Bioleaching of Gold from Silicate Ore by Macrococcus caseolyticus and Acinetobacter calcoaceticus: Effect of Medium, Amino Acids and Growth Supernatant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore Sample Preparation
2.2. Screening and Isolate of Microorganisms for Gold Bioleaching
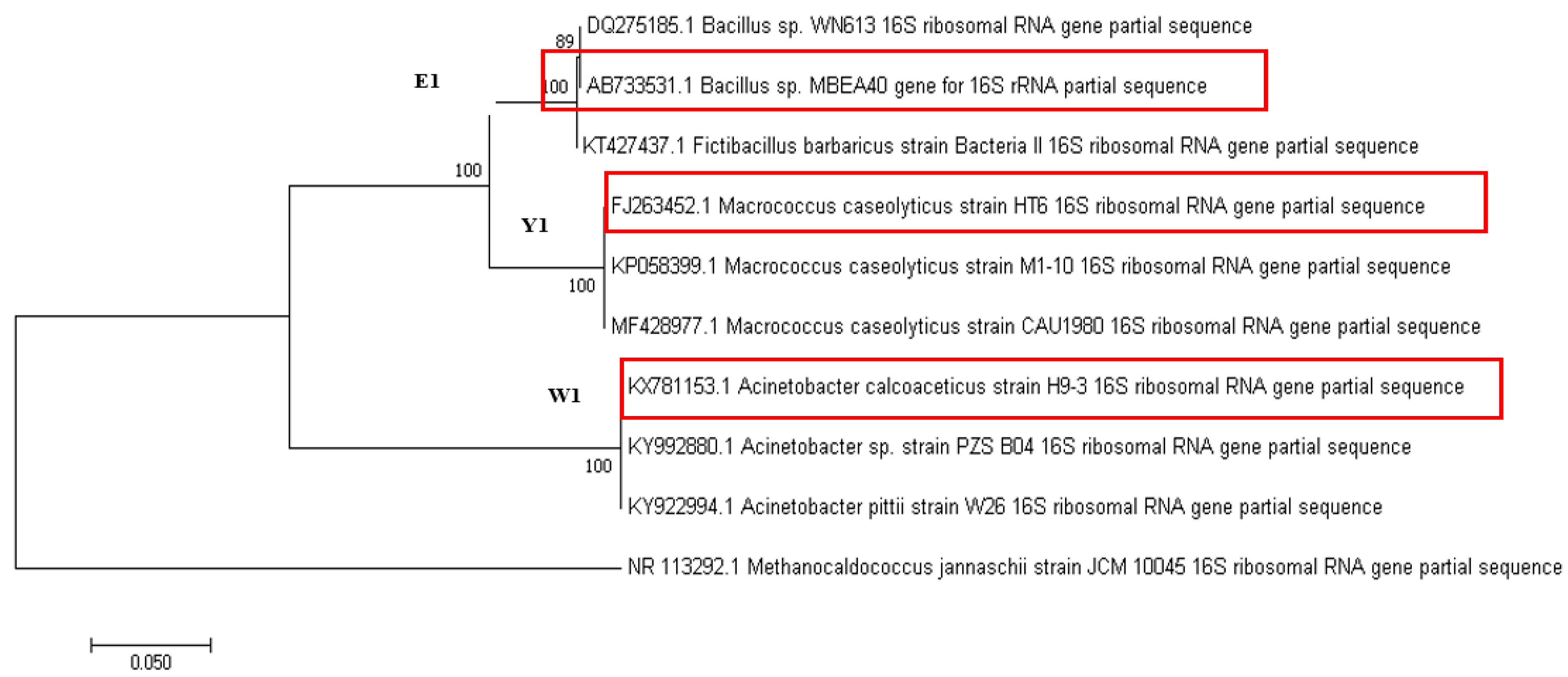
2.3. Microbial Identification
2.4. Gold Concentration Analysis
2.5. Bioleaching of Gold by Isolated Microorganisms
2.5.1. Bioleaching by Microorganisms (in the Presence of the Microorganism)
2.5.2. Bioleaching by Growth Supernatant (in the Absence of the Microorganism)
