An Accurate Model to Calculate CO2 Solubility in Pure Water and in Seawater at Hydrate–Liquid Water Two-Phase Equilibrium
Abstract
1. Introduction
2. Thermodynamic Model of Gas Hydrates
2.1. Model Framework for HLWE
2.2. Method 1: Poynting Correction + Pitzer Model
2.3. Method 2: SAFT-LJ EOS
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Nomenclature | |
| a | activity |
| Cij | the Langmuir constant of gas component j in i-type cavity |
| Cp | isobaric molar heat capacity (J/K/mol) |
| fj | the fugacity of gas component j (Pa) |
| h | molar enthalpy (J/mol) |
| kH | Henry’s law constant |
| P | pressure (Pa) |
| R | universal gas constant (= 8.314 J/mol/K) |
| T | temperature (K) |
| V | molar volume (m3/mol) |
| x | mole fraction |
| θij | the fractional occupancy of i-type cavities with j-type guest molecules |
| μ | molar chemical potential (J/mol) |
| νi | the number of i-type cages per water molecule |
| ρ | density (kg/m3) |
| partial molar volume (m3/mol) | |
| Subscript | |
| sol | solution |
| W | H2O |
| Superscript | |
| aq | aqueous phase |
| car | carbonic phase |
| H | hydrate phase |
| L | liquid water (aqueous phase) |
| sat | saturation |
| V | vapor phase |
| β | hypothetical empty hydrate lattice phase |
Appendix A
Appendix A.1. The Improved SAFT-LJ EOS
Appendix A.2. Lennard–Jones Segment Term
| H2O | CO2 | |
|---|---|---|
| ε/kB (K) | 172.1 − 0.001 × (T-473.15)2 | 173.7 |
| b (cm3/mol) | 16.684 − 0.00476 × (T-423.15) + 1.58 × 10−5 × (T-423.15)2 | 19.97 |
| εassoc/kB (K) | 1430 − 1.07 × (T-273.15) − 10.5 × 10−4 × (T-273.15)2 +7.5 × 10−6 × (T-273.15)3 | |
| Κassoc | 0.004 | |
| m | 1 | 1.5 |
| μ (10−30C·m) | 7.34 | |
| Q (10−40C·m2) | −14.3 |
Appendix A.3. Chain-Forming Term
Appendix A.4. Association Term
Appendix A.5. Multi-Polar Term
Appendix A.6. Charge–Charge Interaction Term
| Parameter | Value |
|---|---|
| d (10−10 m) | 4.65 + 0.00237 × (T-273.15) − 2.572 × 10−5 × (T-273.15)2 |
| εNaCl/kB (K) | 0 |
| bNaCl (cm3/mol) | 11.411 + 0.03052 × (T-273.15) − 2.389 × 10−4 × (T-273.15)2 + 3.048 × 10−7 × (T-273.15)3 |
| εNaCl-H2O/kB (K) | 283.01 − 0.0476 × (T-273.15) + 6.237 × 10−4 × (T-273.15)2 |
| bNaCl-H2O(cm3/mol) | 10.6 − 0.00521 × (T-273.15) − 6.3336 × 10−5 × (T-273.15)2 |
References
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Cao, C.; Liu, H.; Hou, Z.; Mehmood, F.; Liao, J.; Feng, W. A review of CO2 storage in view of safety and cost-effectiveness. Energies 2020, 13, 600. [Google Scholar] [CrossRef]
- Adu, E.; Zhang, Y.; Liu, D. Current situation of carbon dioxide capture, storage, and enhanced oil recovery in the oil and gas industry. Can. J. Chem. Eng. 2019, 97, 1048–1076. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Brewer, P.G.; Friederich, G.; Peltzer, E.T.; Orr, F.M., Jr. Direct experiments on the ocean disposal of fossil fuel CO2. Science 1999, 284, 943–945. [Google Scholar] [CrossRef]
- De Silva, G.; Ranjith, P.; Perera, M. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Blackford, J.; Alendal, G.; Avlesen, H.; Brereton, A.; Cazenave, P.W.; Chen, B.; Dewar, M.; Holt, J.; Phelps, J. Impact and detectability of hypothetical CCS offshore seep scenarios as an aid to storage assurance and risk assessment. Int. J. Greenh. Gas Control 2020, 95, 102949. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef]
