Activation of Peroxymonosulfate by Chrysotile to Degrade Dyes in Water: Performance Enhancement and Activation Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemical Reagents
2.2. Calcination Experiment
2.3. Characterizations
2.4. Catalytic Degradation Experiments
3. Results and Discussion
3.1. Performance Evaluation of PMS Activation by Raw Chrysotile
3.2. Performance Evaluation of PMS Activation by Calcined Chrysotile
3.3. Comparative Characterizations of 850CC and Raw Chrysotile
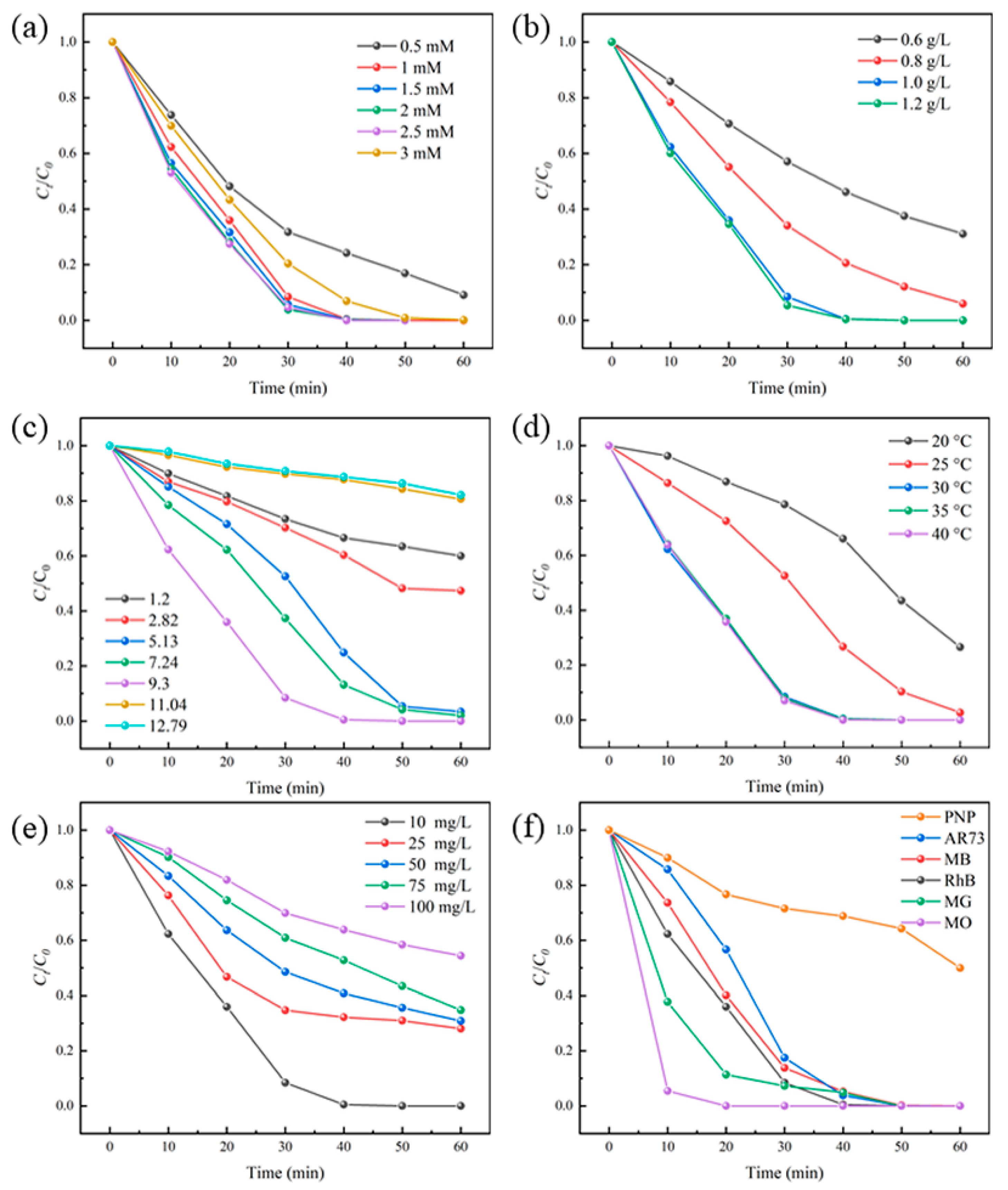
3.4. Effects of Various Parameters on the Degradation of RhB
3.5. Determination of Active Species
3.6. Catalysis Mechanism
3.7. Effects of Inorganic Ions and NOM on the Degradation of RhB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dionysiou, D.D. Degradation of Organic Contaminants in Water with Sulfate Radicals Generated by the Conjunction of Peroxymonosulfate with Cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Kim, M.; Lim, H.; Zhang, M.; You, J.; Yun, J.H.; Bando, Y.; Li, J.; Yamauchi, Y. Nanoarchitectured metal-organic framework-derived hollow carbon nanofiber filters for advanced oxidation processes. J. Mater. Chem. A 2019, 7, 13743–13750. [Google Scholar] [CrossRef]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Huang, G.; Luo, X.; He, D.; Peng, Q.; Huang, J.; Ao, M.; Liu, K. Bismuth MOFs based hierarchical Co3O4-Bi2O3 composite: An efficient heterogeneous peroxymonosulfate activator for azo dyes degradation. Sep. Purif. Technol. 2020, 242, 116825. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.T.; Teng, Y.; Zhao, J.; Kuang, L.; Sun, X. Carbon nanofibers supported Co/Ag bimetallic nanoparticles for heterogeneous activation of peroxymonosulfate and efficient oxidation of amoxicillin. J. Hazard. Mater. 2020, 400, 123290. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, Y.; Li, Y.; Yao, G.; Lai, B. Applied Catalysis B: Environmental Surface Fe (III)/Fe (II) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine. Appl. Catal. B Environ. 2019, 256, 117782. [Google Scholar] [CrossRef]
- Tang, X.; Huang, J.; Liu, K.; Feng, Q.; Li, Z.; Ao, M. Synthesis of magnetically separable MnO2/Fe3O4/silica nanofiber composite with enhanced Fenton-like catalytic activity for degradation of Acid Red 73. Surf. Coat. Technol. 2018, 354, 18–27. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: Structure dependence and mechanism. Appl. Catal. B Environ. 2015, 164, 159–167. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, H.; Li, Y.; Zhang, H. The mechanism and efficiency of MnO2 activated persulfate process coupled with electrolysis. Sci. Total Environ. 2017, 609, 644–654. [Google Scholar] [CrossRef]
