Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Crystals
2.2. Luminescence Study
3. Results
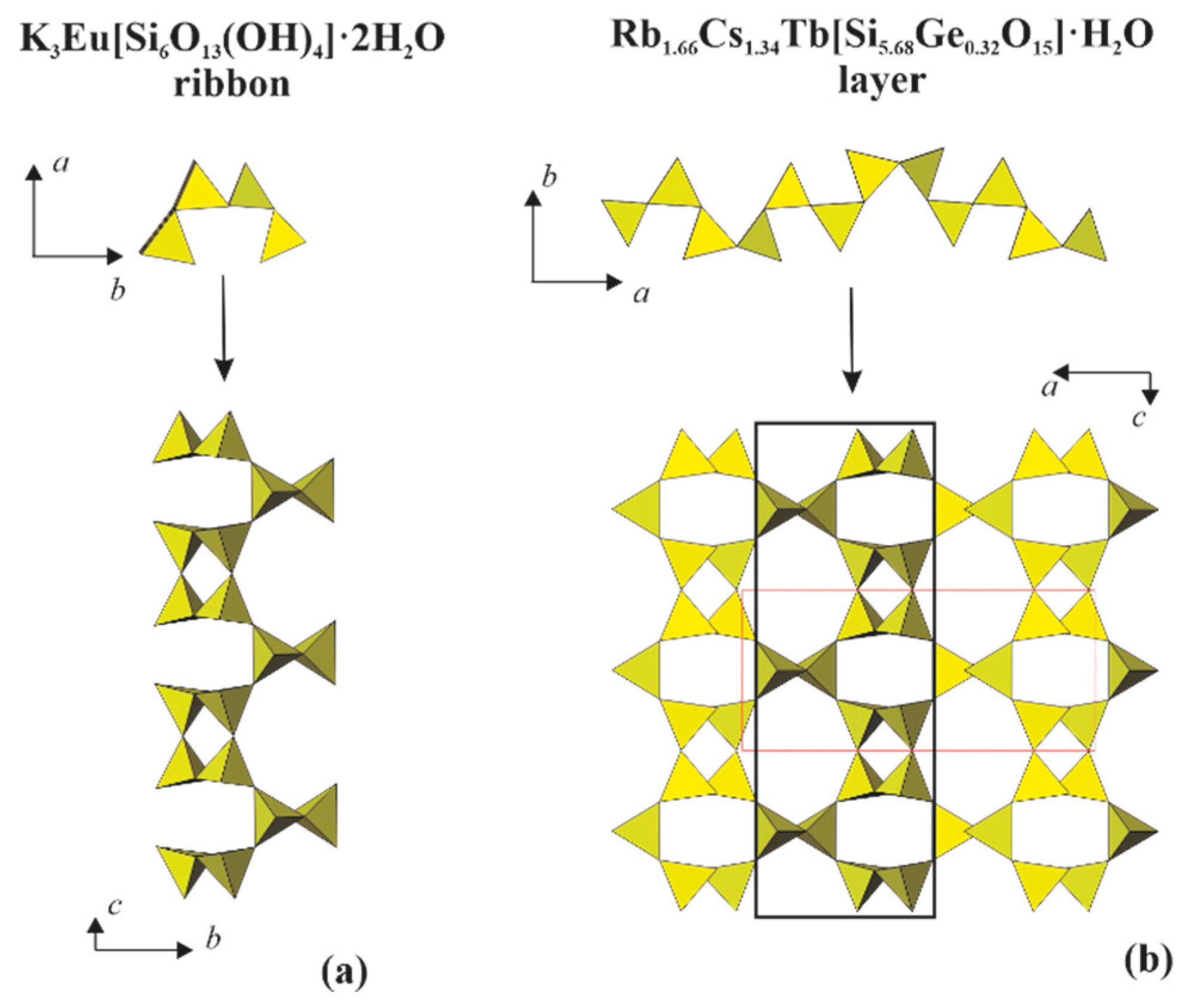
Structure Solving and Description
4. Discussion
4.1. Structural Comparison with the Related Layered Silicates
4.2. Topology-Symmetry Analysis and Structure Prediction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mineralogy Database. Available online: http://www.mindat.org/ (accessed on 20 October 2020).
- ICSD FIZ. Available online: http://www.fiz-karlsruhe.de (accessed on 20 October 2020).
- Crystallography Open Database. Available online: http://www.crystallography.net (accessed on 20 October 2020).
