A Review of Tungsten Resources and Potential Extraction from Mine Waste
Abstract
1. Introduction
2. Tungsten Characteristics and Mineralogy
2.1. Physical and Chemical Characteristics
2.2. Occurrence
2.3. Mineralization
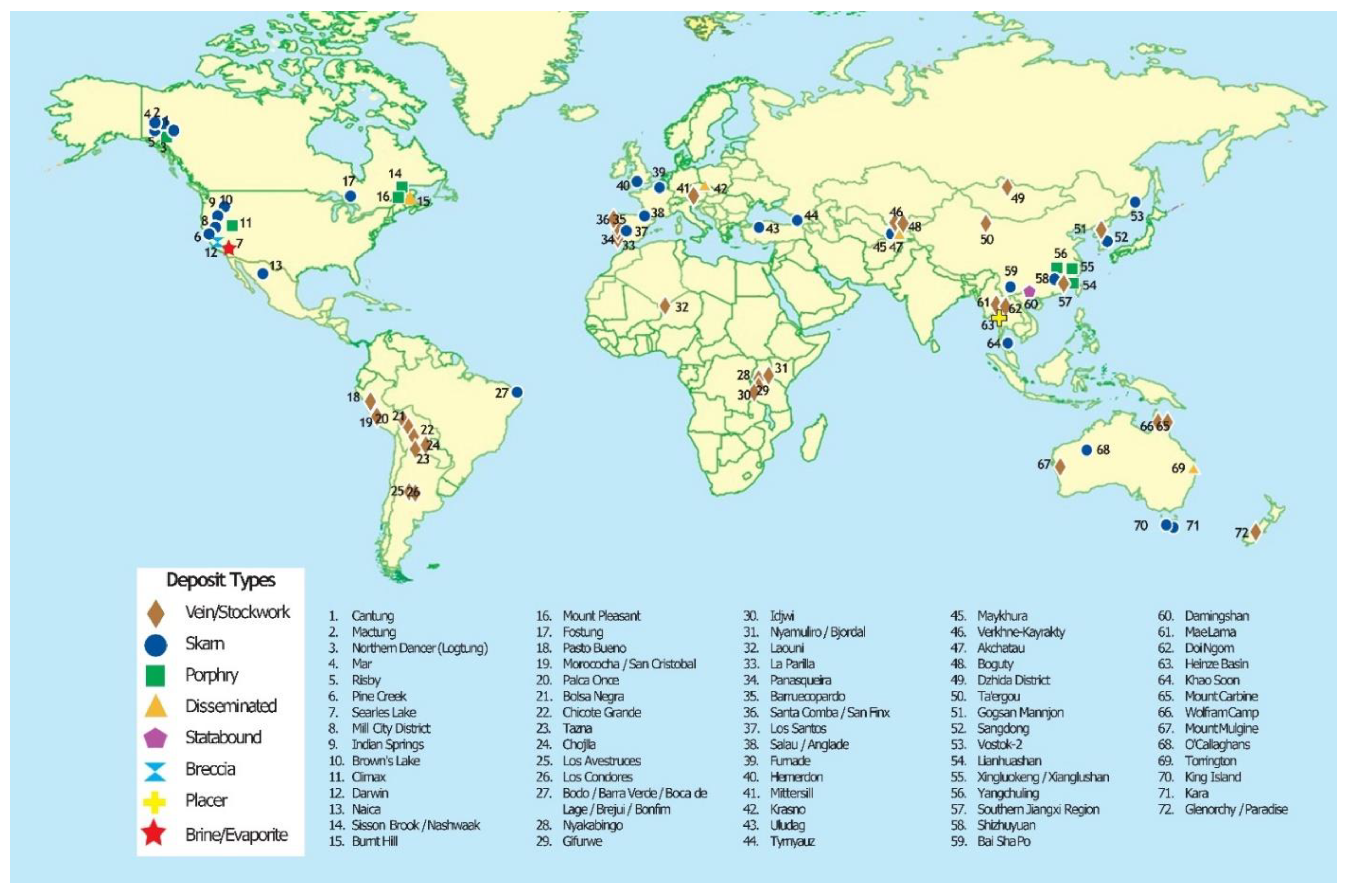
3. Tungsten Resources
3.1. Primary Resources
3.2. Secondary Resources
4. Tungsten Geochemical Mobility, Toxicity and Environmental Risks
4.1. Geochemical Mobility
4.2. Toxicity
4.3. Environmental Risks of Tungsten Waste
5. Potential Reprocessing Approaches for Tungsten Recovery from Tailings
5.1. A Summary of Previous Reprocessing Trials
5.2. Gravity Separation
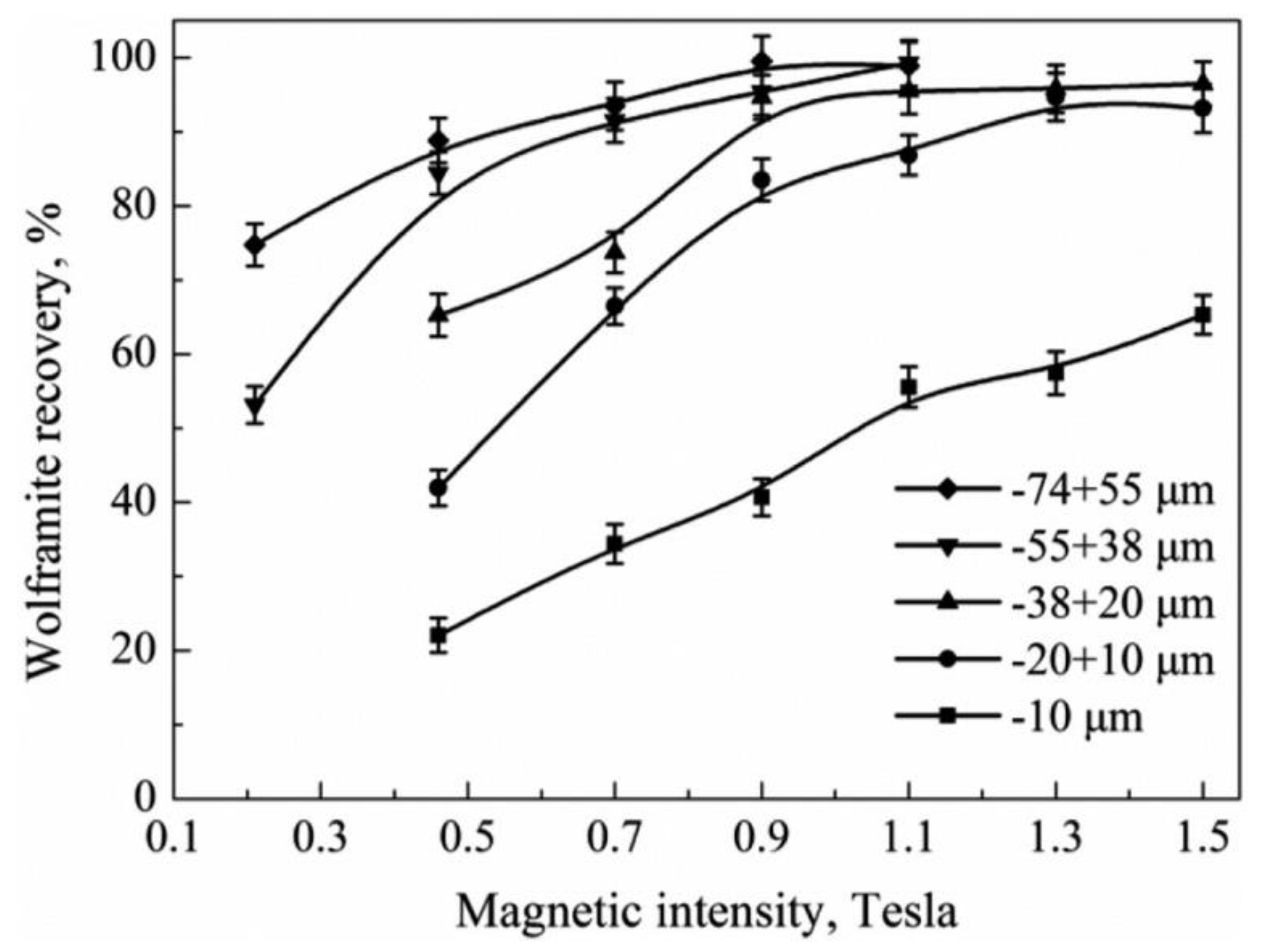
5.3. Magnetic Separation
5.4. Flotation
5.5. Chemical Leaching
5.6. Bioleaching
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Shedd, K.B. 2016 Minerals Yearbook; US Geological Survey: Reston, VA, USA, 2018. [Google Scholar]
- Liu, D.G.; Zheng, L.; Luo, L.M.; Zan, X.; Song, J.-P.; Xu, Q.; Zhu, X.Y.; Wu, Y.C. An overview of oxidation-resistant tungsten alloys for nuclear fusion. J. Alloys Compd. 2018, 765, 299–312. [Google Scholar] [CrossRef]
- Calvo, G.; Valero, A.; Valero, A. How can strategic metals drive the economy? Tungsten and tin production in Spain during periods of war. Extr. Ind. Soc. 2019, 6, 8–14. [Google Scholar] [CrossRef]
- Doi, U. Final list of critical minerals 2018. Fed. Regist. 2018, 83, 23295–23296. [Google Scholar]
- Martins, F.; Castro, H. Significance ranking method applied to some EU critical raw materials in a circular economy—priorities for achieving sustainability. Procedia CIRP 2019, 84, 1059–1062. [Google Scholar] [CrossRef]
- Hayes, S.M.; McCullough, E.A. Critical minerals: A review of elemental trends in comprehensive criticality studies. Resour. Policy 2018, 59, 192–199. [Google Scholar] [CrossRef]
- Brookes, K. What’s new in Chinese tungsten. Met. Powder Rep. 2011, 66, 22–30. [Google Scholar] [CrossRef]
- U.S.G.S. Mineral. Commodity Summaries 2021; USGS: Reston, VA, USA, 2021; pp. 178–179. [Google Scholar]
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.-M.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. J. Environ. Manag. 2020, 270, 110927. [Google Scholar] [CrossRef]
- Dvořáček, J.; Sousedíková, R.; Vrátný, T.; Jureková, Z. Global Tungsten Demand and Supply Forecast. Arch. Min. Sci. 2017, 62, 3–12. [Google Scholar] [CrossRef]