- Kvamme, B.; Graue, A.; Buanes, T.; Kuznetsova, T.; Ersland, G. Storage of CO2 in natural gas hydrate reservoirs and the effect of hydrate as an extra sealing in cold aquifers. Int. J. Greenh. Gas Control. 2007, 1, 236–246. [Google Scholar] [CrossRef]
- Tohidi, B.; Yang, J.; Salehabadi, M.; Anderson, R.; Chapoy, A. CO2 hydrates could provide secondary safety factor in subsurface sequestration of CO2. Environ. Sci. Technol. 2010, 44, 1509–1514. [Google Scholar] [CrossRef]
- Sun, D.; Englezos, P. Storage of CO2 in a partially water saturated porous medium at gas hydrate formation conditions. Int. J. Greenh. Gas Control. 2014, 25, 1–8. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by carbon dioxide from gas hydrates. Phase equilibria for CO2-CH4 mixed hydrate system. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Koh, D.-Y.; Kang, H.; Kim, D.-O.; Park, J.; Cha, M.; Lee, H. Recovery of methane from gas hydrates intercalated within natural sediments using CO2 and a CO2/N2 gas mixture. ChemSusChem 2012, 5, 1443–1448. [Google Scholar] [CrossRef]
- Belosludov, V.R.; Bozhko, Y.Y.; Subbotin, O.S.; Belosludov, R.V.; Zhdanov, R.K.; Gets, K.V.; Kawazoe, Y. Influence of N2 on formation conditions and guest distribution of mixed CO2 + CH4 gas hydrates. Molecules 2018, 23, 3336. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon dioxide sequestration via gas hydrates: A potential pathway toward decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Sloan, E.D.J.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sun, R.; Duan, Z. Prediction of CH4 and CO2 hydrate phase equilibrium and cage occupancy from ab initio intermolecular potentials. Geochim. Cosmochim. Acta 2005, 69, 4411–4424. [Google Scholar] [CrossRef]
- Van der Waals, J.H.; Platteeuw, J.C. Clathrate Solutions. In Advances in Chemical Physics; Prigogine, I., Ed.; Interscience: Geneva, Switzerland, 1959; pp. 1–57. [Google Scholar]
- Cao, Z.T.; Tester, J.W.; Sparks, K.A.; Trout, B.L. Molecular computations using robust hydrocarbon-water potentials for predicting gas hydrate phase equilibria. J. Phys. Chem. B 2001, 105, 10950–10960. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. Ab initio intermolecular potentials for gas hydrates and their predictions. J. Phys. Chem. B 2002, 106, 5722–5732. [Google Scholar] [CrossRef]
- Anderson, B.J.; Tester, J.W.; Trout, B.L. Accurate potentials for argon-water and methane-water interactions via ab initio methods and their application to clathrate hydrates. J. Phys. Chem. B 2004, 108, 18705–18715. [Google Scholar] [CrossRef]
- Lee, J.W.; Yedlapalli, P.; Lee, S. Prediction of hydrogen hydrate equilibrium by integrating ab initio calculations with statistical thermodynamics. J. Phys. Chem. B 2006, 110, 2332–2337. [Google Scholar] [CrossRef] [PubMed]
- Velaga, S.C.; Anderson, B.J. Carbon dioxide hydrate phase equilibrium and cage occupancy calculations using ab initio intermolecular potentials. J. Phys. Chem. B 2014, 118, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Thakre, N.; Jana, A.K. Computing anisotropic cavity potential for clathrate hydrates. J. Phys. Chem. A 2019, 123, 2762–2770. [Google Scholar] [CrossRef]
