.N.; Thevenet, F. Water interaction with mineral dust aerosol: Particle size and hygroscopic properties of dust. ACS Earth Space Chem. 2018, 2, 376–386. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, S. Adsorption and desorption of Sb(III) on goethite. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 12145. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, H. Simultaneous determination of sulfite, sulfate, and hydroxymethanesulfonate in atmospheric waters by ion-pair HPLC technique. Talanta 2003, 59, 875–881. [Google Scholar] [CrossRef]
- Ayris, P.; Delmelle, P. Volcanic and atmospheric controls on ash iron solubility: A review. Phys. Chem. Earth Parts A B C 2012, 45-46, 103–112. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Reynolds, R.L.; Goldstein, H.L.; Moskowitz, B.; Rubasinghege, G. Iron dissolution and speciation in atmospheric mineral dust: Metal-metal synergistic and antagonistic effects. Atmos. Environ. 2018, 187, 417–423. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Reynolds, R.L.; Goldstein, H.L.; Moskowitz, B.; Rubasinghege, G. Bioavailable iron production in airborne mineral dust: Controls by chemical composition and solar flux. Atmos. Environ. 2019, 205, 90–102. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Rubasinghege, G. Mechanistic study on iron solubility in atmospheric mineral dust aerosol: Roles of titanium, dissolved oxygen, and solar flux in solutions containing different acid anions. ACS Earth Space Chem. 2019, 4, 101–111. [Google Scholar] [CrossRef]

| Element | Mýrdalssandur | Dyngjusandur | Hagavatn | Maelifellssandur | Eyjafjallajökull | M’Bour Saharan Dust | Gobi Dust | ATD Dust | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| surf wt.% | bulk wt.% | surf wt.% | bulk wt.% | surf wt.% | bulk wt.% | surf wt.% | bulk wt.% | surf wt.% | bulk wt.% | surf wt.% | Bulk * wt.% | surf wt.% | Bulk * wt.% | surf wt.% | Bulk * wt.% | |
| Si | 47.1 | 31.3 | 49.0 | 32.7 | 42.8 | 27.5 | 43.7 | 28.3 | 60.0 | 49.5 | 57.0 | 94.4 | 54.7 | 57.6 | 57.5 | 74.6 |
| Fe | 20.0 | 23.0 | 15.9 | 19.7 | 10.5 | 19.6 | 22.0 | 23.8 | 7.7 | 13.0 | 6.1 | 1.3 | 6.7 | 5.5 | 8.2 | 3.1 |
| Ca | 8.1 | 13.9 | 9.7 | 16.3 | 9.0 | 19.5 | 6.3 | 14.0 | 5.5 | 7.3 | 2.4 | 1.0 | 7.7 | 16.1 | 5.5 | 4.4 |
| Al | 14.5 | 12.3 | 16.8 | 15.8 | 31.8 | 16.7 | 18.2 | 15.5 | 13.4 | 13.6 | 32.1 | 1.8 | 18.5 | 11 | 18.0 | 9.5 |
| Mg | 3.3 | 5.1 | 4.8 | 7.3 | 2.4 | 10.4 | 3.0 | 5.7 | 1.5 | 3.3 | 0 | 0.1 | 6.1 | 2.3 | 3.9 | 1.0 |
| Ti | 3.8 | 7.6 | 2.0 | 3.3 | 1.8 | 2.4 | 4.3 | 6.1 | 1.0 | 2.4 | 0.2 | 0.7 | 0 | 0.8 | 0.5 | 0.5 |
| Na | 2.3 | 4.8 | 1.0 | 3.9 | 1.4 | 3.1 | 1.3 | 4.6 | 7.5 | 7.3 | 0.3 | 0.2 | 0.8 | 2.5 | 1.3 | 2.5 |
| K | 0.9 | 1.3 | 0 | 0.6 | 0 | 0.2 | 0.4 | 1.5 | 3.3 | 3.1 | 1.9 | 0.1 | 4.7 | 3.5 | 4.4 | 4.0 |
| other | 0 | 0.7 | 0.8 | 0.4 | 0.3 | 0.6 | 0.8 | 0.5 | 0.1 | 0.5 | 0 | 0.1 | 0 | 0.7 | 0.7 | 0.1 |
| Origin of the Icelandic v-Dust Sample | BET Specific Surface Area (m2 g−1) |
|---|---|
| Mýrdalssandur | 1.5 ± 0.38 |
| Dyngjusandur | 7.0 ± 1.8 |
| Hagavatn | 4.5 ± 1.1 |
| Maelifellssandur | 8.2 ± 2.0 |
| Eyjafjallajökull | 0.75 ± 0.19 |
| Origin of the Natural Mineral Dust Sample | |
| M’Bour | 14.5 ± 1.0 |
| Gobi | 10.5 ± 2.0 |
| ATD | 67.2 ± 1.8 |
| Selected Clays | |
| Kaolinite | 9.2 ± 2.8 |
| Montmorillonite | 225 ± 68 |
| Illite | 22.2 ± 6.7 |
| Selected Mineral Oxides | |
| SiO2 (Silica gel) | 402 ± 40 |
| SiO2 (Quartz) | 1.49 ± 0.12 |
| Al2O3 | 118 ± 22 |