- Peng, Q.; Dai, Y.; Liu, K.; Luo, X.; He, D.; Tang, X.; Huang, G. A novel carbon nanotube–magnesium oxide composite with excellent recyclability to efficiently activate peroxymonosulfate for Rhodamine B degradation. J. Mater. Sci. 2020, 55, 11267–11283. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Pang, S.; Li, J.; Lu, X.; Yuan, L. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhou, P.; Nie, G.; Cheng, C.; Duan, X.; Zhang, H.; Wang, S. Hydroxyl radical dominated elimination of plasticizers by peroxymonosulfate on metal-free boron: Kinetics and mechanisms. Water Res. 2020, 186, 116361. [Google Scholar] [CrossRef] [PubMed]
- Solís, R.R.; Mena, I.F.; Nadagouda, M.N.; Dionysiou, D.D. Adsorptive interaction of peroxymonosulfate with graphene and catalytic assessment via non-radical pathway for the removal of aqueous pharmaceuticals. J. Hazard. Mater. 2020, 384, 121340. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Cao, S.; Jin, W.; Zhou, X.; Ding, W.; Tu, R.; Han, S.F.; Wang, C.; Jiang, Q.; Huang, H.; et al. Inactivation of chlorine-resistant bacterial spores in drinking water using UV irradiation, UV/Hydrogen peroxide and UV/Peroxymonosulfate: Efficiency and mechanism. J. Clean. Prod. 2020, 243, 118666. [Google Scholar] [CrossRef]
- Norzaee, S.; Taghavi, M.; Djahed, B.; Kord Mostafapour, F. Degradation of Penicillin G by heat activated persulfate in aqueous solution. J. Environ. Manag. 2018, 215, 316–323. [Google Scholar] [CrossRef]
- Huang, G.; Li, Z.; Liu, K.; Tang, X.; Huang, J.; Zhang, G. Bismuth MOF-derived BiOBr/Bi24O31Br10heterojunctions with enhanced visible-light photocatalytic performance. Catal. Sci. Technol. 2020, 10, 4645–4654. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Tierno, M. Monopersulfate photocatalysis under 365 nm radiation. Direct oxidation and monopersulfate promoted photocatalysis of the herbicide tembotrione. J. Environ. Manag. 2016, 181, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Solís, R.R.; Rivas, F.J.; Chávez, A.M.; Dionysiou, D.D. Peroxymonosulfate/solar radiation process for the removal of aqueous microcontaminants. Kinetic modeling, influence of variables and matrix constituents. J. Hazard. Mater. 2020, 400, 123118. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Nie, W.; Mao, Q.; Ding, Y.; Hu, Y.; Tang, H. Highly efficient catalysis of chalcopyrite with surface bonded ferrous species for activation of peroxymonosulfate toward degradation of bisphenol A: A mechanism study. J. Hazard. Mater. 2019, 364, 59–68. [Google Scholar] [CrossRef]
- Diao, Z.H.; Lin, Z.Y.; Chen, X.Z.; Yan, L.; Dong, F.X.; Qian, W.; Kong, L.J.; Du, J.J.; Chu, W. Ultrasound-assisted heterogeneous activation of peroxymonosulphate by natural pyrite for 2,4-diclorophenol degradation in water: Synergistic effects, pathway and mechanism. Chem. Eng. J. 2020, 389, 123771. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.H. Α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Yu, M.; Teel, A.L.; Watts, R.J. Activation of Peroxymonosulfate by Subsurface Minerals. J. Contam. Hydrol. 