Modulated Monoclinic Hydroxyapatite: The Effect of pH in the Microwave Assisted Method
Abstract
:1. Introduction
2. Materials and Methods
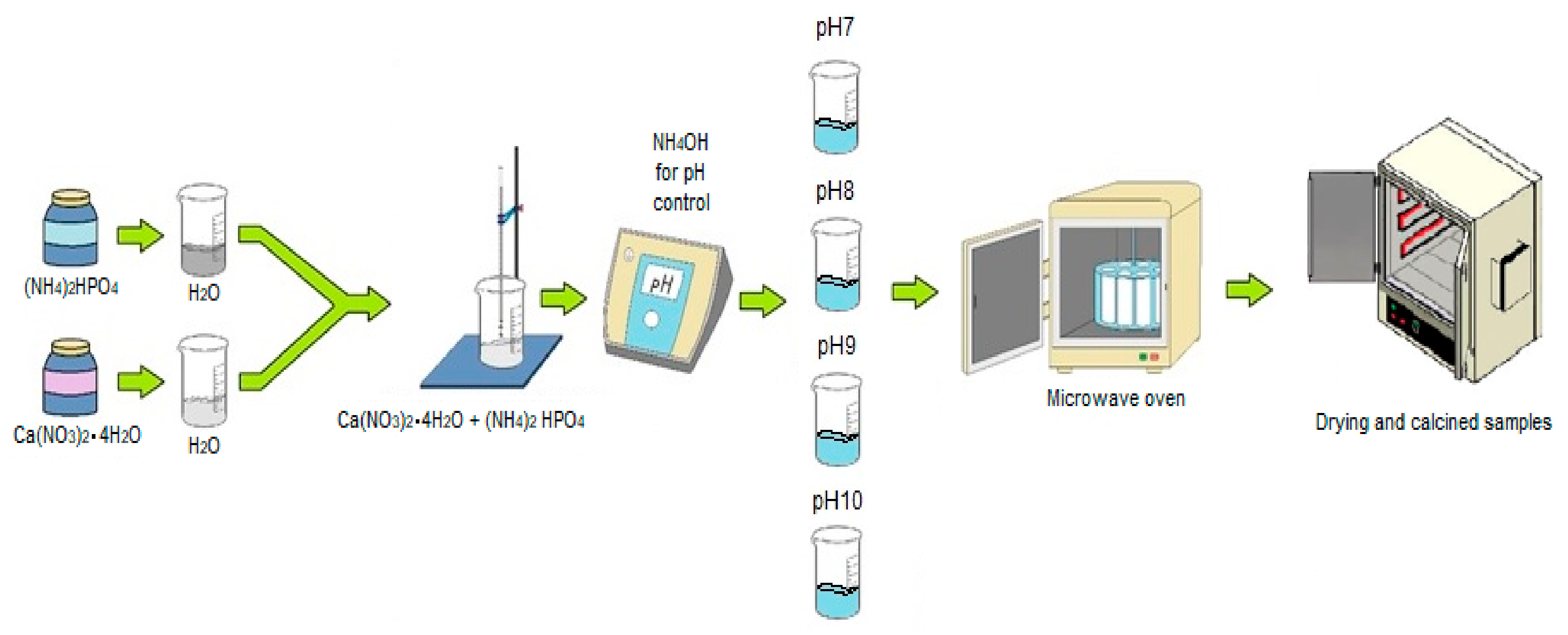
2.1. Microwave Assisted Hydrothermal Synthesis
2.2. Physicochemical Characterization
3. Results
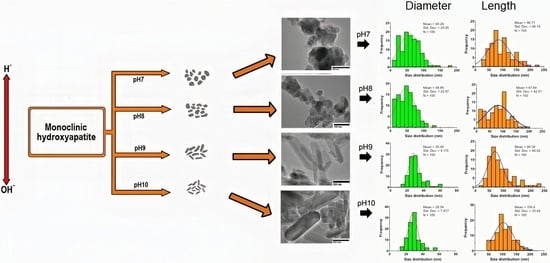
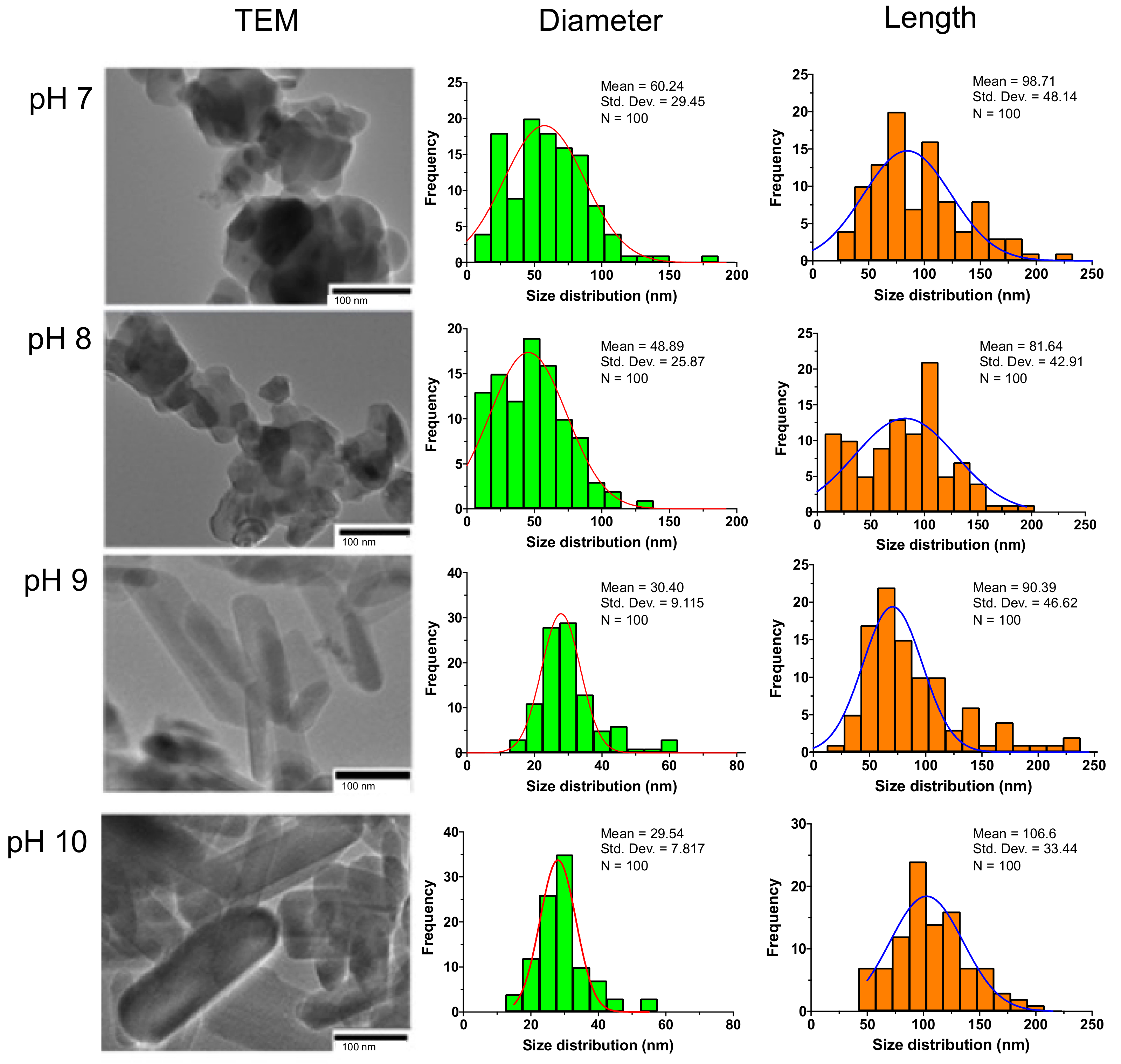
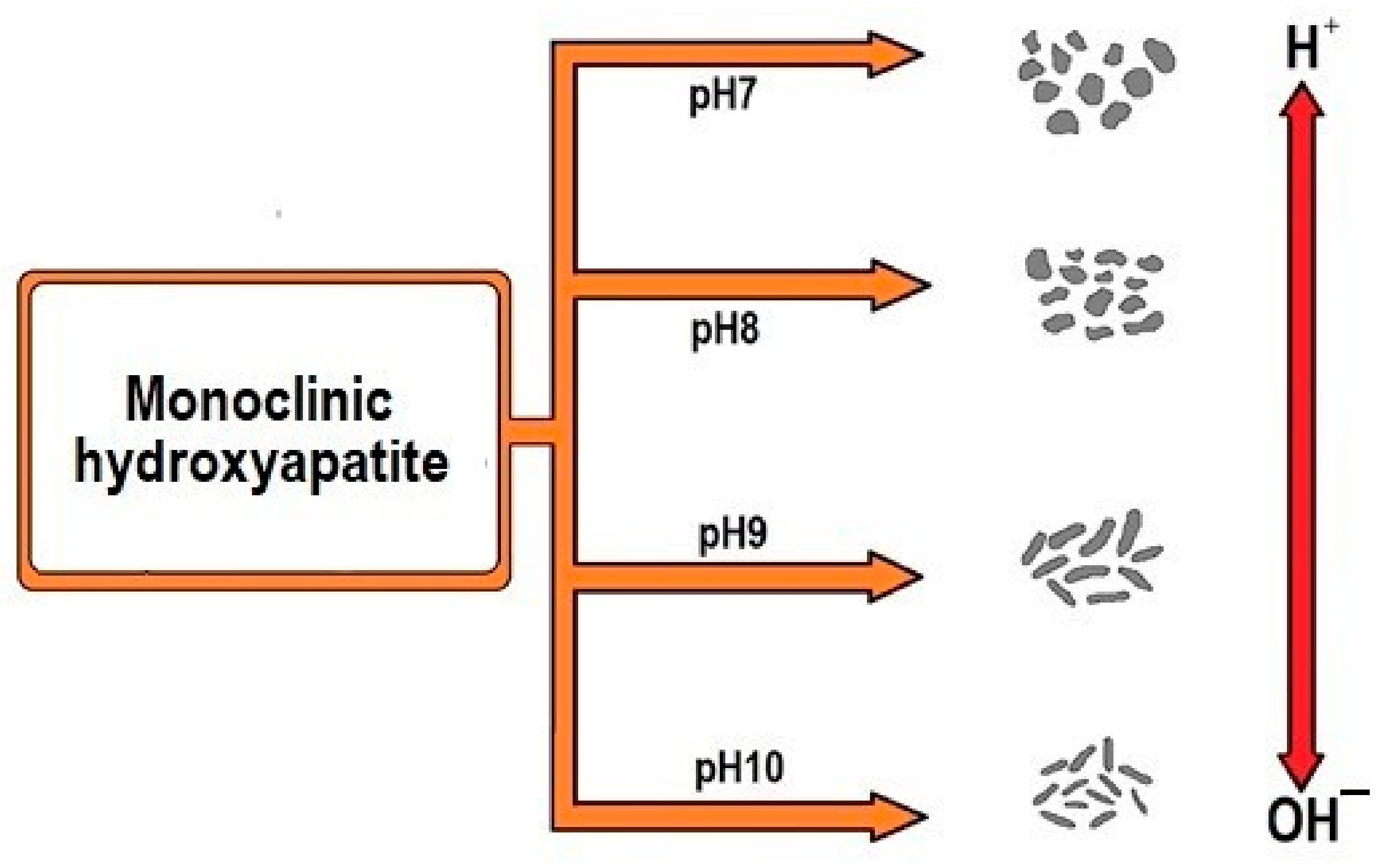
3.1. The Effect of pH on the Size and Shape of HAp
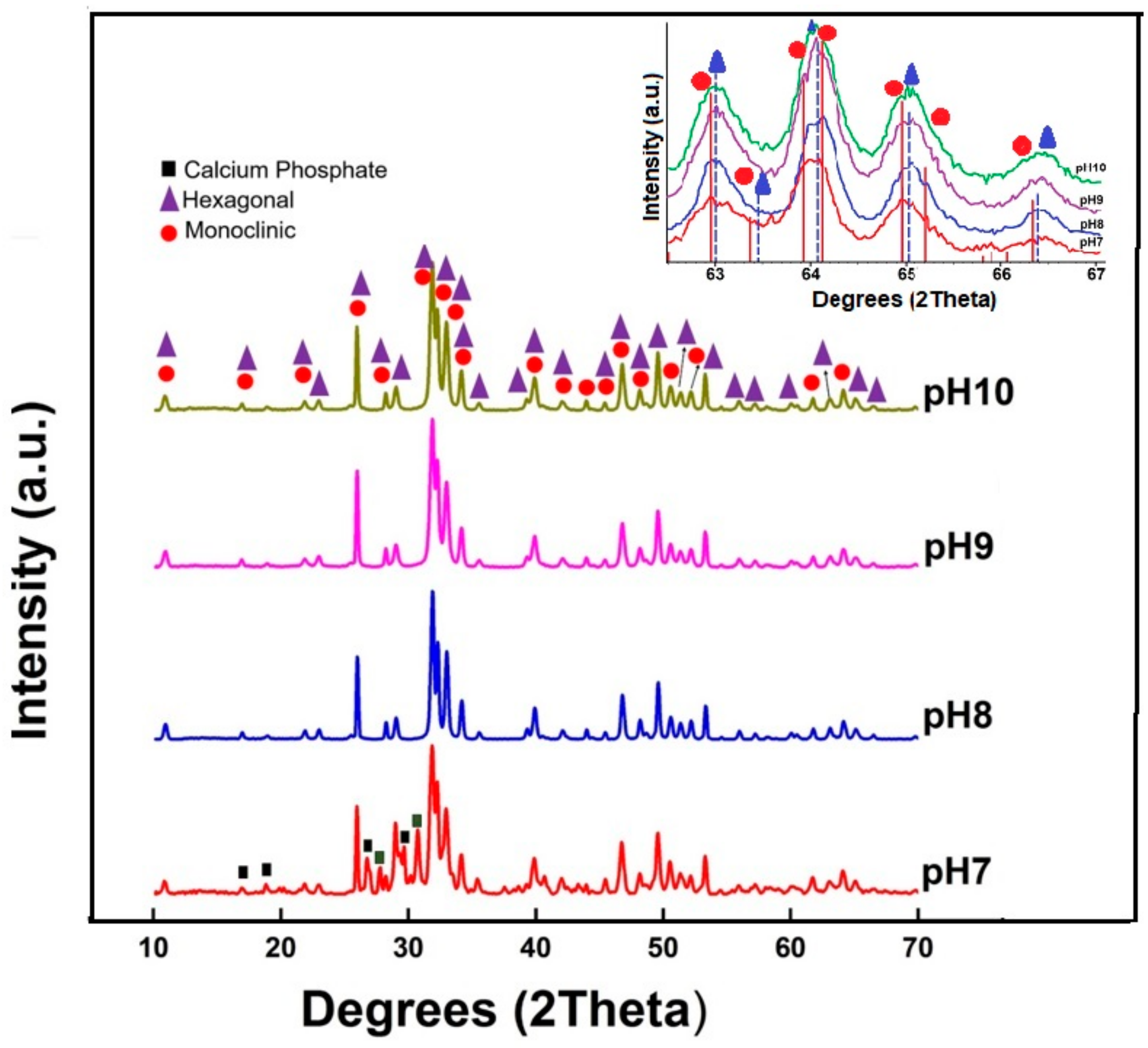
3.2. The Effect of pH on the HAp Crystallite Properties
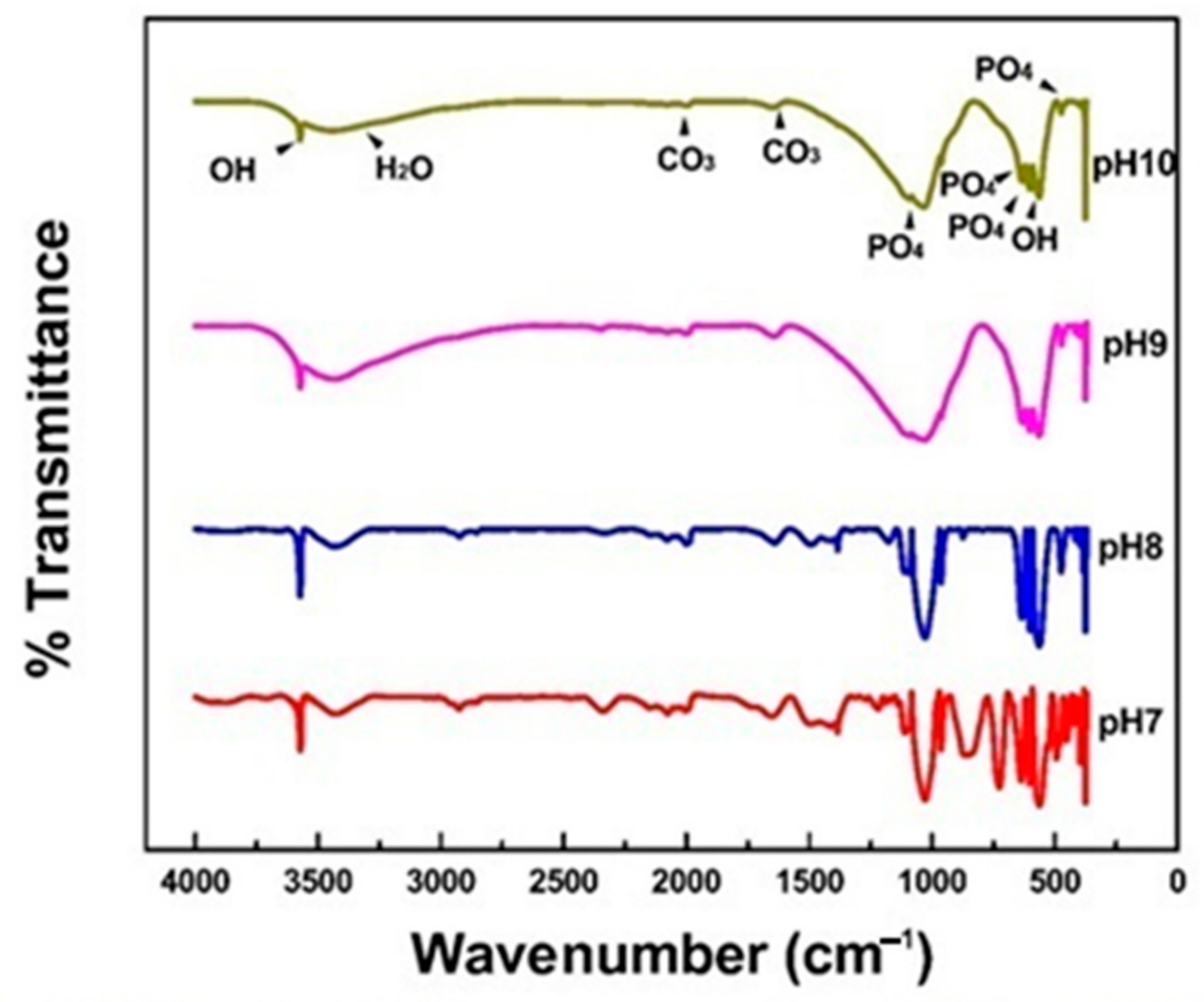
3.3. The Effect of pH on the Presence of HAp Functional Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castaño, V.M. Controlled hydrothermal production of hydroxylapatite from marine skeletons. Mater. Technol. 2001, 16, 97–103. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Jiang, X.; Wang, H.; Li, K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315–321. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, V.; Angeles-Chavez, C.; Mondragon, G.; Recillas-Gispert, S.; Castaño, V.M. Synthesis and structural characterization of hydroxyapatite obtained from CaO and CaHPO4 by a hydrothermal method. Mater. Res. Innov. 2005, 9, 20–22. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, V.; Angeles, C.; de la Isla, A.; Castano, V.M. Effect of bio-calcium oxide on the morphology of hydroxyapatite. Int. J. Basic Appl. Sci. 2015, 4, 395. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Vecchio, K.S. Hydrothermal synthesis of hydroxyapatite rods. J. Cryst. Growth 2007, 308, 133–140. [Google Scholar] [CrossRef]
- Nayak, A.K. Hydroxyapatite Synthesis Methodologies: An Overview Amit. Int. J. ChemTech Res. 2010, 2, 903–907. [Google Scholar] [CrossRef]
