Pyroxenite as a Product of Mafic-Carbonate Melt Interaction (Tazheran Massif, West Baikal Area, Russia)
Abstract
:1. Introduction
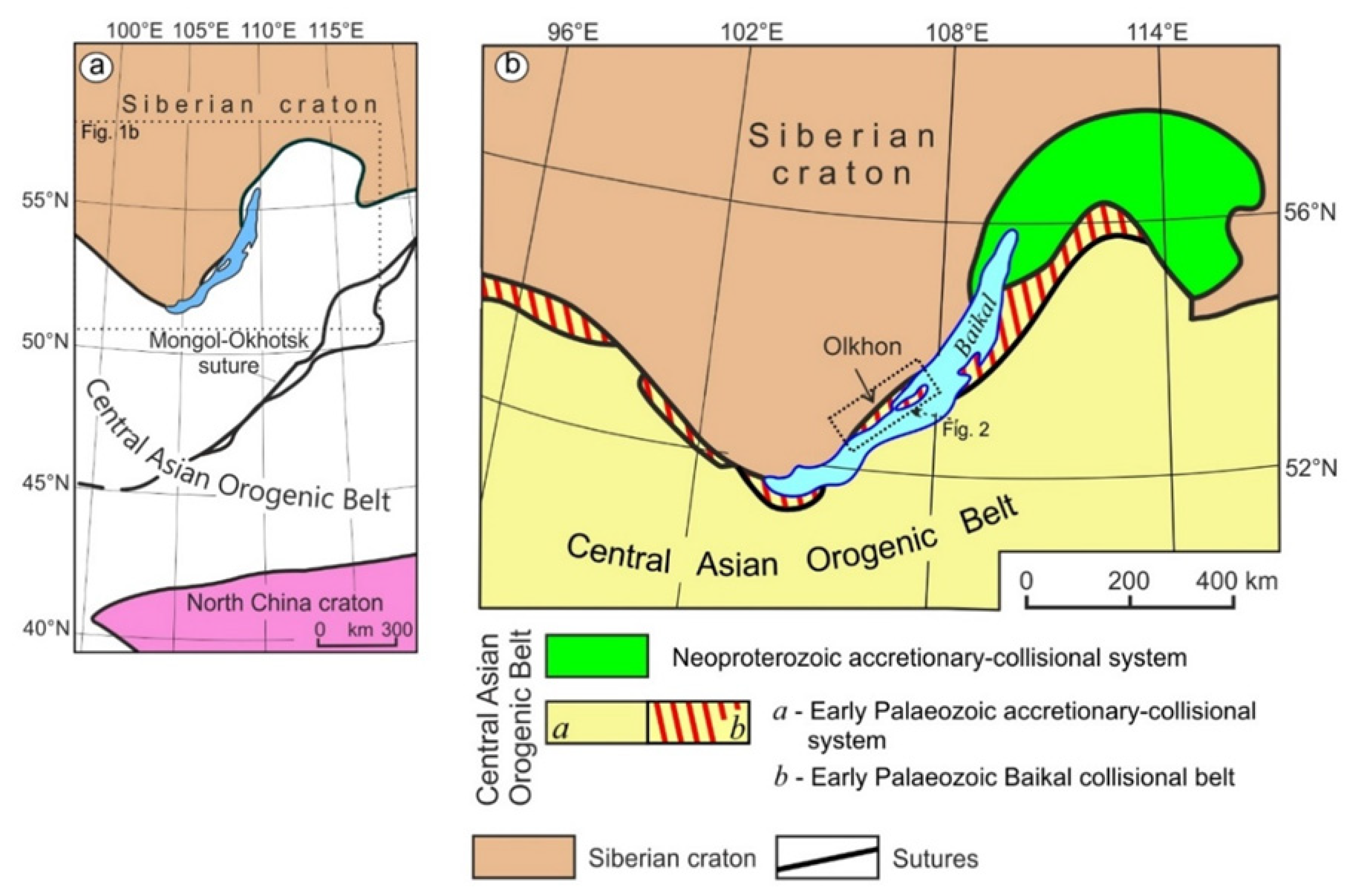
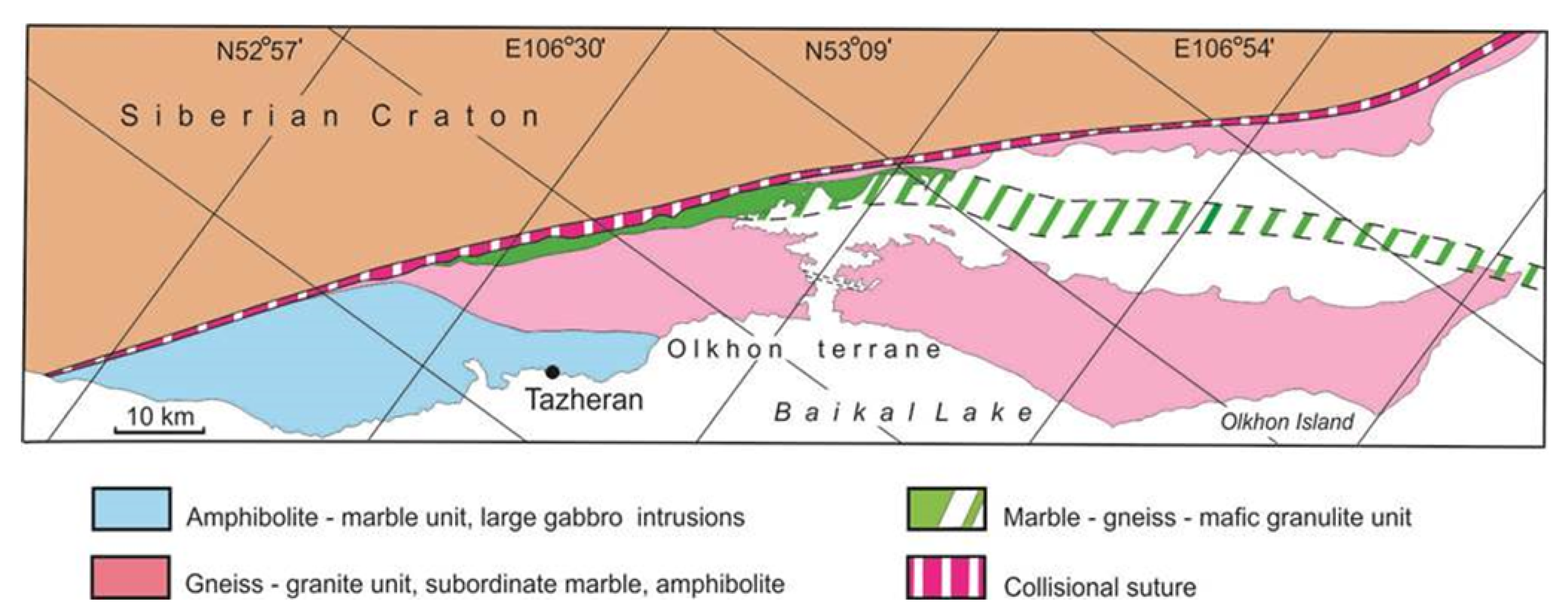
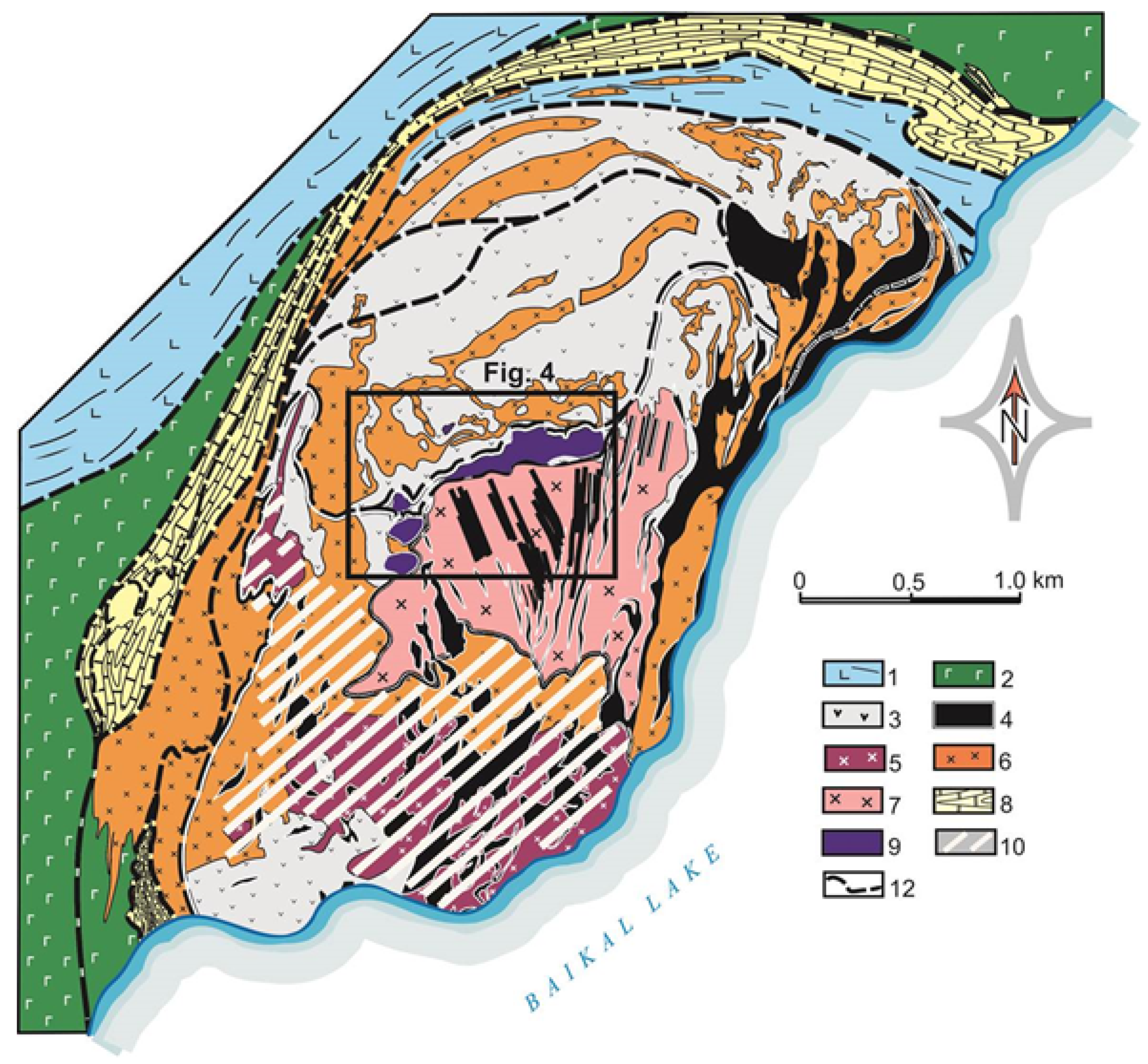
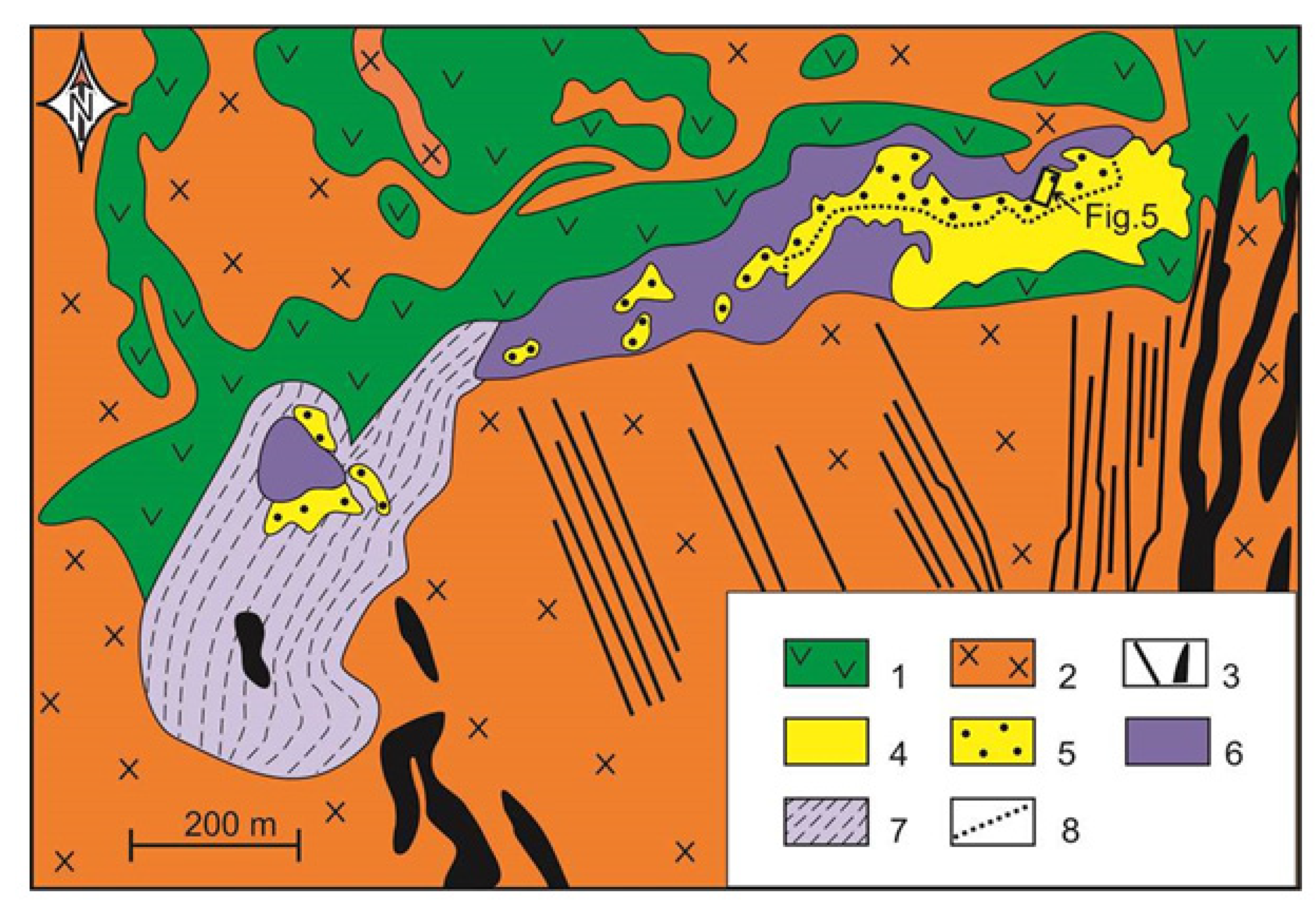
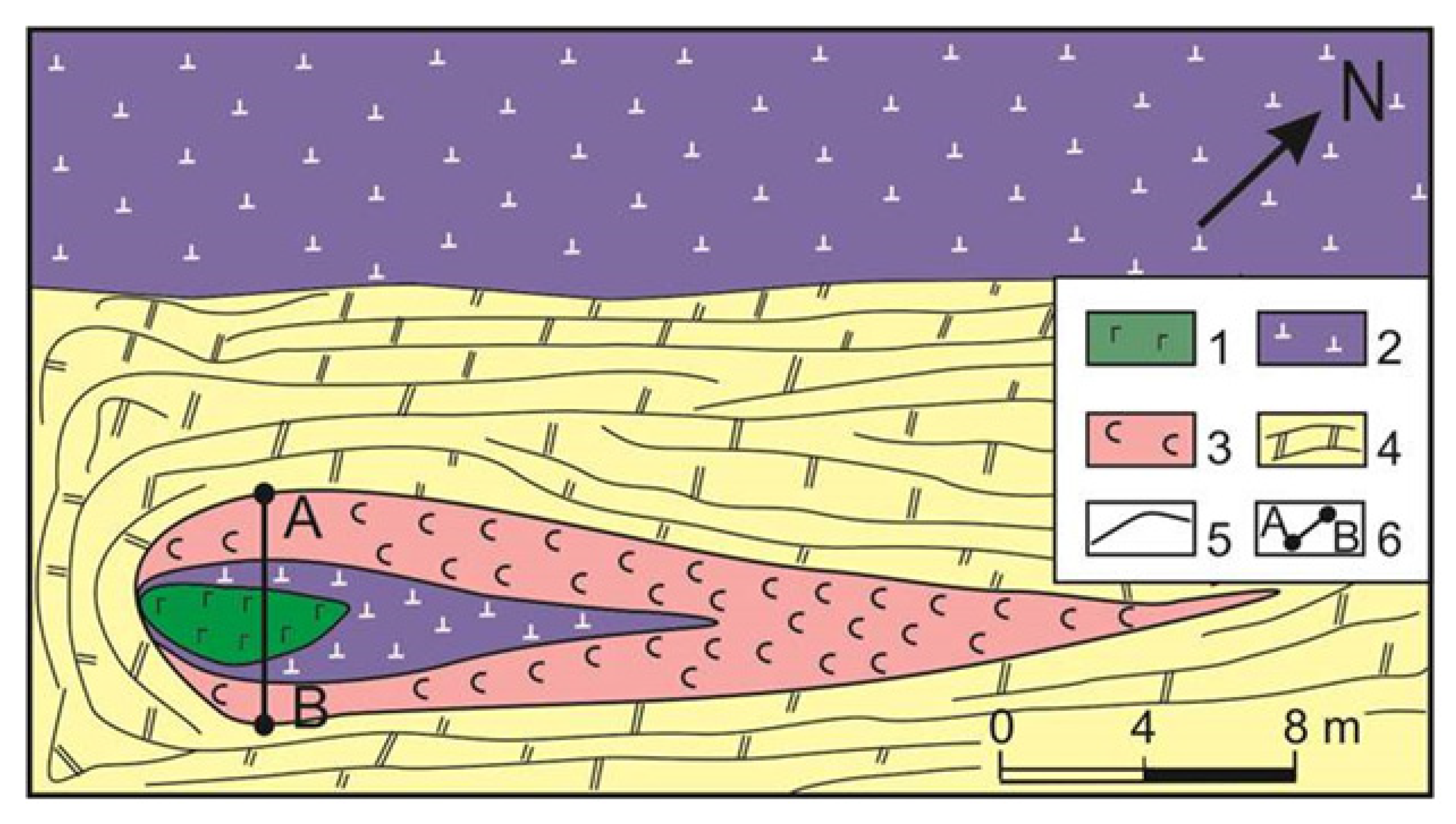
2. Geological Background
3. Analytical Methods
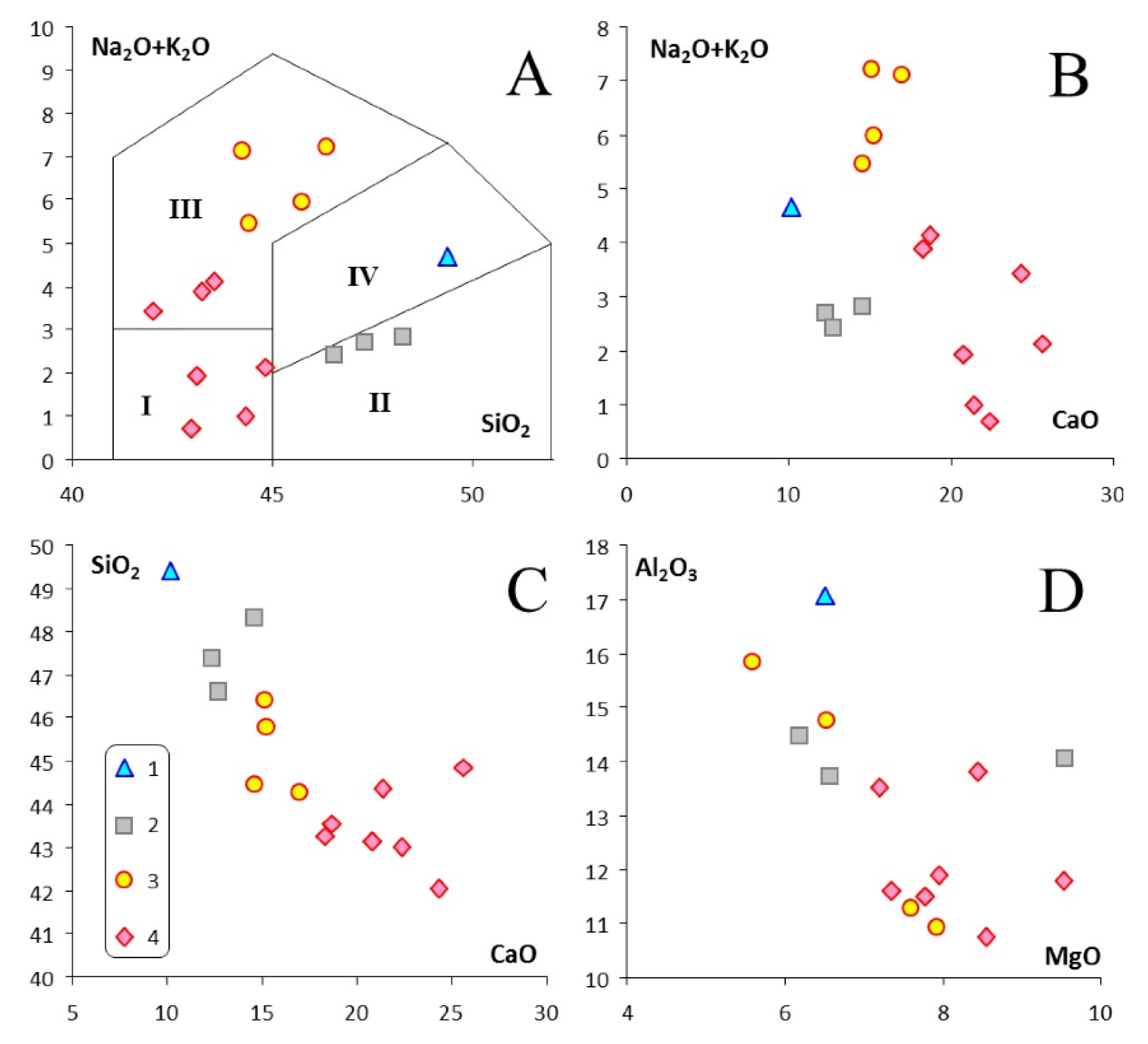
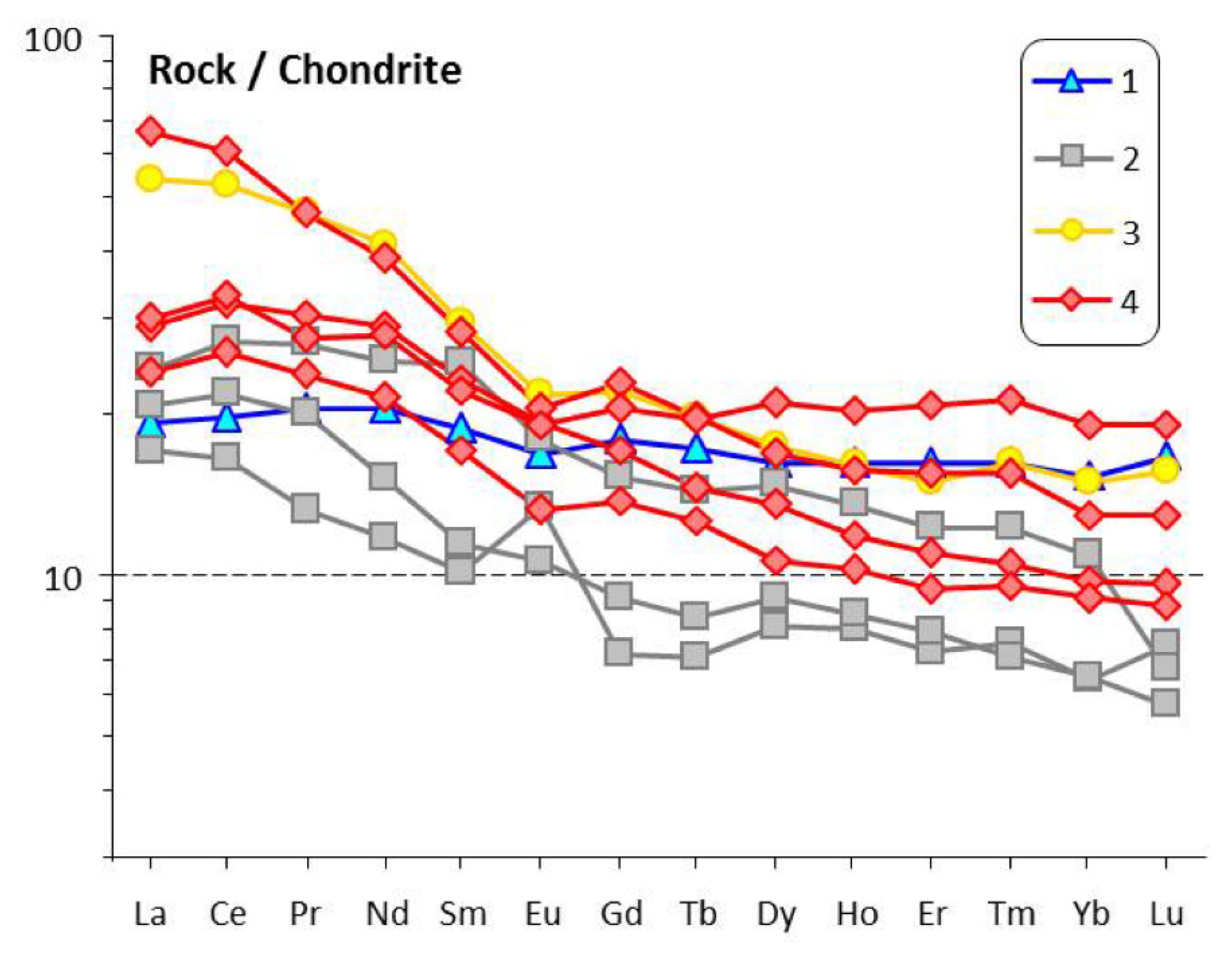
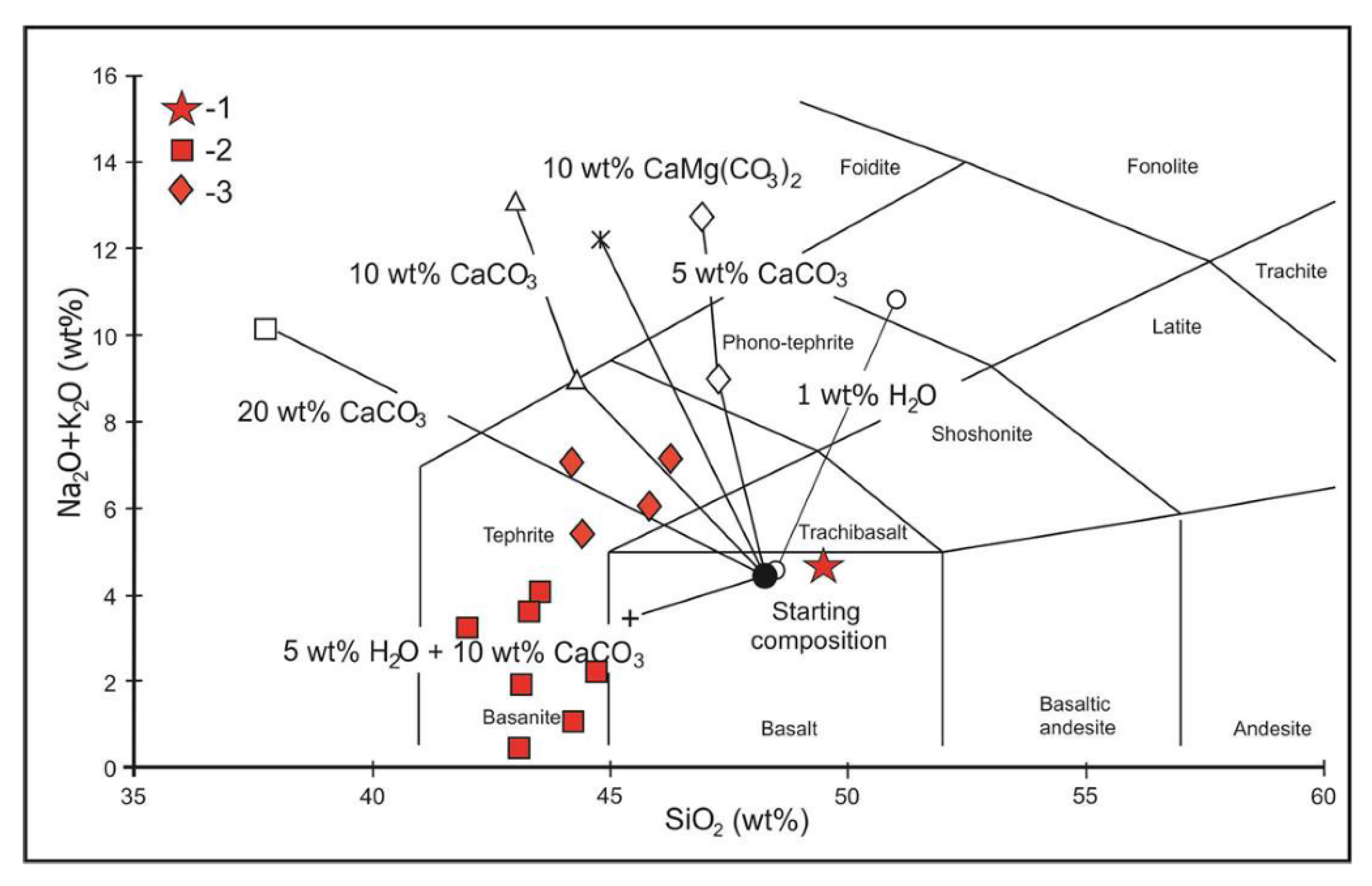
4. Major- and Trace-Element Chemistry of Pyroxenite and Nepheline-Pyroxene Rocks
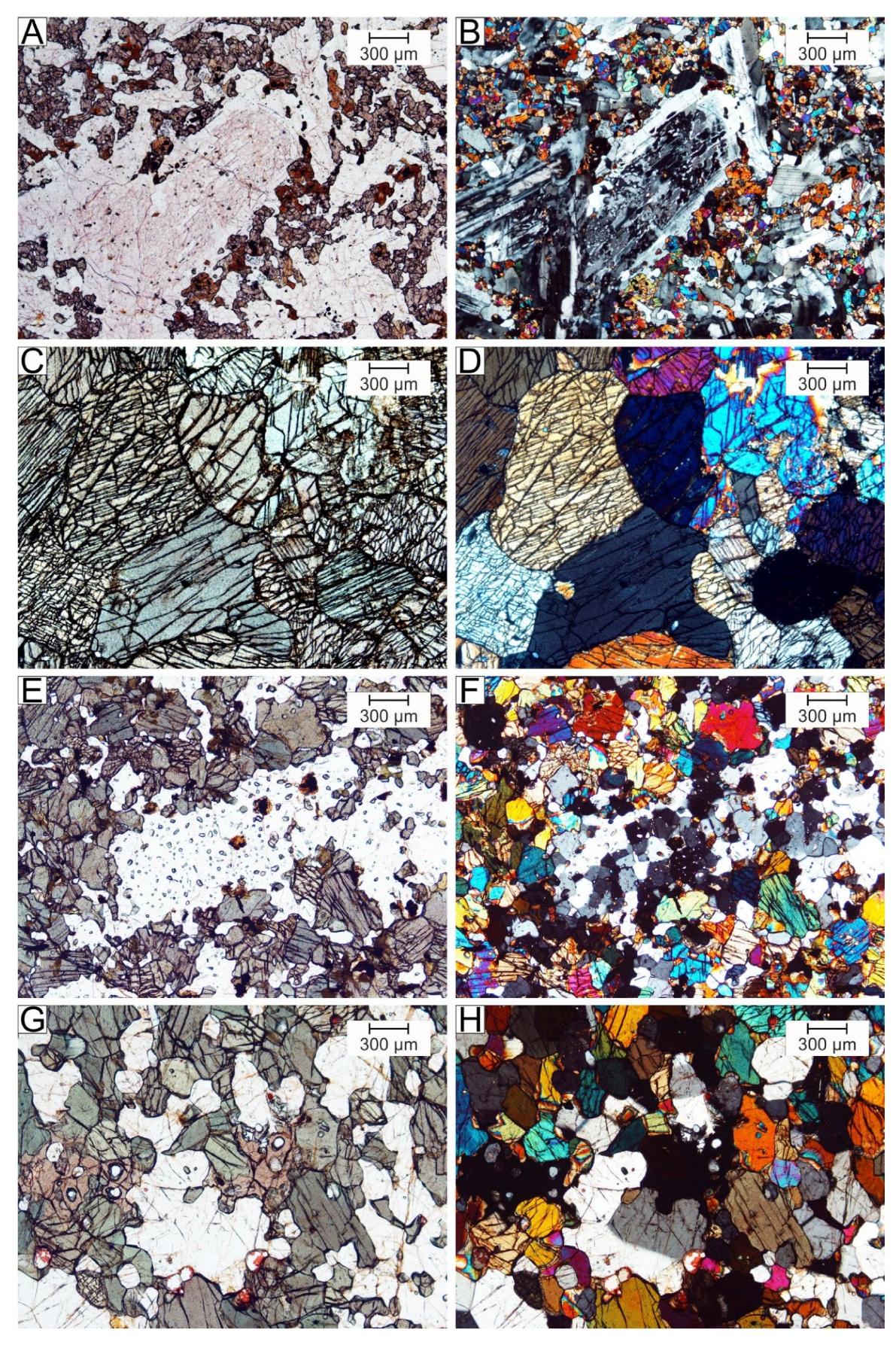
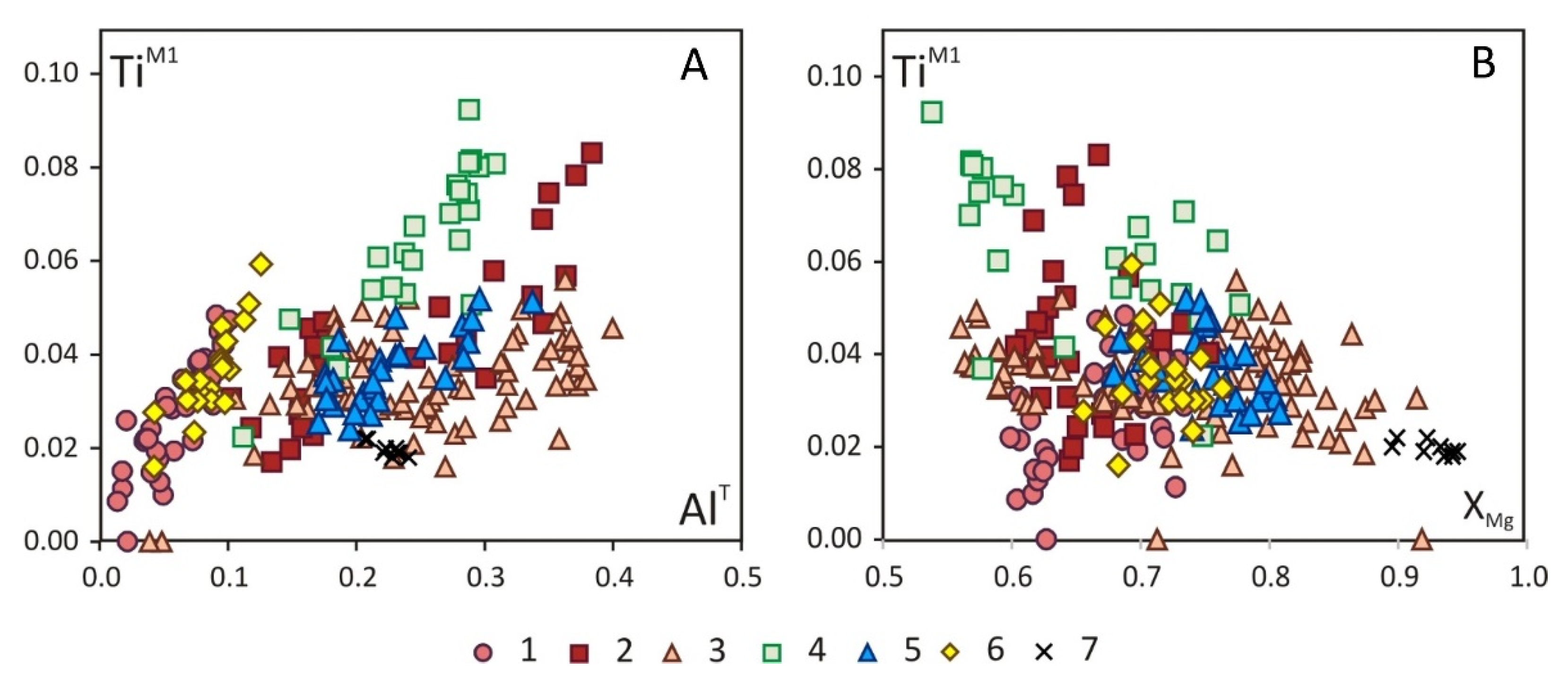
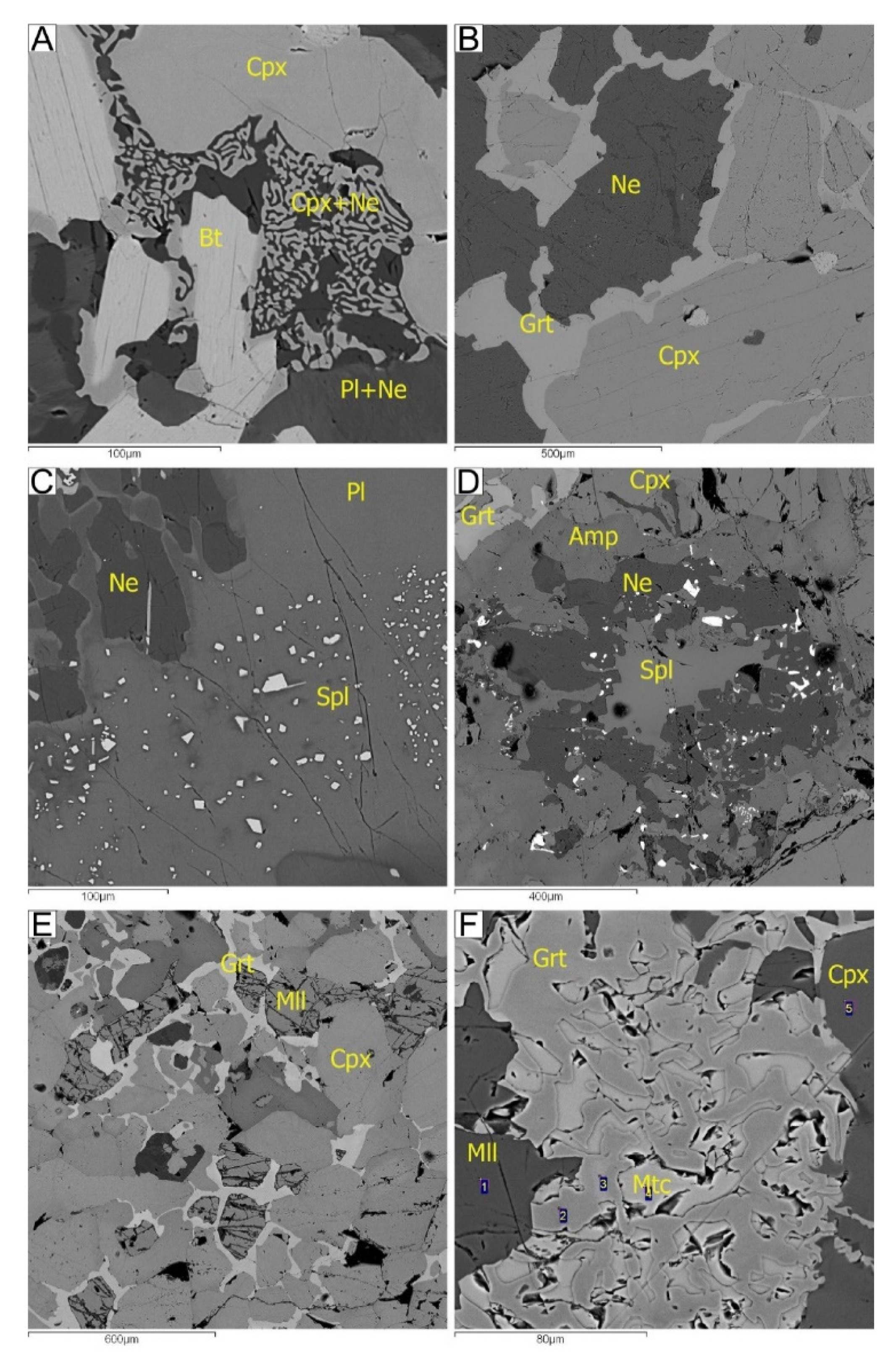
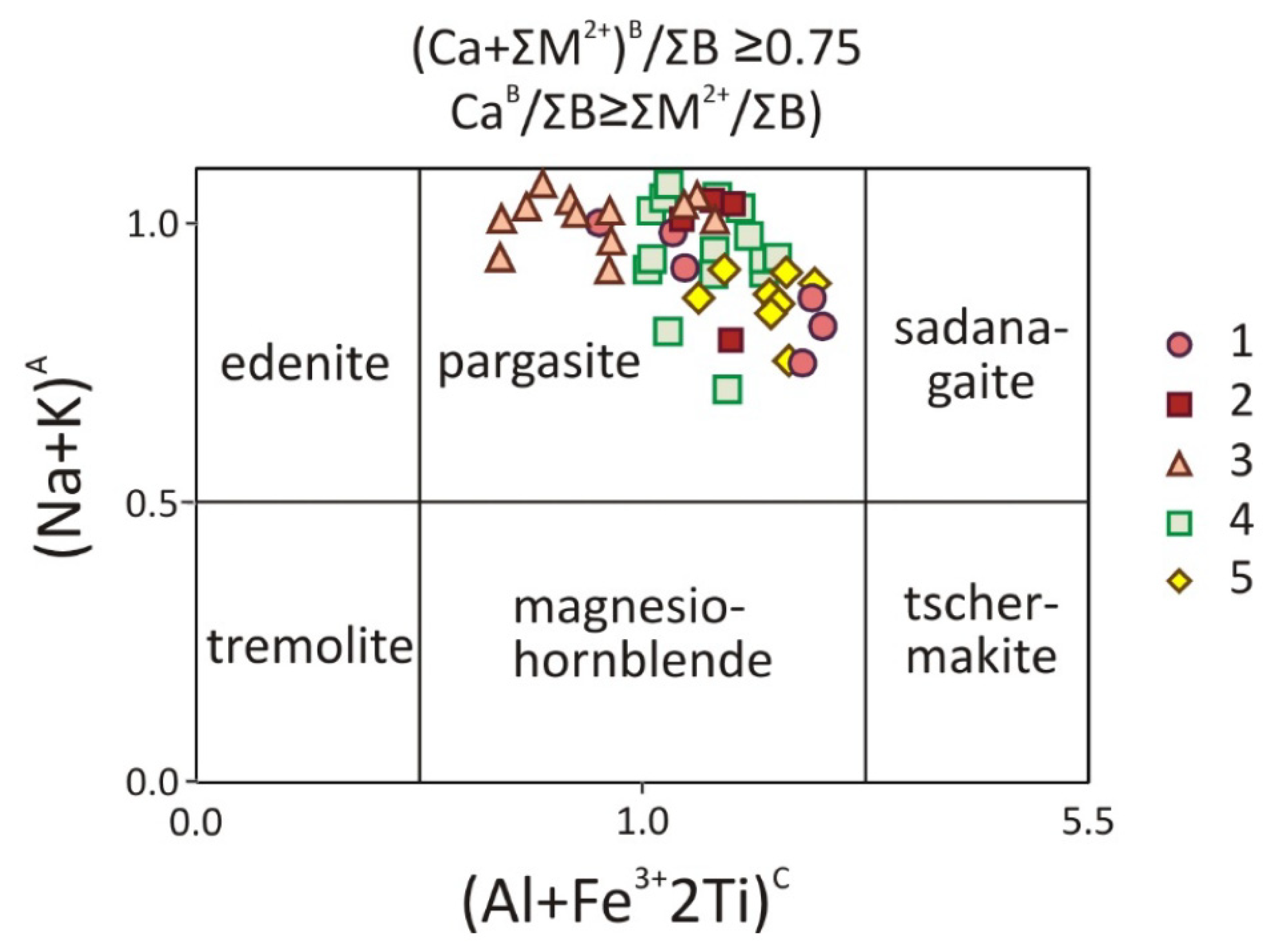
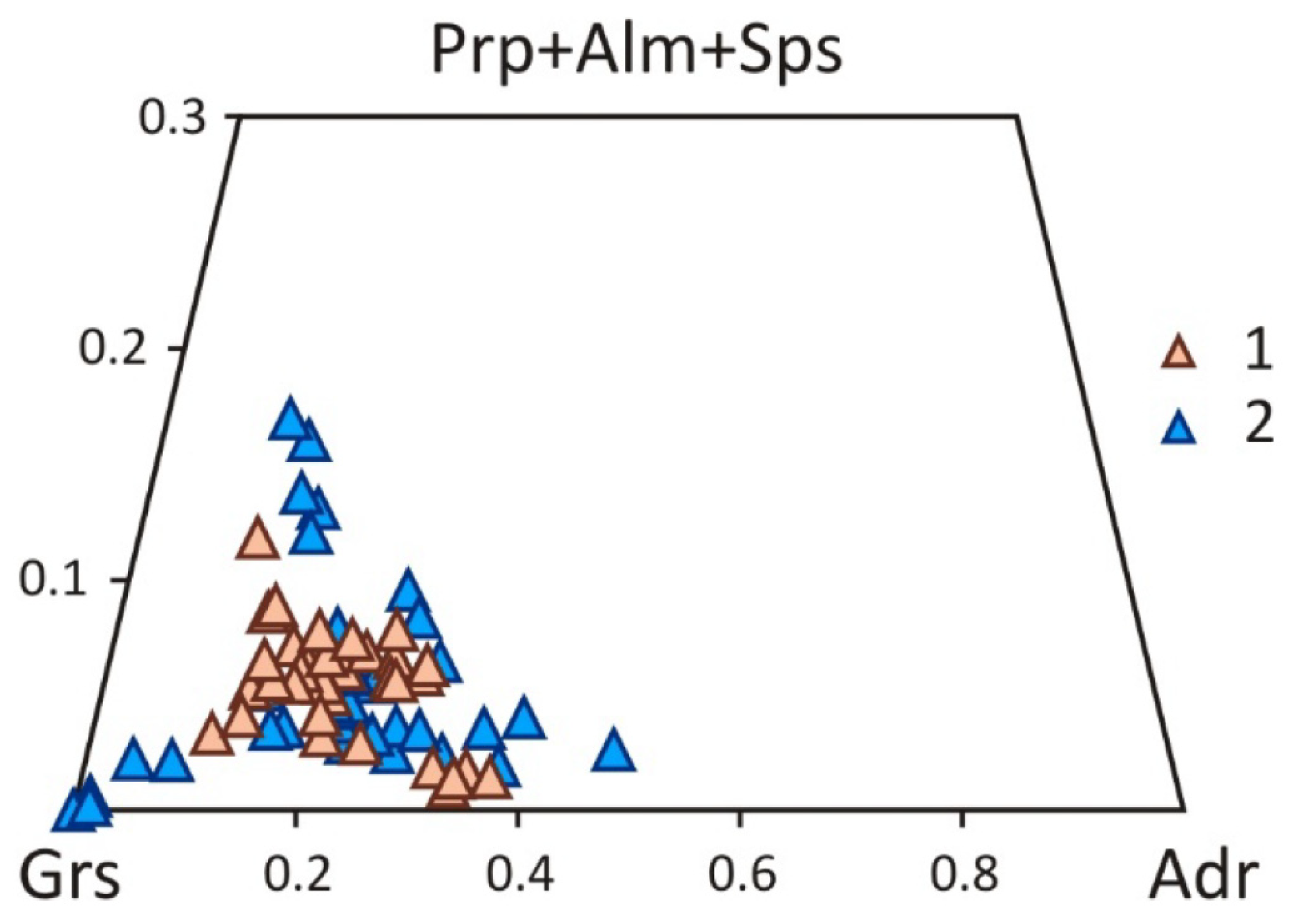
5. Mineralogy of Pyroxenite
6. Stable Isotopes (O, C)
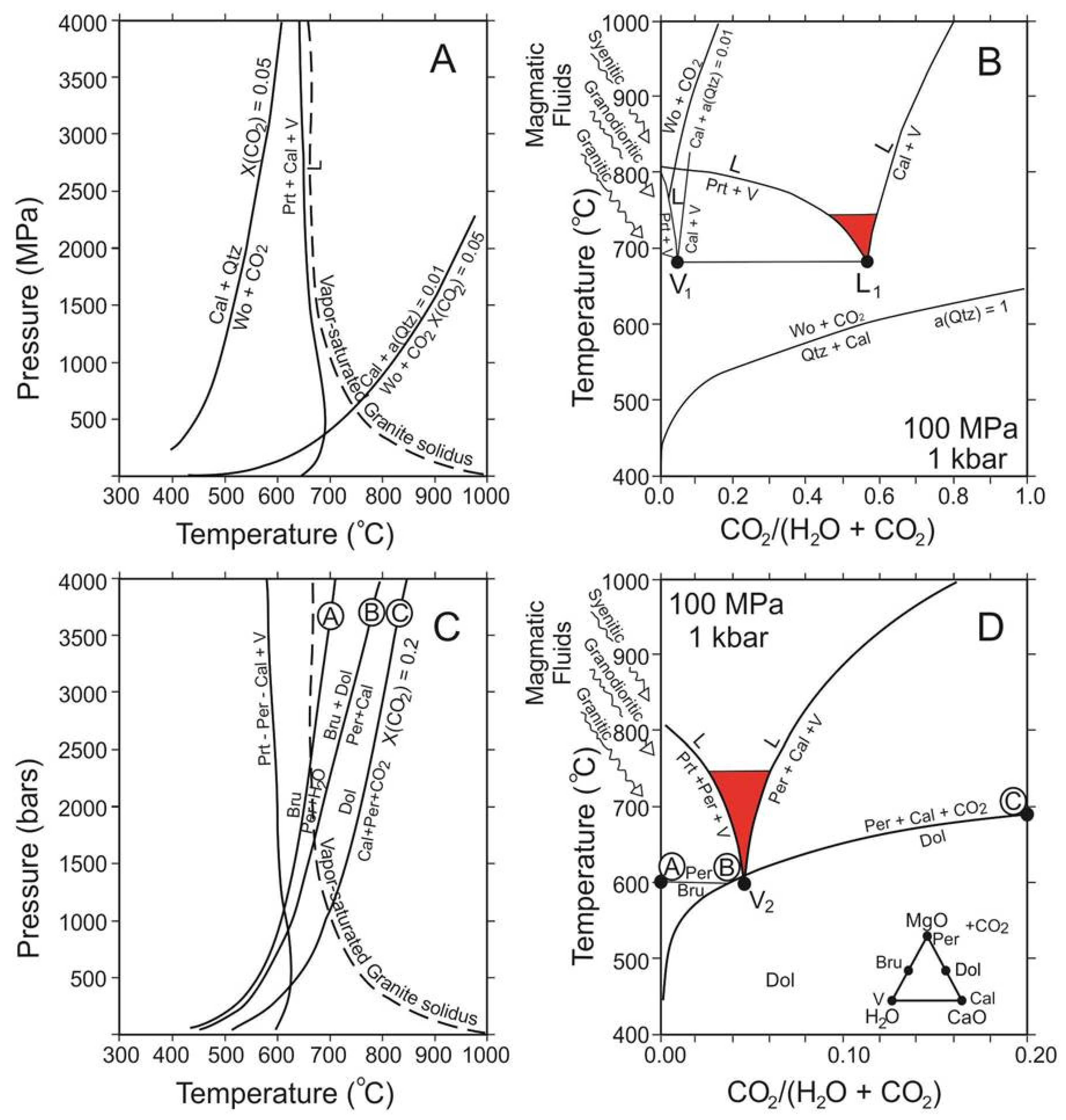
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yardley, B.W.; Kerrick, D.M. Contact Metamorphism. Washington DC (Mineralogical Society of America: Reviews in Mineralogy, Vol. 26), 1992, xvi+ 847 pp. Price $26.00. Mineral. Mag. 1993, 57, 359–360. [Google Scholar] [CrossRef]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19, 145–162. [Google Scholar] [CrossRef]
- Meinert, L.D. Igneous petrogenesis and skarn deposits. Geol. Assoc. Can. Spec. 2016, 40, 569–583. [Google Scholar]
- Freda, C.; Gaeta, M.; Misiti, V.; Mollo, S.; Dolfi, D.; Scarlato, P. Magma-carbonate interaction: An experimental study on ultrapotassic rocks from Alban Hills (Central Italy). Lithos 2010, 101, 397–415. [Google Scholar] [CrossRef]
- Iacono-Marziano, G.; Gaillard, F.; Pichavant, M. Limestone assimilation by basaltic magmas: An experimental re-assessment and application to Italian volcanoes. Contrib. Mineral. Petrol. 2008, 155, 719–738. [Google Scholar] [CrossRef] [Green Version]