- Veesam, S.K.; Ravipati, S.; Punnathanam, S.N. Recent advances in thermodynamics and nucleation of gas hydrates using molecular modeling. Curr. Opin. Chem. Eng. 2019, 23, 14–20. [Google Scholar] [CrossRef]
- Medeiros F de, A.; Segtovich, I.S.V.; Tavares, F.W.; Sum, A.K. Sixty years of the van der Waals and Platteeuw model for clathrate hydrates—A critical review from its statistical thermodynamic basis to its extensions and applications. Chem. Rev. 2020, 120, 13349–13381. [Google Scholar] [CrossRef]
- Thakre, N.; Jana, A.K. Physical and molecular insights to clathrate hydrate thermodynamics. Renew. Sustain. Energy Rev. 2021, 135, 110150. [Google Scholar] [CrossRef]
- Ballard, A.L.; Sloan, E.D. The next generation of hydrate prediction I: Hydrate standard states and incorporation of spectroscopy. Fluid Phase Equilibria 2002, 194−197, 371–383. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. Phase behavior of clathrate hydrates: A model for single and multiple gas component hydrates. Chem. Eng. Sci. 2003, 58, 27–41. [Google Scholar] [CrossRef]
- Belosludov, R.V.; Zhdanov, R.K.; Gets, K.V.; Bozhko, Y.Y.; Belosludov, V.R.; Kawazoe, Y. Role of methane as a second guest component in thermodynamic stability and isomer selectivity of butane clathrate hydrates. J. Phys. Chem. C 2020, 124, 18474–18481. [Google Scholar] [CrossRef]
- Kodera, M.; Matsueda, T.; Belosludov, R.V.; Zhdanov, R.K.; Belosludov, V.R.; Takeya, S.; Alavi, S.; Ohmura, R. Physical properties and characterization of the binary clathrate hydrate with methane + 1,1,1,3,3-pentafluoropropane (HFC-245fa) + water. J. Phys. Chem. C 2020, 124, 20736–20745. [Google Scholar] [CrossRef]
- Belosludov, V.R.; Subbotin, O.S.; Krupskii, D.S.; Belosludov, R.V.; Kawazoe, Y.; Kudoh, J.-I. Physical and chemical properties of gas hydrates: theoretical aspects of energy storage application. Mater. Trans. 2007, 48, 704–710. [Google Scholar] [CrossRef]
- Belosludov, R.V.; Subbotin, O.S.; Mizuseki, H.; Kawazoe, Y.; Belosludov, V.R. Accurate description of phase diagram of clathrate hydrates at the molecular level. J. Chem. Phys. 2009, 131, 244510. [Google Scholar] [CrossRef] [PubMed]
- Belosludov, R.V.; Bozhko, Y.Y.; Subbotin, O.S.; Belosludov, V.R.; Mizuseki, H.; Kawazoe, Y.; Fomin, V.M. Stability and composition of helium hydrates based on Ices I-h and II at low temperatures. J. Phys. Chem. C 2014, 118, 2587–2593. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.-Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Patel, N.C.; Teja, A.S. A new cubic equation of state for fluids and fluid mixtures. Chem. Eng. Sci. 1982, 37, 463–473. [Google Scholar] [CrossRef]
- Trebble, M.; Bishnoi, P. Development of a new four-parameter cubic equation of state. Fluid Phase Equilibria 1987, 35, 1–18. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Pitzer, K.S. (Ed.) Theory and data correlation. In Activity Coefficients in Electrolyte Solutions; CRC Press: London, UK, 1991; pp. 75–153. [Google Scholar]
- Esmaeilzadeh, F.; Hamedi, N.; Karimipourfard, D.; Rasoolzadeh, A. An insight into the role of the association equations of states in gas hydrate modeling: A review. Pet. Sci. 2020, 17, 1432–1450. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. New reference equation of state for associating liquids. Ind. Eng. Chem. Res. 1990, 29, 1709–1721. [Google Scholar] [CrossRef]