- Candeias, C.; Ávila, P.F.; Ferreira da Silva, E.; Ferreira, A.; Salgueiro, A.R.; Teixeira, J.P. Acid mine drainage from the Panasqueira mine and its influence on Zêzere river (Central Portugal). J. Afr. Earth Sci. 2014, 99, 705–712. [Google Scholar] [CrossRef]
- Lin, W.; Chen, C.; Xu, S. Heavy Metal Contamination and Environmental Concerns on Orchard at Abandoned Tungsten Mine, Southern China. Appl. Mech. Mater. 2013, 295–298, 1609–1614. [Google Scholar] [CrossRef]
- Liu, C.-P.; Luo, C.-L.; Gao, Y.; Li, F.-B.; Lin, L.-W.; Wu, C.-A.; Li, X.-D. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China. Environ. Pollut. 2010, 158, 820–826. [Google Scholar] [CrossRef]
- Dori, Z. The coordination chemistry of tungsten. Prog. Inorg. Chem 1981, 28, 239–308. [Google Scholar]
- Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef]
- Lassner, E.; Schubert, W. The Element Tungsten. In Tungsten; Springer: Boston, MA, USA, 1999. [Google Scholar] [CrossRef]
- White, J.S. Wolframite group. In Mineralogy; Springer: Boston, MA, USA, 1983; pp. 520–521. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Weier, M.L.; Duong, L.V.; Frost, R.L. Microwave-assisted synthesis and characterisation of divalent metal tungstate nanocrystalline minerals: Ferberite, hübnerite, sanmartinite, scheelite and stolzite. Mater. Chem. Phys. 2004, 88, 438–443. [Google Scholar] [CrossRef][Green Version]
- Sahama, T.G. The secondary tungsten minerals, a review. Mineral. Rec. 1981, 12, 81–87. [Google Scholar]
- Grey, I.E.; Birch, W.D.; Bougerol, C.; Mills, S.J. Unit-cell intergrowth of pyrochlore and hexagonal tungsten bronze structures in secondary tungsten minerals. J. Solid State Chem. 2006, 179, 3860–3869. [Google Scholar] [CrossRef]
- Steffen, S. From Deposit to Concentrate: The Basics of Tungsten Mining. Part 1: Project Generation and Project Development. In Proceedings of the ITIA’s 25th Anniversary Annual General Meeting, Beijing, China, 17–20 September 2012; pp. 1–20. [Google Scholar]
- Tarassov, M.; Tarassova, E. Structural and chemical evolution of mineral forms of tungsten in the oxidation zone of the Grantcharitza deposit (Western Rhodopes, Bulgaria). Bulg. Chem. Commun. 2018, 50, 270–280. [Google Scholar]
- International Tungsten Industry Association. Mineralogy of the element tungsten. In Proceedings of the 19th Annual General Meeting, Visakhapatnam, India, 1 January 1957. [Google Scholar]
