A Hybrid Device for Enhancing Flotation of Fine Particles by Combining Micro-Bubbles with Conventional Bubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Adsorption Experiments
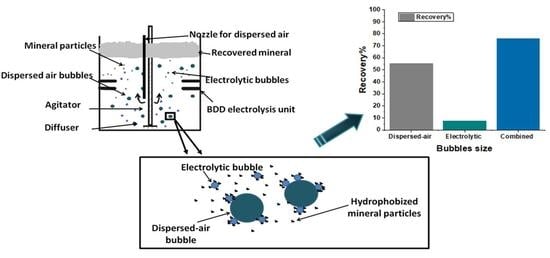
2.3. Experimental Set Up—The Hybrid Flotation Device
2.4. Bubble Size Measurements
2.5. Flotation Experiments
3. Results and Discussion
3.1. Adsorption Results
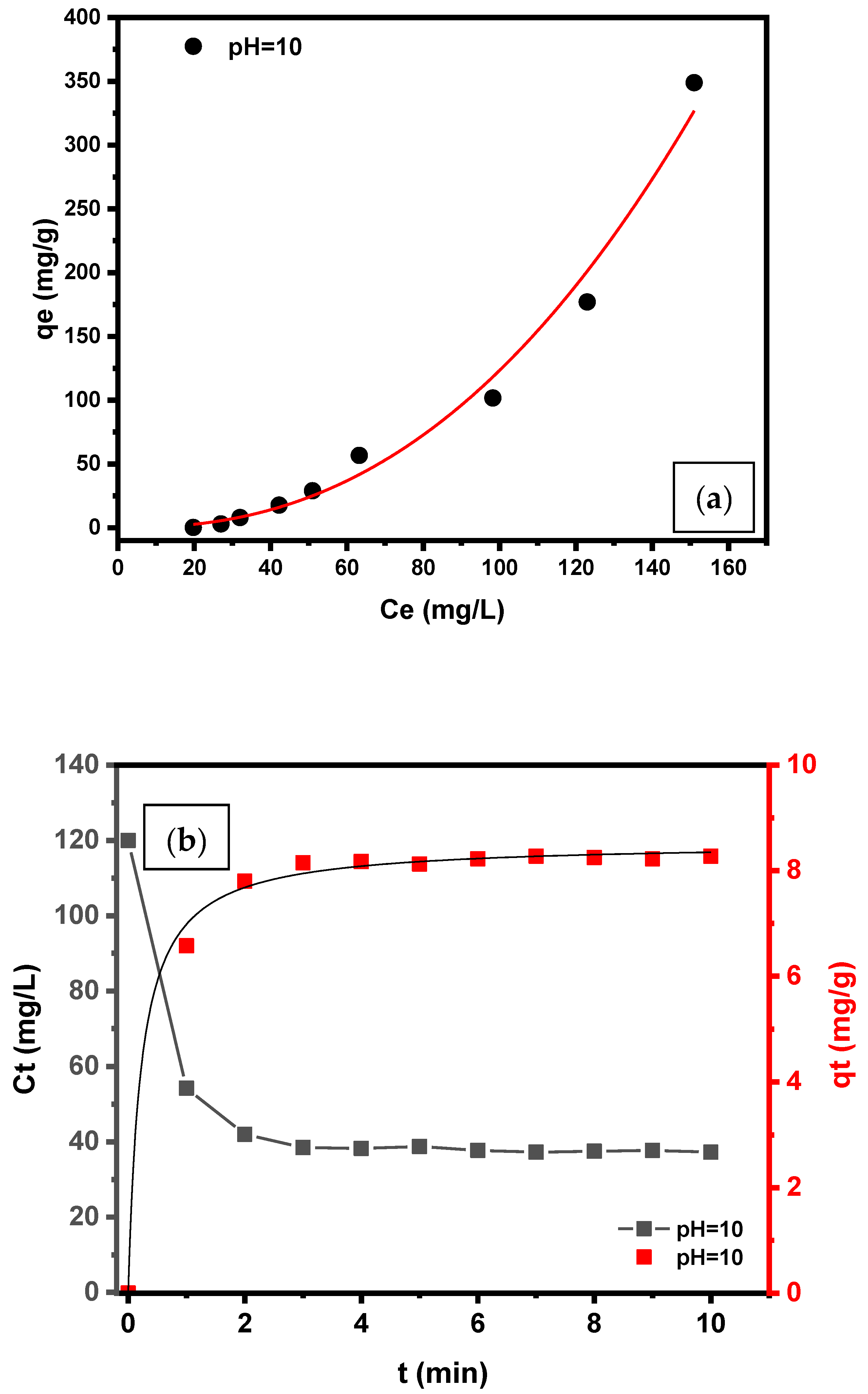
3.1.1. Effect of Initial Sorbate Concentration—Equilibrium Model (Isotherms)
3.1.2. Effect of Contact Time—Kinetic Models
3.1.3. Adsorption Mechanism
3.2. Bubble Size Measurements
3.3. Flotation Results
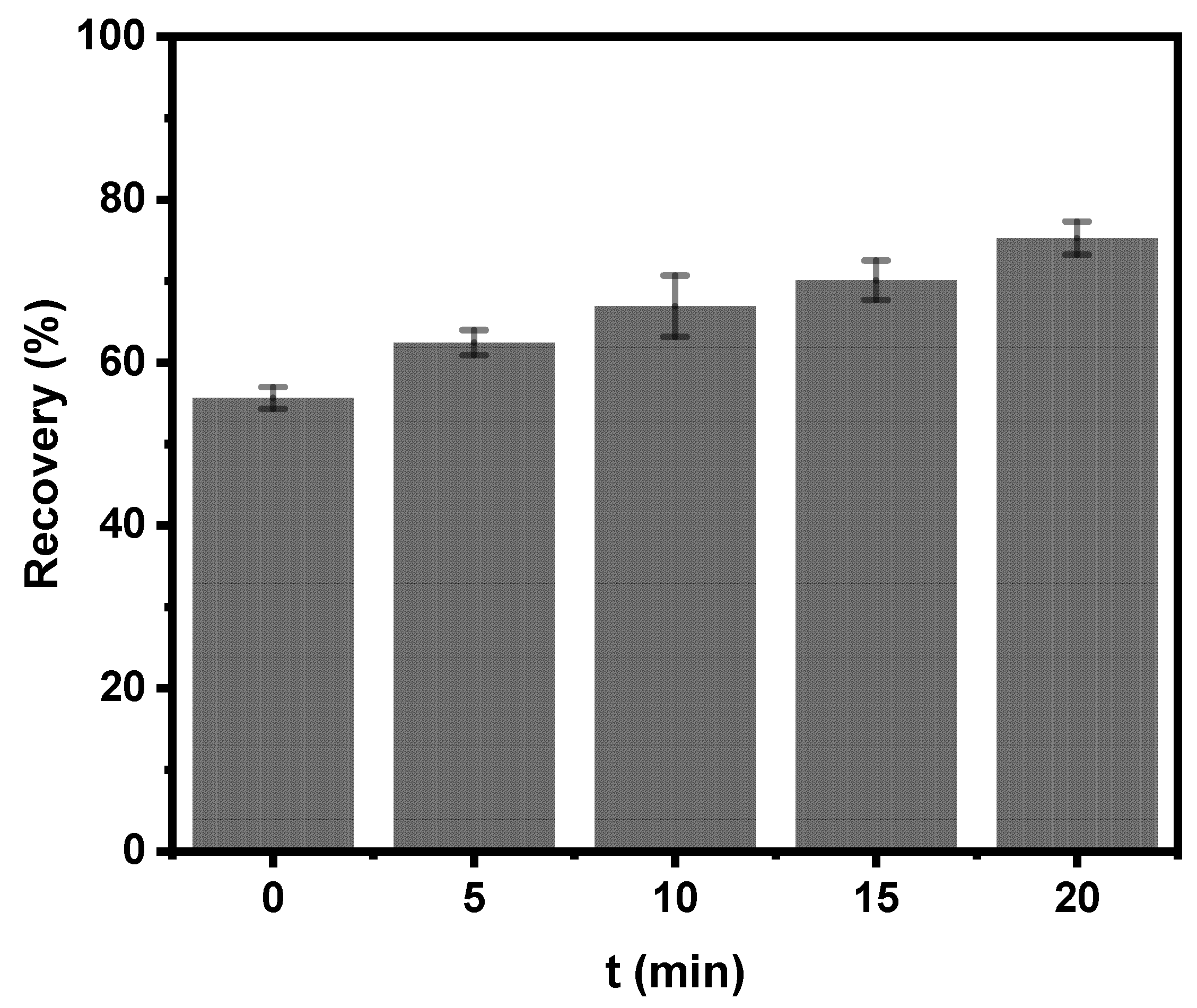
3.3.1. Effect of Conditioning Time with Electrolytic Bubbles
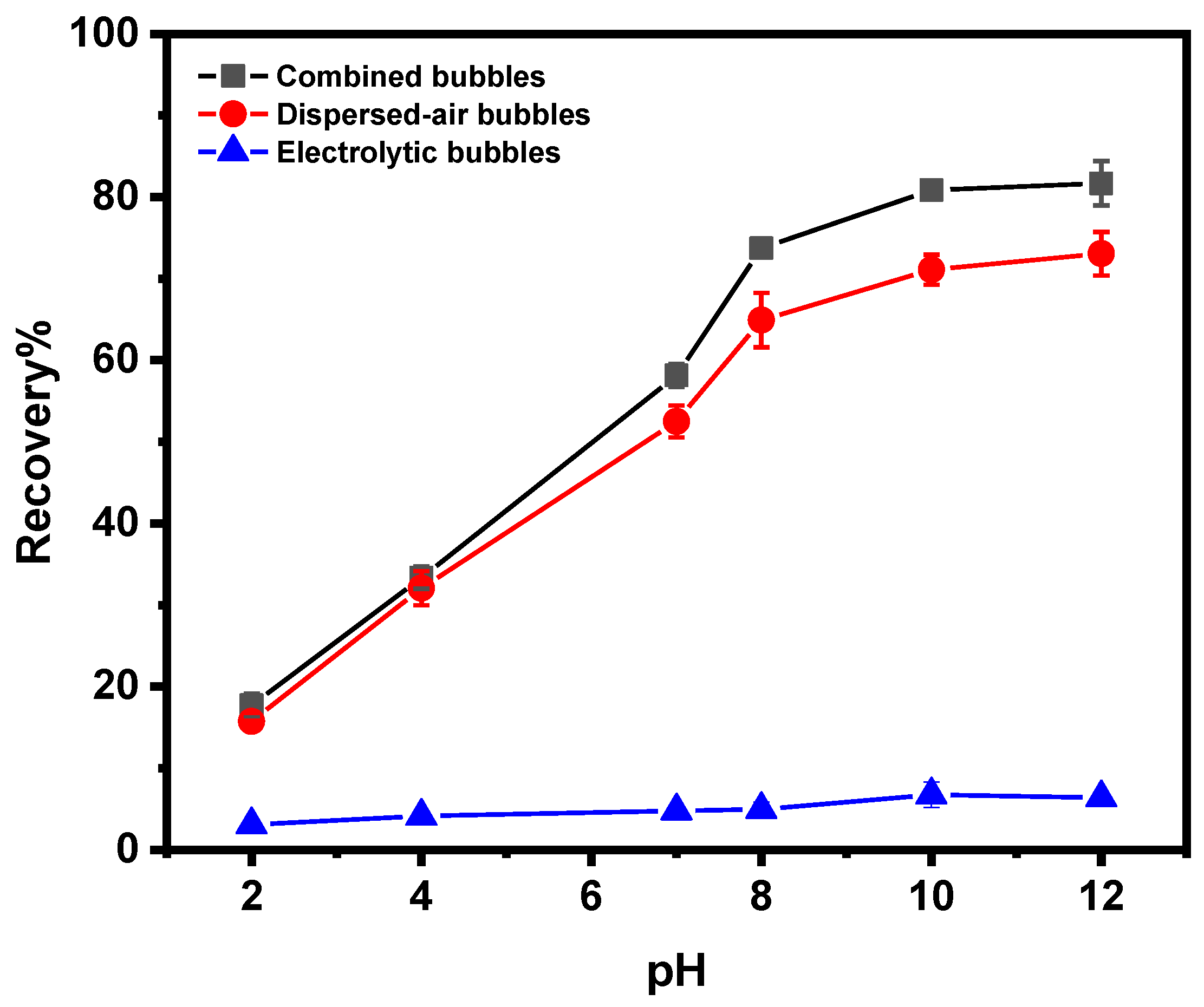
3.3.2. Effect of pH on Magnesite Flotation
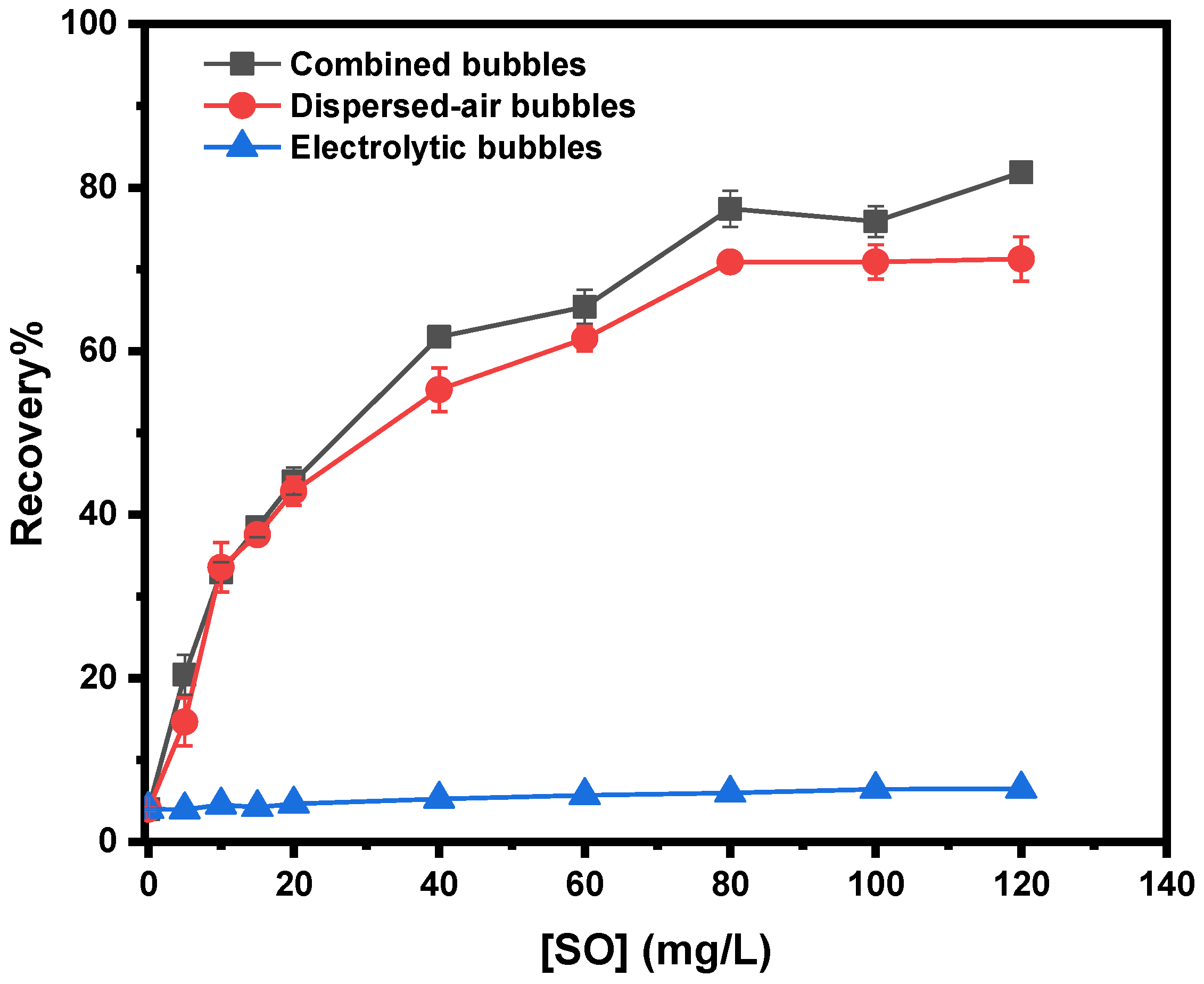
3.3.3. Effect of Collector’s Concentration
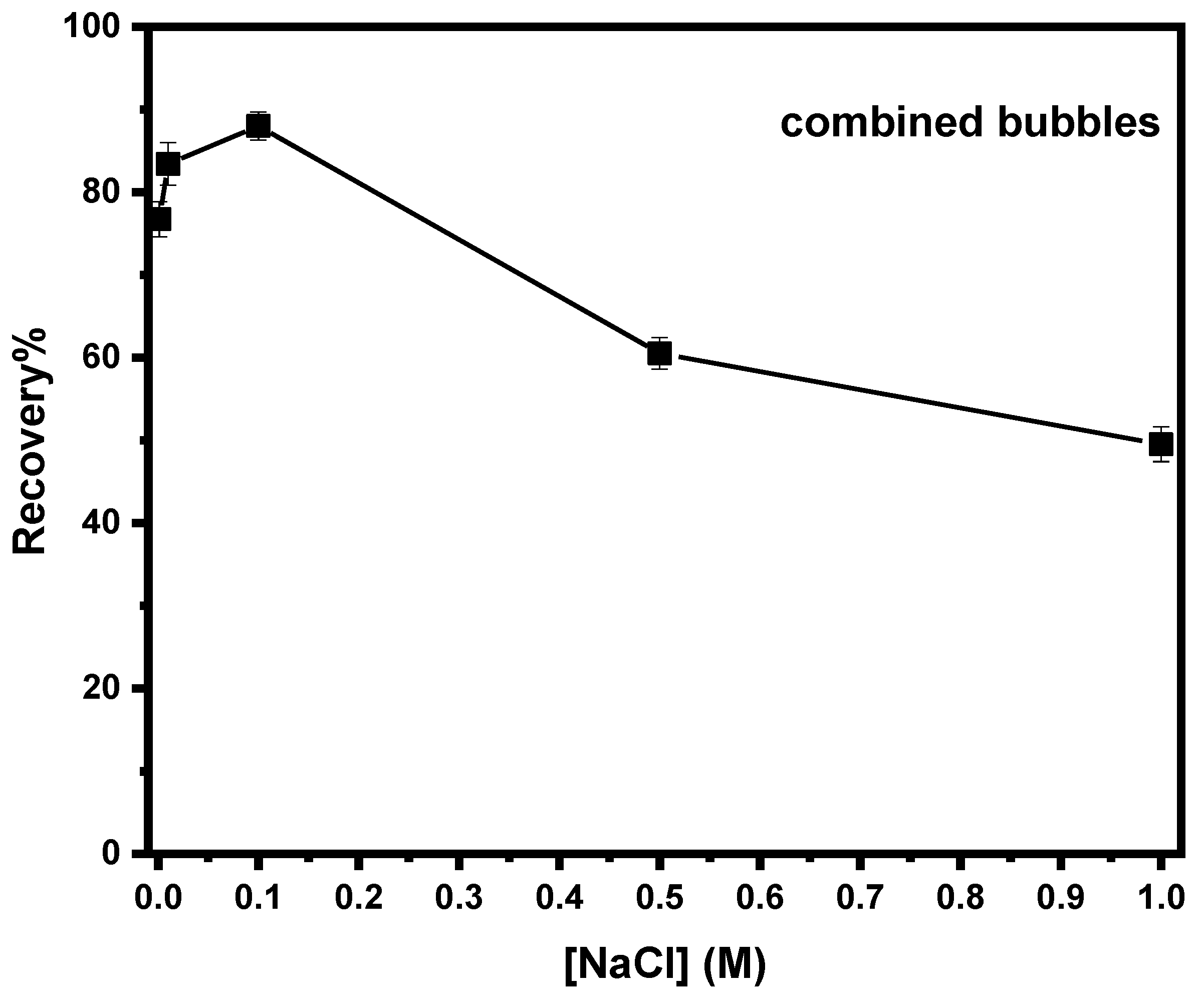
3.3.4. Effect of Electrolyte’s Concentration on Combined Flotation of Magnesite
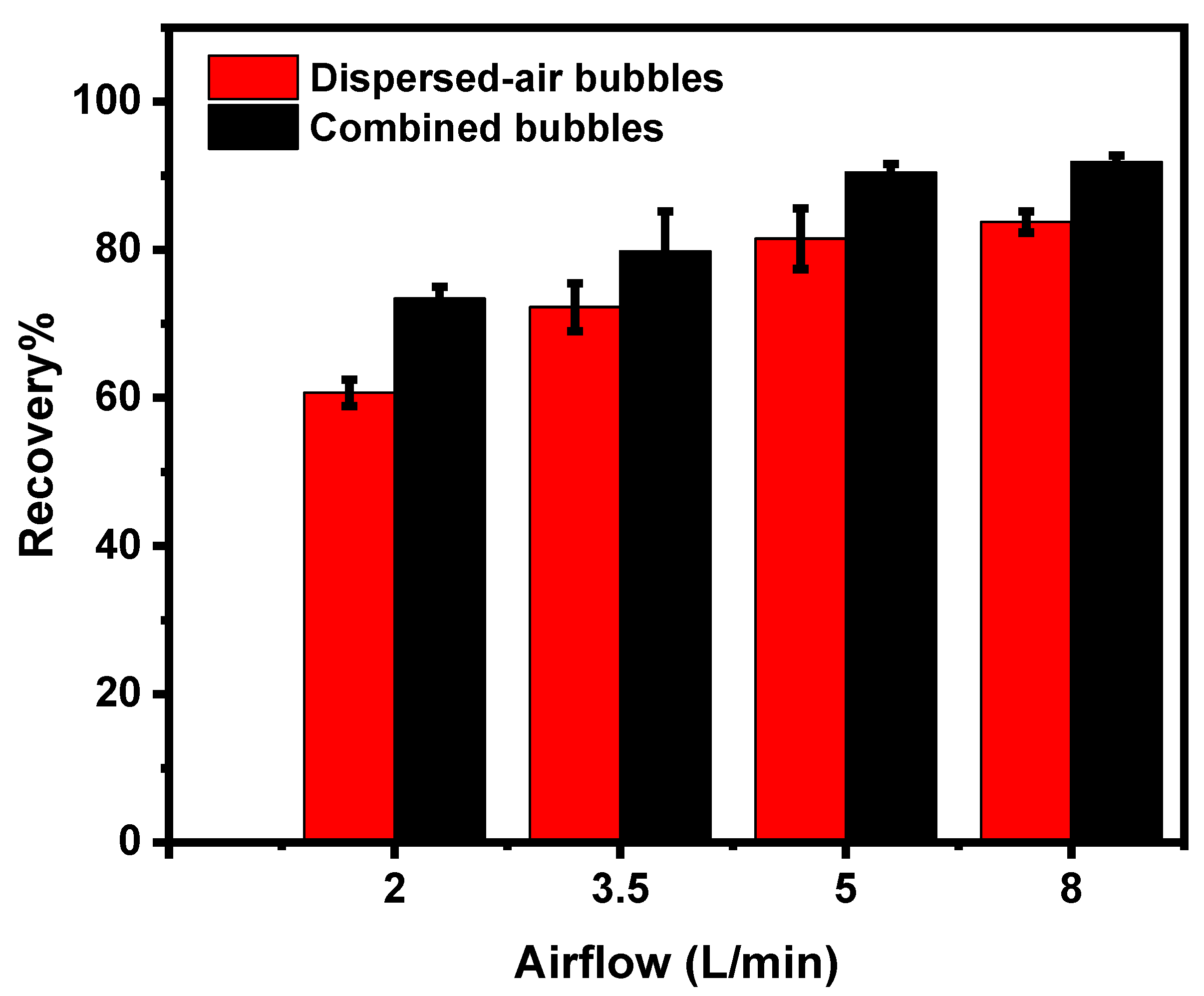
3.3.5. Effect of Airflow Rate on Magnesite Flotation
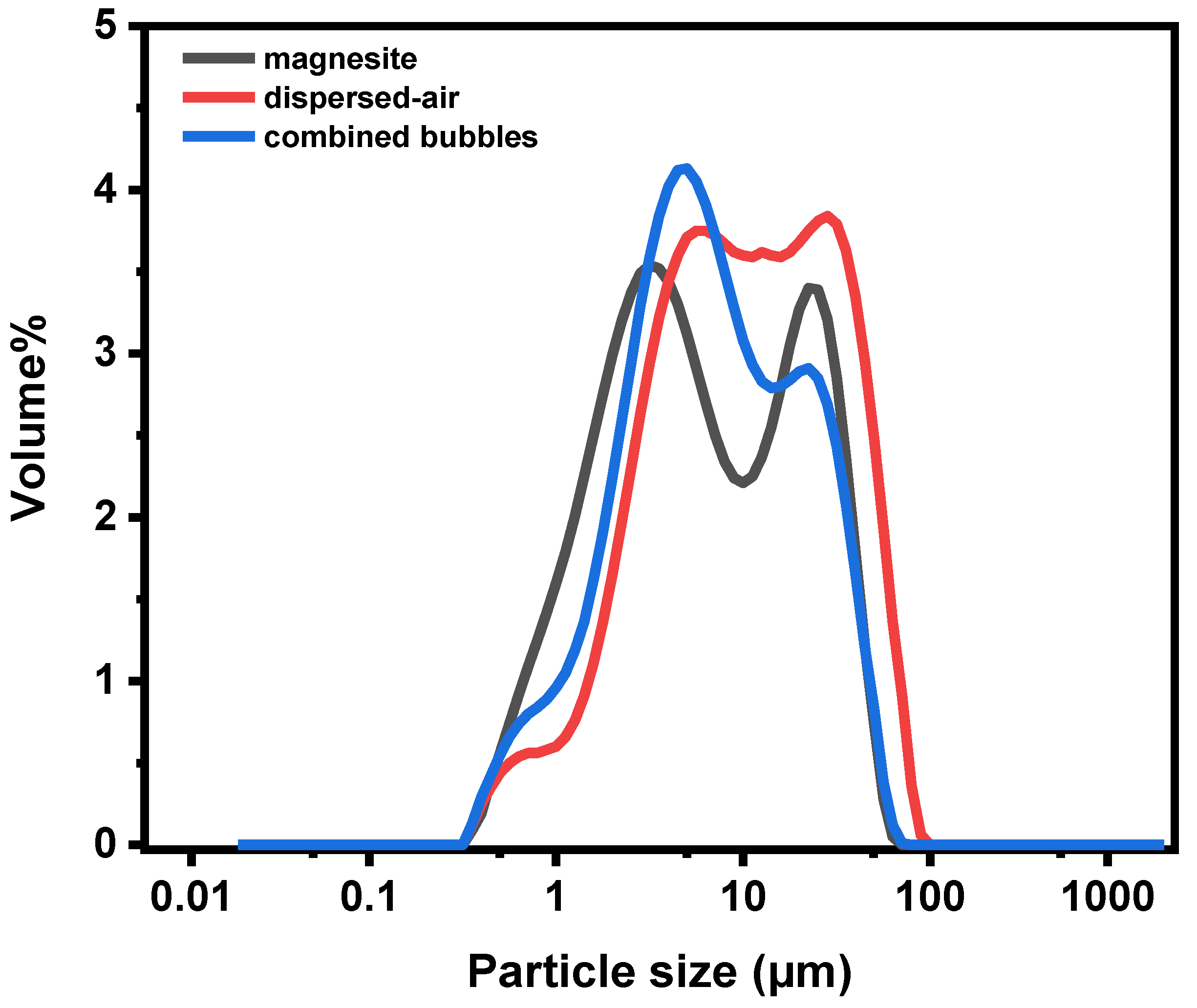
3.3.6. Particle Size Distribution after Flotation
4. Conclusions
- Adsorption of oleate’s molecules onto magnesite particles with the polar part on the surface of the mineral renders magnesite particles hydrophobic.
- Aggregation (or more specific hetero-aggregation) of fine particles with electrolytic bubbles.
- Attachment of the formed aggregates with the dispersed-air bubbles.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glembotskii, V.A.; Klassen, V.I.; Plaksin, I.N. Flotation; Primary Sources: New York, NY, USA, 1972; p. 633. [Google Scholar]
- Grotowski, A.; Witecki, K. Research on the Possibility of Sorting Application for Separation of Shale and/or Gangue from the Feed of Rudna Concentrator; KGHM Cuprum Ltd.; Research and Development Center; Mineral Engineering Conference; MEC2017; EDP Sciences: Wrocław, Poland, 2017. [Google Scholar] [CrossRef] [Green Version]
- Soto, H.; Barbery, G. Separation of fine particles by floc flotation. Production and Processing of Fine Particles. In Proceedings of the International Symposium on the Production and Processing of Fine Particles, Montreal, QC, Canada, 28–31 August 1988. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Forssberg, K.S.E. Mineral solution-interface chemistry in minerals engineering. Miner. Eng. 1993, 6, 439–454. [Google Scholar] [CrossRef]