- Huang, S.H.; Radosz, M. Equation of state for small, large, polydisperse, and associating molecules. Ind. Eng. Chem. Res. 1990, 29, 2284–2294. [Google Scholar] [CrossRef]
- Wertheim, M.S. Fluids with highly directional attractive forces: I. Statistical thermodynamics. J. Stat. Phys. 1984, 35, 19–34. [Google Scholar] [CrossRef]
- Wertheim, M.S. Fluids with highly directional attractive forces. II. Thermodynamic perturbation theory and integral equations. J. Stat. Phys. 1984, 35, 35–47. [Google Scholar] [CrossRef]
- Wertheim, M.S. Fluids with highly directional attractive forces: III. Multiple attraction sites. J. Stat. Phys. 1986, 42, 459–476. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Voutsas, E.C.; Yakoumis, I.V.; Tassios, D.P. An equation of state for associating fluids. Ind. Eng. Chem. Res. 1996, 35, 4310–4318. [Google Scholar] [CrossRef]
- Valtz, A.; Chapoy, A.; Coquelet, C.; Paricaud, P.; Richon, D. Vapor-liquid equilibria in the carbon dioxide-water system, measurement and modelling from 278.2 to 318.2K. Fluid Phase Equilibria 2004, 226, 333–344. [Google Scholar] [CrossRef]
- Ji, X.; Tan, S.P.; Adidharma, H.; Radosz, M. SAFT1-RPM approximation extended to phase equilibria and densities of CO2-H2O and CO2-H2O-NaCl systems. Ind. Eng. Chem. Res. 2005, 44, 8419–8427. [Google Scholar] [CrossRef]
- Dos Ramos, M.C.; Blas, F.J.; Galindo, A. Phase equilibria, excess properties, and Henry’s constants of the water + carbon dioxide binary mixture. J. Phys. Chem. C 2007, 111, 15924–15934. [Google Scholar] [CrossRef]
- Perfetti, E.; Thiery, R.; Dubessy, J. Equation of state taking into account dipolar interactions and association by hydrogen bonding: II: Modelling liquid-vapor equilibria in the H2O-H2S, H2O-CH4 and H2O-CO2 systems. Chem. Geol. 2008, 251, 50–57. [Google Scholar] [CrossRef]
- Sun, R.; Dubessy, J. Prediction of vapor–liquid equilibrium and PVTx properties of geological fluid system with SAFT-LJ EOS including multi-polar contribution. Part I. application to H2O–CO2 system. Geochim. Cosmochim. Acta 2010, 74, 1982–1998. [Google Scholar] [CrossRef]
- Sun, R.; Dubessy, J. Prediction of vapor-liquid equilibrium and PVTx properties of geological fluid system with SAFT-LJ EOS including multi-polar contribution. Part II. Application to H2O-NaCl and CO2-H2O-NaCl System. Geochim. Cosmochim. Acta 2012, 88, 130–145. [Google Scholar] [CrossRef]
- Niño-Amézquita, G.; Van Putten, D.; Enders, S. Phase equilibrium and interfacial properties of water + CO2 mixtures. Fluid Phase Equilibria 2012, 332, 40–47. [Google Scholar] [CrossRef]
- Islam, A.W.; Carlson, E.S. Application of SAFT equation for CO2 + H2O phase equilibrium calculations over a wide temperature and pressure range. Fluid Phase Equilibria 2012, 321, 17–24. [Google Scholar] [CrossRef]
- Courtial, X.; Ferrando, N.; de Hemptinne, J.-C.; Mougin, P. Electrolyte CPA equation of state for very high temperature and pressure reservoir and basin applications. Geochim. Cosmochim. Acta 2014, 142, 1–14. [Google Scholar] [CrossRef]
