Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea)
Abstract
1. Introduction
- to describe the variability of physico-chemical parameters, both measured (pressure (p), temperature (t), electrical conductivity (C), dissolved oxygen (DO), total hydrogen ion scale concentration (pHT), and illuminance (Ev)) and derived (practical salinity (SP), density (ρ), and carbonate parameters), as a result of an in situ high-temporal-resolution monitoring;
- to interpret the measured physical-chemical parameters as a function of sea ice concentration (SIC) and chlorophyll-a (Chl-a) mass concentration obtained from satellite data;
- to estimate the sensitivity of the carbonate system (total alkalinity (TA), partial pressure of carbon dioxide in seawater (pCO2), and calcite and aragonite saturation states (ΩCa, ΩAr)), derived by direct measurements of physical and biogeochemical drivers at the coastal site.
2. Materials and Methods
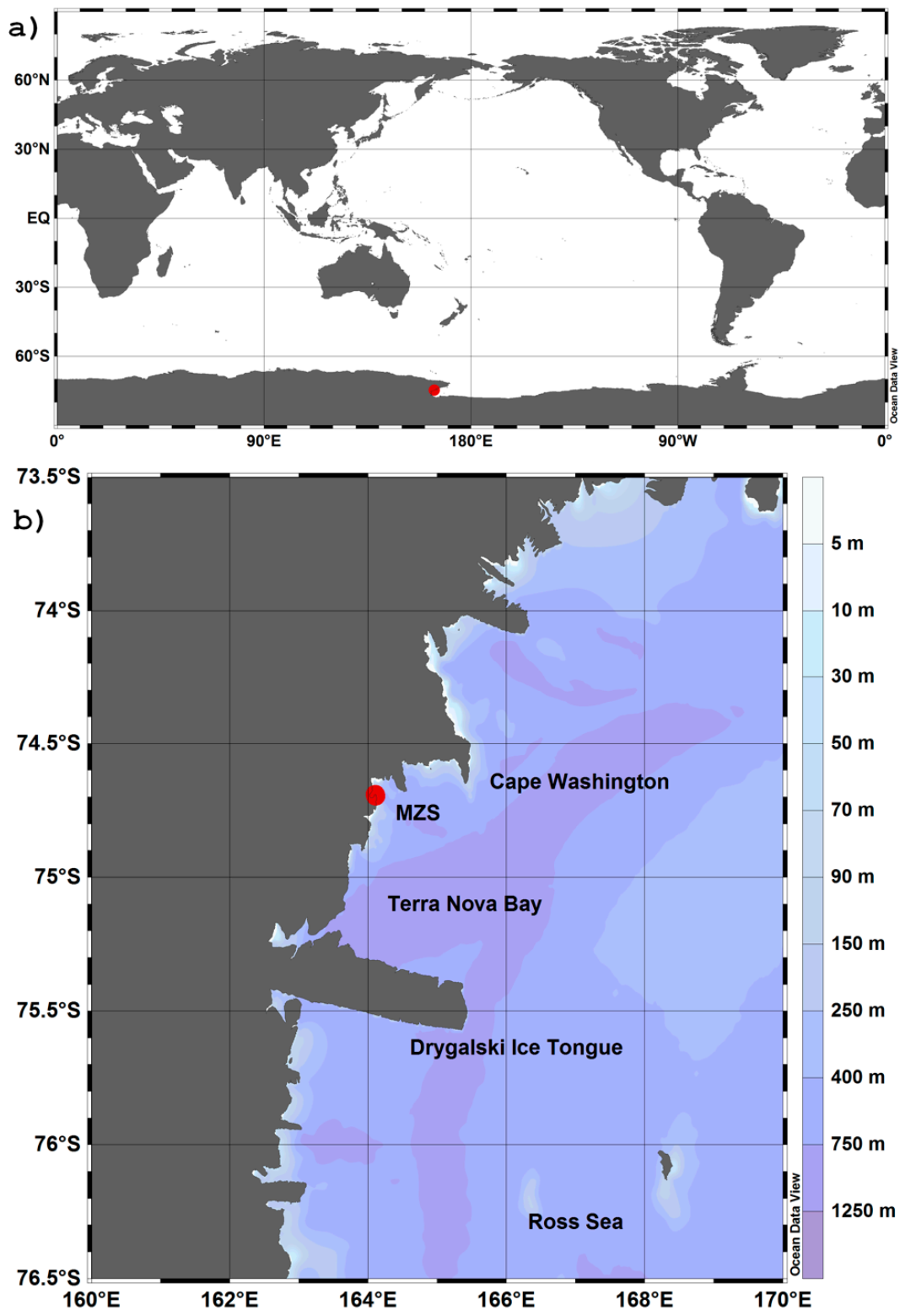
2.1. Study Area: Location and Characteristics
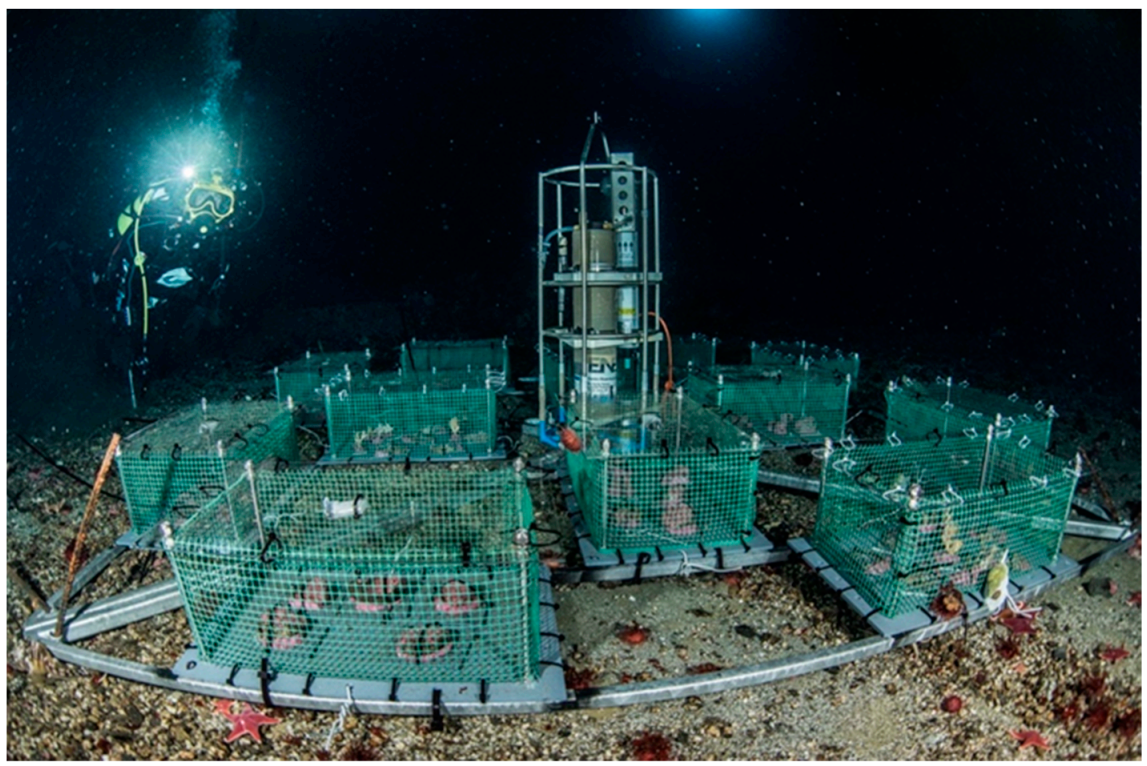
2.2. The Underwater Observatory
2.3. Data Collection, Processing, and Analyses
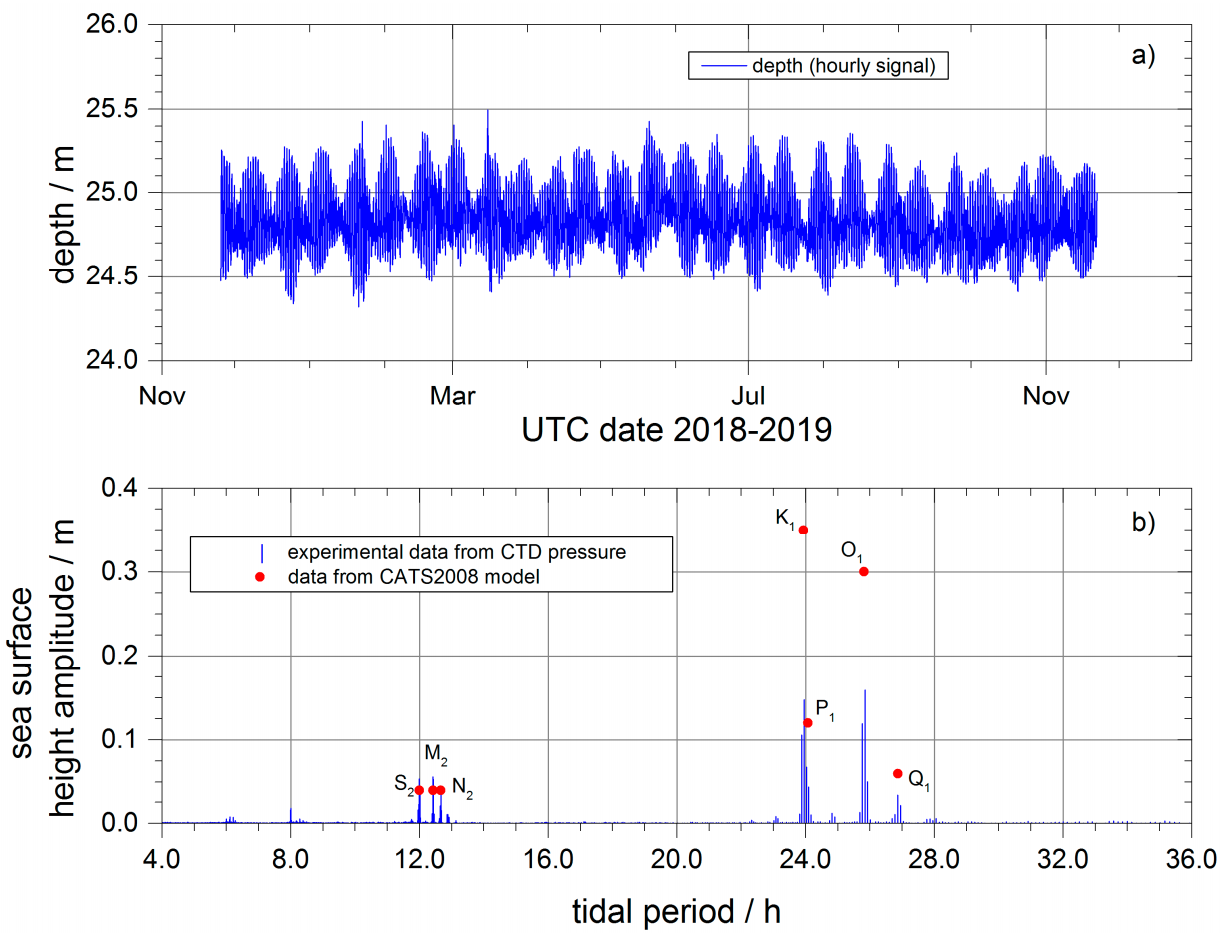
2.3.1. Underwater Observatory Data
2.3.2. Satellite Data
- name: SST_GLO_SST_L4_NRT_OBSERVATIONS_010_001;
- type of data: daily mean satellite observations from 1 January 2007 (near-real-time);
- spatial resolution: 0.05° × 0.05° (surface only, approx. 1.6 km × 5.6 km);
- product level: L4, i.e., a product for which a temporal averaging method or an interpolation procedure is applied to fill out missing data values. Temporal averaging is performed on a monthly basis.
