Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Specimen Preparation
2.2. Methods
2.2.1. SEM/EDS
2.2.2. FTIR
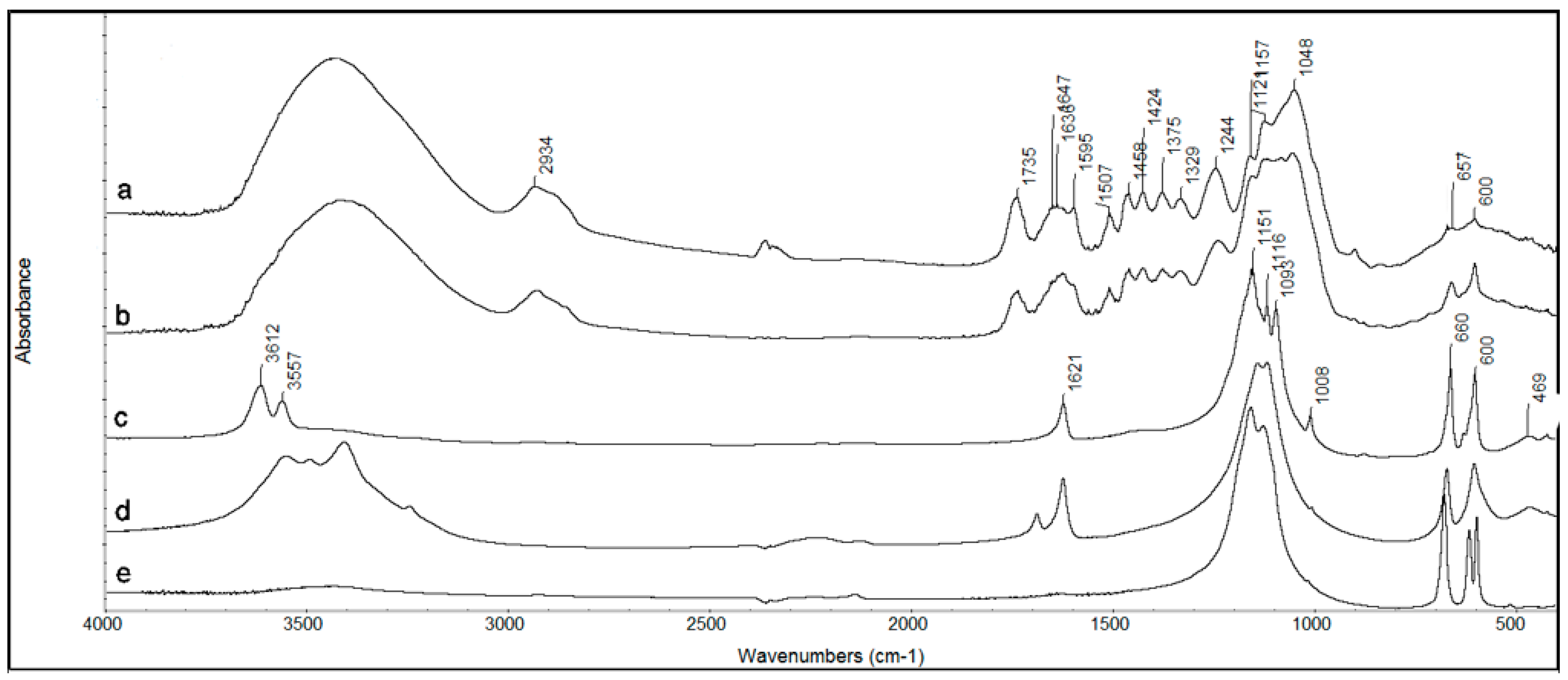
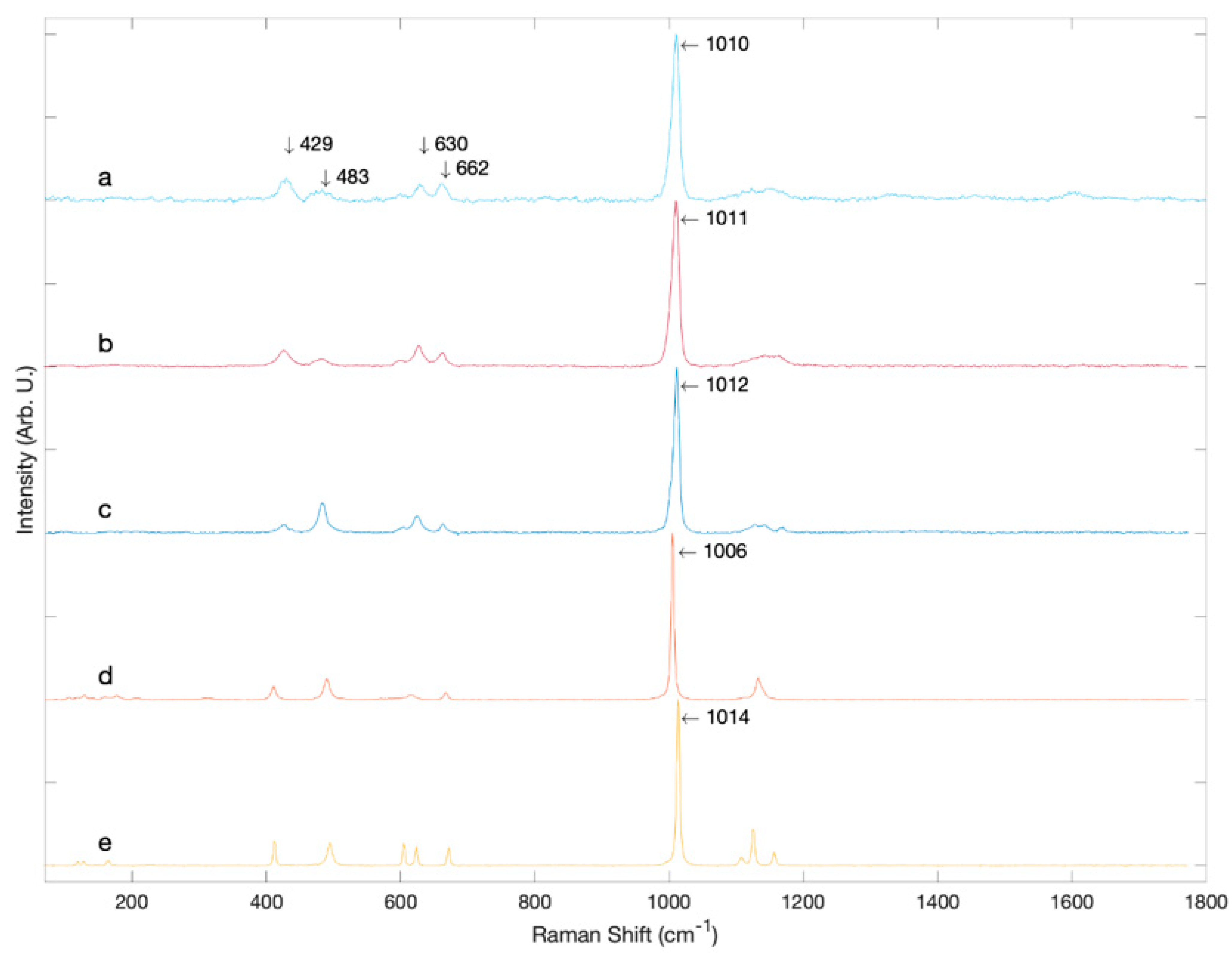
2.2.3. Raman Spectroscopy
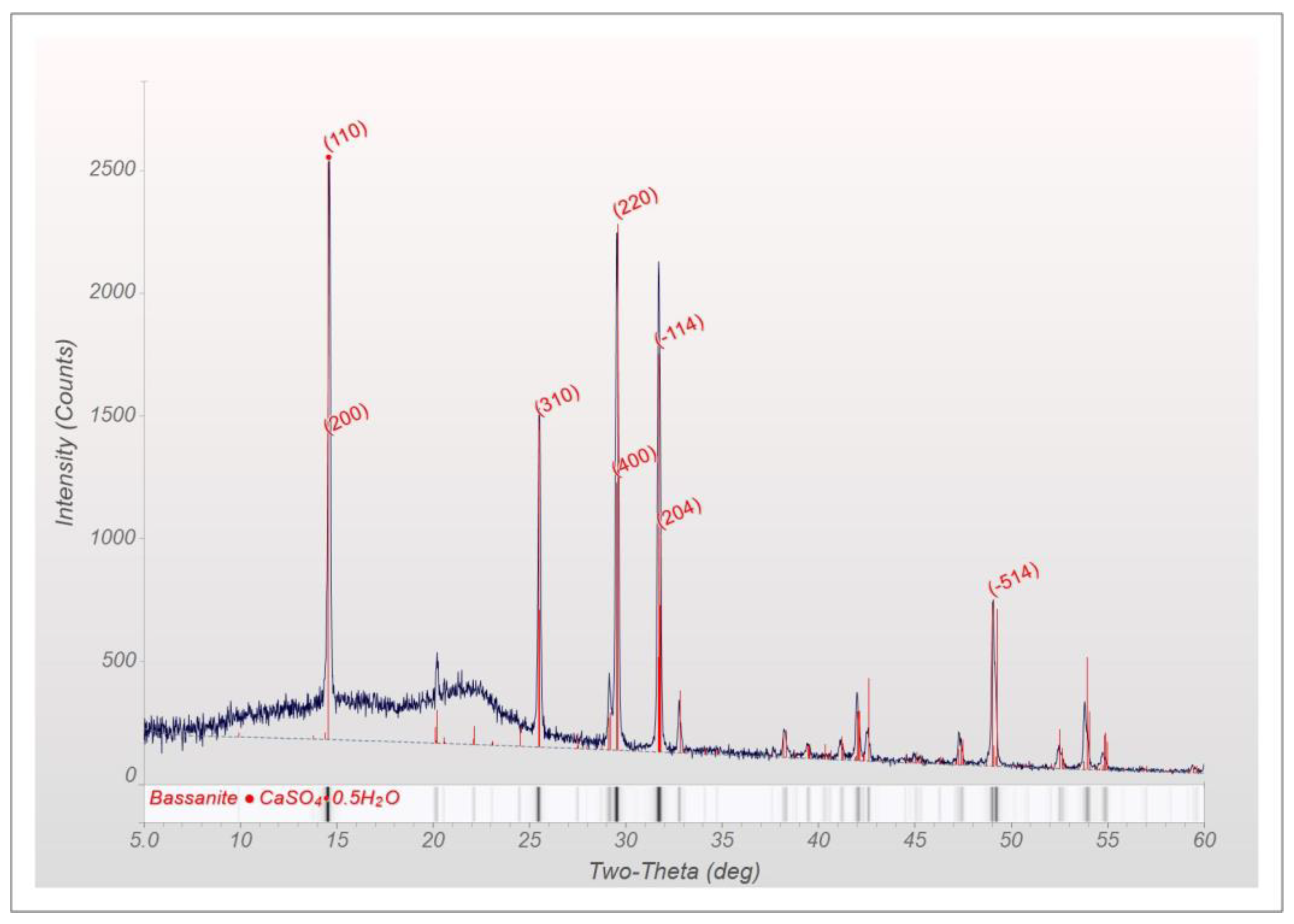
2.2.4. X-ray Powder Diffraction (XRD)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scurfield, G.; Michell, A.J.; Silva, S.R. Crystals in woody stems. Bot. J. Linn. Soc. 1973, 66, 277–289. [Google Scholar] [CrossRef]
- Arnott, H.J.; Pautard, F.G.E. Calcification in plants. In Biological Calcification: Cellular and Molecular Aspects; Schraer, H., Ed.; Appleton-Century-Crofts: New York, NY, USA, 1970; pp. 375–441. [Google Scholar]
- Gal, A.; Brumfeld, V.; Weiner, S.; Addadi, L.; Oron, D. Certain biominerals in leaves function as light scatterers. Adv. Opt. Mat. 2012, 24, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Scurfield, G.; Anderson, C.A.; Segnit, E.R. Silica in woody stems. Aust. J. Bot. 1974, 22, 211–229. [Google Scholar] [CrossRef]
- Schiegl, S.; Lev-Yadun, S.; Bar-Yosef, S.; El Goresy, A.; Weiner, S. Siliceous aggregates from prehistoric wood ash: A major component of sediments in Kebara and Hayonim caves (Israel). Isr. J. Earth Sci. 1994, 43, 267–278. [Google Scholar]