- Zhang, H.; Darvell, B.W. Morphology and structural characteristics of hydroxyapatite whiskers: Effect of the initial Ca concentration, Ca/P ratio and pH. Acta Biomater. 2011, 7, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, Z.; Zhong, S.; Wang, Z.; Wu, J.; Zhang, Q. Synthesis of hydroxyapatite nanorods from abalone shells via hydrothermal solid-state conversion. Mater. Des. 2015, 87, 445–449. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Corrigendum to “Mechanochemical synthesis of hydroxyapatite using cuttlefish bone and chicken eggshell as calcium precursors” (Materials Science & Engineering C (2019) 97 (124–140), (S0928493118314589) (10.1016/j.msec.2018.11.083)). Mater. Sci. Eng. C 2019, 100, 971. [Google Scholar] [CrossRef]
- Kim, W.; Saito, F. Sonochemical synthesis of hydroxyapatite from H3PO4 solution with Ca(OH)2. Ultrason. Sonochem. 2001, 8, 85–88. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, J.C.; Rhee, S.H. Effect of precursor concentration and spray pyrolysis temperature upon hydroxyapatite particle size and density. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.C.; Mackie, P.E.; Young, R.A. Monoclinic hydroxyapatite. Science 1973, 180, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Pu’ad, N.A.S.M.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [Green Version]
- Cerón, L.S.V.; Lugo, V.R.; Alatorre, J.A.A.; Fernández-Garcia, M.E.; Reyes-Valderrama, M.I.; González-Martínez, P.; Anaya, D.M. Characterization of hap nanostructures doped with AgNp and the gamma radiation effects. Results Phys. 2019, 15, 102702. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campos, D.; Mendoza-Anaya, D.; Reyes-Valderrama, M.I.; Esteban-Gómez, S.; Rodríguez-Lugo, V. Cationic surfactant at high pH in microwave HAp synthesis. Mater. Lett. 2020, 265, 3–6. [Google Scholar] [CrossRef]
