Characterization and Suitability of Nigerian Barites for Different Industrial Applications
Abstract
1. Introduction
2. Materials and Methods
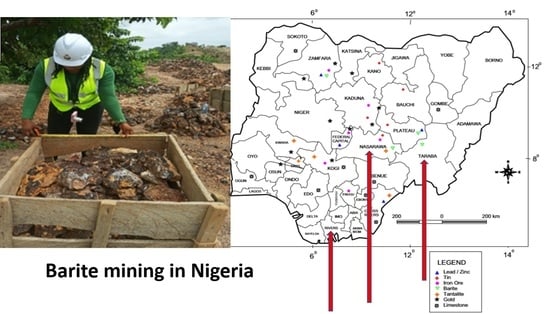
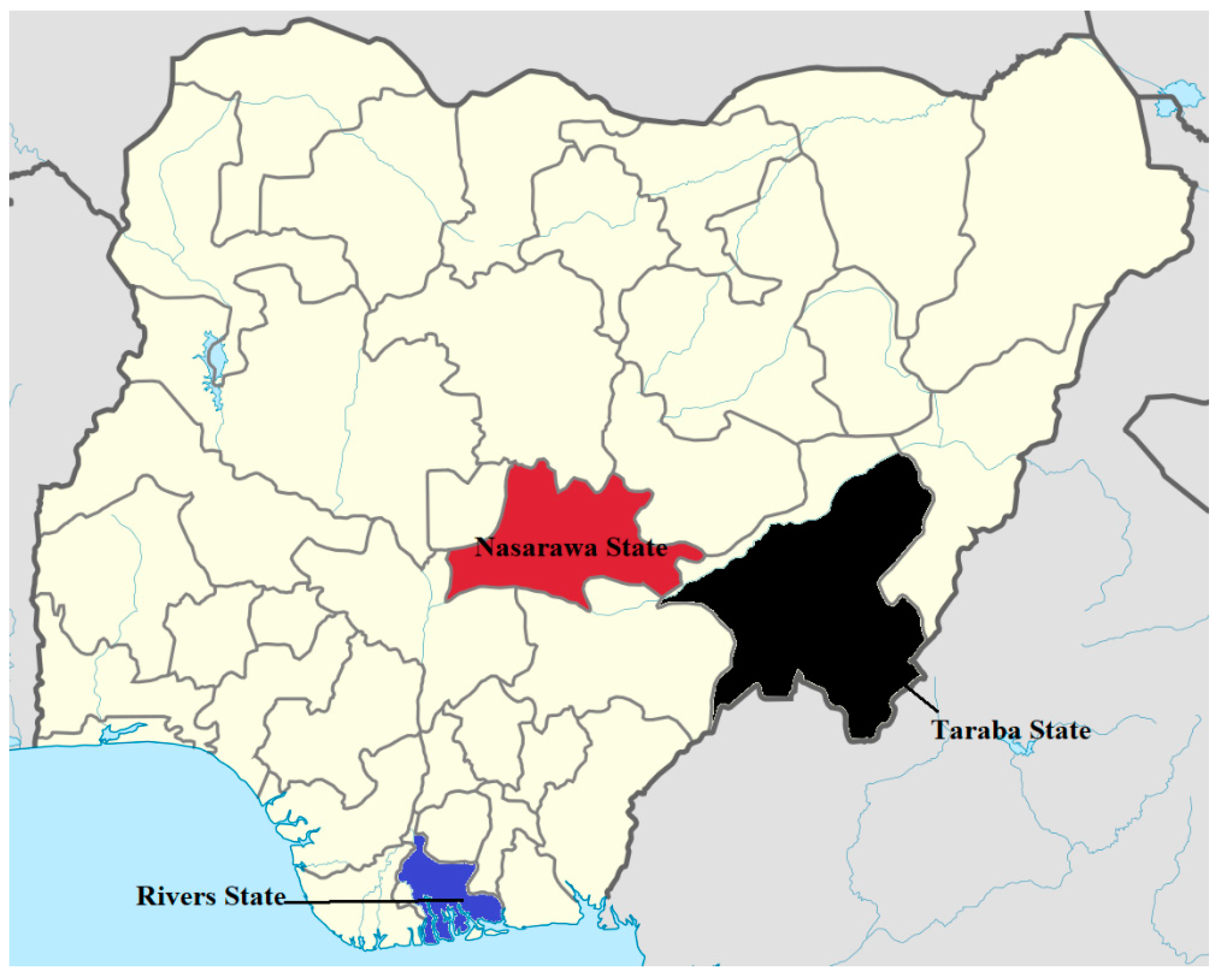
2.1. Study Location
2.2. Sample Collection
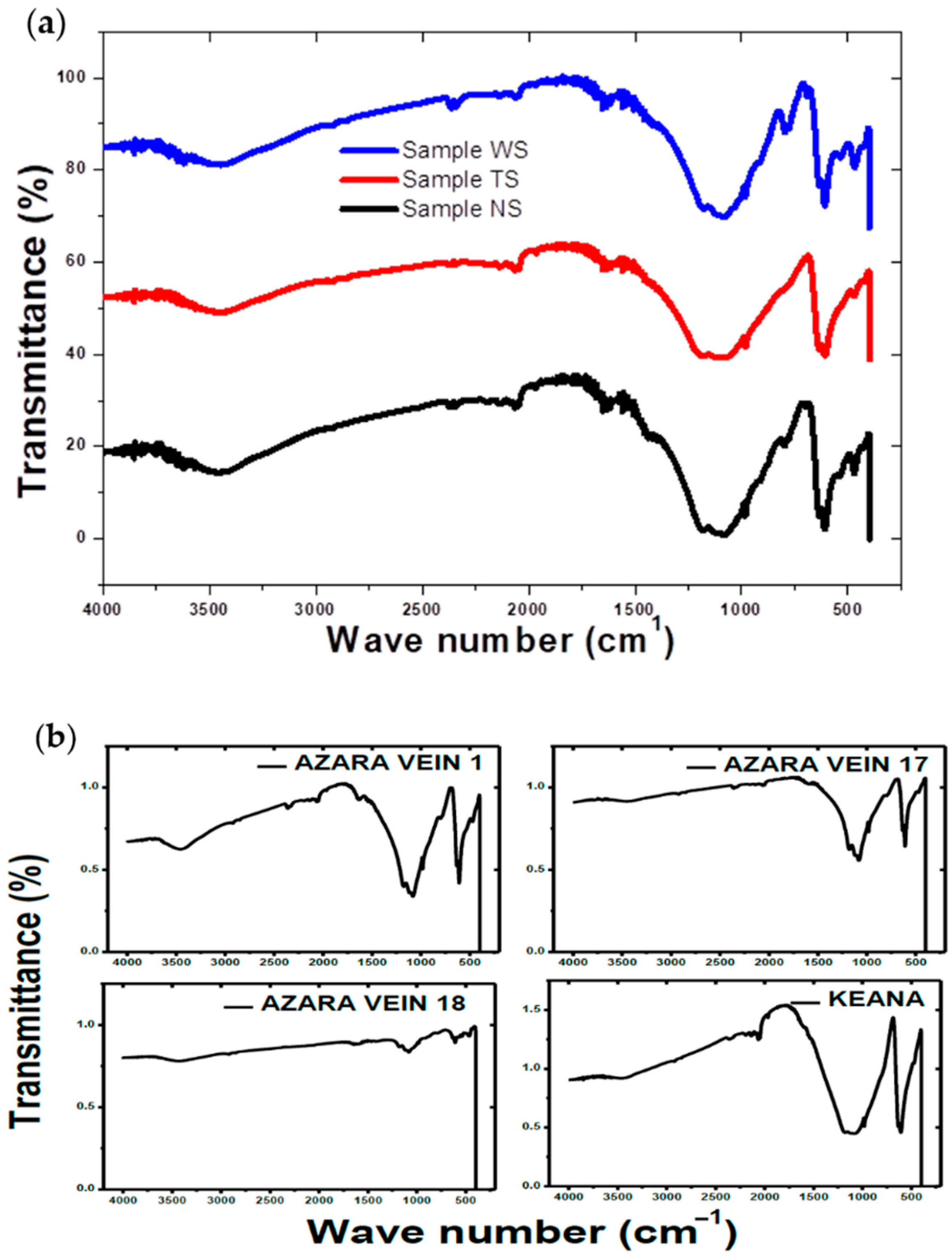
2.3. Sample Characterization
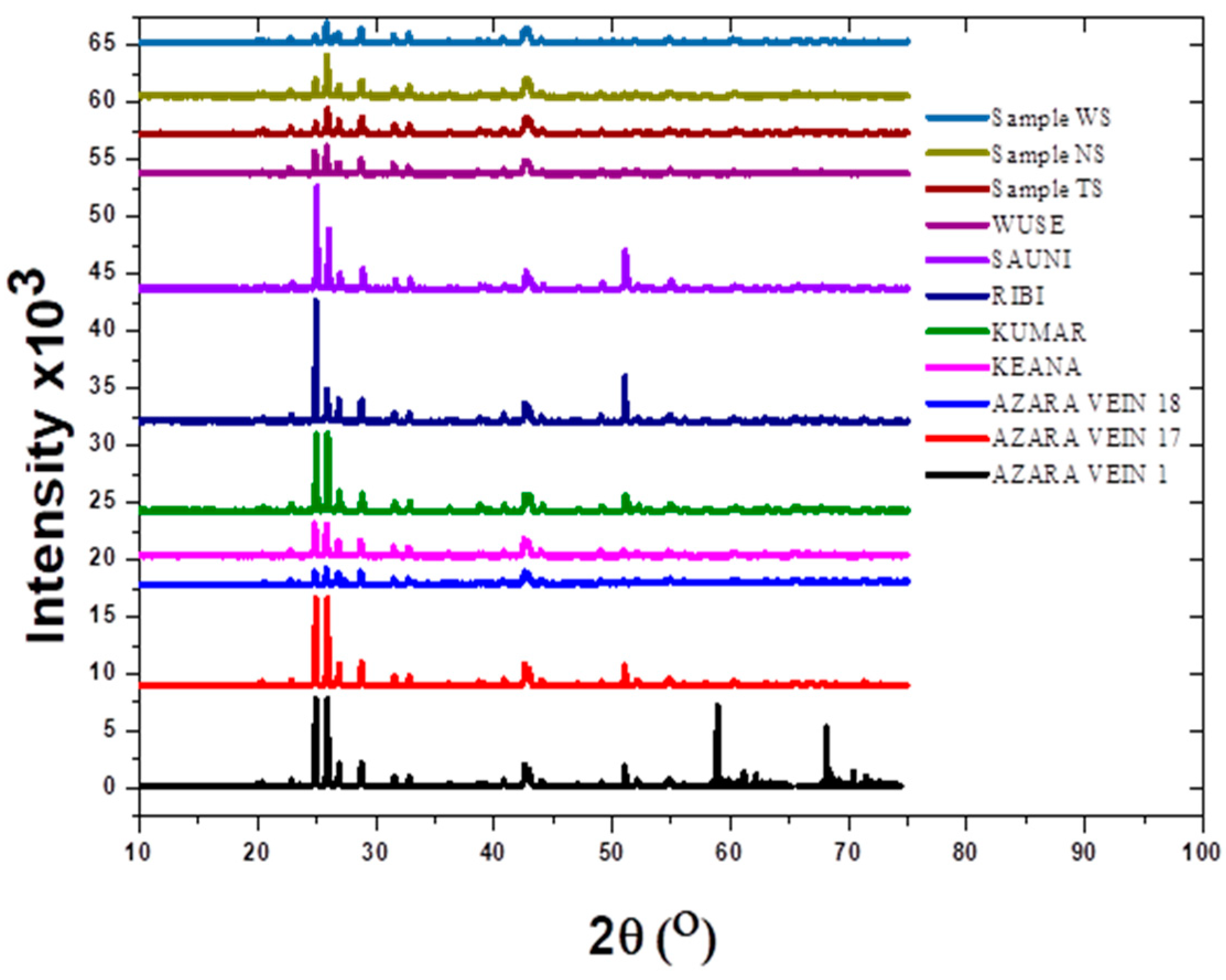
3. Results and Discussion
4. Suitability of Barites for Industrial Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezekwesili, G.E.; Okogbue, C.O.; Chidozie, I.P. Structural styles and ecomic potentials of some barite deposits in the Southern Benue Trough, Nigeria. Rom. J. Earth Sci. 2012, 85, 1. [Google Scholar]
- Onwualu, A.; Ogunwusi, A.; Olife, I.C.; Inyang, A. Raw Materials Development for the Transformation of the Manufacturing Sector in Nigeria; Raw Materials Research and Developmen Council: Garki, Abuja, Nigeria, 2013.
- Onwualu, A.; Obasi, E.; Olife, I.; Inyang, A. Unlocking the Potentials of Nigeria’s Non-Oil Sector; Raw Materials Research and Developmen Council: Garki, Abuja, Nigeria, 2013.
- Olamilekan, A. Overview on the Accelerated Development of the Barite Industry in Nigeria; Raw Materials Research and Developmen Council: Garki, Abuja, Nigeria, 2020.
- MJohnson, M.A.C.; Pictak, M.N. Barite (Barium) in Minerals; U.S. Geological Survey: Reston, VA, USA, 2017.