- Gaeta, M.; Di Rocco, T.; Freda, C. Carbonate assimilation in open magmatic systems: The role of melt-bearing skarns and cumulate-forming processes. J. Petrol. 2009, 50, 361–385. [Google Scholar] [CrossRef] [Green Version]
- Di Rocco, T.; Freda, C.; Gaeta, M.; Mollo, S.; Dallai, L. Magma chambers emplaced in carbonate substrate: Petrogenesis of skarn and cumulate rocks and implications for CO2 degassing in volcanic areas. J. Petrol. 2012, 53, 2307–2332. [Google Scholar] [CrossRef] [Green Version]
- Ganino, C.; Arndt, T.; Chauvel, C.; Jean, A.; Athurion, C. Melting of carbonate wall rocks and formation of the heterogeneous aureole of the Panzhihua intrusion, China. Geosci. Front. 2013, 4, 535–546. [Google Scholar] [CrossRef]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Am. Mineral. 1988, 73, 1123–1133. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals. Volume 2A. Single-Chain Silicates; The Geological Society: London, UK; Alden Press: Oxford, UK, 1997; p. 668. [Google Scholar]
- Gilg, H.A.; Lima, A.; Somma, R.; Belkin, H.E.; De Vivo, B.; Ayuso, R.A. Isotope geochemistry and fluid inclusion study of skarns from Vesuvius. Miner. Petrol. 2001, 73, 145–176. [Google Scholar] [CrossRef]
- Wenzel, T.; Baumgartner, L.P.; Brugmann, G.E.; Konnikov, E.G.; Kislov, E.V.; Orsoev, D.A. Contamination of mafic magma by partial melting of dolomitic xenoliths. Terra Nova 2001, 13, 197–202. [Google Scholar] [CrossRef]
- Konev, A.A.; Samoilov, V.S. Contact Metamorphism and Metasomatism in the Aureole of the Tazheran Alkaline Intrusion; Nauka: Novosibirsk, Russia, 1974; p. 246. (In Russian) [Google Scholar]
- Fedorovsky, V.S.; Sklyarov, E.V.; Mazukabzov, A.M.; Kotov, A.B.; Kargopolov, S.A.; Lavrenchuk, A.V.; Starikova, A.E. Geological Map of the Tazheren Massif; Gruppa Kompanii A: Moscow, Russia, 2009. [Google Scholar]
- Sklyarov, E.V.; Fedorovsky, V.S.; Kotov, A.B.; Lavrenchuk, A.V.; Mazukabzov, A.M.; Levitsky, V.I.; Salnikova, E.B.; Starikova, A.E.; Yakovleva, S.Z.; Anisimova, I.V.; et al. Carbonatites in collisional settings and pseudo-carbonatites of the Early Paleozoic Ol’khon collisional system. Russ. Geol. Geophys. 2009, 50, 1091–1106. [Google Scholar] [CrossRef]