- Garra, W.; Marchetti, F.; Merlino, S. Tb/Na tobermorite: Thermal behaviour and high temperature products. J. Solid State Chem. 2009, 182, 1529–1532. [Google Scholar] [CrossRef]
- Wierzbicka-Wieczorek, M.; Göckeritz, M.; Kolitsch, U.; Lenz, C.; Giester, G. Crystallographic and Spectroscopic Investigations on Nine Metal-Rare-Earth Silicates with the Apatite Structure Type. Eur. J. Inorg. Chem. 2015, 2015, 948–963. [Google Scholar] [CrossRef]
- Latshaw, A.M.; Wilkins, B.O.; Hughey, K.D.; Yeon, J.; Williams, D.E.; Tran, T.T.; Halasyamani, P.S.; Loye, H.-C.Z. A 5 RE 4 X [TO 4] 4 crystal growth and photoluminescence. Fluoride flux synthesis of sodium and potassium rare earth silicate oxyfluorides. CrystEngComm 2015, 17, 4654–4661. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X. Rare earth disilicates R2Si2O7 (R = Gd, Tb, Dy, Ho): Type B. Z. Krist. Cryst. Mater. 2003, 218, 795–801. [Google Scholar] [CrossRef]
- Vidican, I.; Smith, M.D.; Zur Loye, H.-C. Crystal growth, structure determination and optical properties of new potassi-um-rare-earth silicates K3RESi2O7 (RE = Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). J. Solid St. Chem. 2003, 170, 203–210. [Google Scholar] [CrossRef]
- Sieke, C.; Hartenbach, I.; Schleid, T. Sulfidisch derivatisierte Oxodisilicate der schweren Lanthanide vom Formeltyp M4S3(Si2O7) (M = Gd − Tm). Z. Nat. B J. Chem. Sci. 2002, B57, 1427–1432. [Google Scholar]
- Ananias, D.; Kostova, M.; Paz, F.A.A.; Ferreira, A.; Carlos, L.D.; Klinowski, J.; Rocha, J. Photoluminescent Layered Lanthanide Silicates. J. Am. Chem. Soc. 2004, 126, 10410–10417. [Google Scholar] [CrossRef]
- Fulle, K.; Sanjeewa, L.D.; McMillen, C.D.; Kolis, J.W. Crystal chemistry and the role of ionic radius in rare earth tetrasilicates: Ba2RE2Si4O12F2(RE = Er3+–Lu3+) and Ba2RE2Si4O13(RE = La3+–Ho3+). Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 907–915. [Google Scholar] [CrossRef]
- Topnikova, A.P.; Belokoneva, E.L.; Dimitrova, O.V.; Volkov, A.S.; Nelyubina, Yu.V. Na3Tb3[Si6O18]∙H2O, a synthetic analogue of microporous mineral gerenite. Cryst. Rep. 2016, 61, 566–570. [Google Scholar] [CrossRef]
- Lee, C.-S.; Liao, Y.-C.; Hsu, J.-T.; Wang, S.-L.; Lii, K.-H. Rb2REGaSi4O12 (RE = Y, Eu, Gd, Tb): Luminescent Mixed-Anion Double Layer Silicates Containing Chains of Edge-Sharing REO7 Pentagonal Bipyramids. Inorg. Chem. 2008, 47, 1910–1912. [Google Scholar] [CrossRef]
- Bao, X.; Liu, X.; Liu, X. High-pressure synthesis, crystal structure and photoluminescence properties of a new terbium silicate: Na2Tb1.08Ca2.92Si6O18H0.8. RSC Adv. 2017, 7, 50195. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Yu, J.; Chen, P.; Pan, Q.; Song, H.; Xu, R. Na3TbSi3O9·3H2O: A New Luminescent Microporous Terbium(III) Silicate Containing HelicalSechserSilicate Chains and 9-Ring Channels. Chem. Mater. 2006, 18, 5637–5639. [Google Scholar] [CrossRef]
