Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of Surface Tension Isotherms
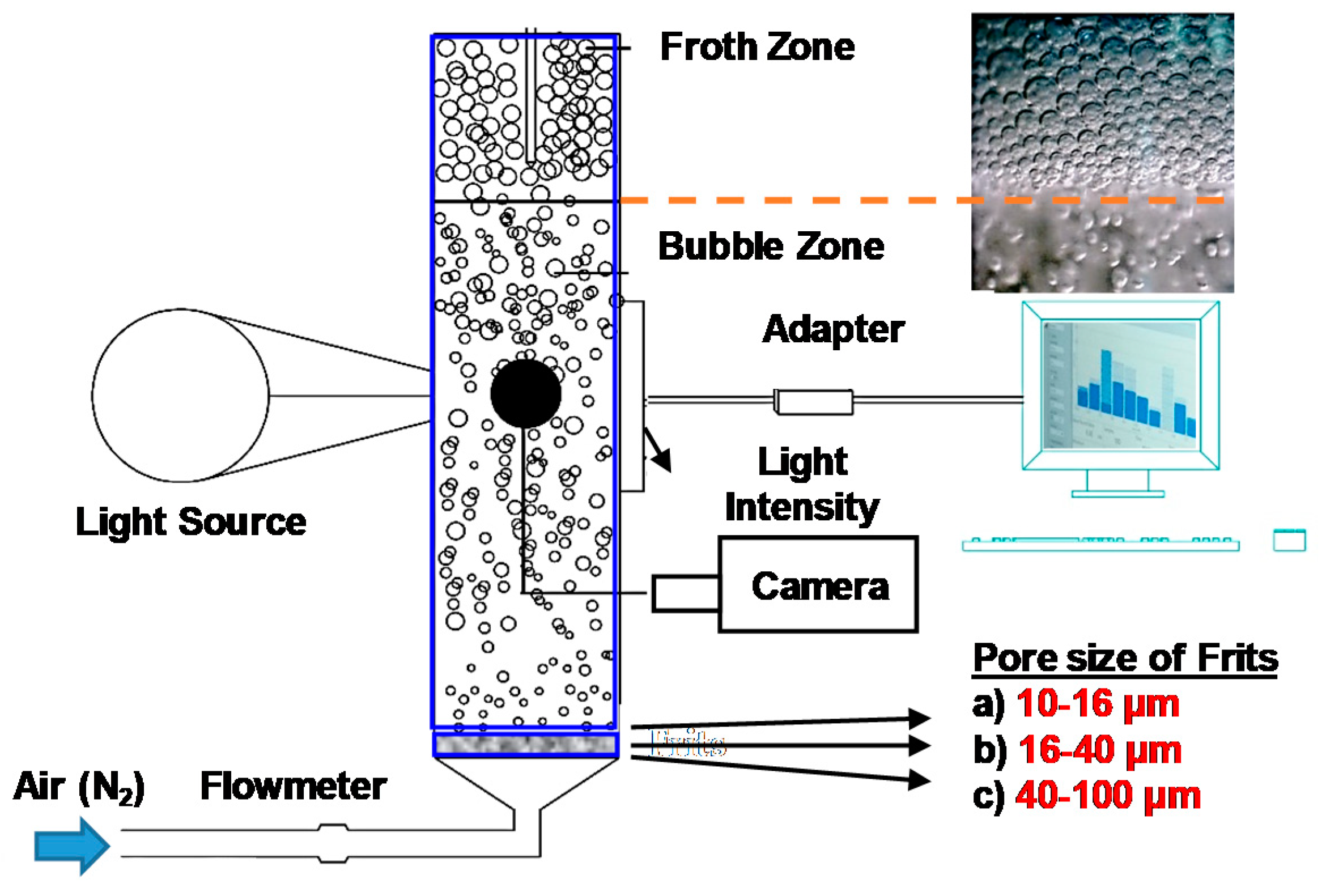
2.2. Bubble Size Measurements
2.3. Kinetics of Frother Adsorption on Rising Bubbles
3. Results and Discussion
3.1. Surface Tension Measurements
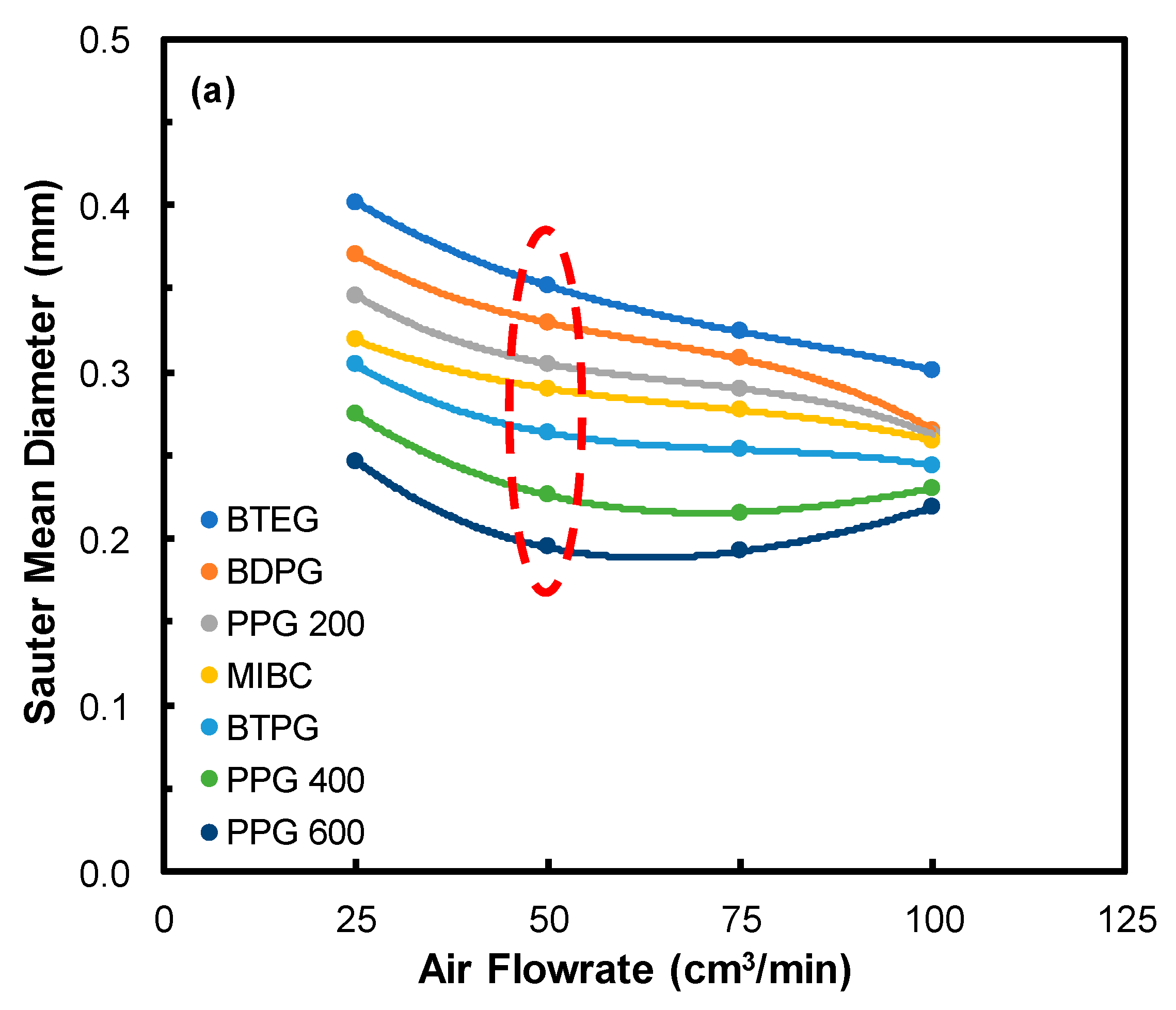
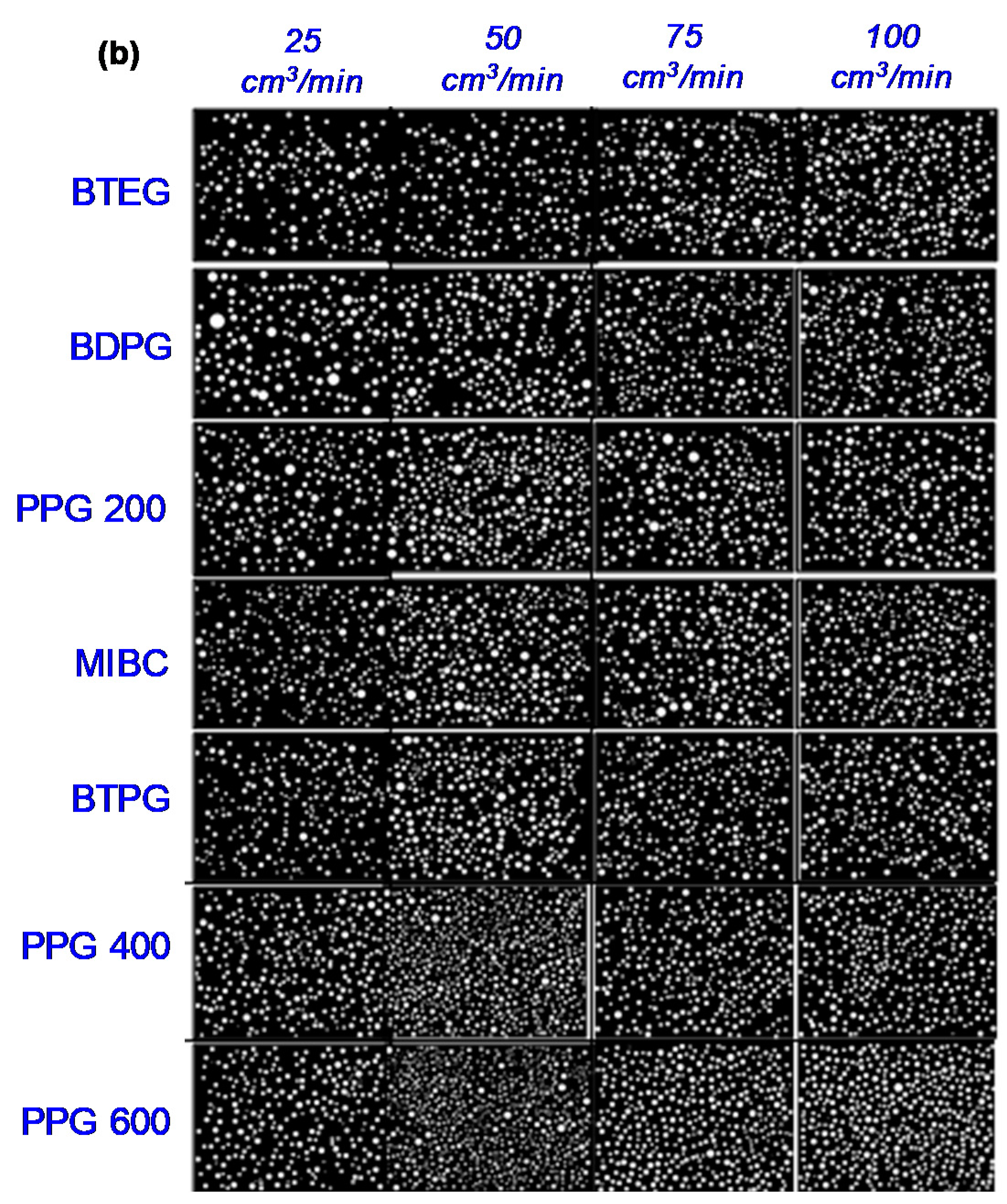
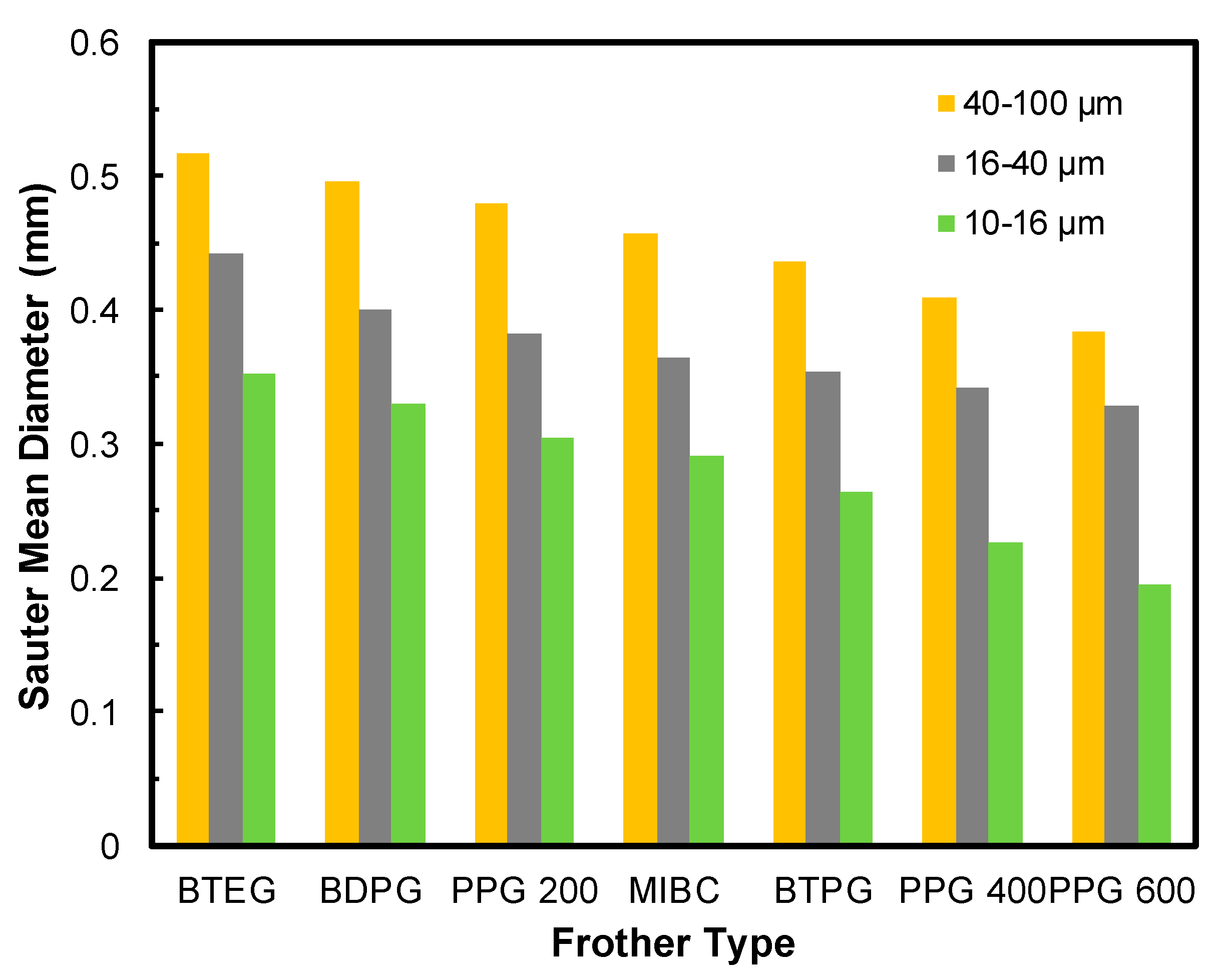
3.2. Bubble Size Measurements
3.2.1. Effect of Air Flowrate
3.2.2. Effect of Frit Pore Size
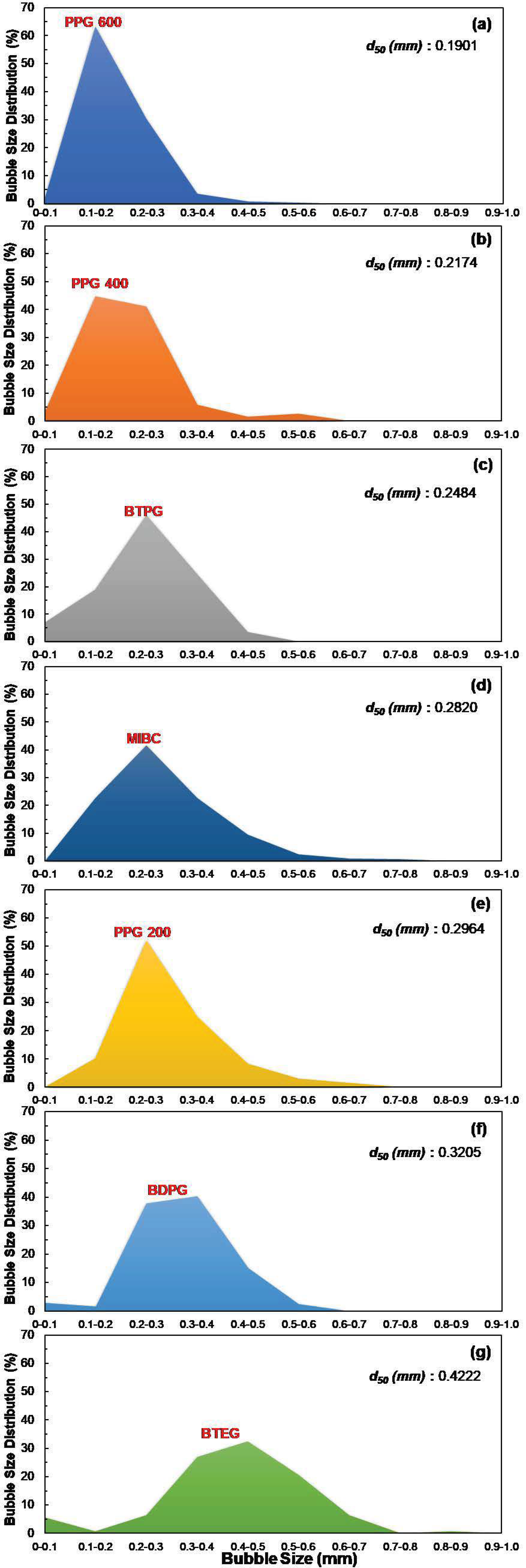
3.3. Bubble Size Distribution (BSD)
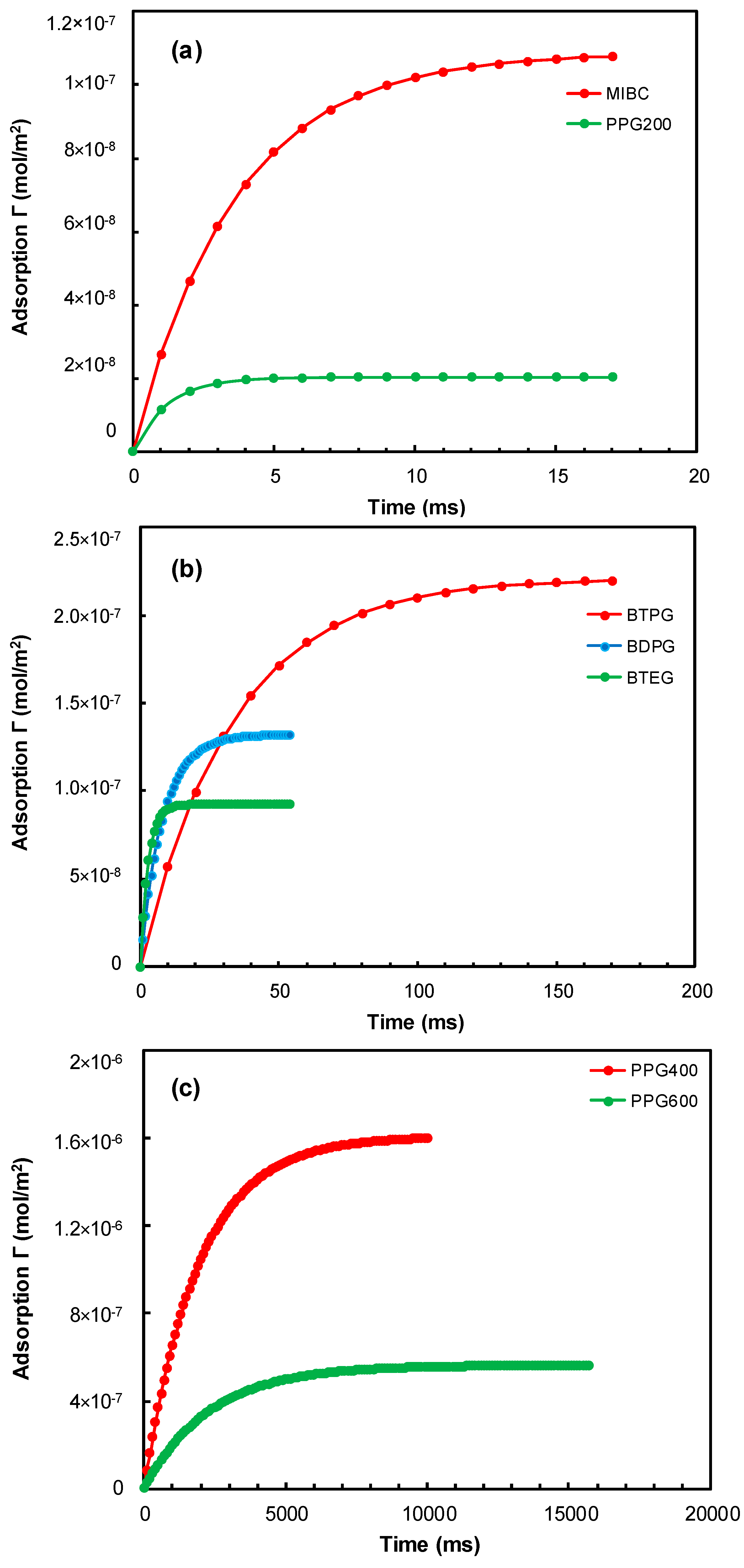
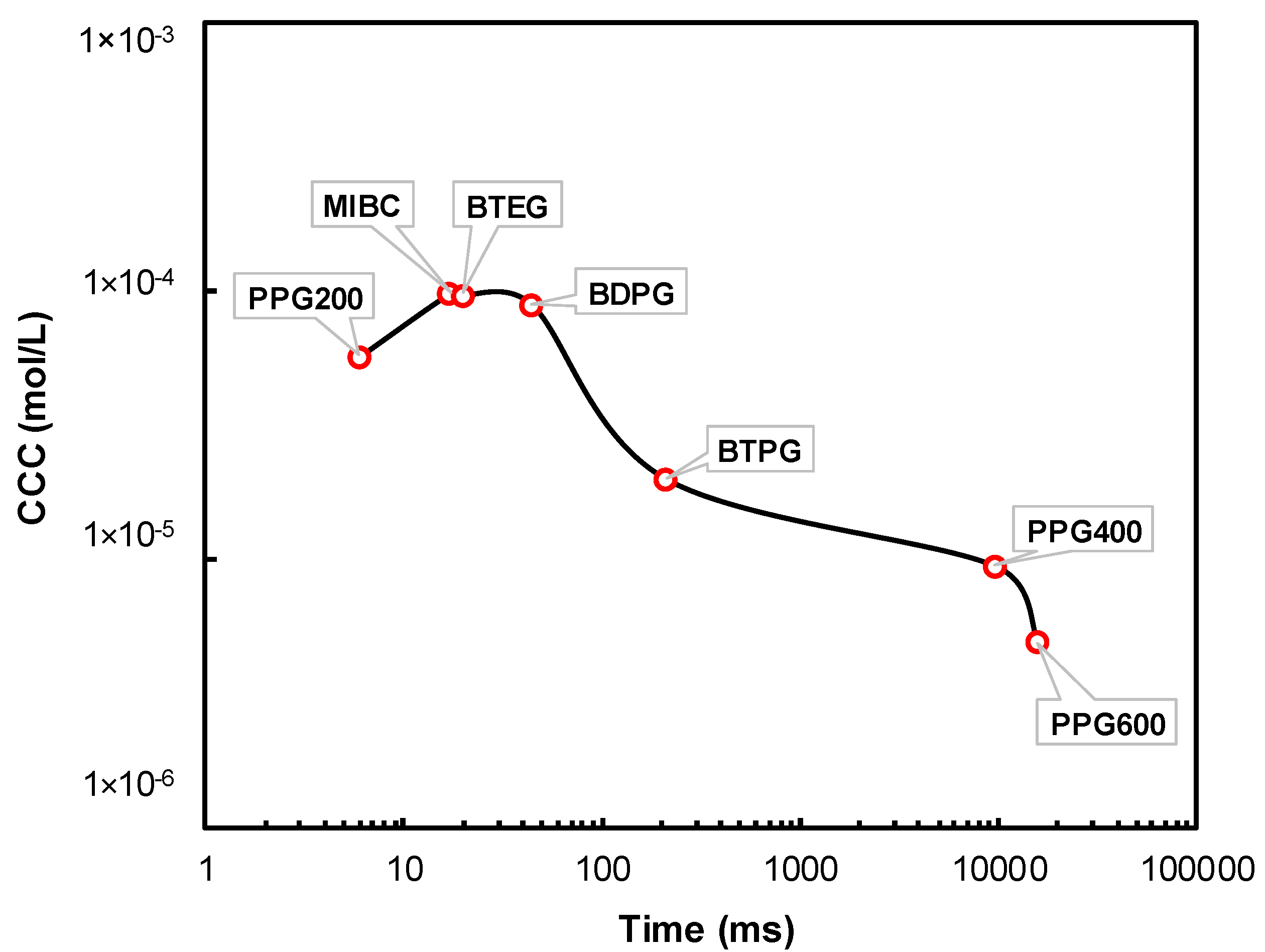
3.4. Kinetics of Frother’s Adsorption on Rising Bubbles at CCC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature to the Appendix
| Speed of bubble | |
| Diffusion coefficient of frother | |
| Radius of the bubble | |
| Frother bulk concentration | |
| Frother concentration at the bubble surface | |
| Peclet number | |
| Henry adsorption constant | |
| Thickness of diffusion boundary layer | |
| Equilibrium adsorption | |
| Temporal adsorption | |
| Henry adsorption constant |
Appendix A. Brief Derivation of the Equation of the Convective Adsorption of the Frothers on the Rising Bubbles
References