- Werner, A.B.T.; Amey, E.B.; Sinclair, W.D. International Strategic Mineral Issues Summary Report—Tungsten; USGPO, U.S. Dept. of the Interior, U.S. Geological Survey: Washington, DC, USA, 1998; 71p. [Google Scholar]
- Sinclair, W.D. Vein-Stockwork Tin, Tungsten; Geological Survey of Canada, Geology of Canada: Ottawa, ON, Canada, 1996; Volume 8, pp. 409–420. [Google Scholar]
- Zhang, Y.; Gao, J.-F.; Ma, D.; Pan, J. The role of hydrothermal alteration in Tungsten mineralization at the Dahutang tungsten deposit, South China. Ore Geol. Rev. 2018, 95. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Xiao, R. Multiple-stage tungsten mineralization in the Silurian Jiepai W skarn deposit, South China: Insights from cathodoluminescence images, trace elements, and fluid inclusions of scheelite. J. Asian Earth Sci. 2019, 181, 103898. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhou, M.-F.; Li, Y.H.M.; Zhao, Z.; Gao, J.-F. Genetic types, mineralization styles, and geodynamic settings of Mesozoic tungsten deposits in South China. J. Asian Earth Sci. 2017, 137, 109–140. [Google Scholar] [CrossRef]
- Harlaux, M.; Mercadier, J.; Marignac, C.; Peiffert, C.; Cloquet, C.; Cuney, M. Tracing metal sources in peribatholitic hydrothermal W deposits based on the chemical composition of wolframite: The example of the Variscan French Massif Central. Chem. Geol. 2018, 479, 58–85. [Google Scholar] [CrossRef]
- Yang, J.-H.; Zhang, Z.; Peng, J.-T.; Liu, L.; Leng, C.-B. Metal source and wolframite precipitation process at the Xihuashan tungsten deposit, South China: Insights from mineralogy, fluid inclusion and stable isotope. Ore Geol. Rev. 2019, 111, 102965. [Google Scholar] [CrossRef]
- Suárez, S.A.; Krzemień, A.; Riesgo, F.P.; Iglesias, R.F.J.; Sánchez, L.F.; de Cos, J.F.J. Investment in new tungsten mining projects. Resour. Policy 2015, 46, 177–190. [Google Scholar] [CrossRef]
- McClenaghan, M.B.; Parkhill, M.A.; Pronk, A.G.; Sinclair, W.D. Indicator mineral and till geochemical signatures of the Mount Pleasant W-Mo-Bi and Sn-Zn-In deposits, New Brunswick, Canada. J. Geochem. Explor. 2017, 172, 151–166. [Google Scholar] [CrossRef]
- Mining Data Online. Panasqueira Mine. Available online: https://miningdataonline.com/property/4482/Panasqueira-Mine.aspx (accessed on 18 February 2021).
