Carbon-Assisted Bioleaching of Chalcopyrite and Three Chalcopyrite/Enargite-Bearing Complex Concentrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals
2.2. Microorganisms
2.3. Carbon-Assisted Bioleaching Tests of the Model Chalcopyrite
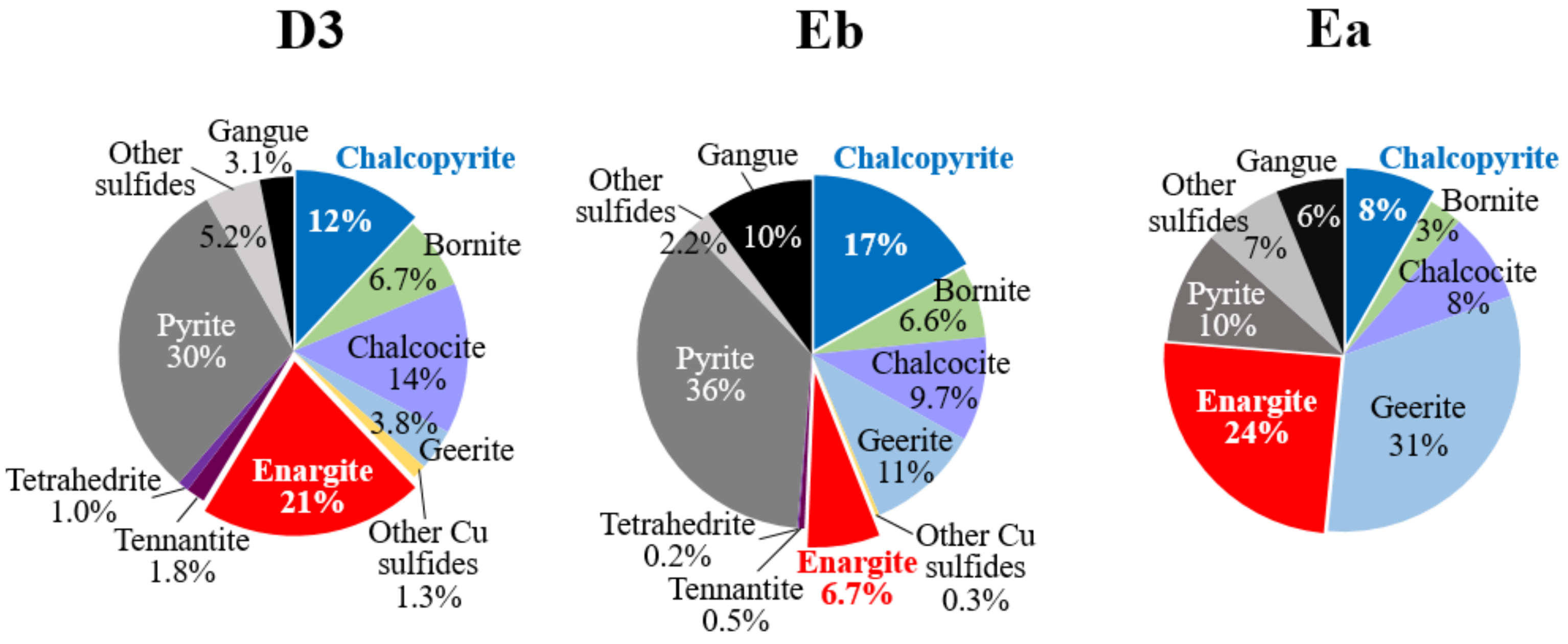