- Guven, O.; Celik, M.S.; Drelich, J.W. Flotation of methylated roughened glass particles and analysis of particle-bubble energy barrier. Miner. Eng. 2015, 79, 125–132. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Nesset, J.E.; Finch, J.A.; Gomez, C.O. Operating variables affecting the bubble size in forced-air mechanical flotation machines. In Proceedings of the 9th Mill Operators’ Conference 2007, Fremantle, WA, Australia, 19–21 March 2007; pp. 55–65. [Google Scholar]
- Zhang, W.; Nesset, J.E.; Finch, J.A. Water recovery and bubble surface area flux in flotation. Can. Metall. Q. 2010, 49, 353–362. [Google Scholar] [CrossRef]
- Welsby, S.D.D. Pilot-Scale Froth Testing at Highland Valley Copper. In Proceedings of the 46th Annual Meeting of the Canadian Mineral Processors Conference, Ottawa, ON, Canada, 21–23 January 2014; pp. 301–314. [Google Scholar]
- Zangooi, A.; Gomez, C.O.; Finch, J.A. Mapping frother distribution in industrial flotation circuits. Miner. Eng. 2017, 113, 36–40. [Google Scholar] [CrossRef]
- Guven, O.; Batjargal, K.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Experimental procedure for the determination of the critical coalescence concentration (CCC) of simple frothers. Minerals 2020, 10, 617. [Google Scholar] [CrossRef]