- Llovell, F.; Vega, L. Accurate modeling of supercritical CO2 for sustainable processes: Water+CO2 and CO2+fatty acid esters mixtures. J. Supercrit. Fluids 2015, 96, 86–95. [Google Scholar] [CrossRef]
- Jiang, H.; Panagiotopoulos, A.Z.; Economou, I.G. Modeling of CO2 solubility in single and mixed electrolyte solutions using statistical associating fluid theory. Geochim. Cosmochim. Acta 2016, 176, 185–197. [Google Scholar] [CrossRef]
- Chen, J.; Lu, J.; Li, Y. Equation of state SAFT-CP for vapour–liquid equilibria and mutual solubility of carbon dioxide and water. Mol. Phys. 2016, 114, 2451–2460. [Google Scholar] [CrossRef]
- Bian, X.-Q.; Xiong, W.; Kasthuriarachchi, D.T.K.; Liu, Y.-B. Phase equilibrium modeling for carbon dioxide solubility in aqueous sodium chloride solutions using an association equation of state. Ind. Eng. Chem. Res. 2019, 58, 10570–10578. [Google Scholar] [CrossRef]
- Sun, L.; Kontogeorgis, G.M.; Von Solms, N.; Liang, X. Modeling of gas solubility using the electrolyte cubic plus association equation of state. Ind. Eng. Chem. Res. 2019, 58, 17555–17567. [Google Scholar] [CrossRef]
- Chabab, S.; Théveneau, P.; Corvisier, J.; Coquelet, C.; Paricaud, P.; Houriez, C.; El Ahmar, E. Thermodynamic study of the CO2 – H2O – NaCl system: Measurements of CO2 solubility and modeling of phase equilibria using Soreide and Whitson, electrolyte CPA and SIT models. Int. J. Greenh. Gas Control. 2019, 91, 102825. [Google Scholar] [CrossRef]
- Pabsch, D.; Held, C.; Sadowski, G. Modeling the CO2 solubility in aqueous electrolyte solutions using ePC-SAFT. J. Chem. Eng. Data 2020, 65, 5768–5777. [Google Scholar] [CrossRef]
- Li, X.-S.; Wu, H.-J.; Englezos, P. Prediction of gas hydrate formation conditions in the presence of methanol, glycol, and triethylene glycol with statistical associating fluid theory equation of state. Ind. Eng. Chem. Res. 2006, 45, 2131–2137. [Google Scholar] [CrossRef]
- Li, X.-S.; Wu, H.-J.; Li, Y.-G.; Feng, Z.-P.; Tang, L.-G.; Fan, S.-S. Hydrate dissociation conditions for gas mixtures containing carbon dioxide, hydrogen, hydrogen sulfide, nitrogen, and hydrocarbons using SAFT. J. Chem. Thermodyn. 2007, 39, 417–425. [Google Scholar] [CrossRef]
- Haghighi, H.; Chapoy, A.; Tohidi, B. Methane and water phase equilibria in the presence of single and mixed electrolyte solutions using the cubic-plus-association equation of state. Oil Gas Sci. Technol. 2009, 64, 141–154. [Google Scholar] [CrossRef]
- Martín, Á.; Peters, C.J. New Thermodynamic model of equilibrium states of gas hydrates considering lattice distortion. J. Phys. Chem. C 2009, 113, 422–430. [Google Scholar] [CrossRef]
- Jiang, H.; Adidharma, H. Hydrate equilibrium modeling for pure alkanes and mixtures of alkanes using statistical associating fluid theory. Ind. Eng. Chem. Res. 2011, 50, 12815–12823. [Google Scholar] [CrossRef]
- Dufal, S.; Galindo, A.; Jackson, G.; Haslam, A.J. Modelling the effect of methanol, glycol inhibitors and electrolytes on the equilibrium stability of hydrates with the SAFT-VR approach. Mol. Phys. 2012, 110, 1223–1240. [Google Scholar] [CrossRef]