2.4. Activated Carbon-Assisted Bioleaching of Three Types of Chalcopyrite/Enargite-Bearing Complex Concentrates (D3, Eb and Ea Concentrates)
2.5. Solution and Solid Residue Analyses
2.6. Electrochemical Galvanic Current Measurement
3. Results and Discussion
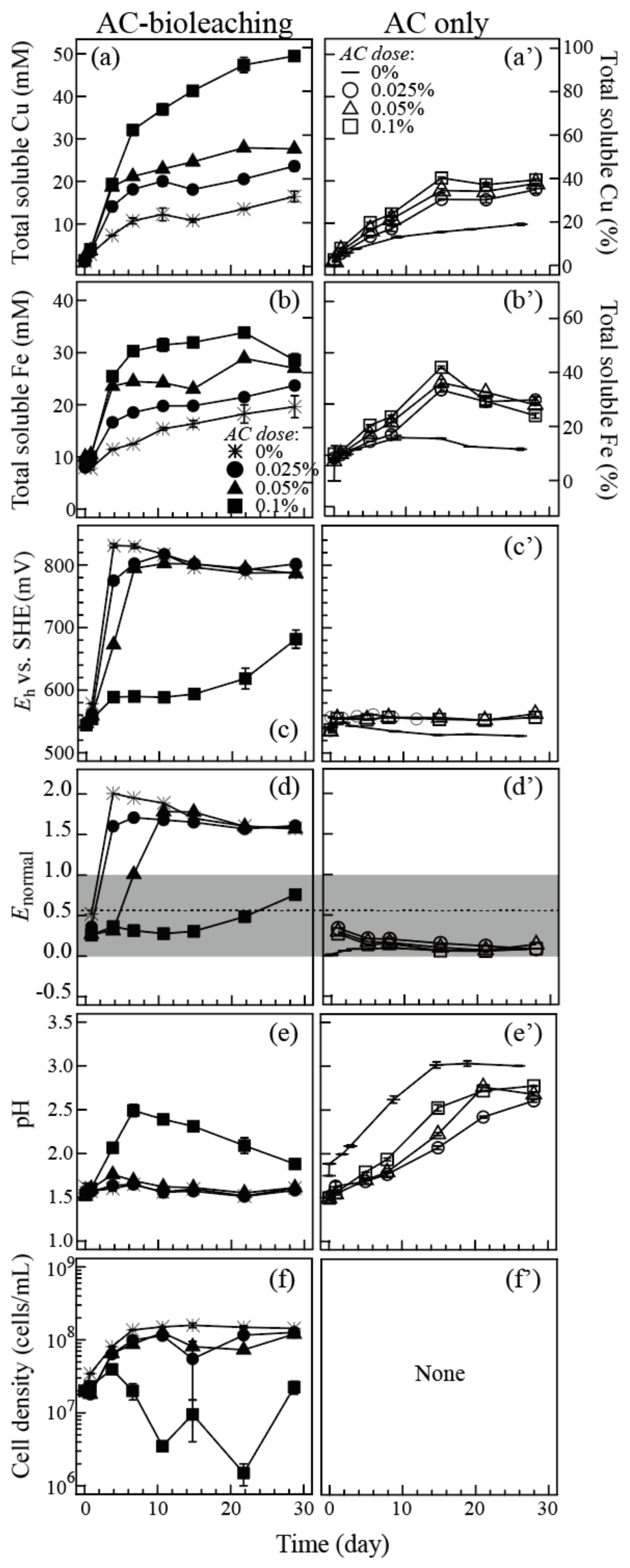
3.1. Carbon-Assisted Bioleaching of the Model Chalcopyrite
