Feasibility of a Mineral Carbonation Technique Using Iron-Silicate Mining Waste by Direct Flue Gas CO2 Capture and Cation Complexation Using 2,2′-Bipyridine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Analytical Methods
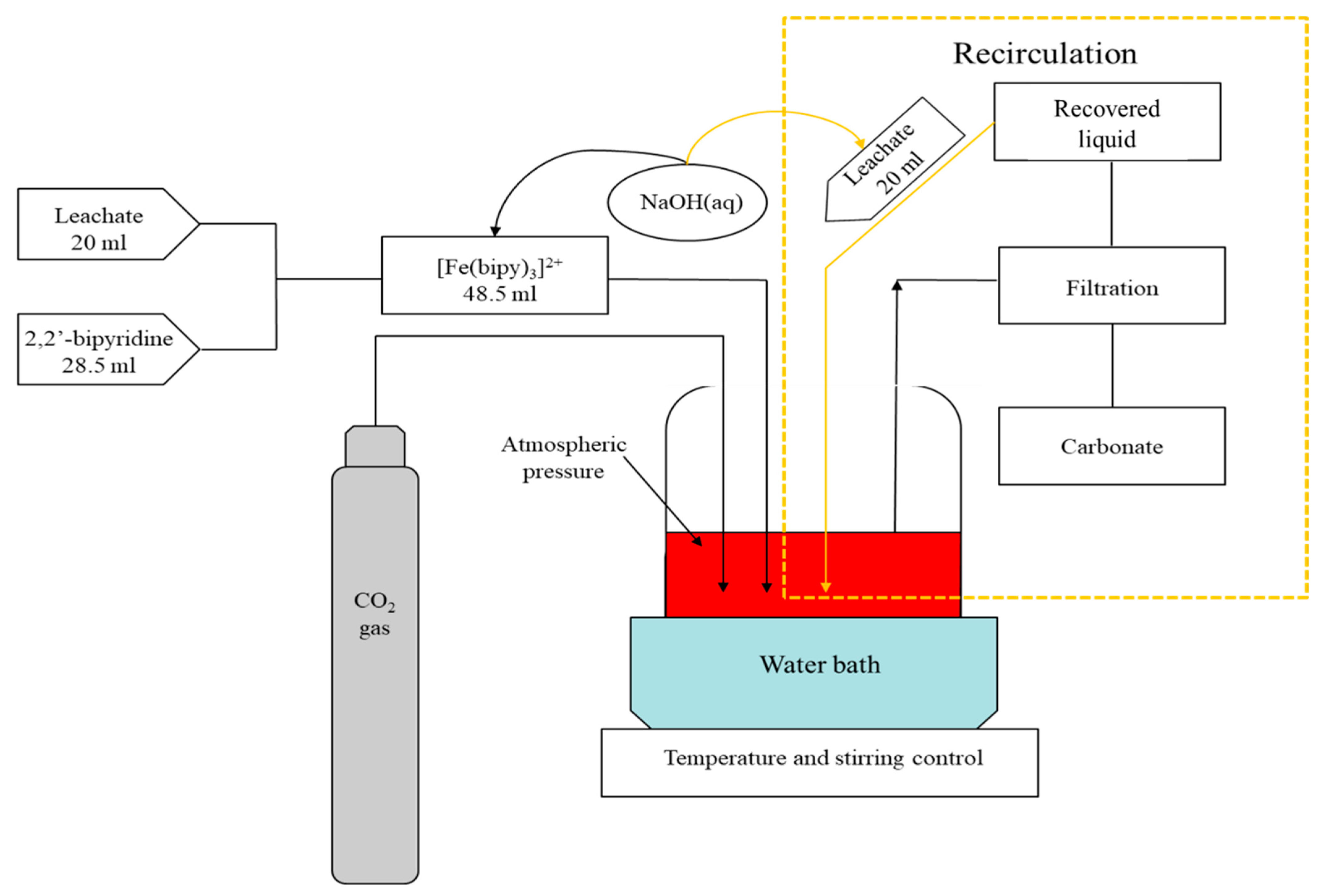
2.4. Methods
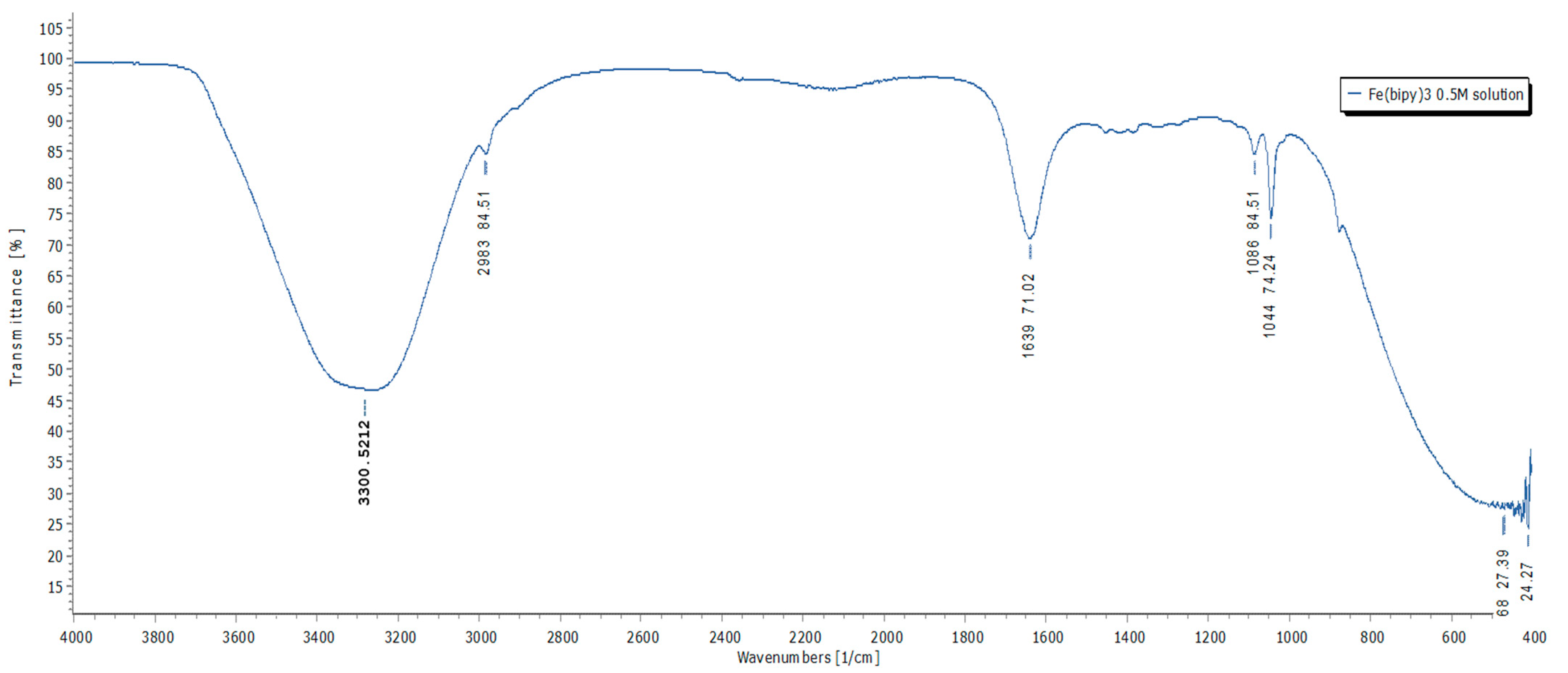
2.4.1. Characterization of the [Fe(bipy)3]2+ and Regeneration of the 2,2′-Bipyridine
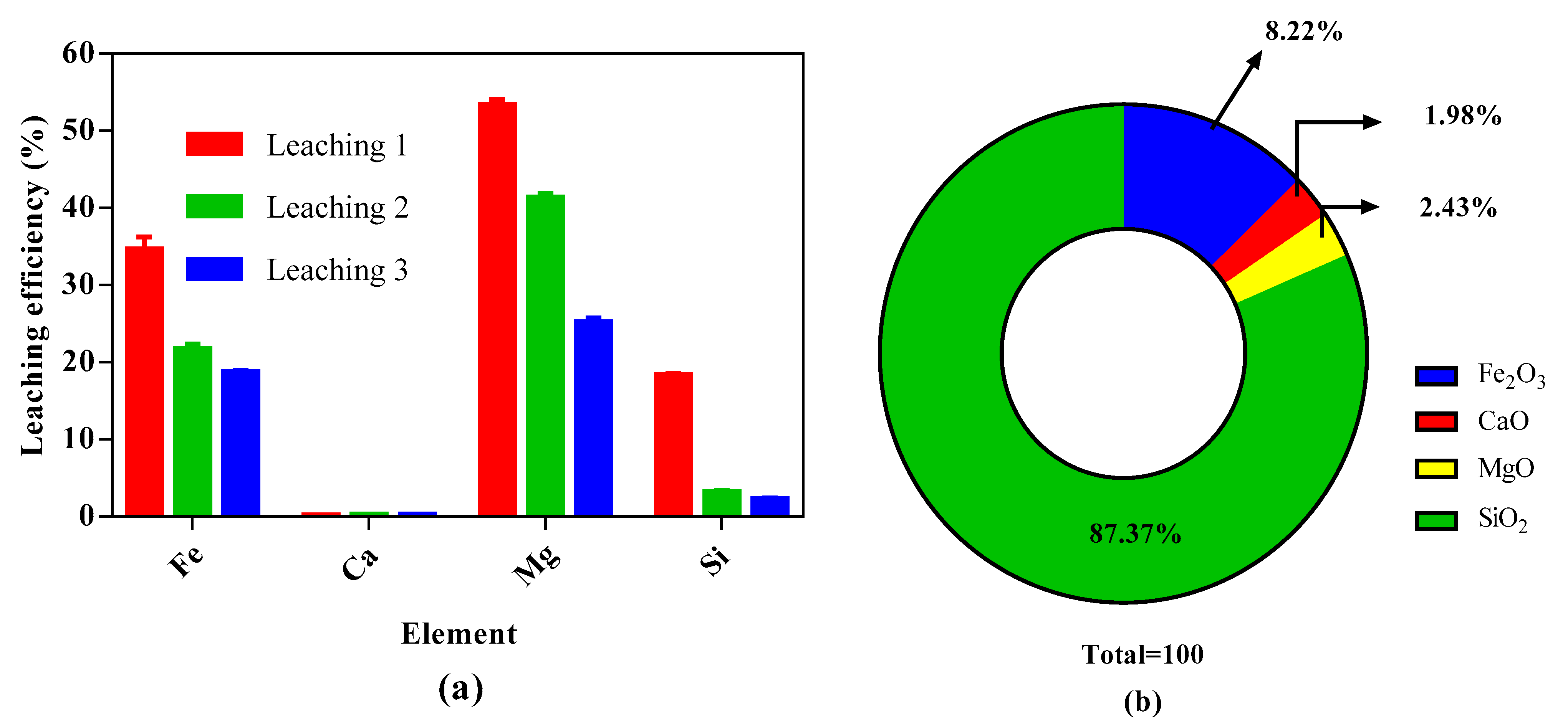
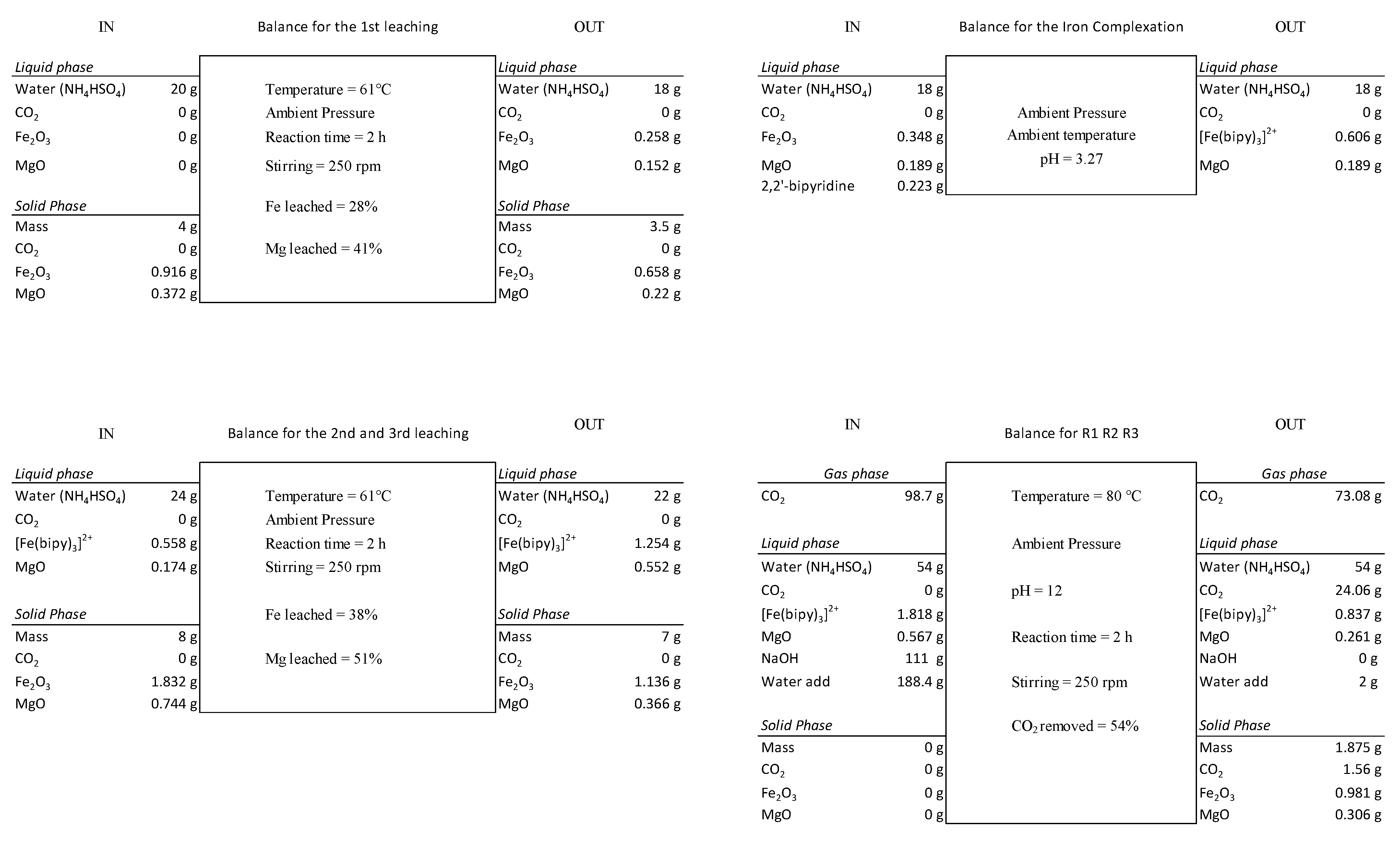
2.4.2. Leaching Process and Valorization of the Mining Residue
2.4.3. Mineral Carbonation Reaction: 2,2′-Bipyridine Recovery Potential and Preferential Reaction Conditions in Recirculation Scenario
2.4.4. Global Evaluation of Mineral Carbonation Approach
2.4.5. GHG and Mass Balance
3. Results and Discussion
3.1. Characterization of the [Fe(bipy)3]2+ and Regeneration of the 2,2′-Bipyridine
3.2. Optimization of Leaching Stage and Valorization of Residue
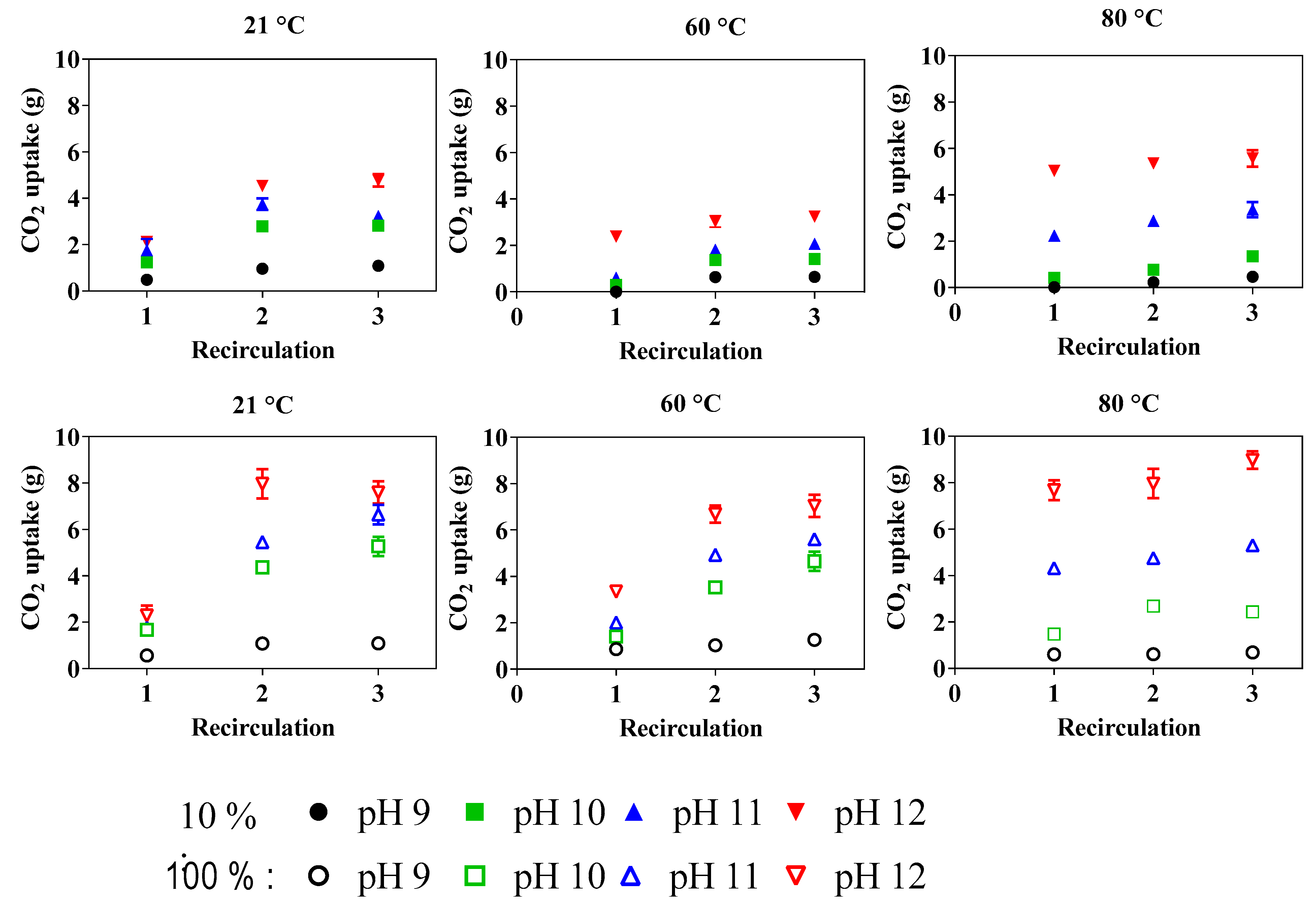
3.3. Mineral Carbonation Reaction
3.3.1. Preferential Reaction Conditions in a Recirculation Scenario
3.3.2. Carbonate Precipitation
3.3.3. Reaction Efficiency
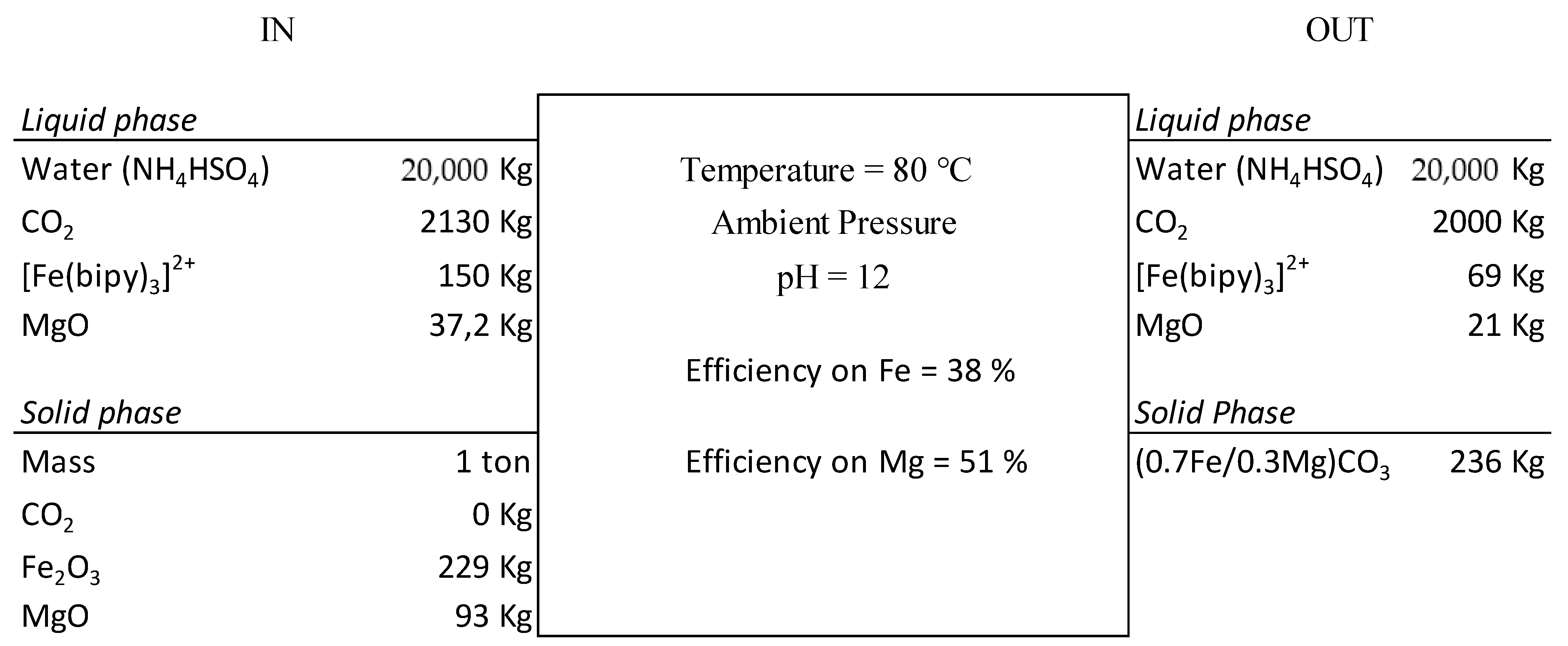
3.4. Global Mineral Carbonation Approach Evaluation
3.4.1. Mass Balance
3.4.2. Global Reaction Efficiency
3.5. GHG Balance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.; Roberts, D.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, R. IPCC, 2019: Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- IPCC. Annex III, Working Group III Contribution to the IPCC 5th Assessment Report “Climate Change 2014: Mitigation of Climate Change”; IPCC: Geneva, Switzerland, 2014; p. 46. [Google Scholar]