2.6. Amino Acid Analysis
2.6.1. Derivatization of Standards and Samples
2.6.2. Chromatographic Conditions
2.7. Gold Leaching by Mixed Amino Acids
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Statistical Analysis
3. Results and Discussion
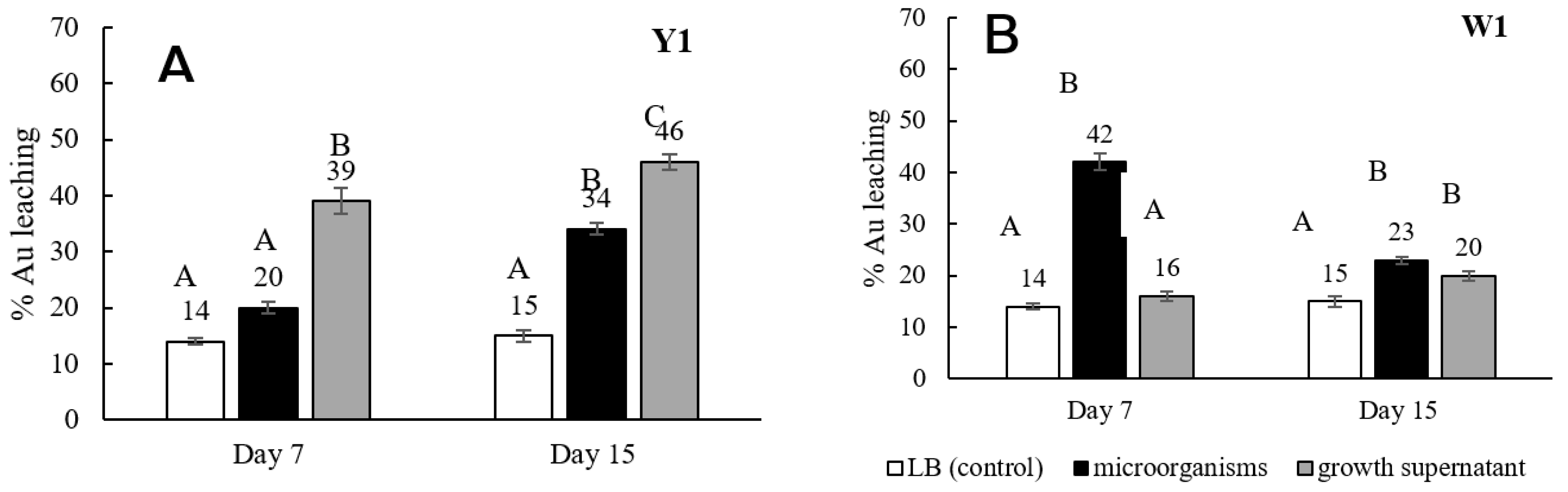
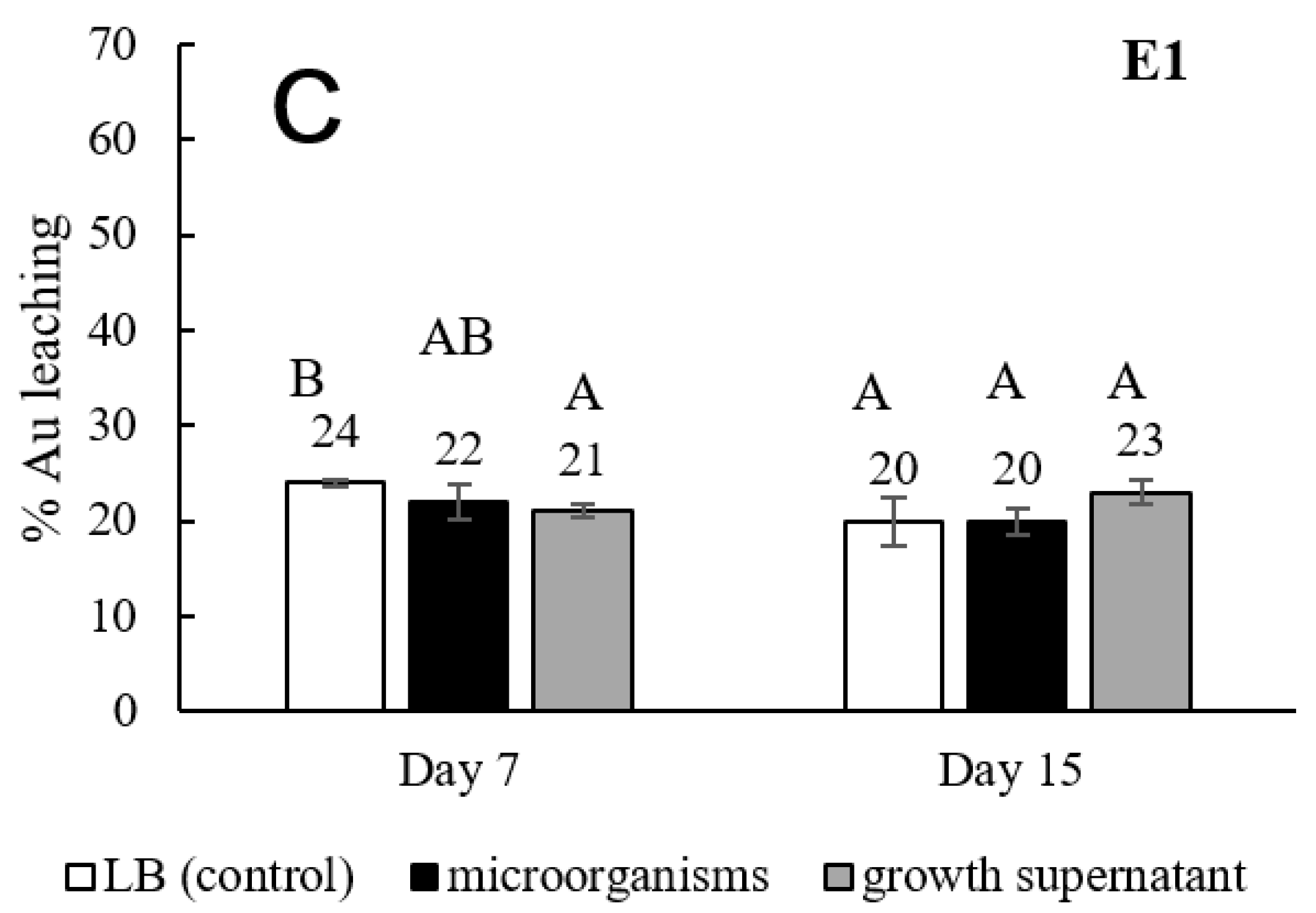
3.1. Screening, Isolation, and Ability of Gold Bioleaching Bacteria
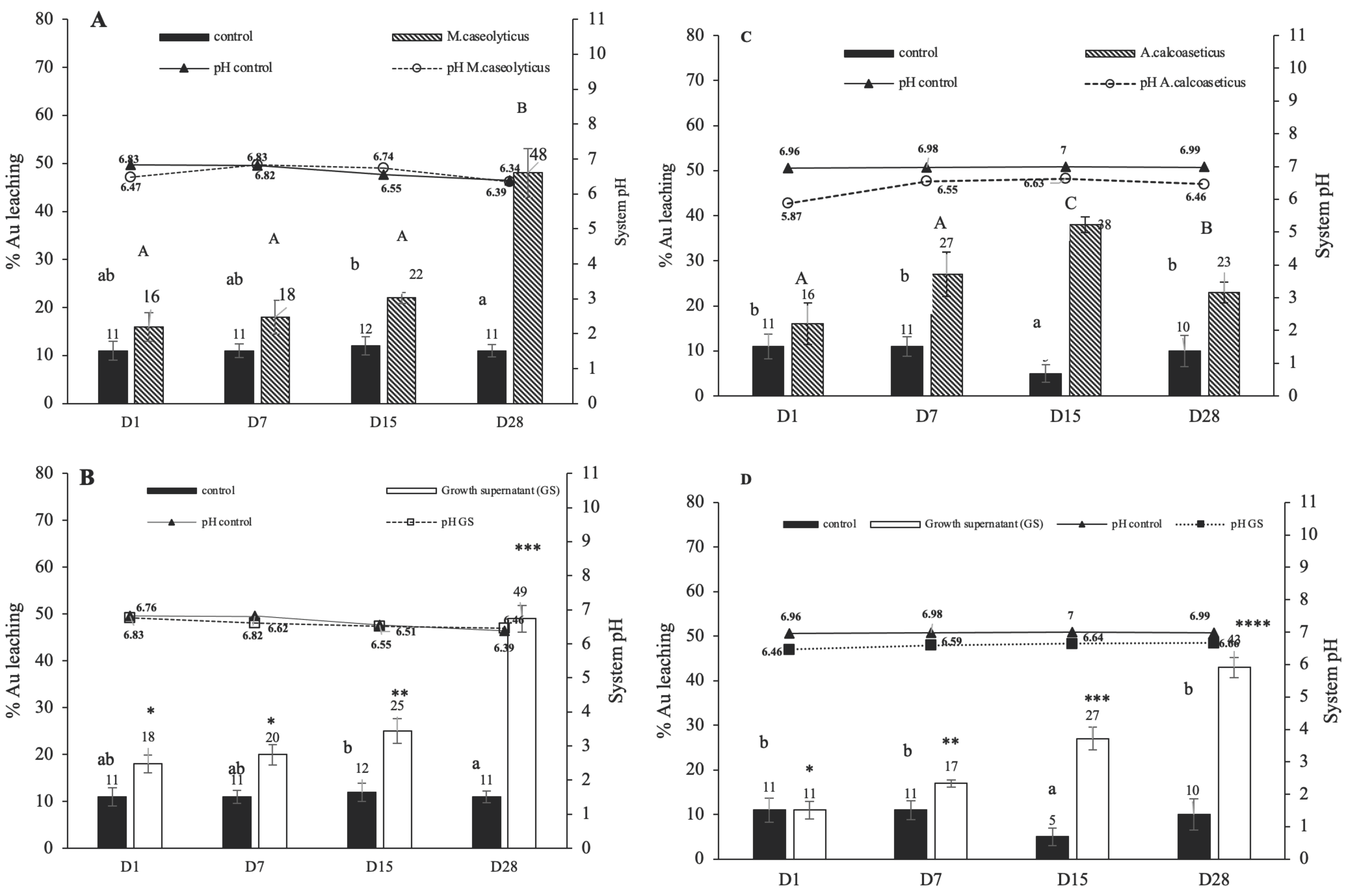
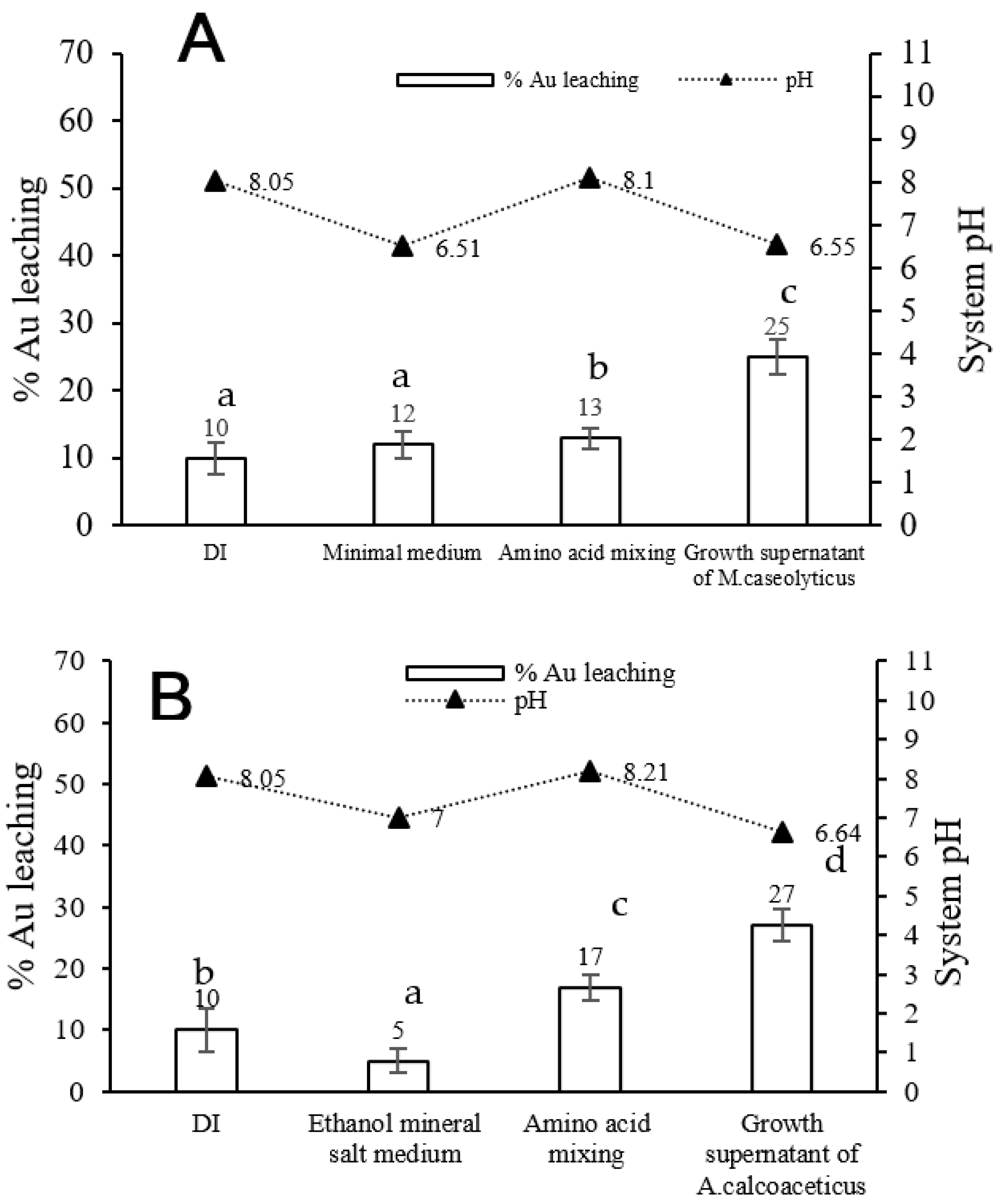
3.2. Effect of Medium Component on Gold Bioleaching from Ores
3.3. Influence of Amino Acids on Gold Bioleaching
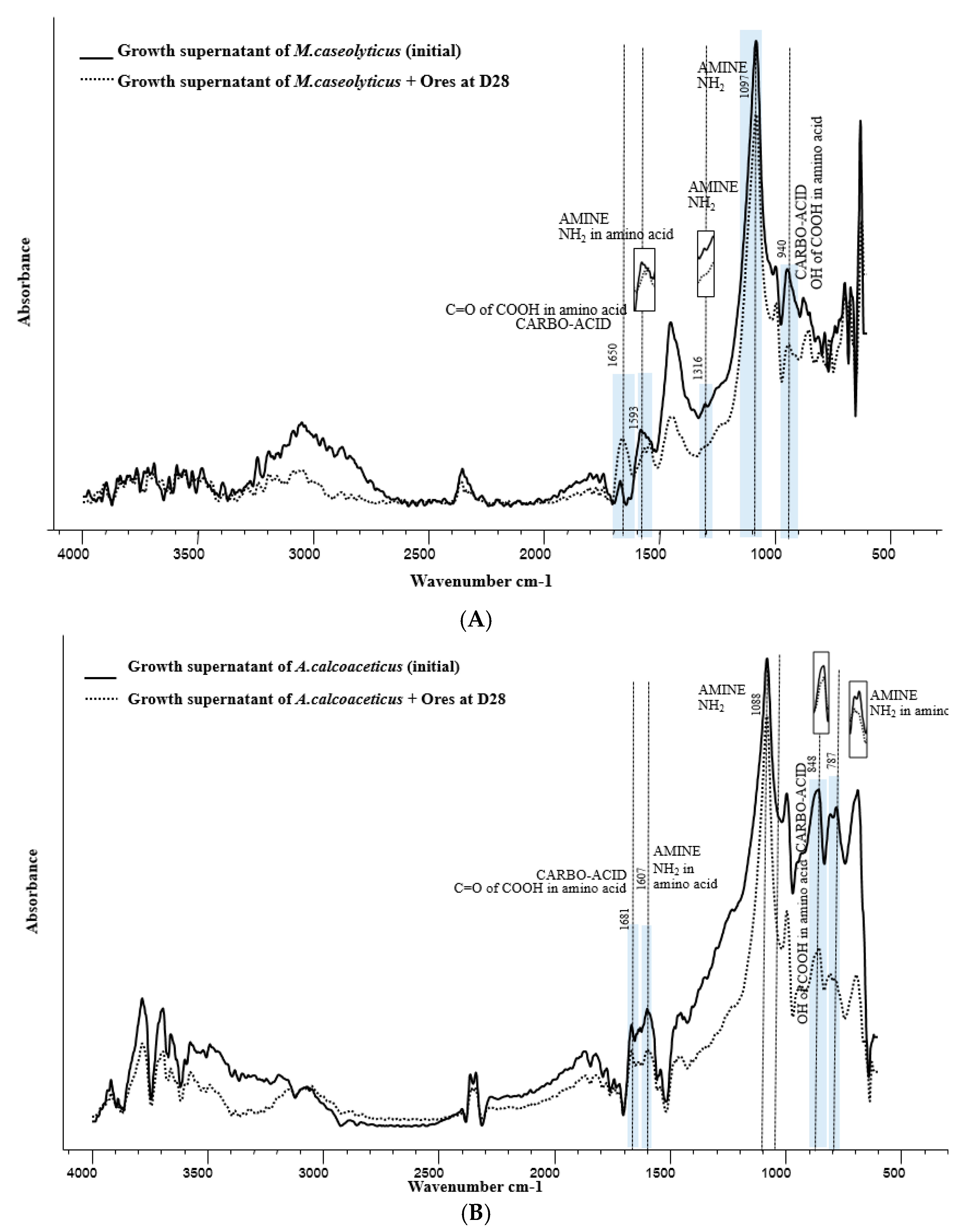