3.1.1. Synergistic Effect of Carbon Material and Microorganisms
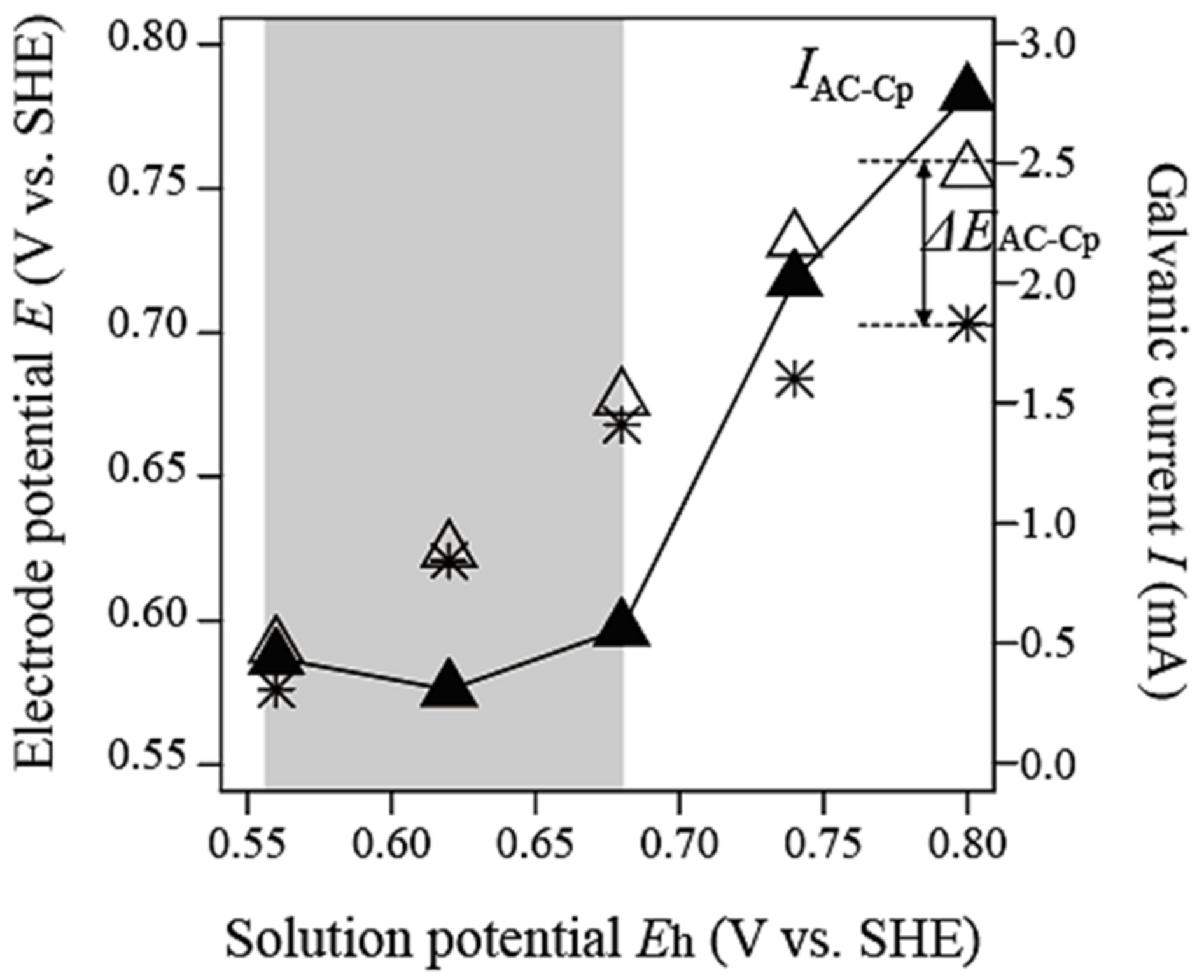
3.1.2. Galvanic Interaction between Carbon Material and Chalcopyrite
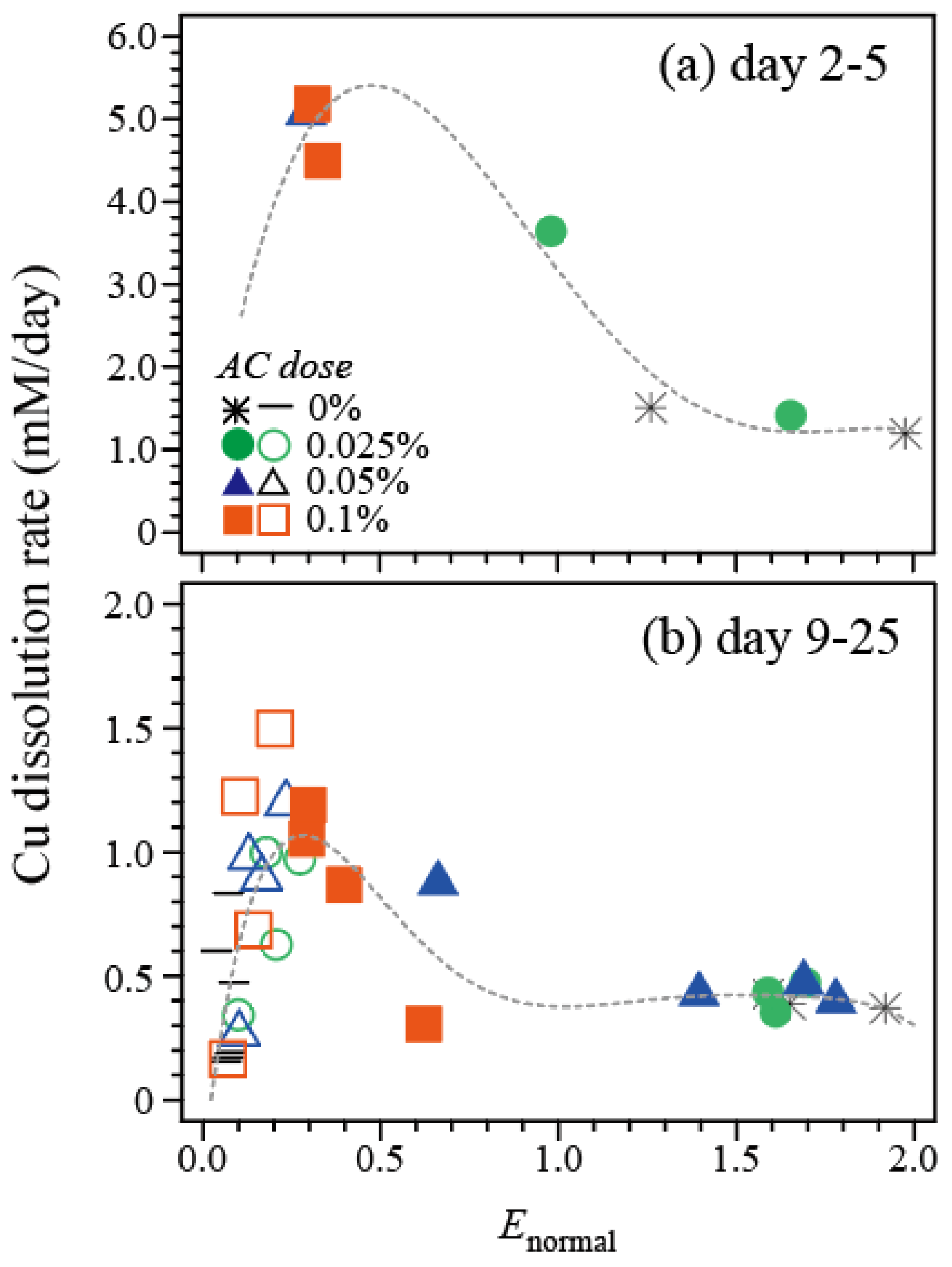
3.1.3. Correlation between the Cu Dissolution Rate and Enormal