- Ripp, G.; Smirnova, O.; Izbrodin, I.; Lastochkin, E.; Rampilov, M.; Posokhov, V. An Isotope Study of the Dzhida Mo–W Ore Field (Western Transbaikalia, Russia). Minerals 2018, 8, 546. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ren, M. Hydrothermal zircon geochronology: Age constraint on Nanling Range tungsten mineralization (Southeast China). Ore Geol. Rev. 2016, 74, 63–75. [Google Scholar] [CrossRef]
- Werner, A.B.T.; Sinclair, W.D.; Amey, E.B. International Strategic Mineral Issues Summary Report-Tungsten; USGS: Reston, VA, USA, 2014. [Google Scholar]
- British Geological Survey. Tungsten. In British Geological Survey; BGS: Keyworth, UK, 2011; pp. 1–34. [Google Scholar]
- Shedd, K.B. Tungsten Recycling in the United States in 2000; US Geological Survey: Reston, VA, USA, 2005. [Google Scholar]
- Luo, L.; Kejun, L.; Shibayama, A.; Yen, W.; Fujita, T.; Shindo, O.; Katai, A. Recovery of tungsten and vanadium from tungsten alloy scrap. Hydrometallurgy 2004, 72, 1–8. [Google Scholar] [CrossRef]
- Kamal, S.S.K.; Sahoo, P.K.; Vimala, J.; Shanker, B.; Ghosal, P.; Durai, L. Synthesis of high purity tungsten nanoparticles from tungsten heavy alloy scrap by selective precipitation and reduction route. J. Alloys Compd. 2016, 678, 403–409. [Google Scholar] [CrossRef]
- Furberg, A.; Arvidsson, R.; Molander, S. Environmental life cycle assessment of cemented carbide (WC-Co) production. J. Clean. Prod. 2019, 209, 1126–1138. [Google Scholar] [CrossRef]
- Peng, K.; Yang, H.; Ouyang, J. Tungsten tailing powders activated for use as cementitious material. Powder Technol. 2015, 286, 678–683. [Google Scholar] [CrossRef]
- Mulenshi, J.; Khavari, P.; Chehreh Chelgani, S.; Rosenkranz, J. Characterization and Beneficiation Options for Tungsten Recovery from Yxsjöberg Historical Ore Tailings. Processes 2019, 7, 895. [Google Scholar] [CrossRef]
- Clemente, D.; Newling, P.; Botelho, d.S.A.; LeJeune, G.; Barber, S.P.; Tucker, P. Reprocessing slimes tailings from a tungsten mine. Miner. Eng. 1993, 6, 831–839. [Google Scholar] [CrossRef]
- Kang, J.; Hu, Y.; Sun, W.; Liu, R.; Yin, Z.; Tang, H.; Meng, X.; Zhang, Q.; Liu, H. A significant improvement of scheelite flotation efficiency with etidronic acid. J. Clean. Prod. 2018, 180, 858–865. [Google Scholar] [CrossRef]
- Doroshkevich, S.G.; Bardamova, I.V. Phytotoxicity of Tailings Dam of the Dzhidinsky Tungsten–Molybdenum Combine (Western Transbaikalia); Springer: Cham, Switzerland, 2015; pp. 277–287. [Google Scholar]
- Bourg, S. Mapping the Secondary Resources in the EU (Mine Tailings, Industrial Waste); ICCRAM: Burgos, Spain, 2016. [Google Scholar]
- Ikramova, Z.O.; Mukhamedzhanova, M.T.; Tukhtaeva, G.G. Tungsten–molybdenum ore flotation tailings for ceramic tile production. Glass Ceram. 2009, 66, 102–103. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, Y.J.; Choi, O.; Lee, K.M.; Lachemi, M. Utilization of tailings from tungsten mine waste as a substitution material for cement. Constr. Build. Mater. 2009, 23, 2481–2486. [Google Scholar] [CrossRef]
- Figueiredo, J.; Vila, M.; Matos, K.; Martins, D.; Futuro, A.; Dinis, M.d.L.; Góis, J.; Leite, A.; Fiúza, A. Tailings reprocessing from Cabeço do Pião dam in Central Portugal: A kinetic approach of experimental data. J. Sustain. Min. 2018, 17, 139–144. [Google Scholar] [CrossRef]
- Chung, A.P.; Coimbra, C.; Farias, P.; Francisco, R.; Branco, R.; Simão, F.V.; Gomes, E.; Pereira, A.; Vila, M.C.; Fiúza, A.; et al. Tailings microbial community profile and prediction of its functionality in basins of tungsten mine. Sci. Rep. 2019, 9, 19596. [Google Scholar] [CrossRef] [PubMed]
- Hällström, L.P.B.; Alakangas, L.; Martinsson, O. Scheelite weathering and tungsten (W) mobility in historical oxidic-sulfidic skarn tailings at Yxsjöberg, Sweden. Environ. Sci. Pollut. Res. 2020, 27, 6180–6192. [Google Scholar] [CrossRef]
- Hallberg, A.; Reginiussen, H. Critical Raw Materials in Ores, Waste Rock and Tailings in Bergslagen; Geological Survey of Sweden: Uppsala, Sweden, 2020. [Google Scholar]
- Pascoe, A. Collingwood plant for tungsten icon. Aust. Paydirt 2009, 1, 69. [Google Scholar]
- Leal-Ayala, D.; Allwood, J.; Petavratzi, E.; Brown, T.; Gunn, G. Mapping the Global Flow of Tungsten to Identify Key Material Efficiency and Supply Security Opportunities. Resour. Conserv. Recycl. 2015, 103, 19–28. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, Q. Tungsten speciation and its geochemical behavior in geothermal water: A review. E3S Web Conf. 2019, 98, 07005. [Google Scholar] [CrossRef]