- Lange, A.G.; Skinner, W.M.; Smart, R.S.C. Fine: Coarse particle interactions and aggregation in sphalerite flotation. Miner. Eng. 1997, 10, 681–693. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C. Effect of particle size on flotation performance of complex sulphide ores. Miner. Eng. 1999, 12, 721–731. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Lara-Valenzuela, C. Floc flotation of galena and sphalerite fines. Miner. Eng. 2001, 14, 87–98. [Google Scholar] [CrossRef]
- Liu, J.; Mak, T.; Zhou, Z.; Xu, Z. Fundamental study of reactive oily- bubble flotation. Miner. Eng. 2002, 15, 667–676. [Google Scholar] [CrossRef]
- Pyke, B.; Fomaserio, D.; Ralston, J. Bubble particle heterocoagulation under turbulent conditions. J. Colloid Interface Sci. 2003, 265, 141–151. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chehreh Chelgani, S.; Osbahr, I.; Möckel, R.; Peuker, U.A.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96–97, 94–98. [Google Scholar] [CrossRef]
- Gorman, M.G.; Smith, R.W. Amine flotation of fine quartz and glass beads. Miner. Eng. 1991, 4, 1219–1226. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. The effect of bubble size on fine coal flotation. Coal Prep. 1986, 2, 179–192. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. The effect of bubble size on fine particle flotation. Miner. Process. Extr. Metall. Rev. 1989, 5, 101–122. [Google Scholar] [CrossRef]
- Yoon, R.H. Microbubble flotation. Miner. Eng. 1993, 6, 619–630. [Google Scholar] [CrossRef]
- Hewitt, D.; Fornasiero, D.; Ralston, J. Bubble-Particle Attachment. Chem. Soc. Faraday Trans. 1995, 91, 1997–2001. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P.; Kydros, K.A. Separation of fines by flotation techniques. Sep. Technol. 1993, 3, 76–90. [Google Scholar] [CrossRef]
- Koh, P.T.L.; Schwarz, M.P. CFD modelling of bubble-particle collision rates and efficiencies in a flotation cell. Miner. Eng. 2003, 16, 1055–1059. [Google Scholar] [CrossRef]
- Mimezami, M.; Hashemi, M.S.; Finch, J.A. Effect of sulphates on aggregation of alumina and pyrite. In Particle Size Enlargement in Mineral Processing, Proceedings of the 5th UBC-McGill International Symposium, Hamilton, ON, Canada, 26 August 2004; Laskowski, J.S., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum: Westmount, QC, Canada, 2004; pp. 159–168. [Google Scholar]
- Zhang, X.; Hu, Y.; Sun, W.; Xu, L. The effect of polystyrene on the carrier flotation of fine smithsonite. Minerals 2017, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Jameson, G.J. The effect ofbubble size on the rate of flotation of fine particles. Int. J. Miner. Process. 1985, 14, 195–215. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle-bubble collision models—A review. Adv. Colloid Interfac. 2000, 85, 231–256. [Google Scholar] [CrossRef]