- name: OCEANCOLOUR_GLO_CHL_L3_REP_OBSERVATIONS_009_085;
- type of data: daily observations of mass concentration of Chl-a in seawater (mg m−3); daily mean reprocessed from 1997;
- spatial resolution of data: 4 km × 4 km (surface only);
- product level: L3, i.e., data are the daily composite products as obtained by merging all the ocean satellite passages.
3. Results
3.1. Physical and Biological Environment
3.2. Calcification Environment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meredith, M.P.; Schofield, O.; Newman, L.; Urban, E.; Sparrow, M. The vision for a Southern Ocean Observing System. Curr. Opin. Environ. Sustain. 2013, 5, 306–313. [Google Scholar] [CrossRef]
- Meredith, M.P.; Sommerkorn, M.; Cassotta, S.; Derksen, C.; Ekaykin, A.; Hollowed, A.; Kofinas, G.; Mackintosh, A.; Mel-bourne-Thomas, J.; Muelbert, M.M.C.; et al. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O.D.C., Roberts, V., Masson-Delmotte, P., Zhai, M., Tignor, E., Poloczanska, K., Mintenbeck, A., Alegría, M., Nicolai, A., Okem, J., et al., Eds.; 2019; in press. [Google Scholar]
- Collins, W.J.; Fry, M.M.; Yu, H.; Fuglestvedt, J.S.; Shindell, D.T.; West, J.J. Global and regional temperature-change potentials for near-term climate forcers. Atmos. Chem. Phys. Discuss. 2013, 13, 2471–2485. [Google Scholar] [CrossRef]
- von Schukmann, K.; Le Traon, P.-Y.; Smith, N.; Pascual, A.; Djavidnia, S.; Gattuso, J.-P.; Grégoire, M.; Nolan, G. Co-pernicus Marine Service Ocean State Report. J. Oper. Ocean 2020, 13, S1–S172. [Google Scholar]
- Berger, A.; Yin, Q.; Nifenecker, H.; Poitou, J. Slowdown of global surface air temperature increase and acceleration of ice melting. Earth’s Futur. 2017, 5, 811–822. [Google Scholar] [CrossRef]
- Convey, P.; Peck, L.S. Antarctic environmental change and biological responses. Sci. Adv. 2019, 5, eaaz0888. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Iceberg killing fields limit huge potential for benthic blue carbon in Antarctic shallows. Glob. Chang. Biol. 2016, 23, 2649–2659. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Polar zoobenthos blue carbon storage increases with sea ice losses, because across-shelf growth gains from longer algal blooms outweigh ice scour mortality in the shallows. Glob. Chang. Biol. 2017, 23, 5083–5091. [Google Scholar] [CrossRef]
- Gutt, J.; Isla, E.; Bertler, A.N.; Bodeker, G.E.; Bracegirdle, T.J.; Cavanagh, R.D.; Comiso, J.C.; Convey, P.; Cummings, V.; De Conto, R.; et al. Cross-disciplinarity in the advance of Antarctic ecosystem research. Mar. Gen. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Henley, S.F.; Schofield, O.M.; Hendry, K.R.; Schloss, I.R.; Steinberg, D.K.; Moffat, C.; Peck, L.S.; Costa, D.P.; Bakker, D.C.; Hughes, C.; et al. Variability and change in the west Antarctic Peninsula marine system: Research priorities and opportunities. Prog. Oceanogr. 2019, 173, 208–237. [Google Scholar] [CrossRef]
- Gutt, J.; Bertler, N.; Bracegirdle, T.J.; Buschmann, A.; Comiso, J.; Hosie, G.; Isla, E.; Schloss, I.R.; Smith, C.R.; Tournadre, J.; et al. The Southern Ocean ecosystem under multiple climate change stresses—An integrated circumpolar assessment. Glob. Chang. Biol. 2015, 21, 1434–1453. [Google Scholar] [CrossRef]
- Hoffmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dy-namics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2012, 6, e28983. [Google Scholar]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing Ocean, Marine Eco-systems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O.D.C., Roberts, V., Masson-Delmotte, P., Zhai, M., Tignor, E., Poloczanska, K., Mintenbeck, A., Alegriía, M., Nicolai, A., Okem, J., Eds.; 2019; in press. [Google Scholar]
- Matson, P.G.; Washburn, L.; Martz, T.R.; Hofmann, G.E. Abiotic versus Biotic Drivers of Ocean pH Variation under Fast Sea Ice in McMurdo Sound, Antarctica. PLoS ONE 2014, 9, e107239. [Google Scholar] [CrossRef]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Caldeira, K.; Duffy, P.B. The role of Southern Ocean in uptake and storage of anthropogenic carbon dioxide. Science 2000, 287, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Sabine, C.L.; Feely, R.A.; Gruber, N.; Key, R.M.; Lee, K.; Bullister, J.L.; Wanninkhof, R.; Wong, C.S.; Wallace, D.W.R.; Tilbrook, B.; et al. The Oceanic Sink for Anthropogenic CO2. Science 2004, 305, 367–371. [Google Scholar] [CrossRef]