- Hansen, J.; Sato, M.; Hearty, P.; Ruedy, R.; Kelley, M.; Masson-Delmotte, V.; Russell, G.; Tselioudis, G.; Cao, J.; Rignot, E. Ice melt, sea level rise and superstorms: Evidence from paleoclimate data, climate modeling, and modern observations that 2 °C global warming is highly dangerous. Atmos. Chem. Phys. Discuss. 2015, 15, 1680–7367. [Google Scholar]
- Dow, K.; Downing, T.E. The Atlas of Climate Change: Mapping the World’s Greatest Challenge; University of California Press: Berkeley, CA, USA, 2016; p. 0520966821. [Google Scholar]
- Jones, M.C.; Cheung, W.W. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 2015, 72, 741–752. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C, an IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Western Climate Initiative (WCI). Cadre de Mise en Oeuvre du Programme Régional de la Western Climate Initiative (WCI). Available online: http://www.westernclimateinitiative.org/the-wci-cap-and-trade-program/program-design (accessed on 27 March 2020).
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Technical & economic evaluation of a mineral carbonation process using southern Québec mining wastes for CO2 sequestration of raw flue gas with by-product recovery. Int. J. Greenh. Gas Control 2016, 50, 147–157. [Google Scholar] [CrossRef]
- ECRA. Deployment of CO2 Capture in the Cement Industry. In Proceedings of the CCS-Conference, Brevik, Norway, 20–21 May 2015. [Google Scholar]