- Tao, D. Role of Bubble Size in Flotation of Coarse and Fine Particles—A Review. Separ. Sci. Tech. 2005, 39, 741–760. [Google Scholar] [CrossRef]
- Dai, Z.; Dukhin, S.S.; Fornasiero, D.; Ralston, J. The inertial hydrodynamic interaction of particles and rising bubbles with mobile surfaces. J. Colloid Interface Sci. 1998, 197, 275–292. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle–bubble attachment in mineral flotation. J. Colloid Interface Sci. 1999, 217, 70–76. [Google Scholar] [CrossRef]
- Bennett, A.J.R.; Chapman, W.R.; Dell, C.C. Studies in froth flotation of coal. In Third International Coal Preparation Conference, Liege, 1958; Editions techniques et scientifiques; Borreng: Bruxelles, Belgium, 1958; pp. 37–39 rue. [Google Scholar]
- Reay, D.; Ratcliff, G.A. Experimental testing of the hydrodynamic collision model of fine particle flotation. Can. J. Chem. Eng. 1975, 53, 481–486. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Venkatachalam, S. Electrogenerated Gas Bubbles in Flotation. Miner. Process. Extr. Metall. Rev. 1992, 8, 47–55. [Google Scholar] [CrossRef]
- Llerena, C.; Ho, J.C.K.; Piron, D.L. Effects of pH on electroflotation of sphalerite. Chem. Eng. Commun. 1996, 155, 217–228. [Google Scholar] [CrossRef]
- Kydros, K.A.; Gallios, G.P.; Matis, K.A. Electrolytic flotation of pyrite. J. Chem. Technol. Biotechnol. 1994, 59, 223–232. [Google Scholar] [CrossRef]
- Tadesse, B.; Albijanic, B.; Makuei, F.; Browner, R. Recovery of fine and ultrafine mineral particles by electroflotation—A review. Miner. Process. Extr. Metall. Rev. 2018, 1–15. [Google Scholar] [CrossRef]
- Montes-Atenas, G.; Garcia-Garcia, F.J.; Mermillod-Blondin, R.; Montes, S. Effect of suspension chemistry onto voltage drop: Application of electro-flotation. Powder Technol. 2010, 204, 1–10. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z.F. Nanobubble generation and its application in froth flotation (part I): Nanobubble generation and its effects on the properties of microbubble and millimeter scale bubble solutions. Min. Sci. Technol. 2010, 20, 1–19. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z.F. Nanobubble generation and its application in froth flotation (part II): Fundamental study and theoretical analysis. Min. Sci. Technol. 2010, 20, 159–177. [Google Scholar] [CrossRef]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Flotation of quartz particles assisted by nanobubbles. Int. J. Miner. Process. 2015, 137, 64–70. [Google Scholar] [CrossRef]