3.4. FTIR Analysis for Functional Groups on Growth Supernatant of M. Caseolyticus and A. Calcoaceticus Involved in Gold Bioleaching
3.5. Comparison of Gold Bioleaching Efficiency by Growth Supernatant of Isolated Strains and Mixed Amino Acids
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avraamides, J. Prospects for alternative leaching systems for gold: A review. In Carbon-in-Pulp Technology for the Extraction of Gold; Australasian Institute of Mining and Metallurgy: Carlton, Australia, 1982; pp. 369–391. [Google Scholar]
- Perea, C.; Restrepo, O. Use of amino acids for gold dissolution. Hydrometallurgy 2018, 177, 79–85. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. Gold leaching in cyanide-starved copper solutions in the presence of glycine. Hydrometallurgy 2015, 156, 81–88. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J. The leaching of gold, silver and their alloys in alkaline glycine–peroxide solutions and their adsorption on carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Adams, M. Chloride as an Alternative Lixiviant to Cyanide for Gold Ores. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 525–531. [Google Scholar]
- Aylmore, M. Alternative Lixiviants to Cyanide for Leaching Gold Ores. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 447–484. [Google Scholar]
- Aylmore, M. Thiosulfate as an Alternative Lixiviant to Cyanide for Gold Ores. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 485–523. [Google Scholar]
- Senanayake, G. Gold leaching in non-cyanide lixiviant systems: critical issues on fundamentals and applications. Miner. Eng. 2004, 17, 785–801. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Olson, G.J. Microbial oxidation of gold ores and gold bioleaching. FEMS Microbiol. Lett. 1994, 119, 1–6. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The role of microorganisms in gold processing and recovery—A review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Sun, J.; Kan, F.; Liu, P.; He, S.; Mou, H.; Xue, C.; Mao, X. Screening of Microorganisms from Deep-Sea Mud for Antarctic Krill (Euphausia superba) Fermentation and Evaluation of the Bioactive Compounds. Appl. Biochem. Biotechnol. 2014, 175, 1664–1677. [Google Scholar] [CrossRef]
- Korobushkina, E.D.; Karavaiko, G.I.; Korobushkin, I.M. Biochemistry of Gold. Ecological Bulletins. 1983. Available online: https://www.jstor.org/stable/20112867 (accessed on 20 December 2020).