- Komarneni, S.; Katsuki, H. Nanophase materials by a novel microwave-hydrothermal process. Pure Appl. Chem. 2002, 74, 1537–1543. [Google Scholar] [CrossRef]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current trends in the development of microwave reactors for the synthesis of nanomaterials in laboratories and industries: A review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Ruys, A.J.; Wei, M.; Sorrell, C.C.; Dickson, M.R.; Brandwood, A.; Milthorpe, B.K. Sintering effects on the strength of hydroxyapatite. Biomaterials 1995, 16, 409–415. [Google Scholar] [CrossRef]
- Hochrein, O.; Kniep, R.; Zahn, D. Atomistic simulation study of the order/disorder (monoclinic to hexagonal) phase transition of hydroxyapatite. Chem. Mater. 2005, 17, 1978–1981. [Google Scholar] [CrossRef]
- Manalu, J.L.; Soegijono, B.; Indrani, D.J. Characterization of Hydroxyapatite Derived from Bovine Bone. Asian J. Appl. Sci. 2015, 03. ISSN:2321-0893. [Google Scholar]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Brès, É.F. Crystallographic structure of human tooth enamel by electron microscopy and x-ray diffraction: Hexagonal or monoclinic? J. Microsc. 2012, 248, 102–109. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Mendoza-Anaya, D.; Sánchez-Campos, D.; Fernandez-García, M.E.; Salinas-Rodríguez, E.; Reyes-Valderrama, M.I.; Rodríguez-Lugo, V. The pH Effect on the Growth of Hexagonal and Monoclinic Hydroxyapatite Synthesized by the Hydrothermal Method. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Sánchez-Pastenes, E.; Reyes-Gasga, J. Point and space groups for hydroxyapatite by computer simulation of CBED electron diffraction patterns. Microsc. Microanal. 2003, 9, 1314–1315. [Google Scholar] [CrossRef] [Green Version]