- Tussupbayev, N.K.; Rulyov, N.N.; Kravtchenco, O.V. Microbubble augmented flotation of ultrafine chalcopyrite from quartz mixtures. Miner. Process. Extr. Metall. Rev. 2016, 125, 5–9. [Google Scholar] [CrossRef]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. An overview of the beneficiation of iron ores via reverse cationic flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Jameson, G.J. New directions in flotation machine design. Miner. Eng. 2010, 23, 835–841. [Google Scholar] [CrossRef]
- Mankosa, M.J.; Kohmuench, J.N.; Christodoulou, L.; Yan, E.S. Improving fine particle flotation using the StackCell™ (raising the tail of the elephant curve). Miner. Eng. 2018, 121, 83–89. [Google Scholar] [CrossRef]
- Xiong, Y.; Peng, F. Optimization of cavitation venturi tube design for pico and nano bubbles generation. Int. J. Min. Sci. Technol. 2015, 25, 523–529. [Google Scholar] [CrossRef]
- Kohmuench, J.N.; Luttrell, G.; Mankosa, M. Coarse particle concentration using the HydroFloat separator. Miner. Metall. Process. 2001, 18, 61–67. [Google Scholar] [CrossRef]
- Clayton, R.; Jameson, G.J.; Manlapig, E.V. The development and application of the Jameson cell. Miner. Eng. 1991, 4, 925–933. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Mohanty, M.K. Enhanced column flotation performance for fine coal cleaning. Miner. Eng. 1996, 9, 931–945. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. Microcel column flotation scale-up and plant practice. In Proceedings of the 26th Annual Meeting Canadian Mineral Processing, CIM, Ottawa, ON, Canada, 18–20 January 1994; Volume 12. [Google Scholar]
- Jameson, G.J.; Nguyen, A.V.; Ata, S. The flotation of fine and coarse particles. In Froth Flotation: A Century of Innovation; Fuerstenau, M.C., Jameson, G.J., Yoon, R.H., Eds.; Society for Mining, Metallurgy & Exploration: Littleton, CO, USA, 2007; pp. 339–372. [Google Scholar]
- Prakash, R.; Majumder, S.K.; Singh, A. Flotation technique: Its mechanisms and design parameters. Chem. Eng. Process. 2018, 127, 249–270. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Filippov, L.O.; Kravchenko, O.V. Combined Microflotation of Glass Beads. Colloid Surf. A Physicochem. Eng. Asp. 2020, 598, 124810. [Google Scholar] [CrossRef]
- Rulyov, N.N. Combined microflotation of fine minerals: Theory and experiment. Miner. Process. Extr. Metall. Rev. 2016, 125, 81–85. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Sajjady, S.A.; Gholami, H.; Amini, S.; Özkan, S.G. An Improvement on Selective Separation by Applying Ultrasound to Rougher and Re-Cleaner Stages of Copper Flotation. Minerals 2020, 10, 619. [Google Scholar] [CrossRef]
- Filippov, L.O.; Matinin, A.S.; Samiguin, V.D.; Filippova, I.V. Effect of ultrasound on flotation kinetics in the reactor-separator. J. Phys. Conf. Ser. 2013, 416, 1–6. [Google Scholar] [CrossRef]