- Gomez, C.O.; Finch, J.A.; Muñoz-Cartes, D. An approach to characterise frother roles in flotation. In Proceedings of the 8th International Mineral Processing Seminar Procemin, Santiago, Chile, 30 November–2 December 2011; pp. 223–231. [Google Scholar]
- Karakashev, S.I.; Grozev, N.A.; Batjargal, K.; Guven, O.; Ozdemir, O.; Boylu, F.; Çelik, M.S. Correlations for easy calculation of the critical coalescence concentration (CCC) of simple frothers. Coatings 2020, 10, 612. [Google Scholar] [CrossRef]
- Vazirizadeh, A.; Bouchard, J.; Chen, Y. Effect of particles on bubble size distribution and gas hold-up in column flotation. Int. J. Miner. Process. 2016, 157, 163–173. [Google Scholar] [CrossRef]
- Kracht, W.; Rebolledo, H. Study of the local critical coalescence concentration (l-CCC) of alcohols and salts at bubble formation in two-phase systems. Miner. Eng. 2013, 50, 77–82. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S. Role of frothers in bubble generation and coalescence in a mechanical flotation cell. Can. J. Chem. Eng. 2006, 84, 170–182. [Google Scholar] [CrossRef]
- Mohagheghian, S.; Elbing, B.R. Characterization of bubble size distributions within a bubble column. Fluids 2018, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, M.; Desbiens, A.; del Villar, R.; Girgin, E.; Gomez, C. On-line