- Donskaya, T.V.; Sklyarov, E.V.; Gladkochub, D.P.; Mazukabzov, A.M. The Baikal collisional metamorphic belt. Doklady Earth Sci. 2000, 374, 1075–1079. [Google Scholar]
- Donskaya, T.V.; Gladkochub, D.P.; Fedorovsky, V.S.; Sklyarov, E.V.; Cho, M.; Sergeev, S.A.; Demonterova, E.I.; Mazukabzov, A.M.; Lepekhina, E.N.; Cheong, W.; et al. Pre-collisional (0.5 Ga) complexes of the Olkhon terrane (southern Siberia) as an echo of events in the Central Asian Orogenic Belt. Gondwana Res. 2017, 42, 243–263. [Google Scholar] [CrossRef]
- Fedorovsky, V.S.; Sklyarov, E.V. The Ol’khon Geodynamic Research Site (Baikal): High-Resolution Aerospace Data and Geological Maps of New Generations. Geodyn. Tectonophys. 2010, 1, 331–418, (in Russian with English abstract). [Google Scholar] [CrossRef]
- Fedorovsky, V.S.; Sklyarov, E.V.; Gladkochub, D.P.; Mazukabzov, A.M.; Donskaya, T.V.; Lavrenchuk, A.V.; Starikova, A.E.; Dobretsov, N.L.; Kotov, A.B.; Tevelev, A.V. Aerospace Geological Map of the Olkhon Region (Baikal, Russia); Copymaster Prof. Center: Moscow, Russia, 2017. [Google Scholar]
- Fedorovsky, V.S.; Sklyarov, E.V.; Gladkochub, D.P.; Mazukabzov, A.M.; Donskaya, T.V.; Lavrenchuk, A.V.; Starikova, A.E.; Dobretsov, N.L.; Kotov, A.B.; Tevelev, A.V. Collision system of West Pribaikalie: Aerospace geological map of Olkhon region (Baikal, Russia). Geodyn. Tectonophys. 2020, 11, 447–452. [Google Scholar] [CrossRef]
- Sklyarov, E.V.; Fedorovsky, V.S.; Kotov, A.B.; Lavrenchuk, A.V.; Mazukabzov, A.M.; Starikova, A.E. Carbonate and silicate-carbonate injection complexes in collision systems: The West Baikal region as an example. Geotectonics 2013, 47, 180–196. [Google Scholar] [CrossRef] [Green Version]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Fedorovsky, V.S.; Sklyarov, E.V.; Izokh, A.E.; Kotov, A.B.; Lavrenchuk, A.V.; Mazukabzov, A.M. Strike-slip tectonics and subalkaline mafic magmatism in the Early Paleozoic collisional system of the western Baikal region. Russ. Geol. Geophys. 2010, 51, 534–547. [Google Scholar] [CrossRef]