- Arts, R.; Chadwick, A.; Eiken, O.; Thibeau, S.; Nooner, S. Ten years’ experience of monitoring CO2 injection in the Utsira Sand at Sleipner, offshore Norway. First Break 2008, 26, 1365–2397. [Google Scholar] [CrossRef]
- Leeson, D.; Mac Dowell, N.; Shah, N.; Petit, C.; Fennell, P.S. A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources. Int. J. Greenh. Gas Control 2017, 61, 71–84. [Google Scholar] [CrossRef]
- Romanov, V.; Soong, Y.; Carney, C.; Rush, G.E.; Nielsen, B.; O’Connor, W. Mineralization of carbon dioxide: A literature review. ChemBioEng Rev. 2015, 2, 231–256. [Google Scholar] [CrossRef]
- Rahmani, O.; Junin, R.; Tyrer, M.; Mohsin, R. Mineral carbonation of red gypsum for CO2 sequestration. Energy Fuels 2014, 28, 5953–5958. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Dunsmore, H. A geological perspective on global warming and the possibility of carbon dioxide removal as calcium carbonate mineral. Energy Convers. Manag. 1992, 33, 565–572. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, B.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Lackner, K.S.; Butt, D.P.; Wendt, C.H. Progress on binding CO2 in mineral substrates. Energy Convers. Manag. 1997, 38, S259–S264. [Google Scholar] [CrossRef]
- Turianicová, E.; Baláž, P.; Tuček, Ľ.; Zorkovská, A.; Zeleňák, V.; Németh, Z.; Šatka, A.; Kováč, J. A comparison of the reactivity of activated and non-activated olivine with CO2. Int. J. Miner. Process. 2013, 123, 73–77. [Google Scholar] [CrossRef]