estimation of bubble size distributions using Gaussian mixture models. In Proceedings of the V International Mineral Processing Seminar, Santiago, Chile, 22–24 October 2008; pp. 389–398. [Google Scholar]
- Kowalczuk, P.B.; Drzymala, J. Physical meaning of the Sauter mean diameter of spherical particulate matter. Particul. Sci. Technol. 2016, 34, 645–647. [Google Scholar] [CrossRef]
- Orpina, G.; Pincovschi, I.; Baran, G. Hidro-gazo-dinamica sistemelor de aerare echipate cu generatoare de bule. Ed. Politech. Press Buch. 2009, 5, 108–128. [Google Scholar]
- Mândrea, L.; Oprina, G.; Chihaia, R.A.; El-Leathey, A.; Mirea, R. Theoretical and Experimental Study of Gas Bubbles Behavior. Int. J. Model. Optim. 2017, 7, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Klimpel, R.R.; Hansen, R.D. Frothers. In Reagents in Mineral Technology; Somasundaran, P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 385–409. [Google Scholar]
- Somasundaran, P.; Moudgil, B.M. Surfactant Science Series, Vol. 27: Reagents in Mineral Technology; Marcel Dekker Inc.: New York, NY, USA, 1988; p. 755. [Google Scholar]
- Gupta, A.K.; Banerjee, P.K.; Mishra, A. Effect of frothers on foamability, foam stability, and bubble size. Coal Prep. 2007, 27, 107–125. [Google Scholar] [CrossRef]