- Labe, N.; Ogunleye, P.; Ibrahim, A.; Fajulugbe, T.; Gbadema, S.T. Review of the occurrence and structural controls of Baryte resources of Nigeria. J. Degrad. Min. Lands Manag. 2018, 5, 1207–1216. [Google Scholar] [CrossRef]
- Muhammad, A.B.; Kazmia, K.R.; Mehmooda, R.; Ahadb, A.; Tabbassumc, A.; Akram, A. Beneficiation Study on Barite Ore of Duddar Area, District Lasbela. Balochistan Province Pakistan. J. Sci. Ind. Res. Ser. A Phys. Sci. 2017, 60, 9–22. [Google Scholar]
- Johnson, C.A.; Piatak, N.M.; Miller, M.M. “Barite (Barium)”. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; U.S. Geological Survey: Reston, VA, USA, 2017. [Google Scholar]
- Singh, R.; Banerjee, B.; Bhattacharyya, J.P.; Srivastava, K.K. Up-gradation of Barite Waste to Marketable Grade Concentrate. In Proceedings of the XXIII International Mineral Processing Congress (IMPC) Mineral Processing Division, National Metallurgical Laboratory, Chicago, IL, USA, 9–12 September 2007; pp. 2303–2307. [Google Scholar]
- Ariffin, K.S. Barite (BARIUM). Miner; EBS 425; Perindustrian: Hyderabad, India, 2016; Volume 1. [Google Scholar]
- Al-Awad, M.N.; Al-Qasabi, A.O. Characterization and Testing of Saudi Barite for Potential Use in Drilling Operations. J. King Saud Univ. Eng. Sci. 2001, 13, 287–298. [Google Scholar] [CrossRef]
- Oden, M.I. Barite Veins in the Benue Trough: Field Characteristics, the Quality Issue and Some Tectonic Implications. Environ. Nat. Resour. Res. 2012, 2, 21. [Google Scholar] [CrossRef]