- Komnitsas, C.; Pooley, F. Bacterial oxidation of an arsenical gold sulphide concentrate from Olympias, Greece. Miner. Eng. 1990, 3, 295–306. [Google Scholar] [CrossRef]
- Reith, F.; Lengke, M.F.; Falconer, D.; Craw, D.; Southam, G. The geomicrobiology of gold. ISME J. 2007, 1, 567–584. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J.; O’Connor, G.M. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 1–10. [Google Scholar] [CrossRef]
- Oraby, E.A.; Li, H.; Eksteen, J.J. An Alkaline Glycine-Based Leach Process of Base and Precious Metals from Powdered Waste Printed Circuit Boards. Waste Biomass Valorization 2019, 11, 3897–3909. [Google Scholar] [CrossRef]
- Altinkaya, P.; Wang, Z.; Korolev, I.; Hamuyuni, J.; Haapalainen, M.; Kolehmainen, E.; Yliniemi, K.; Lundström, M. Leaching and recovery of gold from ore in cyanide-free glycine media. Miner. Eng. 2020, 158, 106610. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J. The selective leaching of copper from a gold–copper concentrate in glycine solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; Tanda, B. Gold and copper leaching from gold-copper ores and concentrates using a synergistic lixiviant mixture of glycine and cyanide. Hydrometallurgy 2017, 169, 339–345. [Google Scholar] [CrossRef]
- Maneesuwannarat, S.; Vangnai, A.S.; Yamashita, M.; Thiravetyan, P. Bioleaching of gallium from gallium arsenide by Cellulosimicrobium funkei and its application to semiconductor/electronic wastes. Process. Saf. Environ. Prot. 2016, 99, 80–87. [Google Scholar] [CrossRef]
- Maneesuwannarat, S.; Teamkao, P.; Vangnai, A.S.; Yamashita, M.; Thiravetyan, P. Possible mechanism of gallium bioleaching from gallium nitride (GAN) by Arthrobacter creatinolyticus: Role of amino acids/peptides/proteins bindings with gallium. Process. Saf. Environ. Prot. 2016, 103, 36–45. [Google Scholar] [CrossRef]
- Brown, D.; Smith, W.; Fox, P.; Sturrock, R. The reactions of gold(0) with amino acids and the significance of these reactions in the biochemistry of gold. Inorganica Chim. Acta 1982, 67, 27–30. [Google Scholar] [CrossRef]
- MacLeod, R.A.; Kuo, S.C.; Gelinas, R. Metabolic injury to bacteria II, Metabolic injury in-duced by distilled water or Cu++ in the plating diluent. J. Bacteriol. 1967, 93, 961–969. Available online: https://jb.asm.org/content/93/3/961/article-info (accessed on 20 December 2020). [CrossRef] [PubMed] [Green Version]
- Sar, N.; Rosenberg, E. Emulsifier production byAcinetobacter calcoaceticus strains. Curr. Microbiol. 1983, 9, 309–313. [Google Scholar] [CrossRef]
- Jingrong, Z.; Jianjun, L.; Fan, Y.; Jingwei, W.; Fahua, Z. An experimental study on gold solubility in amino acid solution and its geological significance. Chin. J. Geochem. 1996, 15, 296–302. [Google Scholar] [CrossRef]
- Maneesuwannarat, S.; Kudpeng, K.; Yingchutrakul, Y.; Roytrakul, S.; Vangnai, A.S.; Yamashita, M.; Thiravetyan, P. A possible protein model involved in gallium arsenide leaching by Cellulosimicrobium funkei. Miner. Eng. 2019, 137, 207–216. [Google Scholar] [CrossRef]
- Fein, J.B.; Martin, A.M.; Wightman, P.G. Metal adsorption onto bacterial surfaces: develop-ment of a predictive approach. Geochimica et Cosmochimica Acta 2001, 65, 4267–4273. [Google Scholar] [CrossRef]