- Morrison, G.; Latshaw, A.M.; Spagnuolo, N.R.; Loye, H.-C.Z. Observation of Intense X-ray Scintillation in a Family of Mixed Anion Silicates, Cs3RESi4O10F2(RE = Y, Eu–Lu), Obtained via an Enhanced Flux Crystal Growth Technique. J. Am. Chem. Soc. 2017, 139, 14743–14748. [Google Scholar] [CrossRef]
- Taroev, V.K.; Kashaev, A.A.; Malcherek, T.; Goettlicher, J.; Kaneva, E.V.; Vasiljev, A.D.; Suvorova, L.F.; Suvorova, D.S.; Tauson, V.L. Crystal structures of new potassium silicates and aluminosilicates of Sm, Tb, Gd, and Yb and their relation to the armstrongite (CaZr(Si6O15)∙3H2O) structure. J. Solid State Chem. 2015, 227, 196–203. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Chen, P.; Li, Y.; Chu, Q.; Liu, X.; Yu, J.; Xu, R. New Lanthanide Silicates Based on Anionic Silicate Chain, Layer, and Framework Prepared under High-Temperature and High-Pressure Conditions. Inorg. Chem. 2010, 49, 9833–9838. [Google Scholar] [CrossRef] [PubMed]
- Ananias, D.; Ferreira, A.; Rocha, J.; Ferreira, P.; Rainho, J.P.; Morais, C.; Carlos, L.D. Novel Microporous Europium and Ter-bium Silicates. J. Am. Chem. Soc. 2001, 123, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Liebau, F. Structural Chemistry of Silicates: Structure, Bonding, and Classification; Springer: Berlin/Heidelberg, Germany, 1985; 347p. [Google Scholar]
- Pushcharovsky, D.Y. Structural Mineralogy of Silicates and Their Synthetic Analogues; Nedra: Moscow, Russia, 1986; 160p. [Google Scholar]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials; Oxford University Press (OUP): Oxford, UK, 2008. [Google Scholar]
- Dornberger-Schiff, K. Grundzuege Einer Theorie der OD-Strukturen aus Schichten; Deutsche Akademie der Wissenschaften, Berlin, Abhandlungen, Klasse fur Chemie, Geologie and Biologie: Halle, Germany, 1964; Volume 3, pp. 1–106. [Google Scholar]
- Merlino, S. OD Structures in Mineralogy. Per. Mineral. 1990, 59, 69–92. [Google Scholar]
- Belokoneva, E.L. Borate crystal chemistry in terms of the exnetded OD theory: Topology and symmetry analysis. Cryst. Rev. 2005, 11, 151–198. [Google Scholar] [CrossRef]
- Ivanova, A.G.; Belokoneva, E.L.; Dimitrova, O.V. New condensed acid diborate GdH[B2O5] with chain radical [B2□B2ΔO10]8-]∞: Synthesis and crystal structure; diborates and their structural system in terms of OD theory. Russ. J. Inorg. Chem. 2004, 49, 816–822. [Google Scholar]
- Belokoneva, E.L.; Reutova, O.V.; Dimitrova, O.V.; Volkov, A.S. Germanosilicate Cs2In2[(Si2.1Ge0.9)2O15](OH)2∙H2O with a New Corrugated Tetrahedral Layer: Topological Symmetry-Based Prediction of Anionic Radicals. Crystallogr. Rep. 2020, 65, 566–572. [Google Scholar] [CrossRef]
- Dorenbos, P. Exchange and crystal field effects on the 4fn 15d levels of Tb3. J. Phys. Condens. Matter 2003, 15, 6249–6268. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Zur, L.; Sołtys, M.; Pisarska, J. Terbium-terbium interactions in lead phosphate glasses. J. Appl. Phys. 2013, 113, 143504. [Google Scholar] [CrossRef]
- Berdowski, P.A.M.; Lammers, M.J.J.; Blasse, G. 5D3-5D4 cross-relaxation of Tb3+ in α-GdOF. Chem. Phys. Lett. 1985, 113, 387–390. [Google Scholar]