- Starikova, A.E.; Sklyarov, E.V.; Kotov, A.B.; Salnikova, E.B.; Fedorovskii, V.S.; Lavrenchuk, A.V.; Mazukabzov, A.M. Vein calciphyre and contact Mg skarn from the Tazheran Massif (Western Baikal area, Russia): Age and genesis. Doklady Earth Sci. 2014, 457, 1003–1007. [Google Scholar] [CrossRef]
- Sklyarov, E.V.; Karmanov, N.S.; Lavrenchuk, A.V.; Starikova, A.E. Perovskites of the Tazheran Massif (Baikal, Russia). Minerals 2019, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Sharp, Z.D. A laser-based microanalytical method for the in situ determination of oxygen isotope ratios of silicates and oxides. Geochim. Cosmochim. Acta 1990, 54, 1353–1357. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Naming materials in the magma/igneous rock system. Earth Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Lavrenchuk, A.V.; Sklyarov, E.V.; Izokh, A.E.; Kotov, A.B.; Sal’nikova, E.B.; Fedorovskii, V.S.; Mazukabzov, A.M. Compositions of gabbro intrusions in the Krestovsky Zone (Western Baikal Region): A record of plume-suprasubduction mantle interaction. Russ. Geol. Geophys. 2017, 58, 1139–1153. [Google Scholar] [CrossRef]
- Boynton, W.V. Cosmochemistry of the rare earth elements: Meteorite studies. Dev. Geochem. 1984, 2, 63–114. [Google Scholar]
- Mollo, S.; Gaeta, M.; Freda, C.; Di Rocco, T.; Misiti, V.; Scarlato, P. Carbonate assimilation in magmas: A reappraisal based on experimental petrology. Lithos 2010, 114, 503–514. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Wiedenmann, D.; Zaitsev, A.N.; Britvin, S.N.; Krivovechev, S.V.; Keller, J. Alumoakermanite, (Ca, Na)2(Al, Mg Fe2+)(Si2O7), a new mineral from the active carbonatite-nephelinite-phonolite volcano Oldonyo Lengai, Northern Tanzania. Min. Mag. 2009, 73, 373–384. [Google Scholar] [CrossRef]