- Bednar, A.J.; Boyd, R.E.; Jones, W.T.; McGrath, C.J.; Johnson, D.R.; Chappell, M.A.; Ringelberg, D.B. Investigations of tungsten mobility in soil using column tests. Chemosphere 2009, 75, 1049–1056. [Google Scholar] [CrossRef]
- van der Voet, G.B.; Todorov, T.I.; Centeno, J.A.; Jonas, W.; Ives, J.; Mullick, F.G. Metals and Health: A Clinical Toxicological Perspective on Tungsten and Review of the Literature. Mil. Med. 2007, 172, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.R.; Ridenour, G.; Speakman, R.J.; Witten, M.L. Elevated tungsten and cobalt in airborne particulates in Fallon, Nevada: Possible implications for the childhood leukemia cluster. Appl. Geochem. 2006, 21, 152–165. [Google Scholar] [CrossRef]
- Datta, S.; Vero, S.E.; Hettiarachchi, G.M.; Johannesson, K. Tungsten Contamination of Soils and Sediments: Current State of Science. Curr. Pollut. Rep. 2017, 3, 55–64. [Google Scholar] [CrossRef]
- Hobson, C.; Kulkarni, H.V.; Johannesson, K.H.; Bednar, A.; Tappero, R.; Mohajerin, T.J.; Sheppard, P.R.; Witten, M.L.; Hettiarachchi, G.M.; Datta, S. Origin of tungsten and geochemical controls on its occurrence and mobilization in shallow sediments from Fallon, Nevada, USA. Chemosphere 2020, 260, 127577. [Google Scholar] [CrossRef]
- Kletzin, A.; Adams, M.W. Tungsten in biological systems. FEMS Microbiol. Rev. 1996, 18, 5–63. [Google Scholar] [CrossRef]
- Agamemnon, D.K.; Demetri, A.K.; Nick, S.; Washington, B.; Christos, C. Tungsten: Environmental Pollution and Health Effects. Encycl. Environ. Health 2011. [Google Scholar] [CrossRef]
- Strigul, N.; Koutsospyros, A.; Arienti, P.; Christodoulatos, C.; Dermatas, D.; Braida, W. Effects of tungsten on environmental systems. Chemosphere 2005, 61, 248–258. [Google Scholar] [CrossRef]
- Lin, C.; Li, R.; Cheng, H.; Wang, J.; Shao, X. Tungsten distribution in soil and rice in the vicinity of the world’s largest and longest-operating tungsten mine in China. PLoS ONE 2014, 9, e91981. [Google Scholar] [CrossRef]
- Strigul, N.; Koutsospyros, A.; Christodoulatos, C. Tungsten speciation and toxicity: Acute toxicity of mono- and poly-tungstates to fish. Ecotoxicol. Environ. Saf. 2010, 73, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Chinde, S.; Grover, P. Toxicological assessment of nano and micron-sized tungsten oxide after 28days repeated oral administration to Wistar rats. Mutat Res. 2017, 819, 1–13. [Google Scholar] [CrossRef]
- Lee, P.-K.; Kang, M.-J.; Jo, H.Y.; Choi, S.-H. Sequential extraction and leaching characteristics of heavy metals in abandoned tungsten mine tailings sediments. Environ. Earth Sci. 2012, 66, 1909–1923. [Google Scholar] [CrossRef]
- Strigul, N.; Koutsospyros, A.; Christodoulatos, C. Tungsten in the former Soviet Union: Review of environmental regulations and related research. Land Contam. Reclam. 2009, 17, 189. [Google Scholar] [CrossRef]
- International Tungsten Industry Association. Tungsten Health and Environment. Available online: https://www.itia.info/health-environment.html (accessed on 18 February 2021).
- Krishna Rao, N. Beneficiation of tungsten ores in India: A review. Bull. Mater. Sci. 1996, 19, 201–265. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective flotation of scheelite from calcite using xanthan gum as depressant. Miner. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Ai, G.; Huang, W.; Yang, X.; Li, X. Effect of collector and depressant on monomineralic surfaces in fine wolframite flotation system. Sep. Purif. Technol. 2017, 176, 59–65. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Pravesh, R.; Pathak, B.P.N. The effect of polymeric dispersant on magnetic separation of tungsten ore slimes. Int. J. Miner. Process. 1996, 47, 213–217. [Google Scholar] [CrossRef]
- Lu, J.; Qu, Z.; Li, M. Industrialized Flotation Experiments on the Fine Mud Discharge of a Wolframite Concentrator. China Tungsten Ind. 2015, 30, 36–40. [Google Scholar]
- Xiao, J.H.; Feng, Q.M.; Fan, S.P.; Xu, L.; Wang, Z. Comprehensive utilization of copper, tungsten and tin polymetallic tailings in Bolivia. Zhongguo Youse Jinshu Xuebao Chin. J. Nonferrous Met. 2013, 23, 2949–2961. [Google Scholar]
- Rao, G.M.; Subrahmanyan, N.N. Beneficiation of tungsten ores in India—problems, processes, applications and demands in general on a global scene. Przeróbka Rud Wolframu W Indict Physicochem. Probl. Miner. Process. 1986, 18, 23–37. [Google Scholar]
- Evdokimov, S.S.; Evdokimov, V. Flotation of wolfram-molybdenum sand tailings at Tyrnyauz processing plant. Gorn. Zhurnal 2015, 2015, 63–68. [Google Scholar] [CrossRef]