- Leventouri, T.; Antonakos, A.; Kyriacou, A.; Venturelli, R.; Liarokapis, E.; Perdikatsis, V. Crystal Structure Studies of Human Dental Apatite as a Function of Age. Int. J. Biomater. 2009, 2009, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, H.; Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P.; Anderson, P. Preparation and characterisation of monoclinic hydroxyapatite and its precipitated carbonate apatite intermediate. Biomaterials 2000, 21, 617–627. [Google Scholar] [CrossRef]
- Pastero, L.; Bruno, M.; Aquilano, D. About the genetic mechanisms of apatites: A survey on the methodological approaches. Minerals 2017, 7, 139. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A.A. TOPAS-Academic, Version 4.1; Coelho Software: Brisbane, QLD, Australia, 2007. [Google Scholar]
- Cullity, B.D. Elements of X-Ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company Inc.: Quezon City, Phillippines, 1978. [Google Scholar]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Solis, R.; Gervacio-Arciniega, J.J.; Joseph, B.; Mendoza, M.E.; Moreno, A. Synthesis and characterization of a monoclinic crystalline phase of hydroxyapatite by synchrotron X-ray powder diffraction and piezoresponse force microscopy. Crystals 2018, 8, 458. [Google Scholar] [CrossRef] [Green Version]
- Salma, K.; Borodajenko, N.; Plata, A.; Berzina-Cimdina, L.; Stunda, A. Fourier Transform Infrared Spectra of Technologically Modified Calcium Phosphates. IFMBE Proc. 2008, 20, 68–71. [Google Scholar] [CrossRef]