- Wiedenmann, D.; Keller, J.; Zaitsev, A.N. Melilite-group minerals at Oldoinyo Lengai, Tanzania. Lithos 2010, 118, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Yoder, H.S. Melilite stability and paragenesis. Fortschr. Miner. 1973, 50, 140–173. [Google Scholar]
- Doroshkevich, A.; Sklyarov, E.; Starikova, A.; Vasiliev, V.; Ripp, G.; Izbrodin, I.; Posokhov, V. Stable isotope (C, O, H) characteristics and genesis of the Tazheran brucite marbles and skarns, Olkhon region, Russia. Miner. Petrol. 2016, 115, 153–169. [Google Scholar] [CrossRef]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in anhydrous silicate minerals. Geochim. Cosmochim. Acta 1993, 57, 1079–1091. [Google Scholar] [CrossRef]
- Valley, J.W. Oxygen isotopes in zircon. Rev. Mineral. Geochem. 2003, 53, 343–385. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Liu, Y.; Wu, F.; Yang, J.; Mitchell, R.H. Precise U–Pb and Th–Pb age determination of kimberlitic perovskites by secondary ion mass spectrometry. Chem. Geol. 2010, 269, 396–405. [Google Scholar] [CrossRef]
- Lavrenchuk, A.V.; Sklyarov, E.V.; Izokh, A.E.; Kotov, A.B.; Vasyukova, E.A.; Fedorovskii, V.S.; Gladkochub, D.P.; Donskaya, T.V.; Mazukabzov, A.M. Birkhin volcanoplutonic association, Ol’khon Region, Western Baikal Area: Petrological criteria of comagmatic origin. Petrology 2019, 27, 291–306. [Google Scholar] [CrossRef]
- Sklyarov, E.V.; Lavrenchuk, A.V.; Fedorovsky, V.S.; Gladkochub, D.P.; Donskaya, T.V.; Kotov, A.B.; Mazukabzov, A.M.; Starikova, A.E. Regional contact metamorphism and autometamorphism of the Olkhon Terrane (West Baikal Area). Petrology 2020, 28, 47–61. [Google Scholar] [CrossRef]
- Zharikov, A. Skarns. Parts I, II, III. Intern. Geol. Rev. 1970, 12, 541–559, 619–647, 760–775. [Google Scholar] [CrossRef]