- Pritchard, S.G.; Prior, S.A.; Rogers, H.H.; Peterson, C.M. Calcium sulfate deposits associated with needle substamoatal cavities of container-grown longleaf pine (Pinus palustris) seedlings. Int. J. Plant Sci. 2000, 161, 917–923. [Google Scholar] [CrossRef]
- He, H.; Bleby, T.M.; Veneklaas, E.J.; Lambers, H.; Kuo, J. Morphologies and elemental compositions of calcium crystals in phyllodes and branchlets of Acacia robeorum (Leguminosae: Mimosoideae). Ann. Bot. 2012, 109, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Tsartsidou, G.; Lev-Yadun, S.; Albert, R.-M.; Miller-Rosen, A.; Efstratiou, N.; Weiner, S. The phytolith archaeological record: Strengths and weaknesses evaluated based on a quantitative modern reference collection from Greece. J. Archaeol. Sci. 2007, 34, 1262–1275. [Google Scholar] [CrossRef]
- Shahack-Gross, R.; Finkelstein, I. Subsistence practices in an arid environment: A geoarchaeological investigation in an Iron Age site, the Negev Highlands, Israel. J. Archaeol. Sci. 2008, 35, 965–982. [Google Scholar] [CrossRef]
- Weiner, S. Microarchaeology: Beyond the Visible Archaeological Record; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Pobequin, T. Les oxalates de calcium chez quelques Angiosperms. Ann. Sci. Nat. Bot. 1943, 4, 1–95. [Google Scholar]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Storey, R.; Thomson, W.W. An X-ray microanalysis study of the salt glands and intracellular calcium crystals of Tamarix. Ann. Bot. 1994, 73, 307–313. [Google Scholar] [CrossRef]
- Shuyskaya, E.V.; Rakhamkulova, Z.F.; Lebedeva, M.P.; Kolesnikov, A.V.; Safarova, A.; Borisochkina, T.I.; Toderich, K.N. Different mechanisms of ion homeostasis are dominant in the recretohalophyte Tamarix ramosissima under different soil salinity. Acta Physiol. Plant 2017, 39, 81–93. [Google Scholar] [CrossRef]
- Chew, M.K. The monstering of tamarisk: How scientists made a plant into a problem. J. Hist. Biol. 2009, 42, 231–266. [Google Scholar] [CrossRef] [PubMed]
- Waly, W. Wood anatomical characters of the Egyptian Tamarix L. species and its taxonomic significance. Taeckholmia 1999, 19, 115–125. [Google Scholar] [CrossRef]
- Mees, F.; Hatert, F.; Rowe, R. Omongwaite, Na2Ca5(SO4)6·3H2O, a new mineral from recent salt lake deposits, Namibia. Mineral. Mag. 2008, 72, 1307–1318. [Google Scholar] [CrossRef]
- Dogan, A.U.; Dogan, M.; Chan, C.N.; Wurster, D.E. Bassanite from Salvadora persica: A new evaporitic biomineral. Carbonates Evaporites 2005, 20, 2–7. [Google Scholar] [CrossRef]
- Tiemann, H.; Sötje, I.; Jarms, G.; Paulmann, C.; Epple, M.; Hasse, B. Calcium sulfate hemihydrate in statoliths of deep-sea medusae. J. Chem. Soc. Dalton Trans. 2002, 1266–1268. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiner, S.; Pinkas, I.; Kossoy, A.; Feldman, Y. Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree. Minerals 2021, 11, 289. https://doi.org/10.3390/min11030289
Weiner S, Pinkas I, Kossoy A, Feldman Y. Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree. Minerals. 2021; 11(3):289. https://doi.org/10.3390/min11030289
Chicago/Turabian StyleWeiner, Steve, Iddo Pinkas, Anna Kossoy, and Yishai (Isai) Feldman. 2021. "Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree" Minerals 11, no. 3: 289. https://doi.org/10.3390/min11030289
APA StyleWeiner, S., Pinkas, I., Kossoy, A., & Feldman, Y. (2021). Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree. Minerals, 11(3), 289. https://doi.org/10.3390/min11030289