- Duru, U.I.; Kerunwa, A.; Omeokwe, I.; Uwaezuoke, N.; Obah, B. Suitability of Some Nigerian Barites in Drilling Fluid Formulations. Pet. Sci. Eng. 2019, 3, 46. [Google Scholar] [CrossRef]
- Abdou, M.; Al-Sabagh, A.; Ahmed, H.E.-S.; Fadl, A. Impact of barite and ilmenite mixture on enhancing the drilling mud weight. Egypt J. Pet. 2018, 27, 955–967. [Google Scholar] [CrossRef]
- Bruton, J.R.; Bacho, J.P.; Newcaster, J. The Future of Drilling-Grade Barite Weight Material. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 24–27 September 2006. [Google Scholar]
- Wellmer, F.-W.; Dalheimer, M.; Wagner, M. Economic Evaluations in Exploration, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Harneit, J. Umwandlung einer GmbH & Co. KG in eine GmbH. Die Bus. Judgement Rule 2019, 1, 1. [Google Scholar] [CrossRef]
- Lar, U.A.; Agene, J.I.; Umar, A.I. Geophagic clay materials from Nigeria: A potential source of heavy metals and human health implications in mostly women and children who practice it. Environ. Geochem. Health 2014, 37, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Nikonow, W.; Rammlmair, D. Risk and benefit of diffraction in Energy Dispersive X-ray fluorescence mapping. Spectrochim. Acta Part B At. Spectrosc. 2016, 125, 120–126. [Google Scholar] [CrossRef]