- Aslani, S.; Samim, B.; Hashemi, H.R.; Arianpour, F. Beneficiation of Iranian magnesite ores by reverse flotation process and its effects on shaped and unshaped refractories properties. Bull. Mater. Sci. 2010, 33, 697–705. [Google Scholar] [CrossRef]
- Amer, A.M.; Staden, V. Hydrometallurgical processing of low grade Egyptian magnesite. Physicochem. Probl. Miner. Process. 2010, 44, 5–12. [Google Scholar]
- Erdoğan, N. The Most Suitable Beneficiation Method for Magnesite Ore. In Proceedings of the 15thInternational Mineral Processing Symposium, Istanbul, Turkey, 19–21 October 2016. [Google Scholar]
- Gence, N.; Ozbay, N. pH dependence of electrokinetic behavior of dolomite and magnesite in aqueous electrolyte solutions. Appl. Surf. Sci. 2006, 252, 8057–8061. [Google Scholar] [CrossRef]
- Moudgil, B.M.; Ince, D.E. Flotation Separation of Apatite from Dolomite Using Dodecylamineand Sodium Chloride. In Particle Technology and Surface Phenomena in Minerals and Petroleum; Springer: Boston, MA, USA, 1991; pp. 191–197. [Google Scholar]
- Matis, K.A.; Balabanidis, T.H.N.; Gallios, G.P. Processing of Magnesium Carbonate Fines by Dissolved-Air Flotation. Colloids Surf. 1988, 29, 191–203. [Google Scholar] [CrossRef]
- Ni, X.; Liu, Q. Adsorption behaviour of Sodium Hexametaphosphate on Pyrochlore and Calcite. Can. Metall. Q. 2013, 52, 473–478. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Wen, S.; Ma, M.; Sun, C.; Yin, W.; Ma, Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Mishchuk, N.; Ralston, J.; Fornasiero, D. Influence of dissolved gas on van der Waals forces between bubbles and particles. J. Phys. Chem. A. 2002, 106, 689–696. [Google Scholar] [CrossRef]
- Mishchuk, N.; Ralston, J.; Fornasiero, D. Influence of very small bubbles on particle/bubble heterocoagulation. J. Colloid Interface Sci. 2006, 301, 168–177. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Finch, J.A.; Hu, H.; Rao, S.R. Role of hydrodynamic cavitation in fine particle flotation. Int. J. Miner. Process. 1997, 51, 139–149. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Bubble coalescence and its effect on dynamic foam stability. Can. J. Chem. Eng. 2002, 80, 299–305. [Google Scholar] [CrossRef]
- Gence, N. Wetting behavior of magnesite and dolomite surfaces. Appl. Surf. Sci. 2006, 252, 3744–3750. [Google Scholar] [CrossRef]