- Van Uitert, L.G.; Johnson, L.F. Energy Transfer between Rare—Earth Ions. J. Chem. Phys. 1966, 44, 3514. [Google Scholar] [CrossRef]
- Tonooka, K.; Nishimura, O. Spectral changes of Tb3+ fluorescence in borosilicate glasses. J. Lumin. 2000, 87, 679–681. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Morozov, V.A.; Vasin, A.A.; Aksenov, S.M.; Dikhtyar, Y.Y.; Stefanovich, S.Y.; Lazoryak, B.I. The crystal site engineering and turning of cross-relaxation in green-emitting β-Ca3(PO4)2-related phosphors. J. Lumin. 2020, 223, 117196. [Google Scholar] [CrossRef]
- CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, Oxfordshire, UK, 2014.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond; Cornell University: Ithaca, NY, USA, 1960; 644p. [Google Scholar]
- Sheldrik, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Dowty, E. ATOMS; Shape Software: Kingsport, TN, USA, 2006. [Google Scholar]
- Cadoni, M.; Ferraris, J. Polytypic and polymorphic relations between sazhinite and isochemical alkali-REE layer silicates. Eur. J. Mineral. 2011, 23, 85–90. [Google Scholar] [CrossRef]
- Shumyatskaya, N.G.; Voronkov, A.A.; Pyatenko, Yu.A. Sazhinite Na2Ce[Si6O14(OH)]∙nH2O, a new member of crystal chemical family of dalyite. Sov. Phys. Cryst. 1980, 25, 728–734. [Google Scholar]
- Cadoni, M.; Cheah, Y.L.; Ferraris, G. New RE microporous heteropolyhedral silicates containing 41516182 tetrahedral sheets. Acta Crystallogr. Sect. B Struct. Sci. 2010, 66, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-K.; Chandrasekaran, A.; Tsapatsis, M. Synthesis of a new open framework cerium silicate and its structure determination by single crystal X-ray diffraction. Chem. Commun. 2002, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Wuensch, B.J. Structure, phase transitions and ionic conductivity of K3NdSi6O15∙xH2O. II. Structure of β-K3NdSi6O15. Acta Cryst. 2000, 56, 349–362. [Google Scholar] [CrossRef]
- Pushcharovsky, D.Y.; Karpov, O.G.; Pobedimskaya, E.A.; Belov, N.V. Crystal structure of K3NdSi6O15. Dokl. AN SSSR 1977, 234, 1323–1326. [Google Scholar]
- Rastsvetaeva, R.K.; Aksenov, S.M.; Taroev, V.K. Crystal Structures of Endotaxic Phases in Europium Potassium Silicate Having a Pellyite Unit Cell. Crystallogr. Rep. 2010, 55, 1041–1049. [Google Scholar] [CrossRef]
- Cámara, F.; Ottolini, L.; Devouard, B.; Garvie, L.A.J.; Hawthorne, F.C. Sazhinite-(La), Na3LaSi6O15(H2O)2, a new mineral from the Aris phonolite, Namibia: Description and crystal structure. Miner. Mag. 2006, 70, 405–418. [Google Scholar] [CrossRef]
- Karpov, O.G.; Pushcharovsky, D.Y.; Pobedimskaya, E.A.; Burshtein, I.F.; Belov, N.V. Crystal structure of rare earth silicate NaNdSi6O13(OH)2∙nH2O. Dokl. AN SSSR 1977, 236, 593–596. [Google Scholar]
- Haile, S.M.; Wuensch, B.J.; Laudise, R.A.; Maier, J. Structure of Na3NdSi6O15∙2H2O—A Layered Silicate with Paths for Possible Fast-Ion Conduction. Acta Cryst. 1997, 53, 7–17. [Google Scholar] [CrossRef]