- Budillon, G.; Pacciaroni, M.; Cozzi, S.; Rivaro, P.; Catalano, G.; Ianni, C.; Cantoni, C. An optimum multiparameter mixing analysis of the shelf waters in the Ross Sea. Antarct. Sci. 2003, 15, 105–118. [Google Scholar] [CrossRef]
- Orsi, A.H.; Wiederwhol, C.L. A recount of Ross Sea waters. Deep Sea Res. Part II Top Stud. Ocean 2009, 56, 778–795. [Google Scholar] [CrossRef]
- Silvano, A.; Foppert, A.; Rintoul, S.R.; Holland, P.R.; Tamura, T.; Kimura, N.; Castagno, P.; Falco, P.; Budillon, G.; Haumann, F.A.; et al. Recent recovery of Antarctic Bottom Water formation in the Ross Sea driven by climate anomalies. Nat. Geosci. 2020, 13, 780–786. [Google Scholar] [CrossRef]
- Barnes, D. Antarctic sea ice losses drive gains in benthic carbon drawdown. Curr. Biol. 2015, 25, R789–R790. [Google Scholar] [CrossRef]
- Barnes, D.K.; Ireland, L.; Hogg, O.T.; Morely, S.; Enederlein, P.; Sands, C.J. Why is the Southern Orkney Island shelf (the world’s first high seas marine protected area) a carbon immobilization hotspot? Glob. Chang. Biol. 2016, 22, 1110–1120. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Roleda, M.Y.; Boyd, P.W.; Hurd, C.L. Before ocean acidification: Calcifier chemistry lessons. J. Phycol. 2012, 48, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Cyronak, T.; Schulz, K.G.; Jokiel, P.L. The Omega myth: What really drives lower calcification rates in an acidifying ocean. ICES J. Mar. Sci. 2016, 73, 558–562. [Google Scholar] [CrossRef]
- Faranda, F.M.; Guglielmo, L.; Ianora, A. The Italian Oceanographic Cruises in the Ross Sea (1987-95): Strategy, General Con-sideration and Description of the sampling sites. In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–14. [Google Scholar]
- Illuminati, S.; Annibaldi, A.; Romagnoli, T.; Libani, G.; Antonucci, M.; Scarponi, G.; Totti, C.; Truzzi, C. Distribution of Cd, Pb and Cu between dissolved fraction, inorganic particulate and phytoplankton in seawater of Terra Nova Bay (Ross Sea, Antarctica) during austral summer 2011–12. Chemosphere 2017, 185, 1122–1135. [Google Scholar] [CrossRef]
- Colacino, M.; Piervitali, E.; Grigioni, P. Climatic Characterization of the Terra Nova Bay Region. In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 15–26. [Google Scholar]
- Nihashi, S.; Ohshima, K.I. Circumpolar Mapping of Antarctic Coastal Polynyas and Landfast Sea Ice: Relationship and Variability. J. Clim. 2015, 28, 3650–3670. [Google Scholar] [CrossRef]
- Sweeney, C. The annual cycle of surface water CO2 And O2 in the Ross Sea: A model for gas exchange on the continental shelves of Antarctica. In Biogeochemistry of the Ross Sea; DiTullio, G.R., Dunbar, R.B., Eds.; American Geophysical Union: Washington, DC, USA, 2003; pp. 295–312. [Google Scholar]
- Rivaro, P.; Messa, R.; Ianni, C.; Magi, E.; Budillon, G. Distribution of total alkalinity and pH in the Ross Sea (Antarctica) waters during austral summer. Polar Res. 2014, 33, 20403. [Google Scholar] [CrossRef]
- Pardo, P.C.; Tilbrook, B.; Langlais, C.; Trull, T.W.; Rintoul, S.R. Carbon uptake and biogeochemical change in the Southern Ocean, south of Tasmania. Biogeosciences 2017, 14, 5217–5237. [Google Scholar] [CrossRef]
- Mahieu, L.; Monaco, C.L.; Metzl, N.; Fin, J.; Mignon, C. Variability and stability of anthropogenic CO2 in Antarctic Bottom Water observed in the Indian sector of the Southern Ocean, 1978–2018. Ocean Sci. 2020, 16, 1559–1576. [Google Scholar] [CrossRef]
- Lazzara, L.; Nardello, I.; Ermanni, C.; Mangoni, O.; Saggiomo, V. Light environment and seasonal dynamics of microalgae in the annual sea ice at Terra Nova Bay, Ross Sea, Antarctica. Antarct. Sci. 2007, 19, 83–92. [Google Scholar] [CrossRef]
- Guglielmo, L.; Carrada, G.C.; Catalano, G.; Dell’Anno, A.; Fabiano, M.; Lazzara, L.; Mangoni, O.; Pusceddu, A.; Saggiomo, V. Structural and functional properties of sympagic communities in the annual sea ice at Terra Nova Bay (Ross Sea, Antarctica). Polar Biol. 2000, 23, 137–146. [Google Scholar] [CrossRef]
- Umani, S.F.; Monti, M.; Bergamasco, A.; Cabrini, M.; De Vittor, C.; Burba, N.; Del Negro, P. Plankton community structure and dynamics versus physical structure from Terra Nova Bay to Ross Ice Shelf (Antarctica). J. Mar. Syst. 2005, 55, 31–46. [Google Scholar] [CrossRef]
- JCGM 100:2008—Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. Available online: https://ncc.nesdis.noaa.gov/documents/documentation/JCGM_100_2008_E.pdf (accessed on 6 January 2021).