- Klimpel, R.R.; Isherwood, S. Some industrial implications of changing frother chemical structure. Int. J. Miner. Process. 1991, 33, 369–381. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Woodburn, E.T.E. Frothing in Flotation II; Gordon and Breach: Amsterdam, The Netherlands, 1998; p. 371. [Google Scholar]
- Laskowski, J.S.; Tlhone, T.; Williams, P.; Ding, K. Fundamental properties of the polyoxypropylene alkyl ether flotation frothers. Int. J. Miner. Process. 2003, 72, 289–299. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Froth Flotation. In Wills’ Mineral Processing Technology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 12, pp. 265–380. [Google Scholar]
- Tan, Y.H.; Zhang, W.; Finch, J.A. Frother structure-property relationship: Effect of polyethylene glycols on bubble rise velocity. Miner. Eng. 2018, 116, 56–61. [Google Scholar] [CrossRef]
- Zhang, W.; Nesset, J.E.; Rao, R.; Finch, J.A. Characterizing frothers through critical coalescence concentration (CCC)95-hydrophile-lipophile balance (HLB) relationship. Minerals 2012, 2, 208–227. [Google Scholar] [CrossRef] [Green Version]
- Kangal, M.O.; Bulut, G.; Guven, O. Physicochemical characterization of natural wollastonite and calcite. Minerals 2020, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Sweet, C.; Van Hoogstraten, J.; Harris, M.; Laskowski, J.S. The effect of frothers on bubble size and frothability of aqueous solutions. In Processing of Complex Ores–Proc. 2nd UBC-Mc Gill Int. Symp; Finch, J.A., Holubec, I., Eds.; Metallurgical Society of CIM: Montreal, QC, Canada, 1997; pp. 235–245. [Google Scholar]
- Comley, B.A.; Harris, P.J.; Bradshaw, D.J.; Harris, M.C. Frother characterisation using dynamic surface tension measurements. Int. J. Miner. Process. 2002, 64, 81–100. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S.; Heiskanen, K. Effect of frothers on bubble size. Int. J. Min. Process. 2005, 76, 225–233. [Google Scholar] [CrossRef]
- Cao, L.; Chen, X.; Peng, Y. The effect of aliphatic alcohol frothers on the dispersion of oily collector. Miner. Eng. 2020, 157, 106552. [Google Scholar] [CrossRef]
- Cao, L.; Chen, X.; Peng, Y. The effect of polyglycol-type frothers on the interfacial characteristics of oily collector dispersion. Miner. Eng. 2020, 157, 106579. [Google Scholar] [CrossRef]
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of frothers on bubble coalescence and foaming in electrolyte solutions and seawater. Int. J. Miner. Process. 