- Ruffini, A.; Sprio, S.; Preti, L.; Tampieri, A. Synthesis of Nanostructured Hydroxyapatite via Controlled Hydrothermal Route. Biomater. Tissue Reconstr. Regen. Ref. 2019, 1, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hadrich, A.; Lautié, A.; Mhiri, T. Monoclinic to hexagonal phase transition and hydroxyl motion in calcium-lead hydroxyapatites studied by Raman spectroscopy. J. Raman Spectrosc. 2001, 32, 33–40. [Google Scholar] [CrossRef]
- Chuprunov, K.; Yudin, A.; Lysov, D.; Kolesnikov, E.; Kuznetsov, D.; Leybo, D.; Ilinykh, I.; Godymchuk, A. The pH Level Influence on Hydroxyapatite Phase Composition Synthesized with Hydrothermal Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 731. [Google Scholar] [CrossRef]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Amalia, T.R.; Nurlely, S.Y.W. Synthesis of Hidroxyapatite Using Microwave Irradiation and Sintering with Variation pH and Time. J. Phys. Conf. Ser. 2020, 1505. [Google Scholar] [CrossRef]
- Goh, K.W.; Wong, Y.H.; Ramesh, S.; Chandran, H.; Krishnasamy, S.; Sidhu, A.; Teng, W.D. Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Yudin, A.; Ilinykh, I.; Chuprunov, K.; Kolesnikov, E.; Kuznetsov, D.; Leybo, D.; Godymchuk, A. Microwave treatment and pH influence on hydroxyapatite morphology and structure. J. Phys. Conf. Ser. 2019, 1145. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.Y.; Peng, F.; Zi, Y.P.; Chen, F.; Qian, Q.R. Microwave-assisted hydrothermal rapid synthesis of calcium phosphates: Structural control and application in protein adsorption. Nanomaterials 2015, 5, 1284–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–Past, Present, and Future in Bone Regeneration Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.L.; Nancollas, G.H. The effect of pH and temperature on the crystal growth of hydroxyapatite. Arch. Oral Biol. 1972, 17, 1623–1627. [Google Scholar] [CrossRef]