- Khuchunaev, B.M.; Tashilova, A.A.; Kesheva, L.A.; Teunova, N.V. The tailing dumps’ reclamation influence evaluation on atmospheric air at the mining enterprise. IOP Conf. Ser. Mater. Sci. Eng. 2020, 913, 052044. [Google Scholar] [CrossRef]
- Yue, T.; Han, H.; Hu, Y.; Wei, Z.; Wang, J.; Wang, L.; Sun, W.; Yang, Y.; Sun, L.; Liu, R.; et al. Beneficiation and Purification of Tungsten and Cassiterite Minerals Using Pb–BHA Complexes Flotation and Centrifugal Separation. Minerals 2018, 8, 566. [Google Scholar] [CrossRef]
- Ai, G.; Liu, Y. A study on recycling fine wolframite slime by “flotation desulfurization-centrifugal preconcentration-flotation wolframite” process. IJSSST. 2016, 17. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Ou, L. Recovery Enhancement of Ultrafine Wolframite through Hydrophobic Flocs Magnetic Separation. Miner. Process. Extr. Metall. Rev. 2017, 38, 298–303. [Google Scholar] [CrossRef]
- Svoboda, J.; Guest, R.N.; Venter, W.J.C. The recovery of copper and lead minerals from Tsumeb flotation tailings by magnetic separation. J. S. Afr. Inst. Min. Metall. 1988, 88, 9–19. [Google Scholar] [CrossRef]
- Tucker, P. Modelling wet high intensity magnetic separation: A case study. Miner. Eng. 1994, 7, 1281–1300. [Google Scholar] [CrossRef]
- Chen, Q.; Kasomo, R.M.; Li, H.; Jiao, X.; Zheng, H.; Weng, X.; Mutua, N.M.; Song, S.; He, D.; Luo, H. Froth flotation of rutile—An overview. Miner. Eng. 2021, 163, 106797. [Google Scholar] [CrossRef]
- Filippov, L.O.; Foucaud, Y.; Filippova, I.V.; Badawi, M. New reagent formulations for selective flotation of scheelite from a skarn ore with complex calcium minerals gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Pradip. Recent advances in the recovery of tungsten values in the fine and ultrafine size range. Bull. Mater. Sci. 1996, 19, 267–293. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Q.; Qiu, X.; Gao, Y.; Xie, Z. Relationship between flotation and Fe/Mn ratio of wolframite with benzohydroxamic acid and sodium oleate as collectors. Physicochem. Probl. Miner. Process. 2014, 50, 747–758. [Google Scholar]
- Yang, S.; Peng, T.; Li, H.; Feng, Q.; Qiu, X. Flotation Mechanism of Wolframite with Varied Components Fe/Mn. Miner. Process. Extr. Metall. Rev. 2015, 37. [Google Scholar] [CrossRef]
- Deng, L.; Zhong, H.; Wang, S.; Liu, G. A novel surfactant N-(6-(hydroxyamino)-6-oxohexyl)octanamide: Synthesis and flotation mechanisms to wolframite. Sep. Purif. Technol. 2015, 145. [Google Scholar] [CrossRef]
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the optimization of heap leaching. Miner. Eng. 2008, 21, 673–678. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap Leaching Technology—Current State, Innovations, and Future Directions: A Review. Miner. Process. Extr. Metall. Rev. 2016, 37, 73–119. [Google Scholar] [CrossRef]
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212. [Google Scholar] [CrossRef]
- Gong, D.; Zhou, K.; Peng, C.; Li, J.; Chen, W. Sequential extraction of tungsten from scheelite through roasting and alkaline leaching. Miner. Eng. 2019, 132, 238–244. [Google Scholar] [CrossRef]
- Queneau, P.B.; Beckstead, L.W.; Huggins, D.K. Autoclave Soda Digestion of Scheelite Concentrates with Feedback Control. U.S. Patent No. 4,325,919, 20 April 1982. [Google Scholar]
- Zhao, Z.; Liang, Y.; Liu, X.; Chen, A.; Li, H. Sodium hydroxide digestion of scheelite by reactive extrusion. Int. J. Refract. Met. Hard Mater. 2011, 29, 739–742. [Google Scholar] [CrossRef]
- Forward, F.A.; Vizsolyi, A.I. Process for the Production of Tungstic Acid. U.S. Patent US3193347A, 6 July 1965. [Google Scholar]
- Shen, L.; Li, X.; Zhou, Q.; Peng, Z.; Liu, G.; Qi, T.; Taskinen, P. Wolframite Conversion in Treating a Mixed Wolframite–Scheelite Concentrate by Sulfuric Acid. JOM 2018, 70, 161–167. [Google Scholar] [CrossRef]
- Leitão, P.; Futuro, A.; Vila, C.; Dinis, L.; Danko, A.; Fiúza, A. Direct Pressure Alkaline Leaching of Scheelite Ores and Concentrates. Min. Metall. Explor. 2019, 36, 993–1002. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.A.; Silva, R.A.; Ngoma, E.; Petersen, J.; Harrison, S.T.L.; Park, J.H.; Kim, H. Continuous bioleaching of arsenopyrite from mine tailings using an adapted mesophilic microbial culture. Hydrometallurgy 2019, 187, 187–194. [Google Scholar] [CrossRef]