- Poonoosamy, J.; Curti, G.; Grolimund, D.; van Loon, L.R.; Mäder, U. Barite precipitation following celestite dissolution in a porous medium: A SEM/BSE and µXRD/XRF study. Geochim. Cosmochim. Acta 2016, 182, 131–144. [Google Scholar] [CrossRef]
- Rammlmair, D.; Tacke, K.; Jung, H. Application of new XRFscanning techniques to monitor crust formation in column experiments, Securing the Future. In Proceedings of the International Conference on Mining and the Environment, San Jose, CA, USA, 29 November–2 December 2001; pp. 683–692. [Google Scholar]
- Pough, F.H.; Scovi, J.A. A field guide to rocks and minerals. Natl. Audubon Soc. 1996, 2. [Google Scholar]
- Ray, F.; Matt, W.; Wayde, M.; Loc, D. Identification of mixite minerals—An SEM and Raman spectroscopic analysis. Mineral. Mag. 2005, 69, 169–177. [Google Scholar]
- Janaki, K.; Velraj, G. Spectroscopic studies of some fired clay Artifacts recently excavated at Tiltagudi in Talmilnadu. Recent Res. Sci. Technol. 2011, 3, 89–91. [Google Scholar]
- Ramaswamy, V.; Vimalathithan, R.M.; Ponnusamy, V. Synthesis of well dispersed elliptical shaped barium sulphate nano particles water chloroform mixed solvent. Arch. Phys. Res. 2010, 1, 217–226. [Google Scholar]
- Dimova, M.; Panczer, G.; Gaft, M. Spectroscopic study of barite from the Kremikovtsi Deposit (Bulgaria) with implication for its origin. Ann. Gologiques Penins. Balk. 2006, 101–108. [Google Scholar] [CrossRef]