- Johnson, N.C.; Thomas, B.; Maher, K.; Rosenbauer, R.J.; Bird, D.; Brown, G.E. Olivine dissolution and carbonation under conditions relevant for in situ carbon storage. Chem. Geol. 2014, 373, 93–105. [Google Scholar] [CrossRef]
- Kemache, N.; Pasquier, L.-C.; Mouedhen, I.; Cecchi, E.; Blais, J.-F.; Mercier, G. Aqueous mineral carbonation of serpentinite on a pilot scale: The effect of liquid recirculation on CO2 sequestration and carbonate precipitation. Appl. Geochem. 2016, 67, 21–29. [Google Scholar] [CrossRef]
- Béarat, H.; McKelvy, M.J.; Chizmeshya, A.V.G.; Gormley, D.; Nunez, R.; Carpenter, R.W.; Squires, K.; Wolf, G.H. Carbon sequestration via aqueous olivine mineral carbonation: Role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, O.; Highfield, J.; Junin, R.; Tyrer, M.; Pour, A.B. Experimental investigation and simplistic geochemical modeling of CO2 mineral carbonation using the mount tawai peridotite. Molecules 2016, 21, 353. [Google Scholar] [CrossRef]
- Pasquier, L.-C. Procédé de Piegeage du CO2 Industriel par Carbonatation Minérale de Résidus Miniers Silicatés (Serpentinite) et Valorisation des Sous-Produits; Institut National de la Recherche Scientifique: Québec, QC, Canada, 2014. [Google Scholar]
- Sanna, A.; Steel, L.; Maroto-Valer, M.M. Carbon dioxide sequestration using NaHSO4 and NaOH: A dissolution and carbonation optimisation study. J. Environ. Manag. 2017, 189, 84–97. [Google Scholar] [CrossRef]
- Werner, M.; Hariharan, S.; Mazzotti, M. Flue gas CO2 mineralization using thermally activated serpentine: From single-to double-step carbonation. Phys. Chem. Chem. Phys. 2014, 16, 24978–24993. [Google Scholar] [CrossRef]