- Raiteri, G.; Bordone, A.; Ciuffardi, T.; Pennecchi, F. Uncertainty evaluation of CTD measurements: A metrological approach to water-column coastal parameters in the Gulf of La Spezia area. Measurement 2018, 126, 156–163. [Google Scholar] [CrossRef]
- SBE Technical Note, Best Practices for the SeaFET V2: Optimizing pH Data Quality. Available online: https://repository.oceanbestpractices.org/handle/11329/876 (accessed on 6 January 2021).
- Schlitzer, R. Ocean Data View. Available online: Odv.awi.de (accessed on 5 February 2021).
- Lee, K.; Tong, L.T.; Millero, F.J.; Sabine, C.L.; Dickson, A.G.; Goyet, C.; Park, G.-H.; Wanninkhof, R.; Feely, R.A.; Key, R.M. Global relationships of total alkalinity with salinity and temperature in surface waters of the world’s oceans. Geophys. Res. Lett. 2006, 33, 19605. [Google Scholar] [CrossRef]
- Robbins, L.L.; Hansen, M.E.; Kleypas, J.A.; Meylan, S.C. CO2calc—A User-friendly Seawater Carbon Calculator for Windows, Max OS X, and iOS (iPhone); U.S. Geological Survey Open-File Report 2010–1280; U.S. Geological Survey: St. Petersburg, FL, USA, 2010.
- Roy, R.N.; Roy, L.N.; Vogel, K.M.; Porter-Moore, C.; Pearson, T.; Good, E.C.; Millero, F.J.; Campbell, D.M. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar. Chem. 1993, 44, 249–267. [Google Scholar] [CrossRef]
- Uppström, L.R. The boron/chlorinity ratio of deep-sea water from the Pacific Ocean. Deep. Sea Res. Oceanogr. Abstr. 1974, 21, 161–162. [Google Scholar] [CrossRef]
- Dickson, A.G. Standard Potential of the Reaction: AgCl(s) + 1/2H2(g) = Ag(s) + HCl(aq), and the Standard Acidity Constant of the Ion HSO4- in Synthetic Seawater from 273.15 to 318.15 K. J. Chem. Thermodyn. 1990, 22, 113–127. [Google Scholar] [CrossRef]
- Kapsenberg, L.; Kelley, A.L.; Shaw, E.C.; Martz, T.R.; Hofmann, G.E. chapter 11 Near-Shore Antarctic pH Variability has Implications for the Design of Ocean Acidification Experiments. Clim. Chang. Ocean Carbon Cycle 2017, 5, 239–266. [Google Scholar] [CrossRef]
- Chereskin, T.; Donohue, K.; Watts, R. cDrake: Dynamics and Transport of the Antarctic Circumpolar Current in Drake Passage. Oceanography 2012, 25, 134–135. [Google Scholar] [CrossRef][Green Version]
- Wåhlin, A.K.; Kalén, O.; Arneborg, L.; Björk, G.; Carvajal, G.K.; Ha, H.K.; Kim, T.W.; Lee, S.H.; Lee, J.H.; Stranne, C. Variability of Warm Deep Water Inflow in a Submarine Trough on the Amundsen Sea Shelf. J. Phys. Oceanogr. 2013, 43, 2054–2070. [Google Scholar] [CrossRef]
- Assmann, K.M.; Darelius, A.K.; Wahlin, A.K.; Kim, T.W.; Lee, S.H. Warm circumpolar deep water at the western gets ice shelf front, Antarctica. Geophys. Res. Lett. 2019, 46, 870–878. [Google Scholar] [CrossRef]
- Pillsbury, R.D.; Jacobs, S.S. Preliminary observations from long-term current meter moorings near the Ross Ice Shelf, Antarctica. In Antarctic Research Series; American Geophysical Union: Washington, DC, USA, 1985; Volume 43, pp. 87–107. [Google Scholar]
- Budillon, G.; Castagno, P.; Aliani, S.; Spezie, G.; Padman, L. Thermoaline variability and Antarctic bottom water formation at the Ross Sea shelf break. Deep Sea Res. Ocean Res. Pap. 2011, 58, 1002–1018. [Google Scholar] [CrossRef]
- Yoon, S.-T.; Lee, W.S.; Stevens, C.; Jendersie, S.; Nam, S.; Yun, S.; Hwang, C.Y.; Jang, G.I.; Lee, J. Variability in high-salinity shelf water production in the Terra Nova Bay polynya, Antarctica. Ocean Sci. 2020, 16, 373–388. [Google Scholar] [CrossRef]