- Gopikrishnan, V.; Vignesh, A.; Radhakrishnan, M.; Joseph, J.; Shanmugasundaram, T.; Doble, M.; Balagurunathan, R. Chapter 10—Microbial leaching of heavy metals from e-waste: Opportunities and challenges. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Krishnaraj, R.N., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 189–216. [Google Scholar] [CrossRef]
- Brierley, J.A. A perspective on developments in biohydrometallurgy. Hydrometallurgy 2008, 94, 2–7. [Google Scholar] [CrossRef]
- Pradhan, N.; Nathsarma, K.C.; Srinivasa, R.K.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Mohanty, S.; Ghosh, S.; Nayak, S.; Das, A.P. Bioleaching of manganese by Aspergillus sp. isolated from mining deposits. Chemosphere 2017, 172, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Guo, Z.; Liu, X.; Yin, H.; Qiu, G.; Pan, F.; Liu, H. The bioleaching feasibility for Pb/Zn smelting slag and community characteristics of indigenous moderate-thermophilic bacteria. Bioresour. Technol. 2009, 100, 2737–2740. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Zhou, M.; Zeng, G.M.; Wang, X.; Li, X.; Fan, T.; Xu, W.H. Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: Effects of substrate concentration. Bioresour. Technol. 2008, 99, 4124–4129. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: Significance of microbial interactions. Biotechnol. Bioeng. 2004, 87, 574–583. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef]
- Li, S.; Zhong, H.; Hu, Y.; Zhao, J.; He, Z.; Gu, G. Bioleaching of a low-grade nickel-copper sulfide by mixture of four thermophiles. Bioresour. Technol. 2014, 153, 300–306. [Google Scholar] [CrossRef]
- Martin, M.; Janneck, E.; Kermer, R.; Patzig, A.; Reichel, S. Recovery of indium from sphalerite ore and flotation tailings by bioleaching and subsequent precipitation processes. Miner. Eng. 2015, 75, 94–99. [Google Scholar] [CrossRef]
- Blazevic, A.; Albu, M.; Mitsche, S.; Rittmann, S.K.R.; Habler, G.; Milojevic, T. Biotransformation of Scheelite CaWO4 by the Extreme Thermoacidophile Metallosphaera sedula: Tungsten-Microbial Interface. Front. Microbiol. 2019, 10, 1492. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Ha, M.G.; Shin, S.; Seo, M.; Jang, J.; Jo, S.; Kim, D.; Lee, S.; Jung, Y.; Kang, P.; et al. Electrochemical effect on bioleaching of arsenic and manganese from tungsten mine wastes using Acidithiobacillus spp. J. Environ. Manag. 2018, 223, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef] [PubMed]


| Name | Formula | Name | Formula |
|---|---|---|---|
| Primary Tungsten Minerals | |||
| Ferberite | FeWO4 | Scheelite | CaWO4 |
| Hübnerite | MnWO4 | Stolzite and Raspite | PbWO4 |
| Sanmartinite | (Zn,Fe)WO4 | Wolframite | (Fe,Mn)WO4 |
| Secondary Tungsten Minerals | |||
| Alumotungstite | (W,Al)(O,OH)3 | Ovamboite | Cu20(Fe,Cu,Zn)6W2Ge6S32 |
| Anthoinite | AlWO3(OH)3 | Paraniite-(Y) | Ca2Y(AsO4)(WO4)2 |
| Catamarcaite | Cu6GeWS8 | Phyllotungstite | CaFe3H[WO4]6·10H2O |
| Cuprotungstite | Cu2[(OH)2|WO4] | Pinalite | Pb3WO3Cl2 |
| Elsmoreite | WO3·0.5H2O | Qitianlingite | (Fe,Mn)2(Nb,Ta)2WO10 |
| Farallonite | Mg2W2SiO9·nH2O | Rankachite | (V4+,V5+)(W,Fe)2O8(OH)·(CaxH2Oy) |
| Ferritungstite | (W,Fe)(O,OH)3 | Mporoite | AlWO3(OH)3·2H2O |
| Hydrotungstite | WO3·2H2O | Russellite | (BiO)2WO4 |
| Jixianite | Pb(W,Fe)2(O,OH)7 | Sanmartinite | (Zn,Fe)WO4 |
| Johnsenite-(Ce) | Na12(Ce,REE,Sr)3Ca6Mn3Zr3W(Si25O73)(OH)3(CO3)·H2O | Tungstenite | WS2 |
| Khomyakovite | Na12Sr3Ca6Fe3Zr3W(Si25O73)(O,OH,H2O)3(OH,Cl)2 | Tungstite | WO3·H2O |
| Kiddcreekite | Cu6SnWS8 | Uranotungstite | (Fe,Ba,Pb)(UO2)2[(OH)2|WO4]·12H2O |
| Koragoite | (Mn,Fe)3(Nb,Ta,Ti)62(Nb,Mn)2(W,Ta)2O20 | Welinite | Mn6(W,Mg)0.7[(O,OH)3|SiO4] |
| Mn-Khomyakovite | Na12Sr3Ca6Mn3Zr3W[Si25O73](O,OH,H2O)3(OH,Cl)2 | Yttrotungstite-(Ce) | (Ce,Nd,Y)W2O6(OH)3 |
| Meymacite | WO3·2H2O | Yttrotungstite-(Y) | YW2O6(OH)3 |
| Deposit | Deposit Type | Reserves, Mt | Grade, WO3 % | Reference |
|---|---|---|---|---|
| Xihuashan (China) | W-Sn | 81.3 | 1.08 | [31] |
| Hemerdon (UK) | W-Sn | 26.7 | 0.19 | [32] |
| Mt Pleasant (Canada) | W-Mo-Bi | 14.4 | 0.26–0.33 | [33] |
| Sangdong (South Korea) | W-Mo | 13.3 | 0.43 | [32] |
| Panasqueira (Portugal) | W-Sn | 10.3 | 0.24 | [34] |
| Barruecopardo (Spain) | W-Mo | 8.7 | 0.30 | [32] |
| Kilba (Australia) | W-Mo | 5.0 | 0.27 | [32] |
| Dzhida (Russia) | W-Mo | 1.4 | 0.15 | [35] |
| Deposit Type | Mining Methods | Processing Methods | Ore Grade, WO3 % | Tungsten Mineral | Accompanying Economic Metals | Typical Mine around the World | Mineralogy and Geology |
|---|---|---|---|---|---|---|---|