2016, 191, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.; Dionysiou, D.D.; Liu, C.; Tong, Y.; Gao, J.; Fang, G.; Zhou, D. Efficient activation of peroxymonosulfate by copper sulfide for diethyl phthalate degradation: Performance, radical generation and mechanism. Sci. Total Environ. 2020, 749, 142387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.M.; Sun, H.J.; Peng, T.J.; Zeng, L. Thermal process and mechanism of phase transition and detoxification of glass-ceramics from asbestos tailings. J. Non Cryst. Solids 2019, 517, 26–31. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lu, A.; Wang, C.; Zheng, X.; Zhao, D.; Liu, R. Nano-fibriform production of silica from natural chrysotile. J. Colloid Interface Sci. 2006, 295, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Bing, W.; Wei, W. Degradation Phenol Wastewater by Heating Activated Persulfate. Int. J. Environ. Monit. Anal. 2019, 7, 14. [Google Scholar] [CrossRef]
- Wypych, F.; Schreiner, W.H.; Richard, E. Grafting of phenylarsonic and 2-nitrophenol-4-arsonic acid onto disordered silica obtained by selective leaching of brucite-like sheet from chrysotile structure. J. Colloid Interface Sci. 2004, 276, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Adamek, J. Thermal decomposition of different types of asbestos. J. Therm. Anal. Calorim. 2012, 109, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Valouma, A.; Verganelaki, A.; Maravelaki-Kalaitzaki, P.; Gidarakos, E. Chrysotile asbestos detoxification with a combined treatment of oxalic acid and silicates producing amorphous silica and biomaterial. J. Hazard. Mater. 2016, 305, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Venkatraman, S.K.; Bulygina, I.; Chatterjee, A.; Abraham, J.; Senatov, F.; Kaloshkin, S.; Ilyasov, A.; Abakumov, M.; Knyazeva, M.; et al. Impact of forsterite addition on mechanical and biological properties of composites. J. Asian Ceram. Soc. 2020, 8, 1051–1065. [Google Scholar] [CrossRef]
- Girotto, C.P.; de Campos, S.D.; de Campos, É.A. Chrysotile asbestos treated with phosphoric acid as an adsorbent for ammonia nitrogen. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, S.; Zhao, F.; Safaei, Z.; Lakshamy, D. Sulfate radical-mediated degradation and mineralization of bisphenol F in neutral medium by the novel magnetic Sr2CoFeO6 double perovskite oxide catalyzed peroxymonosulfate: Influence of co-existing chemicals and UV irradiation. Appl. Catal. B Environ. 2018, 233, 99–111. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, H.; Hou, L. Degradation of c. i. acid orange 7 in aqueous solution by a novel electro/fe3o4/pds process. J. Hazard. Mater. 2014, 276, 182–191. [Google Scholar] [CrossRef]
- Guan, Y.; Ma, J.; Li, X.; Fang, J.; Chen, L. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV / Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Y.; Fang, C.; Xue, Y.; Ai, L.; Liu, J.; Wang, Z. Peroxymonosulfate/base process in saline wastewater treatment: The fight between alkalinity and chloride ions. Chemosphere 2018, 199, 84–88. [Google Scholar] [CrossRef]