- Schram, J.B.; Schoenrock, K.M.; McClintock, J.B.; Amsler, C.D.; Angus, R.A. Multi-frequency observations of seawater car-bonate chemistry on the central coast of the western Antarctic Peninsula. Polar Res 2015, 34, 25582. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, F.; Yang, H.; Lin, L.; He, J. Detection of phytoplankton blooms in Antarctic coastal water with an online mooring system during summer 2010. Antarct. Sci. 2013, 1–8. [Google Scholar] [CrossRef]
- Matson, P.G.; Martz, T.R.; Hofmann, G.E. High-frequency observations of pH under Antarctic sea ice in the southern Ross Sea. Antarct. Sci. 2011, 23, 607–613. [Google Scholar] [CrossRef]
- Fontaneto, D.; Schiaparelli, S. Preface: Biology of the Ross Sea and surrounding areas in Antarctica. Hydrobiologia 2015, 761, 1–3. [Google Scholar] [CrossRef][Green Version]
- Peck, L. Prospects for survival in the Southern Ocean: Vulnerability of benthic species to temperature change. Antarct. Sci. 2005, 17, 497–507. [Google Scholar] [CrossRef]
- Clark, G.F.; Stark, J.S.; Palmer, A.S.; Riddle, M.J.; Johnston, E.L. The Roles of Sea-Ice, Light and Sedimentation in Structuring Shallow Antarctic Benthic Communities. PLoS ONE 2017, 12, e0168391. [Google Scholar] [CrossRef]
- Hunke, E.C.; Dukowicz, J.K. An elastic-viscous-plastic model for sea ice dynamics. J. Phys. Ocean 1997, 27, 1849–1867. [Google Scholar] [CrossRef]
- Padman, L.; Kottmeier, C. High-frequency ice motion and divergence in the Weddell Sea. J. Geophys. Res. Space Phys. 2000, 105, 3379–3400. [Google Scholar] [CrossRef]
- Padman, L.; Siegfried, M.R.; Fricker, H.A. Ocean Tide Influences on the Antarctic and Greenland Ice Sheets. Rev. Geophys. 2018, 56, 142–184. [Google Scholar] [CrossRef]
- Nansen, F. Farthest North; George Newnes, Ltd.: London, UK, 1898. [Google Scholar]
- Koentopp, M.; Eisen, O.; Kottmeier, C.; Padman, L.; Lemke, P. Influence of tides on sea ice in the Weddell Sea: Investigations with a high-resolution dynamic-thermodynamic sea ice model. J. Geophys. Res. Space Phys. 2005, 110, 02014. [Google Scholar] [CrossRef]
- Rivaro, P.; Ianni, C.; Langone, L.; Ori, C.; Aulicino, G.; Cotroneo, Y.; Saggiomo, M.; Mangoni, O. Physical and biological forcing of mesoscale variability in the carbonate system of the Ross Sea (Antarctica) during summer. J. Mar. Syst. 2017, 166, 144–158. [Google Scholar] [CrossRef]
- Caputi, S.S.; Careddu, G.; Calizza, E.; Fiorentino, F.; Maccapan, D.; Rossi, L.; Costantini, M.L. Seasonal Food Web Dynamics in the Antarctic Benthos of Tethys Bay (Ross Sea): Implications for Biodiversity Persistence Under Different Seasonal Sea-Ice Coverage. Front. Mar. Sci. 2020, 7, 594454. [Google Scholar] [CrossRef]
- Rivaro, P.; Ianni, C.; Raimondi, L.; Manno, C.; Sandrini, S.; Castagno, P.; Cotroneo, Y.; Falco, P. Analysis of Physical and Biogeochemical Control Mechanisms on Summertime Surface Carbonate System Variability in the Western Ross Sea (Antarctica) Using In Situ and Satellite Data. Remote. Sens. 2019, 11, 238. [Google Scholar] [CrossRef]
- Clarke, A. Seasonality in the antarctic marine environment. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 90, 461–473. [Google Scholar] [CrossRef]
- Pusceddu, A.; Dell’Anno, A.; Fabiano, M. Organic matter composition in coastal sediments at Terra Nova Bay (Ross Sea) during summer. Polar Biol. 2000, 23, 288–293. [Google Scholar] [CrossRef]
- Leu, E.; Mundy, C.J.; Assmy, P.; Campbell, K.; Gabrielsen, T.M.; Gosselin, M. Arctic spring awakening—Steering prin-ciples behind the phenology of vernal ice algal blooms. Prog. Oceanogr. 2015, 139, 151–170. [Google Scholar] [CrossRef]