- Yin, W.; Sun, Q.; Li, D.; Tang, Y.; Fu, Y.; Yao, J. Mechanism and application on sulphidizing flotation of copper oxide with combined collectors. Trans. Nonferrous Met. Soc. China 2019, 29, 178–185. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, W.; Yang, B.; Li, C.; Zhu, Z.; Li, D. Effect of sodium alginate on reverse flotation of hematite and its mechanism. Int. J. Miner. Metall. Mater. 2018, 251113–251122. [Google Scholar] [CrossRef]
- Rao, T.N.; Fujishima, A. Recent advances in electrochemistry of diamond. Diamond Relat. Mater. 2000, 9, 384–389. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Application of diamond electrodes to electrochemical processes. Electrochim. Acta 2005, 5, 191–199. [Google Scholar] [CrossRef]
- Chen, Q.; Granger, M.C.; Lister, T.E.; Swain, G.M. Morphological and microstructural stability of boron-doped diamond thin film electrodes in an acidic chloride medium at high anodic current densities. J. Electrochem. Soc. 1997, 77, 121–148. [Google Scholar] [CrossRef]
- Farrell, J.; Martin, F.J.; Martin, H.B.; O’Grady, W.E.; Natishan, P. Anodically Generated Short-Lived Species on Boron-Doped Diamond Film Electrodes. J. Electrochem. Soc. 2005, 152, 14–17. [Google Scholar] [CrossRef]
- Zabulis, X.; Papara, M.; Chatziargyriou, A.; Karapantsios, T.D. Detection of densely dispersed spherical bubbles in digital images based on a template matching technique: Application to wet foams. Colloids Surf. A Physicochem. Eng. Asp. 2007, 309, 96–106. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Basic concepts from Equilibrium. In Chemistry for Environmental Engineering and Science; McGraw Hill Companies Inc.: Boston, FL, USA, 2003. [Google Scholar]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.H.; Antti, B.M.; Forssberg, E. Mechanism of oleate interaction on salt-type minerals part I. Adsorption and electrokinetic studies of calcite in the presence of sodium oleate and sodium metasilicate. Colloids Surf. 1988, 34, 227–239. [Google Scholar] [CrossRef]
- Baviere, M.; Ruaux, E.; Defives, D. Sulfonate Retention by Kaolinite at High pH-Effect of Inorganic Anions. SPE Res. Eng. 1991, 8, 123–127. [Google Scholar] [CrossRef]
- Cases, J.M.; Levitz, P.; Poirier, J.E.; Van Damme, H. Adsorption of ionic and nonionic surfactants on mineral solids from aqueous solutions. In Advances in Mineral Processing; Somasundaran, P., Ed.; SME Publication: Littleton, CO, USA, 1986; pp. 171–186. [Google Scholar]
- Fuerstenau, M.C.; Miller, J.D. The role of hydrocarbon chain in anionic flotation of calcite. Trans. AIME 1967, 238, 153–160. [Google Scholar]
- Somasundaran, P. Adsorption of starch and oleate and interaction between them on calcite in aqueous solutions. J. Colloid Interface Sci. 1969, 31, 557–565. [Google Scholar] [CrossRef]