- Karakatsani, E.K.; Kontogeorgis, G.M. Thermodynamic modeling of natural gas systems containing water. Ind. Eng. Chem. Res. 2013, 52, 3499–3513. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Fan, J. The application of CPA-vdWP to the phase equilibrium modeling of methane-rich sour natural gas hydrates. Fluid Phase Equilibria 2016, 409, 291–300. [Google Scholar] [CrossRef]
- Meragawi, S.E.; Diamantonis, N.I.; Tsimpanogiannis, I.N.; Economou, I.G. Hydrate—Fluid phase equilibria modeling using PC-SAFT and Peng–Robinson equations of state. Fluid Phase Equilibria 2016, 413, 209–219. [Google Scholar] [CrossRef]
- Palma, A.M.; Queimada, A.J.; Coutinho, J.A.P. Modeling of hydrate dissociation curves with a modified cubic-plus-association equation of state. Ind. Eng. Chem. Res. 2019, 58, 14476–14487. [Google Scholar] [CrossRef]
- Aya, I.; Yamane, K.; Nariai, H. Solubility of CO2 and density of CO2 hydrate at 30 MPa. Energy 1997, 22, 263–271. [Google Scholar] [CrossRef]
- Yang, S.; Yang, I.; Kim, Y.; Lee, C. Measurement and prediction of phase equilibria for water+CO2 in hydrate forming conditions. Fluid Phase Equilibria 2000, 175, 75–89. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, H.; Lee, J.; Kang, J.M. CO2 hydrate behavior in the deep ocean sediments; phase equilibrium, formation kinetics, and solubility. Geophys. Res. Lett. 2002, 29, 2034. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Effect of temperature and pressure on the solubility of carbon dioxide in water in the presence of gas hydrate. Fluid Phase Equilibria 2001, 190, 127–134. [Google Scholar] [CrossRef]
- Zhang, Y. Formation of Hydrate from Single-Phase Aqueous Solutions. Master’s Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2003. [Google Scholar]
- Someya, S.; Bando, S.; Chen, B.; Song, Y.; Nishio, M. Measurement of CO2 solubility in pure water and the pressure effect on it in the presence of clathrate hydrate. Int. J. Heat Mass Transf. 2005, 48, 2503–2507. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, B.D.; Lee, J.E.; Lee, C.S. Solubilities of carbon dioxide, methane, and ethane in sodium chloride solution containing gas hydrate. J. Chem. Eng. Data 2008, 53, 1351–1354. [Google Scholar] [CrossRef]
- Zhang, Y.; Holder, G.D.; Warzinski, R.P. Phase equilibrium in two-phase, water-rich-liquid, hydrate systems: Experiment and theory. Ind. Eng. Chem. Res. 2008, 47, 459–469. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, H.; Li, Z.; Guo, X.; Liu, A.; Yang, L. Solubility of CO2 in water and NaCl solution in equilibrium with hydrate. Part I: Experimental measurement. Fluid Phase Equilibria 2016, 409, 131–135. [Google Scholar] [CrossRef]
- Geng, L.; Qu, K.; Lu, W.; Jiang, L.; Chou, I.-M. In situ Raman spectroscopic study of the pressure effect on the concentration of CO2 in water at hydrate-liquid water equilibrium up to 900 bar. Fluid Phase Equilibria 2017, 438, 37–43. [Google Scholar] [CrossRef]
- Hashemi, S.; Macchi, A.; Bergeron, S.; Servio, P. Prediction of methane and carbon dioxide solubility in water in the presence of hydrate. Fluid Phase Equilibria 2006, 246, 131–136. [Google Scholar] [CrossRef]
- Parrish, W.R.; Prausnitz, J.M. Dissociation pressures of gas hydrates formed by gas mixtures. Ind. Eng. Chem. Process. Des. Dev. 1972, 11, 26–35. [Google Scholar] [CrossRef]