- Aroke, U.O.; Abdulkarim, A.; Ogubunka, R.O. Fourier transform infrared characterization of kaolin, Granite, Bentonite and barite. ATBU J. Environ. Technol. 2013, 6, 1. [Google Scholar]
- Nordstrom, D.K. What was the groundwater quality before mining in a mineralized region? Lessons from the Questa project. Geosci. J. 2008, 12, 139–149. [Google Scholar] [CrossRef]
- Nagaraju, A. Effects of barite mine on groundwater quality in Andhra Pradesh, India. Mine Water Environ. 2007, 26, 119–123. [Google Scholar]
- Forjanes, P.; Astilleros, J.M.; Fernández-Díaz, L. The Formation of Barite and Celestite through the Replacement of Gypsum. Mineral 2020, 10, 189. [Google Scholar] [CrossRef]
- Bhavan, I. Indian Minerals Yearbook-Barytes, 54th ed.; Indira Bhavan: Kerala, India, 2015. [Google Scholar]
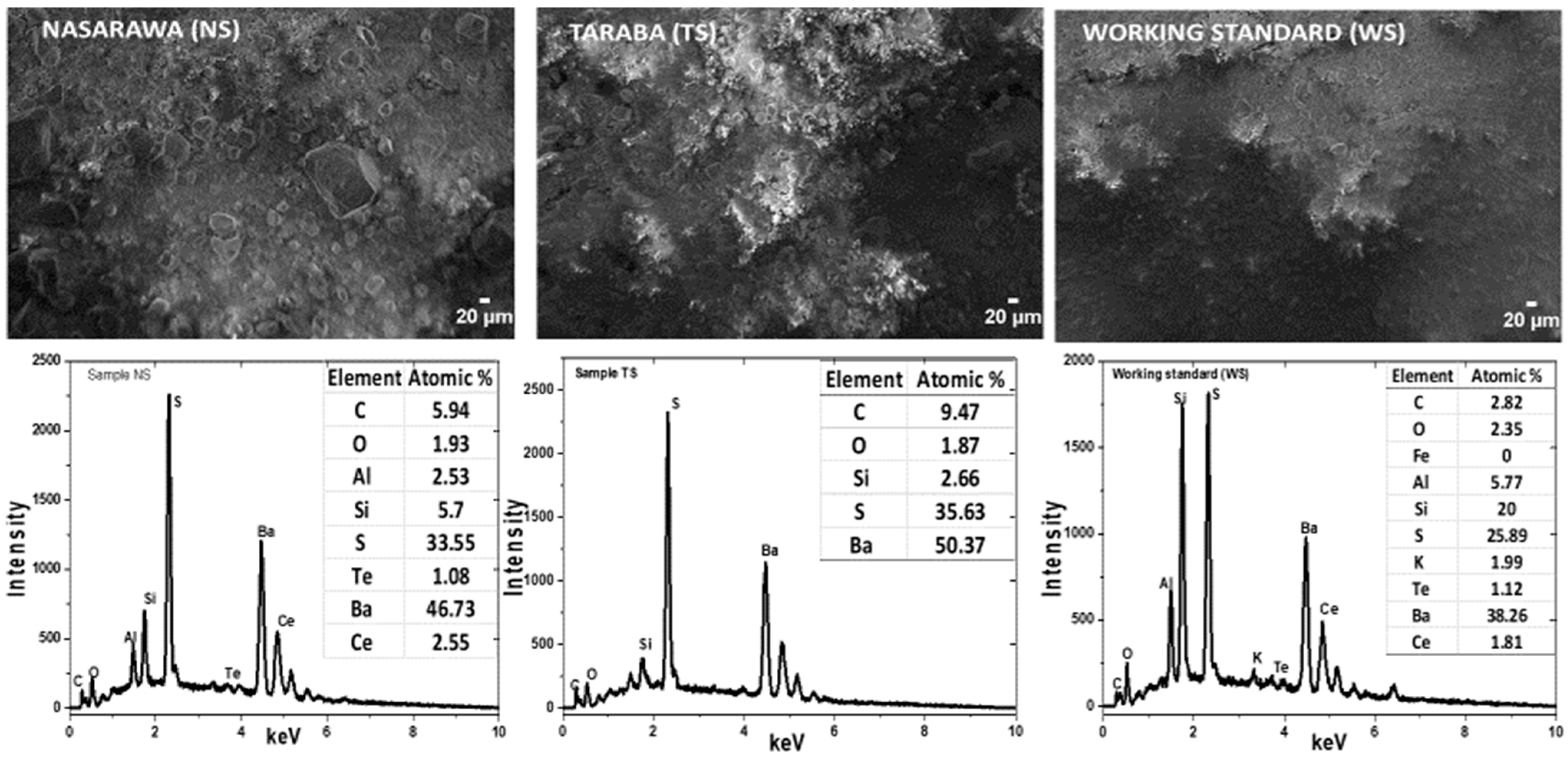
| Samples | EDS Elemental Percentage Composition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba | S | O | Fe | Al | Si | Te | Ce | K | La | Nb | |
| NS | 46.73 | 33.55 | 1.93 | 2.53 | 5.7 | 1.08 | 2.55 | ||||
| TS | 50.37 | 35.63 | 1.87 | 2.66 | |||||||
| Azara Vein 1 | 51.73 | 43.36 | 2.11 | 2.64 | 0.17 | ||||||
| Azara Vein 17 | 38.59 | 36.84 | 2.88 | 21.66 | 0.03 | ||||||
| Azara Vein 18 | 34.79 | 31.48 | 3.74 | 23.89 | 2.2 | 3.25 | 0.29 | 0.36 | |||
| Keana | 50.12 | 42.75 | 3.14 | 2.21 | 0.02 | ||||||
| Kumar | 31.36 | 23.82 | 4.18 | 28.64 | 11.68 | 0.32 | |||||
| Ribi | 51.38 | 44.17 | 2.59 | 0.07 | 1.79 | ||||||