- Miskovic, S.; Luttrell, G. Comparison of Two Bubble Sizing Methods for Performance Evaluation of Mechanical Flotation Cells, Mining and Minerals Engineering; Virginia Tech: Blacksburg, VA, USA, 2012. [Google Scholar]
- Sarkar, M.; Evans, G.M.; Donne, S.W. Bubble size measurement in electroflotation. Miner. Eng. 2010, 23, 1058–1065. [Google Scholar] [CrossRef]
- Xu, Q.; Nakajima, M.; Ichikawa, S.; Nakamura, N.; Roy, P.; Okadome, H.; Shiina, T. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. J. Colloid Interface Sci. 2009, 332, 208–214. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Tussupbayev, N.K.; Turusbekov, D.K.; Semushkina, L.V.; Kaldybaeva, Z.A. Effect of Micro-bubblesas Flotation Carriers on Fine Sulphide Ore Beneficiation. Trans. Inst. Min. Metall. C 2018, 127, 133–139. [Google Scholar] [CrossRef]
- Matis, K.A.; Gallios, G.P. Anionic Flotation of Magnesium Carbonates by Modifiers. Int. J. Miner. Process. 1989, 25, 261–274. [Google Scholar] [CrossRef]
- Yang, B.; Sun, H.R.; Wang, D.H.; Yin, W.Z.; Cao, S.H.; Wang, Y.L.; Zhu, Z.L.; Jiang, K.; Yao, J. Selective adsorption of a new depressant Na2ATP on dolomite: Implications for effective separation of magnesite from dolomite via froth flotation. Sep. Purif. Technol. 2020, 250, 117278. [Google Scholar] [CrossRef]
- Brandão, P.R.G.; George, W. Poling Anionic Flotation of Magnesite. Can. Metall. Q. 1982, 21, 211–220. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z. Nanobubble generation and its application in froth flotation (Part III): Specially designed laboratory scale column flotation of phosphate. Min. Sci. Technol. 2010, 20, 317–338. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Dishon, M.; Zohar, O.; Sivan, U. From repulsion to attraction and back to repulsion: The effect of NaCl, KCl, and CsCl on the force between silica surfaces in aqueous solution. Langmuir 2009, 25, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Marrucci, G.; Nicodemo, L. Coalescence of gas bubbles in aqueous solutions of inorganic electrolytes. Chem. Eng. Sci. 1967, 22, 1257–1265. [Google Scholar] [CrossRef]
- Zieminski, S.A.; Whittemore, R.C. Behavior of gas bubbles in aqueous electrolyte solutions. Chem. Eng. Sci. 1971, 26, 509–520. [Google Scholar] [CrossRef]
- Huang, C.H.; Shen, S.Y.; Chen, C.W.; Dong, C.D.; Kumar, M.; Dakshinamoorthy, B.; Chang, J.H. Effect of Chloride Ions on Electro-Coagulation to Treat Industrial Wastewater Containing Cu and Ni. Sustainability 2020, 12, 7693. [Google Scholar] [CrossRef]
- Laplante, A.R.; Toguri, J.M.; Smith, H.W. The effect of air flow rate on the kinetics of flotation. Part 1: The transfer of material from the slurry to the froth. Int. J. Miner. Process. 1983, 11, 203–219. [Google Scholar] [CrossRef]