| Tb13+ C.N. = 6 0.25* | Tb23+ C.N. = 6 0.25* | Cs1+ C.N. = 11 0.5* | Rb1+ C.N. = 9 0.5* | (Rb,Cs2)+ C.N. = 7 0.5* | T14+ C.N. = 4 0.5* | T24+ C.N. = 4 1.0* | T34+ C.N. = 4 1.0* | T44+ C.N. = 4 0.5* | Σexp | Σtheor | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O12− 0.5 * | 0.045 | 0.056 | 1.0 | −1.1 | −1.0 | ||||||
| O22− 0.5 * | 0.071 | 0.5 | 0.5 | −1.071 | −1.0 | ||||||
| O32− 1.0 * | 0.125 × 4 | 0.045 × 4 | 0.071 × 2 | 1.0 | −1.825 | −2.0 | |||||
| O42− 1.0 * | 0.125 × 4 | 0.056 × 4 | 1.0 | −1.722 | −2.0 | ||||||
| O52− 1.0 * | 0.056 × 2 | 0.5 × 2 | 1.0 | −2.111 | −2.0 | ||||||
| O62− 1.0 * | 0.071 × 2 | 1.0 | 1.0 | −2.143 | −2.0 | ||||||
| O72− 0.5 * | 0.125 × 2 | 0.045 × 2 | 0.071 | 0.5 | −0.912 | −1.0 | |||||
| O82− 1.0 * | 0.045 × 2 | 1.0 | 0.5 × 2 | −2.091 | −2.0 | ||||||
| O92− 0.5 * | 0.125 × 2 | 0.071 | 0.5 | −0.821 | −1.0 | ||||||
| O102− 0.5 * | 0.045 | 0.056 | 1.0 | −1.1 | −1.0 | ||||||
| O11w2− 0.5 * | 0.045 | 0.056 | −0.1 | 0 | |||||||
| Σ | +0.75 | +0.75 | +0.5 | +0.5 | +0.5 | +2 | +4 | +4 | +2 | 15 | 15 |
| Formula | Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O |
|---|---|
| formula weight (g/mol) | 930.48 |
| T (K) | 293(2) |
| crystal system | Orthorhombic |
| space group, Z | Pbam, 4 |
| a (Å) | 15.9429(3) |
| b (Å) | 14.8407(3) |
| c (Å) | 7.2781(1) |
| V (Å 3) | 1722.03(6) |
| crystal size (mm) | 0.10 × 0.05 × 0.04 |
| ρcalc (g/cm 3) | 3.532 |
| μ (mm−1) | 12.610 |
| F(000) | 1677 |
| wavelength (Å) | 0.71073 |
| θ range/deg. | 2.75–30.78 |
| limiting indices | −22 ≤ h ≤ 22, −21≤ k ≤ 20, −10 ≤ l ≤ 10 |
| refl. collected/unique | 28316/2756 [Rint = 0.0695] |
| completeness to theta | 99.9 |
| data/restraints/parameters | 2756/0/143 |
| GOF | 1.187 |
| R1, wR21 [I > 2σ(I)] | 0.0541, 0.0885 |
| R1, wR2 (all data) 1 | 0.0670, 0.0926 |
| Δρmax and Δρmin (e Å−3) | 1.726 and −2.318 |
| Atoms | Wyckoff Position, Point Symm. | S.o.f. | X | Y | Z | Ueq |
|---|---|---|---|---|---|---|
| Cs1 | 4g, m | 1.0 | 0.1306(1) | 0.5459(1) | 0 | 0.0255(2) |
| Rb1 | 4h, m | 1.0 | 0.2830(1) | 0.8920(1) | 0.5 | 0.0408(4) |
| (Rb, Cs)2 | 4g, m | 0.66, 0.34 | 0.4222(1) | 0.4033(1) | 0 | 0.0250(2) |
| Tb1 | 2b, 2/m | 1.0 | 0.5 | 0.5 | 0.5 | 0.00832(14) |
| Tb2 | 2d, 2/m | 1.0 | 0 | 0.5 | 0.5 | 0.00750(14) |
| (Si, Ge)1 | 4h, m | 0.93, 0.07 | 0.0874(1) | 0.7829(2) | 0.5 | 0.0083(7) |
| (Si, Ge)2 | 8i, 1 | 0.88, 0.12 | 0.3535(1) | 0.6345(1) | 0.7838(2) | 0.0077(5) |
| (Si, Ge)3 | 8i, 1 | 0.91, 0.09 | 0.0395(1) | 0.3024(1) | 0.2181(2) | 0.0087(5) |
| (Si, Ge)4 | 4h, m | 0.92, 0.08 | 0.2068(1) | 0.6291(2) | 0.5 | 0.0060(7) |
| O(1) | 4g, m | 1.0 | 0.0165(5) | 0.2918(5) | 0 | 0.0192(16) |
| O(2) | 4h, m | 1.0 | 0.1804(4) | 0.7355(4) | 0.5 | 0.0139(15) |
| O(3) | 8i,1 | 1.0 | 0.0526(3) | 0.4042(3) | 0.2791(7) | 0.0150(10) |
| O(4) | 8i,1 | 1.0 | 0.4291(3) | 0.5701(4) | 0.7326(8) | 0.0176(11) |
| O(5) | 8i, 1 | 1.0 | 0.0392(3) | 0.7465(4) | 0.3168(7) | 0.0184(11) |
| O(6) | 8i, 1 | 1.0 | 0.1258(3) | 0.2425(3) | 0.2554(7) | 0.0141(10) |
| O(7) | 4h, m | 1.0 | 0.1292(4) | 0.5630(5) | 0.5 | 0.0134(14) |