- John, V.T.; Holder, G.D. Contribution of second and subsequent water shells to the potential energy of guest-host interactions in clathrate hydrates. J. Phys. Chem. 1982, 86, 455–459. [Google Scholar] [CrossRef]
- Zele, S.R.; Lee, S.-Y.; Holder, G.D. A theory of lattice distortion in gas hydrates. J. Phys. Chem. B 1999, 103, 10250–10257. [Google Scholar] [CrossRef]
- Lee, S.Y.; Holder, G.D. Model for gas hydrate equilibria using a variable reference chemical potential: Part 1. AICHE J. 2002, 48, 161–167. [Google Scholar] [CrossRef]
- Holder, G.; Zetts, S.; Pradhan, N. Phase behavior in systems containing clathrate hydrates: A review. Rev. Chem. Eng. 1988, 5, 1–70. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, H.; Guo, X.; Liu, A.; Yang, L. Solubility of CO2 in water and NaCl solution in equilibrium with hydrate. Part II: Model calculation. Can. J. Chem. Eng. 2018, 96, 620–624. [Google Scholar] [CrossRef]
- Chen, G.-J.; Guo, T.-M. Thermodynamic modeling of hydrate formation based on new concepts. Fluid Phase Equilibria 1996, 122, 43–65. [Google Scholar] [CrossRef]
- Chen, G.-J.; Guo, T.-M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Bergeron, S.; Macchi, A.; Servio, P. Theoretical temperature dependency of gas hydrate former solubility under hydrate-liquid water equilibrium. J. Chem. Thermodyn. 2007, 39, 737–741. [Google Scholar] [CrossRef]
- Bergeron, S.; Beltrán, J.G.; Macchi, A.; Servio, P. Theoretical pressure dependency of carbon dioxide solubility under hydrate-liquid water equilibrium. Can. J. Chem. Eng. 2010, 88, 307–311. [Google Scholar] [CrossRef]
- Sun, R.; Duan, Z. An accurate model to predict the thermodynamic stability of methane hydrate and methane solubility in marine environments. Chem. Geol. 2007, 244, 248–262. [Google Scholar] [CrossRef]
- Holder, G.D.; Corbin, G.; Papadopoulos, K.D. Thermodynamic and molecular properties of gas hydrates from mixtures containing methane, argon, and krypton. Ind. Eng. Chem. Fundam. 1980, 19, 282–286. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; Gomes de Azevedo, E. Molecular Thermodynamics of Fluid-Phase Equilibria; Prentice-Hall: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Holder, G.D.; Mokka, L.P.; Warzinski, R.P. Formation of gas hydrates from single-phase aqueous solutions. Chem. Eng. Sci. 2001, 56, 6897–6903. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R.; Zhu, C.; Chou, I.-M. An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar. Chem. 2006, 98, 131–139. [Google Scholar] [CrossRef]
- Duan, Z.; Møller, N.; Weare, J.H. An equation of state for the CH4-CO2-H2O system: I. Pure systems from 0 to 1000 °C and 0 to 8000 bar. Geochim. Cosmochim. Acta 1992, 56, 2605–2617. [Google Scholar] [CrossRef]
- Dhima, A.; De Hemptinne, J.-C.; Jose, J. Solubility of hydrocarbons and CO2 mixtures in water under high pressure. Ind. Eng. Chem. Res. 1999, 38, 3144–3161. [Google Scholar] [CrossRef]
- Spencer, R.J.; Moller, N.; Weare, J.H. The prediction of mineral solubilities in natural waters: A chemical equilibrium model for the Na-K-Ca-Mg-Cl-SO4-H2O system at temperatures below 25 °C. Geochim. Cosmochim. Acta 1990, 54, 575–590. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. A model to predict phase equilibrium of CH4 and CO2 clathrate hydrate in aqueous electrolyte solutions. Am. Miner. 2006, 91, 1346–1354. [Google Scholar] [CrossRef]