- Ortiz, S.L.; Rodríguez-Lugo, V.; Villaseñor-Cerón, L.S.; Reyes-Valderrama, M.I.; Salado-Leza, D.E.; Mendoza-Anaya, D. El Potencial de la Hidroxiapatita Dopada como Sensor Termoluminiscente de Radiación ionizante, Pädi Boletín Científico Ciencias Básicas e Ing. Del ICBI 2020, 8, 85–90. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, V.; Karthik, T.V.K.; Mendoza-Anaya, D.; Rubio-Rosas, E.; Cerón, L.S.V.; Reyes-Valderrama, M.I.; Salinas-Rodríguez, E. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Lugo, V.; Salinas-Rodríguez, E.; Vázquez, R.A.; Alemán, K.; Rivera, A.L. Hydroxyapatite synthesis from a starfish and β-tricalcium phosphate using a hydrothermal method. RSC Adv. 2017, 7, 7631–7639. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | Hexagonal HAp | Monoclinic HAp |
|---|---|---|
| Chemical formula | Ca5(PO4)3OH PDF-00-009-0432 | Ca5 (PO4)3 (OH) PDF-01-089-4405 |
| Space group | P63/m | P21/b |
| Lattice parameters unit cell | a= b= 9.432 Å, c= 6.881 Å and γ = 120° | a= 9.421 Å, b= 2a, c= 6.881 Å and γ = 120° |
| OH− position | Opposite direction | Same direction |
| Sample | Diameter Mean ± SD | Length Mean ± SD | Aspect Ratio L/D |
|---|---|---|---|
| pH 7 | 60. 24 ± 29.45 | 98.71 ± 48.14 | 1.637 ± 0.001 |
| pH 8 | 48.89 ± 25.87 | 81.64 ± 42.91 | 1.669 ± 0.003 |
| pH 9 | 30.40 ± 9.115 | 90.39 ± 46.62 | 2.973 ± 0.494 |
| pH 10 | 29.54 ± 7.817 | 106.6 ± 33.44 | 3.608 ± 0.140 |
| Diameter Analysis | |||||
|---|---|---|---|---|---|
| Test | Mean Difference | 99.00% CI of Difference | Significant? | Summary | Adjusted p Value |
| pH 7 vs. pH 8 | 11.35 | −1.869 to 24.57 | No | * | 0.0358 |
| pH 7 vs. pH 9 | 29.84 | 19.99 to 39.70 | Yes | **** | <0.0001 |
| pH 7 vs. pH 10 | 30.70 | 21.16 to 40.25 | Yes | **** | <0.0001 |
| pH 8 vs. pH 9 | 18.49 | 9.615 to 27.37 | Yes | **** | <0.0001 |