- Nookaraju, M.; Rajini, A.; Venkatathri, N.; Reddy, I.A.K. Catalytic degradation of rhodamine B over FeMCM-41. Asian J. Chem. 2012, 24, 5817–5820. [Google Scholar]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Xu, H.; Wang, D.; Ma, J.; Zhang, T.; Lu, X.; Chen, Z. Applied Catalysis B: Environmental A superior active and stable spinel sul fi de for catalytic peroxymonosulfate oxidation of bisphenol S. Appl. Catal. B Environ. 2018, 238, 557–567. [Google Scholar] [CrossRef]
- Rao, P.S.; Hayon, E. Redox potentials of free radicals. IV. Superoxide and hydroperoxy radicals ·O2- and · HO2. J. Phys. Chem. 1975, 79, 397–402. [Google Scholar] [CrossRef]
- Schuchmann, M.N.; Bothe, E.; Sonntag, J.V.; Sonntag, C.V. Reaction of oh radicals with benzoquinone in aqueous solutions. a pulse radiolysis study. J. Chem. Soc. 1988, 4, 791–796. [Google Scholar] [CrossRef]
- Yun, E.; Lee, J.H.; Kim, J.; Park, H.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, H.; Yang, C.; Li, X.; Lin, Y.; Yin, K.; Sun, J.; Teng, Q.; Du, C.; Zhong, Y. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem. Eng. J. 2020, 392, 123683. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.H.; Pignatello, J.J. Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef] [PubMed]
- Fontmorin, J.M.; Burgos Castillo, R.C.; Tang, W.Z.; Sillanpää, M. Stability of 5,5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Désert, A.; Khrouz, L.; Páez, C.A.; Parola, S.; Heinrichs, B. Heterogeneous singlet oxygen generation: In-operando visible light EPR spectroscopy. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Lyu, L.; Hu, C. Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl. Catal. B Environ. 2020, 270, 118874. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.; Liu, K.; Luo, X.; He, D.; Dai, Y.; Huang, G. High-efficiency catalysis of peroxymonosulfate by mgo for the degradation of organic pollutants. Minerals 2020, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, Z.; Chen, J.; Cheng, L.; Chang, J.; Sheng, W.; Hu, C.; Cao, S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Guo, L.; Chen, F.; Fan, X.; Cai, W.; Zhang, J. S-doped α-Fe2O3 as a highly active heterogeneous Fenton-like catalyst towards the degradation of acid orange 7 and phenol. Appl. Catal. B Environ. 2010, 96, 162–168. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bouibes, A.; Zaoui, A. High-pressure phase transitions of forsterite from first-principles. J. Phys. Chem. Solids 2020, 136, 109161. [Google Scholar] [CrossRef]
- Valencia, E.M.; Worth, C.J.; Fortenberry, R.C. Enstatite (MgSiO3) and forsterite (Mg2SiO4) monomers and dimers: Highly detectable infrared and radioastronomical molecular building blocks. Mon. Not. R. Astron. Soc. 2020, 492, 276–282. [Google Scholar] [CrossRef]