| SiO2 | CaO | Al2O3 | MgO | Fe2O3 | K2O | SO3 | MnO | TiO2 | ZnO |
| 65 | 9.61 | 7.6 | 6.67 | 4.2 | 3.35 | 1.69 | 0.42 | 0.238 | 0.191 |
| Na2O | P2O5 | BaO | PbO | CuO | V2O5 | Ag | SrO | Cr2O3 | Rb2O |
| 0.143 | 0.0942 | 0.0811 | 0.0437 | 0.0348 | 0.031 | 0.0269 | 0.0079 | 0.00722 | 0.00575 |
| Amino Acid | Minimal Medium | Initial Growth Supernatant of M. caseolyticus (nmol) | Growth Supernatant of M. caseolyticus + Ores at Various Time | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 15 | Day 28 | |||
| Aspartic acid | nd | nd | nd | nd | nd | nd |
| Serine | nd | nd | nd | nd | nd | nd |
| Glutamic acid | nd | nd | nd | nd | nd | nd |
| Glycine | nd | nd | nd | nd | nd | nd |
| Histidine | nd | nd | nd | nd | nd | nd |
| Arginine | nd | 3.96 ± 5.21 | nd | nd | nd | nd |
| Threonine | nd | 13.22 ± 10.11 | nd | nd | nd | nd |
| Alanine | nd | 54.15 ± 39.52 | nd | nd | nd | nd |
| Proline | nd | nd | nd | nd | nd | nd |
| Cysteine | nd | nd | nd | nd | nd | nd |
| Tyrosine | nd | 7.09 ± 3.19 | nd | nd | nd | nd |
| Valine | nd | 11.89 ± 4.54 | nd | nd | nd | nd |
| Methionine | nd | nd | nd | nd | nd | nd |
| Lysine | nd | 3.68 ± 5.2 | nd | nd | nd | nd |
| Isoleucine | nd | 7.71 ± 4.37 | nd | nd | nd | nd |
| Leucine | nd | 11.38 ± 6.01 | nd | nd | nd | nd |
| Phenylalanine | nd | 7.33 ± 4.56 | nd | nd | nd | nd |
| Amino Acid | Ethanol Mineral Salt Medium | Initial Growth Supernatant of A. calcoaceticus (nmol) | Growth Supernatant of A. calcoaceticus + Ores at Various Time | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 15 | Day 28 | |||
| Aspartic acid | nd | nd | nd | nd | nd | nd |
| Serine | nd | 104.825 | nd | nd | 21.1 | 31.925 |
| Glutamic acid | nd | 986.7 | 106.05 | 45.7 | 36.2 | 65.875 |
| Glycine | nd | 356.15 | 81.325 | nd | 95.125 | 139.425 |
| Histidine | nd | nd | nd | nd | nd | nd |
| Arginine | nd | 110.525 | nd | nd | nd | nd |
| Threonine | nd | 130.475 | 16.075 | nd | nd | nd |
| Alanine | nd | 589.875 | nd | nd | nd | nd |
| Proline | nd | 90.375 | nd | nd | nd | nd |
| Cysteine | nd | nd | nd | nd | nd | nd |
| Tyrosine | nd | 88.2 | 24.45 | 22.8 | nd | 35.15 |
| Valine | nd | 286.2 | nd | nd | nd | nd |
| Methionine | nd | 46.5 | nd | nd | nd | nd |
| Lysine | nd | 125.725 | nd | nd | nd | nd |
| Isoleucine | nd | 101.025 | nd | nd | nd | nd |
| Leucine | nd | 121.125 | nd | nd | nd | nd |
| Phenylalanine | nd | 110.85 | nd | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudpeng, K.; Thayanukul, P.; Thiravetyan, P. Bioleaching of Gold from Silicate Ore by Macrococcus caseolyticus and Acinetobacter calcoaceticus: Effect of Medium, Amino Acids and Growth Supernatant. Minerals 2021, 11, 580. https://doi.org/10.3390/min11060580
Kudpeng K, Thayanukul P, Thiravetyan P. Bioleaching of Gold from Silicate Ore by Macrococcus caseolyticus and Acinetobacter calcoaceticus: Effect of Medium, Amino Acids and Growth Supernatant. Minerals. 2021; 11(6):580. https://doi.org/10.3390/min11060580
Chicago/Turabian StyleKudpeng, Kanjana, Parinda Thayanukul, and Paitip Thiravetyan. 2021. "Bioleaching of Gold from Silicate Ore by Macrococcus caseolyticus and Acinetobacter calcoaceticus: Effect of Medium, Amino Acids and Growth Supernatant" Minerals 11, no. 6: 580. https://doi.org/10.3390/min11060580