- Gil-Villegas, A.; Galindo, A.; Whitehead, P.J.; Mills, S.J.; Jackson, G.; Burgess, A.N. Statistical associating fluid theory for chain molecules with attractive potentials of variable range. J. Chem. Phys. 1997, 106, 4168–4186. [Google Scholar] [CrossRef]
- Adidharma, H.; Radosz, M. Prototype of an engineering equation of state for heterosegmented polymers. Ind. Eng. Chem. Res. 1998, 37, 4453–4462. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Kraska, T.; Gubbins, K.E. Phase equilibria calculations with a modified saft equation of state. 1. Pure Alkanes, Alkanols, and Water. Ind. Eng. Chem. Res. 1996, 35, 4727–4737. [Google Scholar] [CrossRef]
- Kolafa, J.; Nezbeda, I. The Lennard-Jones fluids. An accurate analytic and theoretically-based equation of state. Fluid Phase Equilibria 1994, 100, 1–34. [Google Scholar] [CrossRef]
- Twu, C.; Gubbins, K. Thermodynamics of polyatomic fluid mixtures—II. Chem. Eng. Sci. 1978, 33, 879–887. [Google Scholar] [CrossRef]
- Blum, L.; Hoeye, J.S. Mean spherical model for asymmetric electrolytes. 2. Thermodynamic properties and the pair correlation function. J. Phys. Chem. 1977, 81, 1311–1316. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: evaluation of literature data and thermodynamic modelling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
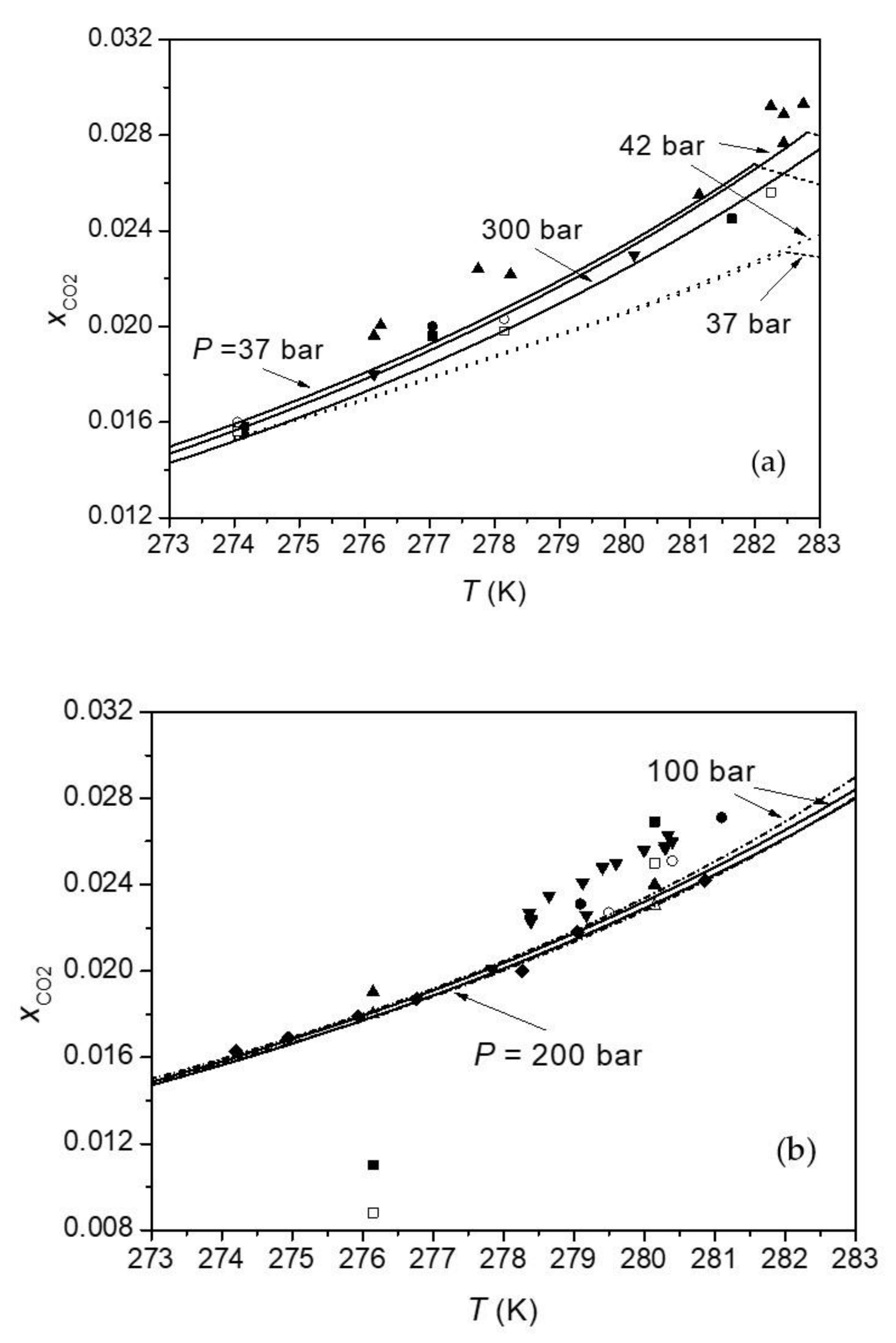

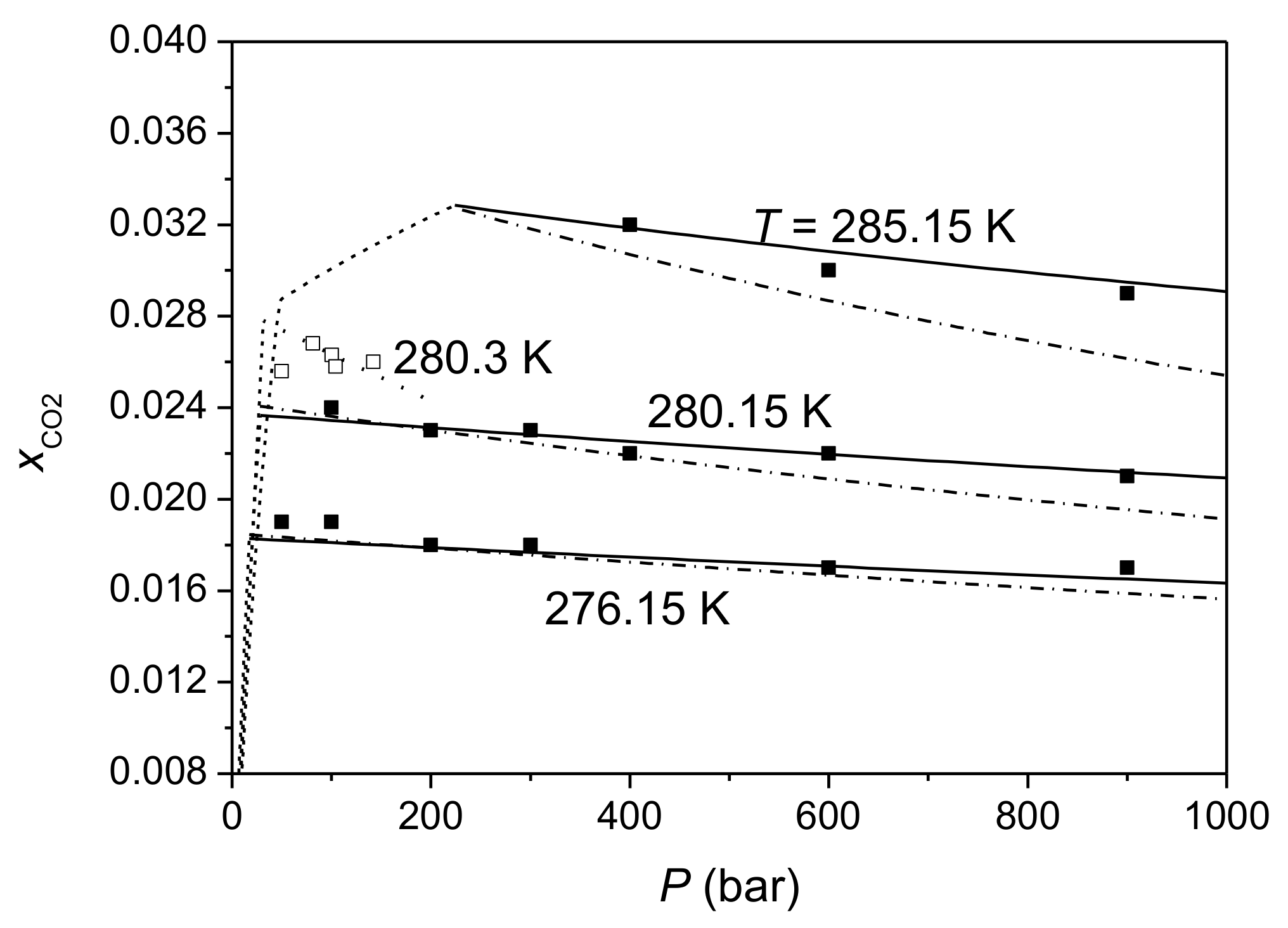

| Parameter | Value |
|---|---|
| k1,ij | 0.00518 + 0.001 × (T-273.15) − 5.695 × 10−7 × (T-273.15)2 |
| k2,ij | 0.9671 + 9.997 × 10−4 × (T-273.15) − 9.304 × 10−6 × (T-273.15)2 |
| Data Source | T (K) | P (bar) | N * | AAD a% | AAD b% |
|---|---|---|---|---|---|
| [76] | 276.2–282.8 | 300 | 9 | 9.4 | 9.8 |
| [79] | 274.0–283.0 | 20–60 | 15 | 3.5 | 4.3 |
| [78] | 273.2–280.2 | 50–200 | 11 | 65.1 | 67.4 |
| [77] | 277.8–281.0 | 50–142 | 32 | 7.7 | 6.9 |
| [80] | 274.0–279.3 | 66–506 | 9 | 6.1 | 4.8 |
| [81] | 275.8–282.3 | 40–120 | 52 | 7.7 | 7.1 |
| [82] | 279.1–281.5 | 101–201 | 10 | 4.2 | 3.9 |
| [83] | 274.1–281.1 | 19–236 | 30 | 1.6 | 1.5 |
| [85] | 276.2–285.2 | 50–900 | 15 | 1.8 | 3.6 |
| [84] | 274.0–282.9 | 20–50 | 7 | 2.5 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, M.; Sun, R.; Geng, L.; Lu, W. An Accurate Model to Calculate CO2 Solubility in Pure Water and in Seawater at Hydrate–Liquid Water Two-Phase Equilibrium. Minerals 2021, 11, 393. https://doi.org/10.3390/min11040393
Di M, Sun R, Geng L, Lu W. An Accurate Model to Calculate CO2 Solubility in Pure Water and in Seawater at Hydrate–Liquid Water Two-Phase Equilibrium. Minerals. 2021; 11(4):393. https://doi.org/10.3390/min11040393
Chicago/Turabian StyleDi, Mengyao, Rui Sun, Lantao Geng, and Wanjun Lu. 2021. "An Accurate Model to Calculate CO2 Solubility in Pure Water and in Seawater at Hydrate–Liquid Water Two-Phase Equilibrium" Minerals 11, no. 4: 393. https://doi.org/10.3390/min11040393
APA StyleDi, M., Sun, R., Geng, L., & Lu, W. (2021). An Accurate Model to Calculate CO2 Solubility in Pure Water and in Seawater at Hydrate–Liquid Water Two-Phase Equilibrium. Minerals, 11(4), 393. https://doi.org/10.3390/min11040393