- Wu, C.Y.; Tu, K.J.; Deng, J.P.; Lo, Y.S.; Wu, C.H. Markedly enhanced surface hydroxyl groups of TiO2 nanoparticles with Superior water-dispersibility for photocatalysis. Materials 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Schott, J. Forsterite surface composition in aqueous solutions: A combined potentiometric, electrokinetic, and spectroscopic approach. Geochim. Cosmochim. Acta 2000, 64, 3299–3312. [Google Scholar] [CrossRef]
- Muir, J.M.R.; Jollands, M.; Zhang, F.; Walker, A.M. Explaining the dependence of M-site diffusion in forsterite on silica activity: A density functional theory approach. Phys. Chem. Miner. 2020, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Moon, W.; Roh, Y. Characterization of Mineralogical Changes of Chrysotile and its Thermal Decomposition by Heat Treatment. Econ. Environ. Geol. 2016, 49, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ao, Z.; Sun, H.; Duan, X.; Wang, S. Activation of peroxymonosulfate by carbonaceous oxygen groups: Experimental and density functional theory calculations. Appl. Catal. B Environ. 2016, 198, 295–302. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. B Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhang, H.; Saunders, M.; Sun, H.; Shao, Z.; Wang, S. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: Core-shell layer dependence. Appl. Catal. B Environ. 2018, 222, 176–181. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef]
- Rivas, F.J.; Solís, R.R. Chloride promoted oxidation of tritosulfuron by peroxymonosulfate. Chem. Eng. J. 2018, 349, 728–736. [Google Scholar] [CrossRef]
- Luo, R.; Liu, C.; Li, J.; Wang, J.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Nanostructured CoP: An efficient catalyst for degradation of organic pollutants by activating peroxymonosulfate. J. Hazard. Mater. 2017, 329, 92–101. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 73307339. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Peng, Q.; Liu, K.; Tang, X.; Zhou, M.; Jiang, K.; Zhu, B. Activation of Peroxymonosulfate by Chrysotile to Degrade Dyes in Water: Performance Enhancement and Activation Mechanism. Minerals 2021, 11, 400. https://doi.org/10.3390/min11040400
Dai Y, Peng Q, Liu K, Tang X, Zhou M, Jiang K, Zhu B. Activation of Peroxymonosulfate by Chrysotile to Degrade Dyes in Water: Performance Enhancement and Activation Mechanism. Minerals. 2021; 11(4):400. https://doi.org/10.3390/min11040400
Chicago/Turabian StyleDai, Ying, Qian Peng, Kun Liu, Xuekun Tang, Muyang Zhou, Kun Jiang, and Binnan Zhu. 2021. "Activation of Peroxymonosulfate by Chrysotile to Degrade Dyes in Water: Performance Enhancement and Activation Mechanism" Minerals 11, no. 4: 400. https://doi.org/10.3390/min11040400
APA StyleDai, Y., Peng, Q., Liu, K., Tang, X., Zhou, M., Jiang, K., & Zhu, B. (2021). Activation of Peroxymonosulfate by Chrysotile to Degrade Dyes in Water: Performance Enhancement and Activation Mechanism. Minerals, 11(4), 400. https://doi.org/10.3390/min11040400