3.2. Activated Carbon (AC)-Assisted Bioleaching of Three Types of Chalcopyrite/Enargite-Bearing Complex Concentrates
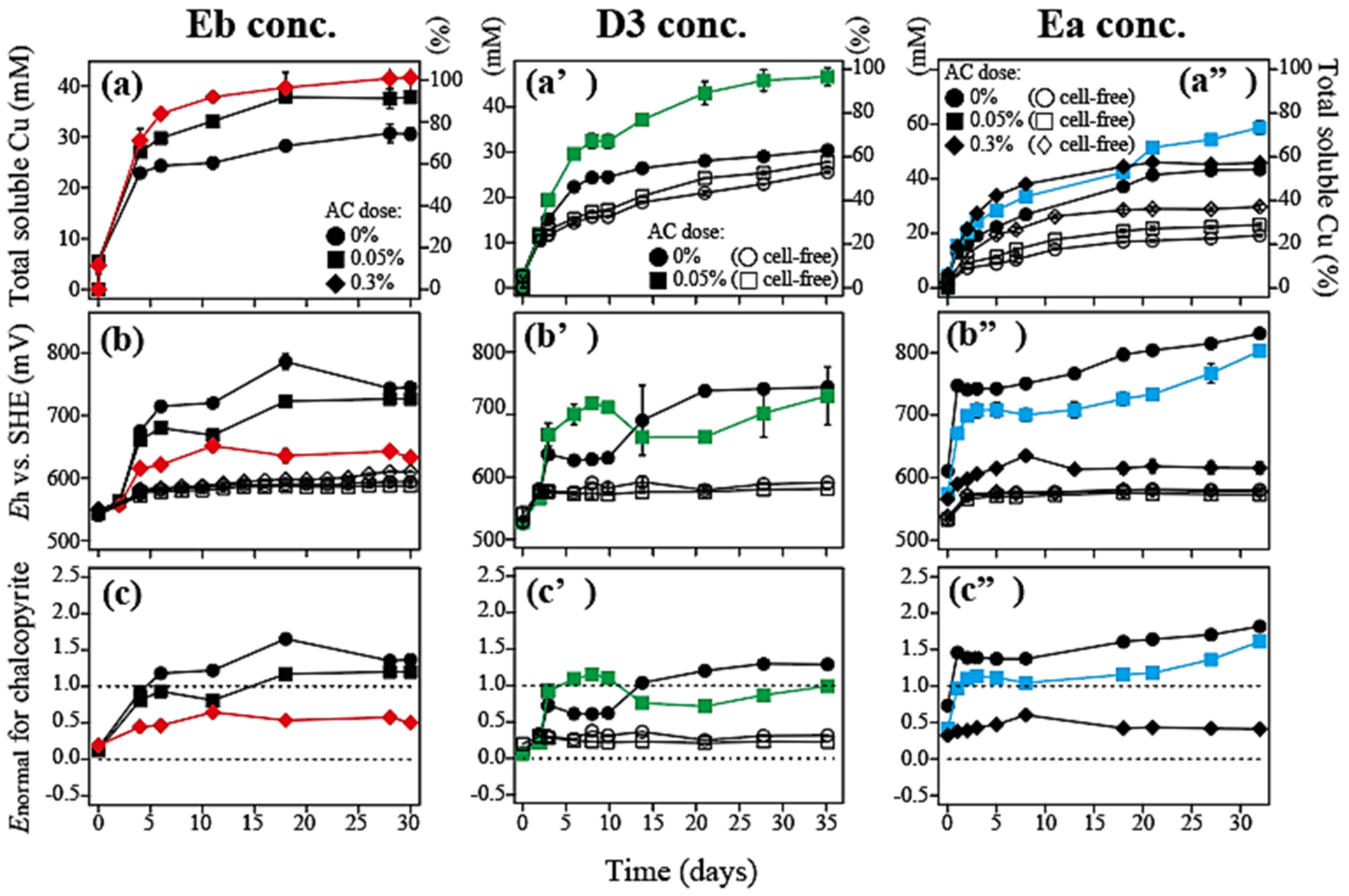
3.2.1. Eb Concentrate (Weight Cha/Ena Ratio = 2.5)
3.2.2. D3 Concentrate (Weight Cha/Ena Ratio = 0.6)
3.2.3. Ea Concentrate (Weight Cha/Ena Ratio = 0.3)
4. Conclusions
- The carbon catalyst (AC and CF) effectively controlled the Eh level in bioleaching liquors, which would otherwise exceed its optimal range (0 ≤ Enormal ≤ 1) due to regeneration of Fe3+ by microbial activity.
- Enormal of ~0.3 was shown to maximize the AC-assisted bioleaching of the model chalcopyrite mineral.