| pH 8 vs. pH 10 | 19.35 | 10.49 to 28.22 | Yes | **** | <0.0001 |
| pH 9 vs. pH 10 | 0.8603 | −3.120 to 4.840 | No | ns | 0.9005 |
| Length analysis | |||||
| Test | Mean Difference | 99.00% CI of Difference | Significant? | Summary | Adjusted pValue |
| pH 7 vs. pH 8 | 17.07 | −1.145 to 35.29 | No | * | 0.0180 |
| pH 7 vs. pH 9 | 8.324 | −9.797 to 26.45 | No | ns | 0.4610 |
| pH 7 vs. pH 10 | −7.886 | −26.64 to 10.87 | No | ns | 0.5379 |
| pH 8 vs. pH 9 | −8.751 | −28.41 to 10.90 | No | ns | 0.4885 |
| pH 8 vs. pH 10 | −24.96 | −40.98 to −8.944 | Yes | **** | <0.0001 |
| pH 9 vs. pH 10 | −16.21 | −35.51 to 3.095 | No | * | 0.0419 |
| Hexagonal (PDF-00-009-0432) | Monoclinic (PDF-01-089-4405) | ||
|---|---|---|---|
| Plane | Peak | Plane | Peak |
| (100) | 10.839° | (100) | 10.830° |
| (101) | 16.846° | (101) | 16.830° |
| (002) | 25.880° | (002) | 25.853° |
| (102) | 28.131° | (102) | 28.102° |
| (211) | 31.785° | (221) | 31.740° |
| (112) | 32.201° | (222) | 32.165° |
| (300) | 32.921° | (060) | 32.872° |
| (221) | 40.463° | (023) | 40.797° |
| (213) | 49.499° | (223) | 49.437° |
| Sample | Hexagonal HAp | Monoclinic HAp | (χ2) | ||||
|---|---|---|---|---|---|---|---|
| Phase (%) | CrystalliteSize (nm) | Phase (%) | CrystalliteSize (nm) | Rwp | Rexp | ||
| pH 7 | 14.96 | 21.3 | 85.04 | 43.80 | 5.81 | 4.68 | 1.54 |
| pH 8 | 3.26 | 34.3 | 96.74 | 36.80 | 5.58 | 4.46 | 1.56 |
| pH 9 | 3.28 | 38.8 | 96.72 | 26.20 | 5.20 | 4.33 | 1.44 |
| pH 10 | 4.76 | 40.8 | 95.24 | 22.90 | 4.82 | 4.36 | 1.22 |
| Functional Group | Bands | Vibrational Mode |
|---|---|---|
| OH− | 3570 and 633 cm−1 | Stretching and bending |
| PO4−3 | 472, 566, 603, 960, 1035 and 1092 cm−1 | Stretching |
| CO3−2 | 1640 and 1950 cm−1 | Bending |