- Saggiomo, M.; Poulin, M.; Mangoni, O.; Lazzara, L.; De Stefano, M.; Sarno, D.; Zingone, A. Spring-time dynamics of diatom communities in landfast and underlying platlet ice in Terra Nova Bay, Ross Sea, Antarctica. J. Mar. Sys. 2017, 166, 26–36. [Google Scholar] [CrossRef]
- Mendes, C.R.B.; Tavano, V.M.; Dotto, T.S.; Kerr, R.; De Souza, M.S.; Garcia, C.A.E.; Secchi, E.R. New insights on the dominance of cryptophytes in Antarctic coastal waters: A case study in Gerlache Strait. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 149, 161–170. [Google Scholar] [CrossRef]
- Alonso-Saez, L.; Sanchez, O.; Gasol, J.; Balaguè, V.; Pedros-Alio, C. Winter-to-summer changes in the composition and sin-gle-cell activity of near-surface Arctic prokaryotes. Environ. Microbiol. 2008, 10, 2444–2454. [Google Scholar] [CrossRef]
- Cantero Álvaro, L.P.; Boero, F.; Piraino, S. Shallow-water benthic hydroids from Tethys Bay (Terra Nova Bay, Ross Sea, Antarctica). Polar Biol. 2013, 36, 731–753. [Google Scholar] [CrossRef]
- Calizza, E.; Rossi, L.; Careddu, G.; Caputi, S.S.; Costantini, M.L. Species richness and vulnerability to disturbance propagation in real food webs. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Michel, L.N.; Danis, B.; Dubois, P.; Eleaume, M.; Fournier, J.; Gallut, C.; Jane, P.; Lepoint, G. Increased sea ice cover alters food web structure in East Antarctica. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Pearse, J.S.; McClintock, J.B.; Bosch, I. Reproduction of Antarctic Benthic Marine Invertebrates: Tempos, Modes, and Timing. Am. Zoöl. 1991, 31, 65–80. [Google Scholar] [CrossRef]
- Knox, G.A. Biology of the Southern Ocean; Apple Academic Press: Palm Bay, FL, USA, 2006. [Google Scholar]
- Santagata, S.; Ade, V.; Mahon, A.R.; Wisocki, P.A.; Halanych, K.M. Compositional Differences in the Habitat-Forming Bryozoan Communities of the Antarctic Shelf. Front. Ecol. Evol. 2018, 6, 116. [Google Scholar] [CrossRef]
- Bresnahan, P.J.; Martz, T.R.; Takeshita, Y.; Johnson, K.S.; LaShomb, M. Best practices for autonomous measurement of seawater pH with the Honeywell Durafet. Methods Oceanogr. 2014, 9, 44–60. [Google Scholar] [CrossRef]
- Miller, C.A.; Pocock, K.; Evans, W.; Kelley, A.L. An evaluation of the performance of Sea-Bird Scientific’s SeaFET™ autono-mous pH sensor: Considerations for the broader oceanographic community. Ocean Sci. 2018, 14, 751–768. [Google Scholar] [CrossRef]
- Pérez, F.F.; Fraga, F. The pH measurements in seawater on the NBS scale. Mar. Chem. 1987, 21, 315–327. [Google Scholar] [CrossRef]
- Chierici, M.; Fransson, A. Calcium carbonate saturation in the surface water of the Arctic Ocean: Undersaturation in freshwater influenced shelves. Biogeosciences 2009, 6, 2421–2431. [Google Scholar] [CrossRef]
- Luchetta, A.; Cantoni, C.; Catalano, G. New observations of CO2-induced acidification in the northern Adriatic Sea over the last 696 quarter century. Chem. Ecol. 2010, 26, 1–17. [Google Scholar] [CrossRef]
- Hoel, P.G. Introduction to Mathematical Statistics, 5th ed.; Wiley: New York, NY, USA, 1984. [Google Scholar]






| Probe Model | Quantity | Units | Accuracy | Stability | uc |
|---|---|---|---|---|---|
| SBE SeaFET V2 | Total scale pH | --- | ±0.05 pHT | 0.005 pHT month−1 | 0.06 |
| Temperature * | °C | n/a | n/a | n/a | |
| CTD SBE37-SMP-ODO | Temperature | °C | ±0.002 | 0.0002 month−1 | 0.023 |
| Pressure | dbar | ±0.35 | 0.01 month−1 | 0.24 | |
| Electrical Conductivity | mS cm−1 | ±0.003 | 0.003 month−1 | 0.032 | |
| Dissolved oxygen | mL L−1 | ±0.07 | 0.0002 month−1 | n/a | |
| Practical salinity | PSU | n/a | n/a | 0.009 | |
| Density | kg m−3 | n/a | n/a | n/a | |