| O(8) | 8i, 1 | 1.0 | 0.2653(3) | 0.6115(4) | 0.6809(7) | 0.0189(11) |
| O(9) | 4h, m | 1.0 | 0.0990(4) | 0.8882(5) | 0.5 | 0.0142(15) |
| O(10) | 4g, m | 1.0 | 0.3262(5) | 0.6260(6) | 0 | 0.0187(16) |
| O(11)w | 4g, m | 1.0 | 0.2446(9) | 0.3400(10) | 0 | 0.079(4) |
| Atoms | Bonds (Å) | Atoms | Bonds (Å) |
|---|---|---|---|
| Tb1O6 Octahedron | Tb2O6 Octahedron | ||
| Tb1-O4 x4 | 2.286(5) | Tb2-O7 ×2 | 2.262(7) |
| Tb1-O9 ×2 | 2.290(7) | Tb2-O3 ×4 | 2.304(5) |
| Average | 2.287 | Average | 2.290 |
| (Si,Ge)1O4 Tetrahedron | (Si,Ge)2O4 Tetrahedron | ||
| (Si,Ge)1-O9 | 1.573(7) | (Si,Ge)2-O4 | 1.582(5) |
| (Si,Ge)1-O5 ×2 | 1.632(5) | (Si,Ge)2-O8 | 1.629(5) |
| (Si,Ge)1-O2 | 1.640(7) | (Si,Ge)2-O10 | 1.637(3) |
| Average | 1.619 | (Si,Ge)2-O6 | 1.662(5) |
| (Si,Ge)3O4 Tetrahedron | Average | 1.628 | |
| (Si,Ge)3-O3 | 1.588(5) | (Si,Ge)4O4 Tetrahedron | |
| (Si,Ge)3-O5 | 1.617(5) | (Si,Ge)4-O7 | 1.579(7) |
| (Si,Ge)3-O1 | 1.637(3) | (Si,Ge)4-O8 x2 | 1.634(5) |
| (Si,Ge)3-O6 | 1.660(5) | (Si,Ge)4-O2 | 1.635(7) |
| Average | 1.626 | Average | 1.621 |
| Chemical Formula | Space Group | Unit CellParameters, Å | Reference |
| Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]· H2O | Pbam | a = 15.943 b = 14.841 c = 7.278 | [this work] |
| K3Nd[Si6O15]·2H2O | Pbam | a = 16.008 b = 15.004 c = 7.279 | [44] |
| K3Nd[Si6O15] | Pbam | a = 16.011 b = 14.984 c = 7.276 | [45] |
| K3Eu[Si6O15]·2H2O | P21212 | a = 14.852 b = 15.902 c = 7.243 | [46] |
| Na2Ce[Si6O14(OH)2]·nH2O Ce-sazhinite | Pmm2 | a = 7.500 b = 15.620 c = 7.350 | [41] |
| Na3La[Si6O15]·2H2O La-sazhinite | Pmm2 | a = 7.415 b = 15.515 c = 7.164 | [47] |
| β-K3Nd[Si6O15] | Bb21m | a = 14.370 b = 15.518 c = 14.265 | [44] |
| Na2.4Ce[Si6O15]·2H2O | Pman | a = 7.309 b = 14.971 c = 7.135 | [43] |
| NaNd[Si6O13(OH)2]·H2O | Cmm2 | a = 30.870 b = 7.387 c = 7.120 | [48] |
| NaNd[Si6O15]·2H2O | Cmm2 | a = 7.385 b = 30.831 c = 7.117 | [49] |
| Na2.74K0.26Ce[Si6O15]·2H2O | Cmm2 | a = 7.413 b = 30.965 c = 7.167 | [42] |
| Na3La[Si6O15]·2.25H2O | Cmm2 | a = 7.415 b = 31.008 c = 7.153 | [42] |
| Na2.72K0.25LaSi6O15·2.25H2O | Cmm2 | a
= 7.422 b = 31.039 c = 7.196 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topnikova, A.; Belokoneva, E.; Dimitrova, O.; Volkov, A.; Deyneko, D. Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction. Minerals 2021, 11, 395. https://doi.org/10.3390/min11040395
Topnikova A, Belokoneva E, Dimitrova O, Volkov A, Deyneko D. Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction. Minerals. 2021; 11(4):395. https://doi.org/10.3390/min11040395
Chicago/Turabian StyleTopnikova, Anastasiia, Elena Belokoneva, Olga Dimitrova, Anatoly Volkov, and Dina Deyneko. 2021. "Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction" Minerals 11, no. 4: 395. https://doi.org/10.3390/min11040395
APA StyleTopnikova, A., Belokoneva, E., Dimitrova, O., Volkov, A., & Deyneko, D. (2021). Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction. Minerals, 11(4), 395. https://doi.org/10.3390/min11040395