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Finch, J.A.; Nesset, J.E.; Acuña, C. Role of frother on bubble production and behaviour in flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Szyszka, D. Critical Coalescence Concentration (CCC) for surfactants in aqueous solutions. Minerals 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Levich, V.G. Physicochemical Hydrodynamics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1962; p. 512. [Google Scholar]
- Karakashev, S.I.; Ivanova, D.S.; Angarska, Z.K.; Manev, E.D.; Tsekov, R.; Radoev, B.; Slavchov, R.; Nguyen, A.V. Comparative validation of the analytical models for the Marangoni effect on foam film drainage. Coll. Surf. A 2010, 365, 122–136. [Google Scholar] [CrossRef]
- Park, S.H.; Park, C.; Lee, J.; Lee, B. A Simple Parameterization for the Rising Velocity of Bubbles in a Liquid Pool. Nucl. Eng. Technol. 2017, 49, 692–699. [Google Scholar] [CrossRef]


| Frothers | Chemical Formula | CCC (mol/L) | a (mm) | Γeq (mol/m2) | U (m/s) | D (m2/s) | K (m) | Pe |
|---|---|---|---|---|---|---|---|---|
| MIBC | CH3CH2(CH3)CH2(OH)CH3 | 9.78 × 10−5 | 0.20 | 1.09 × 10−7 | 0.13 | 7.62 × 10−10 | 1.17 × 10−6 | 3.43 × 104 |
| PPG 600 | HO(C3H6O)10H | 5.00 × 10−6 | 0.19 | 5.61 × 10−7 | 0.12 | 3.51 × 10−10 | 5.00 × 10−4 | 6.54 × 104 |
| PPG 400 | HO(C3H6O)6.5H | 9.52 × 10−6 | 0.22 | 1.60 × 10−6 | 0.16 | 4.52 × 10−10 | 5.00 × 10−4 | 7.53 × 104 |