| Sauni | 51.17 | 44.75 | 3.94 | 0.14 | |||||||
| Wuse | 51.42 | 42.58 | 2.98 | 2.05 | |||||||
| WS | 38.26 | 25.89 | 2.35 | 5.77 | 20 | 1.12 | 1.81 | 1.99 | |||
| OXIDES/Elements | Percentage of Elemental Oxide Composition for Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WS | NS | TS | AZARA VEIN 1 | AZARA VEIN 17 | AZARA VEIN 18 | KEANA | KUMAR | SAUNI | RIBI | WUSE | |
| Fe2O3 | 0.175 | 0.307 | 0.028 | 0.101 | 0.292 | 9.76 | 0.028 | 0.553 | 0.165 | 0.102 | 0.015 |
| SiO2 | 1.831 | 1.878 | 1.164 | 6.752 | 5.036 | 10.11 | 1.709 | 2.989 | 0.592 | 4.355 | 1.362 |
| Al2O3 | 0.722 | 0.681 | 0.667 | 1.193 | 0.914 | 2.415 | 0.783 | 1.281 | 0.397 | 1.229 | 0.463 |
| MgO | 1.731 | 0.34 | 1.14 | 0.18 | 0.19 | 0.05 | 1.69 | 0.53 | 0.28 | 1.18 | |
| P2O5 | 0.023 | 0.05 | 0.023 | 0.021 | 0.056 | 0.033 | 0.05 | ||||
| SO3 | 17.3 | 7.609 | 7.687 | 13.73 | 16.3 | 10.81 | 16.65 | 16.53 | 12.3 | 16.29 | 13.44 |
| TiO2 | 6.353 | 6.572 | |||||||||
| MnO | 0.013 | 0.421 | 0.014 | 0.069 | |||||||
| CaO | 0.389 | 0.166 | 0.01 | 0.004 | 0.113 | 0.002 | |||||
| K2O | 0.030 | 0105 | 0.092 | 0.092 | 0.048 | 0.496 | 0.029 | 0.055 | 0.015 | 0.116 | 0.008 |
| CuO | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.015 | 0.002 | 0.003 | 0.005 | 0.001 | 0.003 |
| ZnO | 0.001 | 0.045 | |||||||||
| Cr2O3 | 0.023 | 0.03 | 0.028 | 0.002 | 0.032 | 0.018 | 0.011 | 0.034 | 0.02 | ||