| HOBO Pendant Logger | Illuminance | lux | n/a | n/a | n/a |
| Temperature * | °C | ±0.53 °C | n/a | n/a |
| Annual | pHT | p dbar | t °C | Sp PSU | ρ kg m−3 | DO mL L−1 | Ev lux | TA * μmol kgSW−1 | pCO2 ** μatm | ΩCa ** | ΩAr ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | 8.09 ± 0.08 | 25.10 ± 0.19 | −1.63 ± 0.56 | 34.61 ± 0.24 | 1027.98 ± 0.20 | 7.42 ± 0.71 | 4 | 2336 ± 13 | 367 ± 74 | 2.44 ± 0.46 | 1.53 ± 0.29 |
| median | 8.05 | 25.07 | −1.91 | 34.72 | 1028.06 | 7.17 | 0 | 2340 | 395 | 2.22 | 1.39 |
| min | 7.97 | 24.57 | −1.95 | 33.72 | 1027.25 | 6.61 | 0 | 2292 | 176 | 1.91 | 1.20 |
| max | 8.35 | 25.76 | 1.08 | 34.87 | 1028.20 | 10.61 | 201 | 2350 | 466 | 4.42 | 2.78 |
| range | 0.38 | 1.19 | 3.03 | 1.15 | 0.95 | 4.00 | 201 | 59 | 290 | 2.51 | 1.58 |
| Monthly | |||||||||||
| Dec-18 | 8.09 ± 0.09 | 25.07 ± 0.20 | −1.09 ± 0.45 | 34.73 ± 0.02 | 1028.05 ± 0.03 | 8.04 ± 0.84 | 0 | 2340 ± 3 | 355 ± 79 | 2.55 ± 0.54 | 1.60 ± 0.34 |
| Jan-19 | 8.24 ± 0.04 | 25.09 ± 0.20 | −0.13 ± 0.37 | 34.50 ± 0.20 | 1027.82 ± 0.15 | 8.85 ± 0.54 | 8 | 2324 ± 9 | 242 ± 24 | 3.41 ± 0.27 | 2.14 ± 0.17 |
| Feb-19 | 8.24 ± 0.03 | 25.14 ± 0.19 | −1.31 ± 0.15 | 33.99 ± 0.11 | 1027.47 ± 0.09 | 8.26 ± 0.26 | 12 | 2305 ± 5 | 240 ± 21 | 3.13 ± 0.19 | 1.96 ± 0.12 |
| Mar-19 | 8.16 ± 0.02 | 25.12 ± 0.18 | −1.74 ± 0.18 | 34.35 ± 0.07 | 1027.77 ± 0.06 | 7.72 ± 0.11 | 21 | 2323 ± 4 | 291 ± 16 | 2.74 ± 0.11 | 1.72 ± 0.07 |
| Apr-19 | 8.09 ± 0.02 | 25.11 ± 0.17 | −1.89 ± 0.02 | 34.54 ± 0.05 | 1027.93 ± 0.04 | 7.39 ± 0.09 | 3 | 2333 ± 2 | 353 ± 18 | 2.39 ± 0.09 | 1.50 ± 0.05 |
| May-19 | 8.07 ± 0.01 | 25.14 ± 0.19 | −1.91 ± 0.00 | 34.61 ± 0.02 | 1027.98 ± 0.02 | 7.24 ± 0.05 | 0 | 2337 ± 1 | 379 ± 5 | 2.27 ± 0.02 | 1.42 ± 0.01 |
| Jun-19 | 8.05 ± 0.01 | 25.12 ± 0.20 | −1.92 ± 0.01 | 34.69 ± 0.06 | 1028.05 ± 0.05 | 7.15 ± 0.05 | 0 | 2341 ± 3 | 398 ± 8 | 2.21 ± 0.02 | 1.39 ± 0.01 |
| Jul-19 | 8.03 ± 0.00 | 25.10 ± 0.22 | −1.93 ± 0.00 | 34.74 ± 0.02 | 1028.09 ± 0.02 | 6.98 ± 0.06 | 0 | 2344 ± 1 | 419 ± 5 | 2.14 ± 0.02 | 1.34 ± 0.01 |
| Aug-19 | 8.03 ± 0.00 | 25.09 ± 0.20 | −1.93 ± 0.00 | 34.74 ± 0.02 | 1028.10 ± 0.02 | 6.90 ± 0.03 | 0 | 2344 ± 1 | 423 ± 3 | 2.13 ± 0.01 | 1.34 ± 0.01 |
| Sep-19 | 8.02 ± 0.01 | 25.03 ± 0.17 | −1.92 ± 0.00 | 34.81 ± 0.02 | 1028.15 ± 0.02 | 6.80 ± 0.07 | 0 | 2347 ± 1 | 435 ± 6 | 2.11 ± 0.02 | 1.32 ± 0.02 |
| Oct-19 | 8.03 ± 0.00 | 25.04 ± 0.18 | −1.92 ± 0.01 | 34.82 ± 0.01 | 1028.16 ± 0.01 | 6.81 ± 0.02 | 0 | 2348 ± 1 | 430 ± 2 | 2.14 ± 0.01 | 1.34 ± 0.01 |
| Nov-19 | 8.03 ± 0.00 | 25.09 ± 0.19 | −1.88 ± 0.03 | 34.79 ± 0.01 | 1028.13 ± 0.01 | 6.82 ± 0.04 | 0 | 2346 ± 1 | 430 ± 2 | 2.14 ± 0.01 | 1.34 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, C.; Kuklinski, P.; Bordone, A.; Spirandelli, E.; Raiteri, G. Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea). Minerals 2021, 11, 374. https://doi.org/10.3390/min11040374
Lombardi C, Kuklinski P, Bordone A, Spirandelli E, Raiteri G. Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea). Minerals. 2021; 11(4):374. https://doi.org/10.3390/min11040374
Chicago/Turabian StyleLombardi, Chiara, Piotr Kuklinski, Andrea Bordone, Edoardo Spirandelli, and Giancarlo Raiteri. 2021. "Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea)" Minerals 11, no. 4: 374. https://doi.org/10.3390/min11040374
APA StyleLombardi, C., Kuklinski, P., Bordone, A., Spirandelli, E., & Raiteri, G. (2021). Assessment of Annual Physico-Chemical Variability via High-Temporal Resolution Monitoring in an Antarctic Shallow Coastal Site (Terra Nova Bay, Ross Sea). Minerals, 11(4), 374. https://doi.org/10.3390/min11040374