- Farhang, F.; Oliver, T.; Rayson, M.; Brent, G.; Stockenhuber, M.; Kennedy, E. Experimental study on the precipitation of magnesite from thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2016, 303, 439–449. [Google Scholar] [CrossRef]
- Kashim, M.Z.; Tsegab, H.; Rahmani, O.; Abu Bakar, Z.A.; Aminpour, S.M. Reaction Mechanism of Wollastonite In Situ Mineral Carbonation for CO2 Sequestration: Effects of Saline Conditions, Temperature, and Pressure. ACS Omega 2020, 5, 28942–28954. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils–An Ontario field study. Int. J. Greenh. Gas Control 2020, 97, 103017. [Google Scholar] [CrossRef]
- Ayub, S.A.; Tsegab, H.; Rahmani, O.; Beiranvand Pour, A. Potential for CO2 Mineral Carbonation in the Paleogene Segamat Basalt of Malaysia. Minerals 2020, 10, 1045. [Google Scholar] [CrossRef]
- Daval, D.; Martinez, I.; Corvisier, J.; Findling, N.; Goffe, B.; Guyot, F. Carbonation of Ca-bearing silicates, the case of wollastonite: Experimental investigations and kinetic modeling. Chem. Geol. 2009, 265, 63–78. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, R.; Geerlings, H.; Bi, J.C. A Novel Indirect Wollastonite Carbonation Route for CO2 Sequestration. Chem. Eng. Technol. 2010, 33, 1177–1183. [Google Scholar] [CrossRef]
- Qafoku, O.; Kovarik, L.; Kukkadapu, R.K.; Ilton, E.S.; Arey, B.W.; Tucek, J.; Felmy, A.R. Fayalite dissolution and siderite formation in water-saturated supercritical CO2. Chem. Geol. 2012, 332–333, 124–135. [Google Scholar] [CrossRef]