| PbO | 0.281 | 0.006 | 0.001 | 0.001 | 0.274 | 0.001 | 0.001 | ||||
| Rb2O | 0.002 | 0.001 | 0.001 | 0.002 | |||||||
| Cl | 0.451 | 0.494 | 0.644 | 0.432 | 0.526 | 0.469 | 0.447 | 0.556 | 0.314 | 0.515 | 0.322 |
| BaO | 30.45 | 18.04 | 18.62 | 26.32 | 31.42 | 24.18 | 32.57 | 31.32 | 22.94 | 31.64 | 24.33 |
| Ta2O5 | 0.017 | 0.003 | 0.003 | 0.001 | 0.002 | ||||||
| WO3 | 0.312 | 0.016 | 0.106 | 0.304 | |||||||
| SrO | 2.855 | 0.546 | 4.687 | 4.258 | 3.309 | 2.72 | 2.834 | 5.68 | 2.666 | 1.421 | 6.148 |
| CeO2 | 1.712 | 1.42 | 1.744 | 1.513 | 1.661 | 1.772 | 1.3 | 1.658 | 1.234 | ||
| ThO3 | 0.001 | 0.001 | 0 | 0.001 | 0.001 | ||||||
| Y2O3 | 0.002 | 0.017 | 0.002 | ||||||||
| Nb2O5 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.02 | |
| SnO2 | 0.017 | ||||||||||
| Sb2O3 | 0.001 | 0.11 | 0.001 | 0.001 | 0.001 | 0.001 | |||||
| Cs | 0.156 | 0.055 | |||||||||
| Sample | Specific Gravity (g/cm3) |
|---|---|
| SAMPLE TS | 4.0087 |
| SAMPLE NS | 3.8122 |
| AZARA VEIN 1 | 4.2138 |
| AZARA VEIN 17 | 4.3761 |
| AZARA VEIN 18 | 4.0106 |
| KEANA | 4.4052 |
| KUMAR | 4.4000 |
| RIBI | 4.4200 |
| SAUNI | 4.3800 |
| WUSE | 4.4000 |
| WORKING SAMPLE (WS) | 3.6001 |
| Barite Application | (%) BaSO4 Sinimum std | Constituents | Specific Gravity Minimum std (g/cm3) | Study Samples Suitable for Application after Purification |
|---|---|---|---|---|
| Oil well drilling | 90 | 4.15 | All samples apart from TS | |