- Burt, D.M. Mineralogy and petrology of skarn deposits. Soc. Ital. Mineral. Petrol. Rendic. 1977, 33, 859–873. [Google Scholar]
- Einaudi, M.T.; Meinert, L.D.; Newberry, R.I. Skarn deposits. Econ. Geol. 1981, 12, 327–391. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry; Springer Science & Business Media: Berlin, Germany, 2015; p. 404. [Google Scholar]
- Mitchell, R.H. Carbonatites and carbonatites and carbonatites. Can. Mineral. 2005, 43, 2049–2068. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Tuttle, O.F. The system CaO-CO2-H2O and the origin of carbonatites. J. Petrol. 1960, 1, 1–46. [Google Scholar] [CrossRef]
- Fanelli, M.T.; Cava, N.; Wyllie, P.J. Calcite and Dolomite without Portlandite at a New Eutectic in CaO–MgO–CO2–H2O with Applications to Carbonatites. Morphology and Phase Equilibria of Minerals. In Proceedings of the 13th General Meeting of the International Mineralogical Association, Varna, Bulgaria, 19–25 September 1986; Bulgarian Academy of Science: Sofia, Bulgaria; pp. 313–322.
- Drabek, M.; Fryda, J.; Janoushek, V.; Sarbach, M. Regionally Metamorphosed Carbonatite-Like Marbles from the Varied Group, Molanubian Unit, Bohemian Massif, Czech Republic, and their Mo–Th–Nb–REE Mineralization. In Mineral Deposits: Processes to Processing; Stanley, C.J., Ed.; Balkema: Rotterdam, The Netherlands, 1999; Volume 1, pp. 635–638. [Google Scholar]
- Le Bas, M.J.; Babattat, M.A.O.; Taylor, R.N.; Milton, J.A.; Windley, B.F.; Evins, P.M. The carbonatite–marble dykes of Abyan province, Yemen Republic: The mixing of mantle and crustal carbonate materials revealed by isotope and trace element analysis. Miner. Petrol. 2004, 82, 105–135. [Google Scholar] [CrossRef]
- Liu, Y.; Berner, Z.; Massonne, H.-J.; Zhong, D. Carbonatite-like dykes from the eastern Himalayan syntaxis: Geochemical, isotopic, and petrogenetic evidence for melting of metasedimentary carbonate rocks within the orogenic crust. J. Asian Earth Sci. 2006, 26, 105–120. [Google Scholar] [CrossRef]