| CaO | 66.01 ± 0.54 |
| CaCO3 | 0.6 ± 0.1 |
| Fe3O4 | 3.4 ± 0.4 |
| FeOOH | 15.8 |
| TiO2 | 52 ± 6.4 |
| MgO | 49.26 ± 0.23 |
| Group | Samples | Comments |
|---|---|---|
| 1 | Silica gel (SiO2) quartz (SiO2), montmorillonite | Sulfites and sulfates formation are below detection limits. |
| 2 | Al2O3, Fe3O4, and TiO2 | Only sulfites are detected. |
| 3 | FeOOH, MgO, illite, Dyngjusandur, Hagavatn, Mýrdalssandur, Maelifellssandur, Gobi and M’Bour | Both sulfites and sulfates are detected. |
| 4 | CaO, CaCO3, Eyjafjallajökull, kaolinite and Arizona Test Dust | Detection of sulfates only. Fast conversion of sulfites to sulfates prevents their detection. |
| Dust Sample | SiO2 | Al2O3 | CaO | Na2O | MgO | TiO2 | K2O | Fe2O3 | MnO | Calculated from Individual Contributions of Pure Oxides | Experimentally Measured | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myrdalssandur | wt.% | 59.96 | 17.61 | 4.89 | 2.36 | 3.86 | 2.27 | 0.47 | 8.59 | 0 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 20.5 | 95 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 2.7 | 34 | ||||
| Dyngjusandur | wt.% | 60.14 | 19.67 | 5.68 | 0.95 | 5.56 | 1.18 | 0.00 | 6.59 | 0.23 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 20.6 | 56 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 3.4 | 4 | ||||
| Hagavatn | wt.% | 50.24 | 35.50 | 5.03 | 1.35 | 2.65 | 1.00 | 0.00 | 4.14 | 0.08 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 17.2 | 54 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 2.1 | 25 | ||||
| Maelifellsandur | wt.% | 56.23 | 22.34 | 3.86 | 1.33 | 3.59 | 2.63 | 0.24 | 9.53 | 0.25 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 22.5 | 101 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 2.4 | 24 | ||||
| Eyjafjallajokull | wt.% | 68.76 | 14.63 | 3.00 | 6.88 | 1.64 | 0.55 | 1.59 | 2.96 | 0.00 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 9.3 | 0 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 1.7 | 39 | ||||
| Gobi | wt.% | 63.03 | 20.30 | 4.22 | 0.76 | 6.56 | 0.00 | 2.27 | 2.60 | 0.24 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 17.0 | 27 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 3.6 | 53 | ||||
| M’Bour | wt.% | 62.01 | 33.27 | 1.26 | 0.24 | 0.00 | 0.12 | 0.87 | 2.24 | 0.00 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 10.2 | 8 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 0.8 | 53 | ||||
| ATD | wt.% | 66.10 | 19.77 | 2.98 | 1.20 | 4.12 | 0.30 | 2.14 | 3.19 | 0.23 | ||
| Sulfites (µg/m2) | 0 | 23 | 0 | 144 | 61 | 111 | 14.2 | 0 | ||||
| Sulfates (µg/m2) | 1 | 0 | 12 | 38 | 0 | 0 | 2.6 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urupina, D.; Romanias, M.N.; Thevenet, F. How Relevant Is It to Use Mineral Proxies to Mimic the Atmospheric Reactivity of Natural Dust Samples? A Reactivity Study Using SO2 as Probe Molecule. Minerals 2021, 11, 282. https://doi.org/10.3390/min11030282
Urupina D, Romanias MN, Thevenet F. How Relevant Is It to Use Mineral Proxies to Mimic the Atmospheric Reactivity of Natural Dust Samples? A Reactivity Study Using SO2 as Probe Molecule. Minerals. 2021; 11(3):282. https://doi.org/10.3390/min11030282
Chicago/Turabian StyleUrupina, Darya, Manolis N. Romanias, and Frederic Thevenet. 2021. "How Relevant Is It to Use Mineral Proxies to Mimic the Atmospheric Reactivity of Natural Dust Samples? A Reactivity Study Using SO2 as Probe Molecule" Minerals 11, no. 3: 282. https://doi.org/10.3390/min11030282