- When three types of chalcopyrite/enargite-bearing complex concentrates were tested, the optimal Eh level for a maximum Cu solubilization increased in response to the decreasing chalcopyrite/enargite ratio in the concentrate.
- Modification of the Eh level by adjusting AC doses was useful to maximize Cu solubilization from the concentrate of complex mineralogy.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Stott, M.; Watling, H.; Franzmann, P.; Sutton, D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Miner. Eng. 2000, 13, 1117–1127. [Google Scholar] [CrossRef]
- Córdoba, E.; Muñoz, J.; Blázquez, M.; González, F.; Ballester, A. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C. Miner. Eng. 2009, 22, 229–235. [Google Scholar] [CrossRef]
- Viramontes-Gamboa, G.; Peña-Gomar, M.M.; Dixon, D.G. Electrochemical hysteresis and bistability in chalcopyrite passivation. Hydrometallurgy 2010, 105, 140–147. [Google Scholar] [CrossRef]
- Parker, A.; Paul, R.; Power, G. Electrochemistry of the oxidative leaching of copper from chalcopyrite. J. Electroanal. Chem. Interfacial Electrochem. 1981, 118, 305–316. [Google Scholar] [CrossRef]
- Antonijević, M.; Janković, Z.; Dimitrijević, M. Investigation of the kinetics of chalcopyrite oxidation by potassium dichromate. Hydrometallurgy 1994, 35, 187–201. [Google Scholar] [CrossRef]
- Córdoba, E.; Muñoz, J.; Blázquez, M.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Viramontes-Gamboa, G.; Rivera-Vasquez, B.F.; Dixon, D.G. The active-passive behavior of chalcopyrite: Comparative study between electrochemical and leaching responses. J. Electrochem. Soc. 2007, 154, C299. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Tsunekawa, M.; Okamoto, H.; Nakayama, R.; Kuroiwa, S. Improved chalcopyrite leaching through optimization of redox potential. Can. Metall. Q. 2008, 47, 253–258. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Kitagawa, H.; Tsunekawa, M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2008, 91, 144–149. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. A model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 2000, 57, 31–38. [Google Scholar] [CrossRef]
- Okamoto, H.; Nakayama, R.; Hiroyoshi, N.; Tsunekawa, M. Redox potential dependence and optimum potential of chalcopyrite leaching in sulfuric acid solutions. J. MMIJ 2004, 120, 592–599. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Okamoto, H.; Nakayama, R.; Kuroiwa, S.; Hiroyoshi, N.; Tsunekawa, M. Normalized redox potential used to assess chalcopyrite column leaching. J. MMIJ 2005, 121, 246–254. (In Japanese) [Google Scholar] [CrossRef]
- Gericke, M.; Govender, Y.; Pinches, A. Tank bioleaching of low-grade chalcopyrite concentrates using redox control. Hydrometallurgy 2010, 104, 414–419. [Google Scholar] [CrossRef]
- Ahmadi, A.; Schaffie, M.; Manafi, Z.; Ranjbar, M. Electrochemical bioleaching of high grade chalcopyrite flotation concentrates in a stirred bioreactor. Hydrometallurgy 2010, 104, 99–105. [Google Scholar] [CrossRef]
- Ahmadi, A.; Schaffie, M.; Petersen, J.; Schippers, A.; Ranjbar, M. Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density. Hydrometallurgy 2011, 106, 84–92. [Google Scholar] [CrossRef]
- Masaki, Y.; Hirajima, T.; Sasaki, K.; Miki, H.; Okibe, N. Microbiological redox potential control to improve the efficiency of chalcopyrite bioleaching. Geomicrobiol. J. 2018, 35, 648–656. [Google Scholar] [CrossRef]