- Roberts, D.; Zwaan, K.B. Marble dykes emanating from marble layers in an amphibolite-facies, multiply-deformed carbonate succession, Troms, northern Norway. Geol. Mag. 2007, 144, 883–888. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, D.; Xu, Z.; Dong, C.; Wang, Z.; Zhou, H.; Yang, Z.; Liu, Z.; Wu, J. Paleoproterozoic crustally derived carbonate-rich magmatic rocks from the Daqinshan area, North China Craton: Geological, petrographical, geochronological and geochemical (Hf, Nd, O and C) evidence. Am. J. Sci. 2008, 308, 351–378. [Google Scholar] [CrossRef] [Green Version]
- Proskurnin, V.F.; Petrov, O.V.; Gavrish, A.V.; Paderin, P.G.; Mozoleva, I.N.; Petrushkov, B.S.; Bagaeva, A.A. The Early Mesozoic carbonatite belt in the Taimyr Peninsula. Litosfera 2010, 3, 95–102. [Google Scholar]
- Schumann, D.; Martin, R.F.; Fuchs, S.; De Fourestier, J. Silicocarbonatitic melt inclusions in fluorapatite from the Yates Prospect, Otter Lake, Quebec: Evidence of marble anataxis in the Central Metasidementary Belt of the Grenville Province. Can. Mineral. 2019, 57, 583–604. [Google Scholar] [CrossRef]
- Lentz, D.R. Carbonatite genesis: A reexamination of the role of intrusion-related pneumatolytic skarn processes in limestone melting. Geology 1999, 27, 335–338. [Google Scholar] [CrossRef]
- Barnes, C.G.; Prestvik, T.; Sundvoll, B.; Surratt, D. Pervasive assimilation of carbonate and silicate rocks in the Hortavaer igneous complex, north-central Norway. Lithos 2005, 80, 179–199. [Google Scholar] [CrossRef]
- Bowen, N.L. The Evolution of the Igneous Rocks; Princeton University Press: Princeton, NJ, USA, 1928; p. 332. [Google Scholar]
- Spandler, C.M.; Lukas, H.J.; Pettke, T. Carbonate assimilation during magma evolution at Nisyros (Greece), South Aegean Arc; evidence from clinopyroxenite xenoliths. Lithos 2012, 146–147, 18–33. [Google Scholar] [CrossRef]