- Penner, L.; O’Connor, W.K.; Dahlin, D.C.; Gerdemann, S.; Rush, G.E. Mineral Carbonation: Energy Costs of Pretreatment Options and Insights Gained from Flow Loop Reaction Studies; Albany Research Center, Office of Fossil Energy, US DOE: Albany, OR, USA, 2004; p. 18. [Google Scholar]
- Qafoku, O.; Ilton, E.S.; Bowden, M.E.; Kovarik, L.; Zhang, X.; Kukkadapu, R.K.; Engelhard, M.H.; Thompson, C.J.; Schaef, H.T.; McGrail, B.P.; et al. Synthesis of nanometer-sized fayalite and magnesium-iron(II) mixture olivines. J. Colloid Interface Sci. 2018, 515, 129–138. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. A Review on Integrated Mineral Carbonation Process in Ultramafic Mine Deposit. Geo-Resour. Environ. Eng. (GREE) 2017, 2, 148–154. [Google Scholar] [CrossRef]
- Rahmani, O. CO2 sequestration by indirect mineral carbonation of industrial waste red gypsum. J. CO2 Util. 2018, 27, 374–380. [Google Scholar] [CrossRef]
- Mine Arnaud. Groupe-conseil, Consultants externes, Roche ltée and Ausenco Sandwell. In Projet Minier Arnaud—Étude d´Impact sur l´Environnement; Mine Arnaud: Sept-Îles, QC, Canada, 2012; p. 726. [Google Scholar]
- Ministère du Développement Durable, d.l.E.e.d.l.L.c.l.c.c. Émissions de gaz à Effet de Serre Déclarées et Vérifiées des Établissements Visés par le Règlement Concernant le Système de Plafonnement et D’échange de Droits D’émission de gaz à Effet de Serre (RSPEDE). Available online: http://www.mddelcc.gouv.qc.ca/changements/carbone/ventes-encheres/liste-etablissements-visesRSPEDE.pdf (accessed on 13 October 2017).