| Chemical manufacturing | 97 | SiO2, CaCO3, Al, Fe | 4.0 | NS, TS, Azara 1, Azara 17, Azara 18, Ribi |
| Paint manufacturing | 95 | 4.45 | All samples | |
| Glass | 90–96 | SiO2, Al, Fe | All samples | |
| Pharmaceuticals | 97 | Fe2O3, SiO2, Al2O3 | All samples | |
| Rubber | 99.5 | SiO2 | All samples | |
| Asbestos products | 90 | Fe2O3, SiO2, Al2O3 | All samples | |
| Plastering | 95 | SiO2, Al2O3 | All samples | |
| Cement | 95 | SiO2 | All samples |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otoijamun, I.; Kigozi, M.; Adetunji, A.R.; Onwualu, P.A. Characterization and Suitability of Nigerian Barites for Different Industrial Applications. Minerals 2021, 11, 360. https://doi.org/10.3390/min11040360
Otoijamun I, Kigozi M, Adetunji AR, Onwualu PA. Characterization and Suitability of Nigerian Barites for Different Industrial Applications. Minerals. 2021; 11(4):360. https://doi.org/10.3390/min11040360
Chicago/Turabian StyleOtoijamun, Itohan, Moses Kigozi, Adelana Rasak Adetunji, and Peter Azikiwe Onwualu. 2021. "Characterization and Suitability of Nigerian Barites for Different Industrial Applications" Minerals 11, no. 4: 360. https://doi.org/10.3390/min11040360
APA StyleOtoijamun, I., Kigozi, M., Adetunji, A. R., & Onwualu, P. A. (2021). Characterization and Suitability of Nigerian Barites for Different Industrial Applications. Minerals, 11(4), 360. https://doi.org/10.3390/min11040360