| Initial pH | Obtained Phase | Morphology | Rietveld Analysis | Reference |
|---|---|---|---|---|
| 7, 9 and 11 | Hexagonal and chlorapatite | Rounded shape | Without | [40] |
| 8, 9, 10, 11 and 12 | Hexagonal | Needle-like shape | Without | [41] |
| 8, 10 and 13 | Hexagonal and calcium carbonate | Irregular shape | Without | [42] |
| 5, 7, 9 and 11 | Hexagonal tri-calcium phosphate | Nanorods and hierarchical shape | Without | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Campos, D.; Reyes Valderrama, M.I.; López-Ortíz, S.; Salado-Leza, D.; Fernández-García, M.E.; Mendoza-Anaya, D.; Salinas-Rodríguez, E.; Rodríguez-Lugo, V. Modulated Monoclinic Hydroxyapatite: The Effect of pH in the Microwave Assisted Method. Minerals 2021, 11, 314. https://doi.org/10.3390/min11030314
Sánchez-Campos D, Reyes Valderrama MI, López-Ortíz S, Salado-Leza D, Fernández-García ME, Mendoza-Anaya D, Salinas-Rodríguez E, Rodríguez-Lugo V. Modulated Monoclinic Hydroxyapatite: The Effect of pH in the Microwave Assisted Method. Minerals. 2021; 11(3):314. https://doi.org/10.3390/min11030314
Chicago/Turabian StyleSánchez-Campos, Daniel, Maria Isabel Reyes Valderrama, Susana López-Ortíz, Daniela Salado-Leza, María Eufemia Fernández-García, Demetrio Mendoza-Anaya, Eleazar Salinas-Rodríguez, and Ventura Rodríguez-Lugo. 2021. "Modulated Monoclinic Hydroxyapatite: The Effect of pH in the Microwave Assisted Method" Minerals 11, no. 3: 314. https://doi.org/10.3390/min11030314
APA StyleSánchez-Campos, D., Reyes Valderrama, M. I., López-Ortíz, S., Salado-Leza, D., Fernández-García, M. E., Mendoza-Anaya, D., Salinas-Rodríguez, E., & Rodríguez-Lugo, V. (2021). Modulated Monoclinic Hydroxyapatite: The Effect of pH in the Microwave Assisted Method. Minerals, 11(3), 314. https://doi.org/10.3390/min11030314