- Gerbet, T. (Ed.) Gaz à Effet de Serre: La Carte des Émissions Industrielles au Québec; Radio-Canada: Toronto, ON, Canada, 2014. [Google Scholar]
- Tanupabrungsun, T.; Young, D.; Brown, B.; Nešic, S. Construction and verification of pourbaix diagrams for CO2 corrosion of mild steel valid up to 250 °C. In Proceedings of the CORROSION, Salt Lake City, UT, USA, 11–15 March 2012. [Google Scholar]
- Den Boef, G.; Zuur, A.P. Theoretische Grondslagen van de Analyse in Waterige Oplossingen; Elsevier: Amsterdam, The Netherlands, 1980; p. 9010103730. [Google Scholar]
- Josceanu, A.M.; Moore, P. Comparison of the rates and mechanisms of formation and solvolysis of [Fe(bipy)3]2+ (bipy = 2,2′-bipyridine) and [FeL]2+ [L = 1,4,7-tris(2,2′-bipyridyl-5-ylmethyl)-1,4,7-triazacyclononane] and their stabilities in dimethylformamide solution. J. Chem. Soc. Dalton Trans. 1998, 24, 369–374. [Google Scholar] [CrossRef]
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Parameters optimization for direct flue gas CO2 capture and sequestration by aqueous mineral carbonation using activated serpentinite based mining residue. Appl. Geochem. 2014, 50, 66–73. [Google Scholar] [CrossRef]
- Sanna, A.; Lacinska, A.; Styles, M.; Maroto-Valer, M.M. Silicate rock dissolution by ammonium bisulphate for pH swing mineral CO2 sequestration. Fuel Process. Technol. 2014, 120, 128–135. [Google Scholar] [CrossRef]
- Office de L’éfficacité Énérgétique. Annexe B Coefficient D’émission de CO2; Office de L’éfficacité Énérgétique: Québec, QC, Canada, 2018. [Google Scholar]
- Khattak, R.; Naqvi, I.I. Addition product of iron (II) complex of aromatic diimine with sulphuric acid. J. Res. (Sci.) 2007, 18, 219–235. [Google Scholar]
- Tangstad, M. Ferrosilicon and silicon technology. In Handbook of Ferroalloys; Elsevier: Amsterdam, The Netherlands, 2013; pp. 179–220. [Google Scholar] [CrossRef]
- Gasik, M. Handbook of Ferroalloys: Theory and Technology; Butterworth-Heinemann: Oxford, UK, 2013; p. 0080977669. [Google Scholar]
- Patnaik, P. Dean’s Analytical Chemistry Handbook; McGraw-Hill: New York, NY, USA, 2004; Volume 1143. [Google Scholar]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. (Oxford) 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; p. 1482208687. [Google Scholar]
- Schott, J.; Mavromatis, V.; Fujii, T.; Pearce, C.R.; Oelkers, E.H. The control of magnesium aqueous speciation on Mg isotope composition in carbonate minerals: Theoretical and experimental modeling. Chem. Geol. 2016, 445, 120–134. [Google Scholar] [CrossRef]
- Gadikota, G.; Swanson, E.J.; Zhao, H.; Park, A.-H.A. Experimental Design and Data Analysis for Accurate Estimation of Reaction Kinetics and Conversion for Carbon Mineralization. Ind. Eng. Chem. Res. 2014, 53, 6664–6676. [Google Scholar] [CrossRef]
- Mora Mendoza, E.Y.; Sarmiento Santos, A.; Vera López, E.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent. Processes 2019, 7, 735. [Google Scholar] [CrossRef]
- Llorens, I.; Fattahi, M.; Grambow, B. New synthesis route and characterization of siderite (FeCO3) and coprecipitation of 99Tc. MRS Online Proc. Libr. Arch. 2006, 985, 1208. [Google Scholar] [CrossRef]
- Lammers, K.; Murphy, R.; Riendeau, A.; Smirnov, A.; Schoonen, M.A.A.; Strongin, D.R. CO2 Sequestration through Mineral Carbonation of Iron Oxyhydroxides. Environ. Sci. Technol. 2011, 45, 10422–10428. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Canadian Environmental Sustainability Indicators: Greenhouse Gas Emissions. Available online: www.ec.gc.ca/indicateurs-indicators/default.asp?lang=En&n=FBF8455E-1 (accessed on 16 October 2017).




| Mining Residue before Leaching (mg/40 g of Sample) | ||||
| Sample | Ca(II) | Fe(II) | Mg(II) | Si(IV) |
| 1 | 2449 | 6646 | 2334 | 8187 |
| 2 | 2525 | 6518 | 2318 | 8328 |
| 3 | 2460 | 6750 | 2369 | 8286 |
| 4 | 2442 | 6731 | 2357 | 8130 |
| 5 | 2405 | 6839 | 2347 | 8061 |
| 6 | 2503 | 6775 | 2366 | 8127 |
| 7 | 2476 | 6595 | 2336 | 8243 |
| 8 | 2397 | 6822 | 2355 | 8065 |
| Mean | 2457 ± 46 | 6710 ± 123 | 2348 ± 20 | 8178 ± 99 |