- Nakazawa, H.; Fujisawa, H.; Sato, H. Effect of activated carbon on the bioleaching of chalcopyrite concentrate. Int. J. Miner. Process. 1998, 55, 87–94. [Google Scholar] [CrossRef]
- Zhang, W.-M.; Gu, S.-F. Catalytic effect of activated carbon on bioleaching of low-grade primary copper sulfide ores. Trans. Nonferr. Met. Soc. China 2007, 17, 1123–1127. [Google Scholar] [CrossRef]
- Liang, C.-L.; Xia, J.-L.; Zhao, X.-J.; Yang, Y.; Gong, S.-Q.; Nie, Z.-Y.; Ma, C.-Y.; Zheng, L.; Zhao, Y.-D.; Qiu, G.-Z. Effect of activated carbon on chalcopyrite bioleaching with extreme thermophile Acidianus manzaensis. Hydrometallurgy 2010, 105, 179–185. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Liu, H.-C.; Xia, J.-L.; Nie, Z.-Y.; Zhu, H.-R.; Zhao, Y.-D.; Zheng, L.; Hong, C.-H.; Wen, W. Relatedness between catalytic effect of activated carbon and passivation phenomenon during chalcopyrite bioleaching by mixed thermophilic Archaea culture at 65 °C. Trans. Nonferr. Met. Soc. China 2017, 27, 1374–1384. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhu, P.; Chen, A.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Carbon material with high specific surface area improves complex copper ores’ bioleaching efficiency by mixed moderate thermophiles. Minerals 2018, 8, 301. [Google Scholar] [CrossRef]
- Lattanzi, P.; Da Pelo, S.; Musu, E.; Atzei, D.; Elsener, B.; Fantauzzi, M.; Rossi, A. Enargite oxidation: A review. Earth-Sci. Rev. 2008, 86, 62–88. [Google Scholar] [CrossRef]
- Oyama, K.; Shimada, K.; Ishibashi, J.-I.; Sasaki, K.; Miki, H.; Okibe, N. Catalytic mechanism of activated carbon-assisted bioleaching of enargite concentrate. Hydrometallurgy 2020, 196, 105417. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamaji, Y.; Fukano, Y.; Shimada, K.; Ishibashi, J.-I.; Hirajima, T.; Sasaki, K.; Sawada, M.; Okibe, N. Biooxidation of gold-, silver, and antimony-bearing highly refractory polymetallic sulfide concentrates, and its comparison with abiotic pretreatment techniques. Geomicrobiol. J. 2015, 32, 538–548. [Google Scholar] [CrossRef]
- Oyama, K.; Shimada, K.; Ishibashi, J.-I.; Miki, H.; Okibe, N. Silver-catalyzed bioleaching of enargite concentrate using moderately thermophilic microorganisms. Hydrometallurgy 2018, 177, 197–204. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, K.; Takamatsu, K.; Hayashi, K.; Aoki, Y.; Kuroiwa, S.; Hirajima, T.; Okibe, N. Carbon-Assisted Bioleaching of Chalcopyrite and Three Chalcopyrite/Enargite-Bearing Complex Concentrates. Minerals 2021, 11, 432. https://doi.org/10.3390/min11040432
Oyama K, Takamatsu K, Hayashi K, Aoki Y, Kuroiwa S, Hirajima T, Okibe N. Carbon-Assisted Bioleaching of Chalcopyrite and Three Chalcopyrite/Enargite-Bearing Complex Concentrates. Minerals. 2021; 11(4):432. https://doi.org/10.3390/min11040432
Chicago/Turabian StyleOyama, Keishi, Kyohei Takamatsu, Kaito Hayashi, Yuji Aoki, Shigeto Kuroiwa, Tsuyoshi Hirajima, and Naoko Okibe. 2021. "Carbon-Assisted Bioleaching of Chalcopyrite and Three Chalcopyrite/Enargite-Bearing Complex Concentrates" Minerals 11, no. 4: 432. https://doi.org/10.3390/min11040432
APA StyleOyama, K., Takamatsu, K., Hayashi, K., Aoki, Y., Kuroiwa, S., Hirajima, T., & Okibe, N. (2021). Carbon-Assisted Bioleaching of Chalcopyrite and Three Chalcopyrite/Enargite-Bearing Complex Concentrates. Minerals, 11(4), 432. https://doi.org/10.3390/min11040432