- Mollo, S.; Vona, A. The geochemical evolution of clinopyroxene in the Roman Province: A window on decarbonation from wallrocks to magma. Lithos 2014, 192–195, 1–7. [Google Scholar] [CrossRef]


| Sample | Rock | Mineral | δ13C ‰V-PDB | δ18O‰ V-SMOW | Δ18Occ-min | T (°C) |
|---|---|---|---|---|---|---|
| T-9p | Dolerite | Plagioclase | 8.0 | |||
| groundmass | 6.2 | |||||
| T-8 | Remnant dolerite | groundmass | 10.9 | |||
| T-8a | 10.3 | |||||
| T-6 | Beerbachite | groundmass | 10.3 | |||
| T-10 | Pyroxenite | pyroxene | 10.3 | 1.4 | 1038 | |
| nepheline | 11 | 0.7 | 1245 | |||
| biotite | 9.2 | 2.5 | 568 | |||
| carbonate | −0.5 | 11.7 | ||||
| T-5 | Marble (veins, Tazheran) | carbonate | −0.1 | 13.3 | ||
| T-20 | −0.1 | 17.7 | ||||
| T-22 | 0.3 | 15.6 | ||||
| T-13 | Marble (country rock) | carbonate | −0.6 | 23.7 | ||
| T-14 | 2.3 | 27.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sklyarov, E.V.; Lavrenchuk, A.V.; Doroshkevich, A.G.; Starikova, A.E.; Kanakin, S.V. Pyroxenite as a Product of Mafic-Carbonate Melt Interaction (Tazheran Massif, West Baikal Area, Russia). Minerals 2021, 11, 654. https://doi.org/10.3390/min11060654
Sklyarov EV, Lavrenchuk AV, Doroshkevich AG, Starikova AE, Kanakin SV. Pyroxenite as a Product of Mafic-Carbonate Melt Interaction (Tazheran Massif, West Baikal Area, Russia). Minerals. 2021; 11(6):654. https://doi.org/10.3390/min11060654
Chicago/Turabian StyleSklyarov, Eugene V., Andrey V. Lavrenchuk, Anna G. Doroshkevich, Anastasia E. Starikova, and Sergei V. Kanakin. 2021. "Pyroxenite as a Product of Mafic-Carbonate Melt Interaction (Tazheran Massif, West Baikal Area, Russia)" Minerals 11, no. 6: 654. https://doi.org/10.3390/min11060654
APA StyleSklyarov, E. V., Lavrenchuk, A. V., Doroshkevich, A. G., Starikova, A. E., & Kanakin, S. V. (2021). Pyroxenite as a Product of Mafic-Carbonate Melt Interaction (Tazheran Massif, West Baikal Area, Russia). Minerals, 11(6), 654. https://doi.org/10.3390/min11060654