| Leachate (mg/40 g of sample) | ||||
| Sample | Ca(II) | Fe(II) | Mg(II) | Si(IV) |
| 1 | 130 | 2344 | 1276 | 1642 |
| 2 | 145 | 2344 | 1295 | 1659 |
| 3 | 146 | 2332 | 1262 | 1640 |
| 4 | 140 | 2329 | 1199 | 1660 |
| 5 | 160 | 2308 | 1278 | 1640 |
| 6 | 152 | 2303 | 1220 | 1691 |
| 7 | 136 | 2307 | 1291 | 1693 |
| 8 | 150 | 2380 | 1298 | 1624 |
| Mean | 145 ± 10 | 2331 ± 58 | 1264 ± 36 | 1656 ± 26 |
| T (°C) | pH | Reaction | % Carbonate (Purity) | % FeCO3 | % MgCO3 | Fe/Mg Ratio | % Silica | Stoichiometry |
|---|---|---|---|---|---|---|---|---|
| 21 | 9 | 1 | 76.9 | 86.5 | 13.5 | 0.90 | 24.5 | 1.13 |
| 2 | 77.2 | 93.7 | 6.3 | 22.7 | 1.16 | |||
| 3 | 75.7 | 90.8 | 9.2 | 21.1 | 1.08 | |||
| 10 | 1 | 75.7 | 82.9 | 17.1 | 0.85 | 21.1 | 1.05 | |
| 2 | 77.3 | 86.6 | 13.4 | 21.1 | 1.05 | |||
| 3 | 78.6 | 85.9 | 14.1 | 20.5 | 1.03 | |||
| 11 | 1 | 75.3 | 82.8 | 17.2 | 0.80 | 22.6 | 1.05 | |
| 2 | 71.2 | 78.6 | 21.4 | 15.5 | 0.98 | |||
| 3 | 70.2 | 77.9 | 22.1 | 16.3 | 0.94 | |||
| 12 | 1 | 70.6 | 74.3 | 25.7 | 0.74 | 18.7 | 0.94 | |
| 2 | 71.6 | 76.5 | 23.5 | 17.8 | 0.94 | |||
| 3 | 72.1 | 72.1 | 27.9 | 16.5 | 0.87 | |||
| 60 | 9 | 1 | 72.0 | 90.2 | 9.8 | 0.83 | 19.3 | 1.11 |
| 2 | 73.1 | 73.3 | 26.7 | 14.5 | 0.95 | |||
| 3 | 69.4 | 84.8 | 15.2 | 17.8 | 1.02 | |||
| 10 | 1 | 70.2 | 80.6 | 19.4 | 0.77 | 13.7 | 0.99 | |
| 2 | 68.6 | 77.3 | 22.7 | 13.9 | 0.96 | |||
| 3 | 71.9 | 72.3 | 27.7 | 14.8 | 0.94 | |||
| 11 | 1 | 69.4 | 73.9 | 26.1 | 0.71 | 16.2 | 0.95 | |
| 2 | 71.7 | 68.7 | 31.3 | 15.4 | 0.90 | |||
| 3 | 69.1 | 69.1 | 30.9 | 13.2 | 0.91 | |||
| 12 | 1 | 68.9 | 71.5 | 28.5 | 0.71 | 16.9 | 0.92 | |
| 2 | 70.0 | 73.4 | 26.6 | 14.2 | 0.95 | |||
| 3 | 71.0 | 69.1 | 30.9 | 14.4 | 0.91 | |||
| 80 | 9 | 1 | 70.4 | 77.7 | 22.3 | 0.71 | 15.6 | 0.98 |
| 2 | 69.1 | 68.9 | 31.1 | 11.0 | 0.95 | |||
| 3 | 68.9 | 67.7 | 32.3 | 12.0 | 0.94 | |||
| 10 | 1 | 72.8 | 68.7 | 31.3 | 0.68 | 15.2 | 0.93 | |
| 2 | 71.0 | 68.8 | 31.2 | 13.3 | 0.90 | |||
| 3 | 73.5 | 68.0 | 32.0 | 14.3 | 0.92 | |||
| 11 | 1 | 69.9 | 70.9 | 29.1 | 0.69 | 16.5 | 0.90 | |
| 2 | 68.9 | 68.0 | 32.0 | 12.2 | 0.93 | |||
| 3 | 72.8 | 69.5 | 30.5 | 13.7 | 0.93 | |||
| 12 | 1 | 71.7 | 67.7 | 32.3 | 0.68 | 14.9 | 0.91 | |
| 2 | 70.7 | 70.5 | 29.5 | 17.4 | 0.89 | |||
| 3 | 72.0 | 67.1 | 32.9 | 14.1 | 0.91 |
| Temperature | pH | g CO2 Uptake/g Residue | Reaction Efficiency (%) |
|---|---|---|---|
| 21 | 9 | 0.011 ± 0.007 | 5.00 ± 0.8 |
| 10 | 0.031 ± 0.02 | 14.1 ± 2 | |
| 11 | 0.039 ± 0.03 | 17.5 ± 3 | |
| 12 | 0.056 ± 0.03 | 37.4 ± 3 | |
| 60 | 9 | 0.004 ± 0.008 | 2.01 ± 1 |
| 10 | 0.012 ± 0.02 | 5.24 ± 1 | |
| 11 | 0.018 ± 0.02 | 8.25 ± 2 | |
| 12 | 0.045 ± 0.01 | 20.7 ± 4 | |
| 80 | 9 | 0.002 ± 0.005 | 1.05 ± 0.8 |
| 10 | 0.010 ± 0.01 | 4.66 ± 0.6 | |
| 11 | 0.042 ± 0.01 | 19.1 ± 4 | |
| 12 | 0.125 ± 0.02 | 57.3 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynes, J.F.; Mercier, G.; Blais, J.-F.; Pasquier, L.-C. Feasibility of a Mineral Carbonation Technique Using Iron-Silicate Mining Waste by Direct Flue Gas CO2 Capture and Cation Complexation Using 2,2′-Bipyridine. Minerals 2021, 11, 343. https://doi.org/10.3390/min11040343
Reynes JF, Mercier G, Blais J-F, Pasquier L-C. Feasibility of a Mineral Carbonation Technique Using Iron-Silicate Mining Waste by Direct Flue Gas CO2 Capture and Cation Complexation Using 2,2′-Bipyridine. Minerals. 2021; 11(4):343. https://doi.org/10.3390/min11040343
Chicago/Turabian StyleReynes, Javier F., Guy Mercier, Jean-François Blais, and Louis-César Pasquier. 2021. "Feasibility of a Mineral Carbonation Technique Using Iron-Silicate Mining Waste by Direct Flue Gas CO2 Capture and Cation Complexation Using 2,2′-Bipyridine" Minerals 11, no. 4: 343. https://doi.org/10.3390/min11040343
APA StyleReynes, J. F., Mercier, G., Blais, J.-F., & Pasquier, L.-C. (2021). Feasibility of a Mineral Carbonation Technique Using Iron-Silicate Mining Waste by Direct Flue Gas CO2 Capture and Cation Complexation Using 2,2′-Bipyridine. Minerals, 11(4), 343. https://doi.org/10.3390/min11040343