| BTPG | C4H9(C3H6O)3OH | 2.01 × 10−5 | 0.22 | 2.21 × 10−7 | 0.16 | 5.57 × 10−10 | 9.72 × 10−6 | 6.12 × 104 |
| PPG 200 | HO(C3H6O)3.5H | 5.73 × 10−5 | 0.21 | 2.04 × 10−8 | 0.14 | 6.12 × 10−10 | 3.60 × 10−7 | 4.69 × 104 |
| BDPG | C4H9(C3H6O)2OH | 8.93 × 10−5 | 0.22 | 1.32 × 10−7 | 0.15 | 6.24 × 10−10 | 2.42 × 10−6 | 5.23 × 104 |
| BTEG | C4H9(C2H4O)3OH | 9.70 × 10−5 | 0.28 | 9.23 × 10−8 | 0.26 | 6.24 × 10−10 | 9.72 × 10−7 | 1.15 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals 2021, 11, 304. https://doi.org/10.3390/min11030304
Batjargal K, Guven O, Ozdemir O, Karakashev SI, Grozev NA, Boylu F, Çelik MS. Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals. 2021; 11(3):304. https://doi.org/10.3390/min11030304
Chicago/Turabian StyleBatjargal, Khandjamts, Onur Guven, Orhan Ozdemir, Stoyan I. Karakashev, Nikolay A. Grozev, Feridun Boylu, and Mehmet Sabri Çelik. 2021. "Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions" Minerals 11, no. 3: 304. https://doi.org/10.3390/min11030304
APA StyleBatjargal, K., Guven, O., Ozdemir, O., Karakashev, S. I., Grozev, N. A., Boylu, F., & Çelik, M. S. (2021). Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals, 11(3), 304. https://doi.org/10.3390/min11030304