- Laplante, A.R.; Smith, H.W.; Toguri, J.M. The effect of air flow rate on the kinetics of flotation. Part 2: The transfer of material from the froth over the cell lip. Int. J. Miner. Process. 1983, 11, 221–234. [Google Scholar] [CrossRef]
- Yang, X.S.; Aldrich, C. Effects of impeller speed and aeration rate on flotation performance of sulfide ore. Trans. Nonferrous Met. Soc. China 2006, 16, 185–190. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of Fine Particles: A Review. Miner. Process. Extr. Metall. Rev. 2020, 1–11. [Google Scholar] [CrossRef]
- Abdo, M.S.E.; Darwish, A.M. Effect of Some Operating Variables on the Flotation of Aluminium Particles. Chem. Eng. Technol. 1991, 14, 118–121. [Google Scholar] [CrossRef]


| Chemical Component | Content |
|---|---|
| MgCO3 | <95% |
| Al2O3 | 0.031% |
| SiO2 | 1.047% |
| SO3 | <0.001% |
| CaO | 1.462% |
| MnO | 0.305% |
| Fe2O3 | 0.051% |
| NiO | 0.760% |
| Size (μm) | Volume % |
|---|---|
| 0–5 | 49.53 |
| 6–10 | 12.69 |
| 11–15 | 9.38 |
| 17–20 | 5.84 |
| 22–25 | 6.67 |
| 28–31 | 6.60 |
| 35–39 | 5.21 |
| 45–63 | 4.08 |
| Model Isotherm | Isotherm Parameters | |
|---|---|---|
| Langmuir-Freundlich | Ks (L/mg)n | 9.023 ± 2.712 × 10−6 |
| qm (mg/g) | 474.63 ± 281.45 | |
| ns | 2.366 ± 0.416 | |
| R2 | 0.999 | |
| Kinetic Model | Kinetic Parameters | |
|---|---|---|
| Pseudo-first order | k1 (min−1) | 0.01 |
| qe (mg/g) | 7.280 ± 0.783 | |
| R2 | 0.695 | |
| Pseudo-second order | k2 (min−1(mg/g)−1) | 0.528 ± 0.086 |
| qe (mg/g) | 8.535 ± 0.074 | |
| R2 | 0.999 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsave, P.K.; Kostoglou, M.; Karapantsios, T.D.; Lazaridis, N.K. A Hybrid Device for Enhancing Flotation of Fine Particles by Combining Micro-Bubbles with Conventional Bubbles. Minerals 2021, 11, 561. https://doi.org/10.3390/min11060561
Tsave PK, Kostoglou M, Karapantsios TD, Lazaridis NK. A Hybrid Device for Enhancing Flotation of Fine Particles by Combining Micro-Bubbles with Conventional Bubbles. Minerals. 2021; 11(6):561. https://doi.org/10.3390/min11060561
Chicago/Turabian StyleTsave, Polyxeni K., Margaritis Kostoglou, Thodoris D. Karapantsios, and Nikolaos K. Lazaridis. 2021. "A Hybrid Device for Enhancing Flotation of Fine Particles by Combining Micro-Bubbles with Conventional Bubbles" Minerals 11, no. 6: 561. https://doi.org/10.3390/min11060561
APA StyleTsave, P. K., Kostoglou, M., Karapantsios, T. D., & Lazaridis, N. K. (2021). A Hybrid Device for Enhancing Flotation of Fine Particles by Combining Micro-Bubbles with Conventional Bubbles. Minerals, 11(6), 561. https://doi.org/10.3390/min11060561