| Skarn (deposit size < 104 –5 × 107 t) | Underground/ Open-pit | Magnetic, gravity, flotation | 0.3–1.4 | Scheelite | Cu, Mo, Zn, and Bi | Vostok-2 (Russia), Uludag (Turkey), Mactung and Cantung (Canada), Sang Dong (South Korea), King Island (Australia) | Tabular or lenticular scheelite-dominated ore bodies in calc-silicate rocks formed by replacement of carbonate rocks and more rarely carbonaceous rocks at contacts with S- and I-type granitoid intrusions |
| Vein/stockwork (deposit size < 105–108 t) | Underground/ Open-pit | Gravity, flotation, magnetic, dense media, chemical leaching | Variable | Wolframite | Sn, Cu, Mo, Bi, and Au | Panasqueira (Portugal), Xihuashan (China), Bolsa Negra (Bolivia), Erzgebirge (Czech Rep.), Hemerdon (UK) | Single and multiple systems of simple or complex fissure filling and replacement veins of quartz + wolframite at margins of felsic plutonic rocks in clastic (meta-) sedimentary country rocks |
| Porphyry (deposit size < 107–108 t) | Open-pit | Gravity, flotation | 0.1–0.4 | Wolframite or/and scheelite | Mo, Bi, and Sn | Xingluokeng (China), Yangchulin (China), Northern Dancer (Canada),Climax (USA) | Medium to large, low-grade stockwork of quartz veinlets and disseminations in subvolcanic felsic intrusive rocks ± country rocks |
| Disseminated (deposit size < 107–108 t) | Underground | Magnetic, gravity, flotation | 0.1–0.5 | Wolframite and scheelite | Sn, Bi, and Mo | Shizhuyuan, Xihuashan, and Dangping (China), Akchatau, Kara-Oba, and Lultin (Russia) | Low-grade greisen deposits formed by pervasive metasomatic (endoskarn) alteration in the cupolas of granitic stocks |
| Stratabound (deposit size < 106–107 t) | Underground/ Open-pit | Gravity, flotation | 0.2–1.0 | Scheelite | Mo | Mittersill (Austria), Damingshan (China), Mount Mulgine (Australia) | Concordant lenses of stratiform scheelite in submarine volcano sedimentary sequences. Volcanogenic exhalative origin |
| Breccia (small, little production from them) | Open-pit | Magnetic, gravity, flotation | 0.14–1.0 | Wolframite | Cu, Mo, Ag, Sb, and Sn | Wolfram camp (Australia), Doi Ngom, and Khao Soon (Thailand), Washington (Mexico) | Near-vertical bodies of fragmented rock formed either by hydraulic fracturing or steam-dominated volcanic explosions marginal to I- or A-type granitic intrusions |
| Pegmatite (deposit size < 106–107 t) | Underground | Flotation | 0.5–0.8 | Scheelite or/and Wolframite | Li, Be, Nb, Ta, REEs, and Sn | Okbang mine (South Korea), Mawchi mine (Myanmar) | Dyke-like masses around granitic bodies. Simple unzoned to complex strongly zoned types with more varied mineralogy |
| Placer (deposit size < 3 × 104–107 t) | Open-pit | Magnetic, gravity, flotation | 0.43 | Wolframite and scheelite | Sn | Heinze Basin (Myanmar), Andrew mine (USA), Mergui district (Myanmar), Dzhida district (Russia), Bodmin Moor (UK) | Heavy mineral concentrations in alluvial, eluvial, or marine sediments derived from proximal bedrock sources of tungsten. |
| Brine/evaporate (deposit size < 104–105 t) | Salt flats | Chemical, ion-exchange | 7 × 10−4 | Lake brines | Salts of a complex mixture | Searles Lake (USA), other examples in the CIS and the western USA | Tungsten-bearing brines in lakes and the saline deposits of palaeolakes in arid continental regions |
| Tungsten Tailings Deposit | Type | Tailings, Mt | Grade (WO3), % | WO3, kt | Known Reuse Examples | Reference |
|---|---|---|---|---|---|---|
| Dzhidinsky (Russia) | Mo-W | 40 | 0.1 | 40 | Nil | [47] |
| Luanchuan (China) | W-Mo | 20 | 0.14 | 28 | Reprocessing for tungsten recovery | [48] |
| Kaitashskoe (Uzbekistan) | W-Mo | 12 | - | - | Flotation tailings reuse trials for ceramic tile production | [49] |
| Sangdong (South Korea) | W-Mo | 12 | 0.1 | 12 | Feasibility study for reuse in cement production | [50] |
| Panasqueira (Portugal) | W-Sn | 8 | 0.12 | 9.6 | Reprocessing trials for tungsten recovery | [45,48,51,52] |
| Yxsjöberg (Sweden) | W-Cu-F | 5.2 | 0.08 | 4.2 | Nil | [53,54] |
| Mount Carbine (Australia) | Sn-W | 2 | 0.1 | 2 | Reprocessing for tungsten recovery | [55] |
| Wolfram Camp (Australia) | W-Mo | 1 | 0.06 | 0.6 | Nil | Authors’ estimate |
| Total | 100.2 | 96.4 |
| Substance | Hazard Class (EC1272/2008) | Hazard Warning |
|---|---|---|
| Ammonium Metatungstate | Acute oral toxicity 4 | Harmful if swallowed |
| Ammonium Paratungstate | Not classified | None |
| Sodium Tungstate | Acute oral toxicity 4 | Harmful if swallowed |
| Tungsten Powder (0.6–0.9 µm) | Flammable solid 1; Self-heating 2 | Flammable solid; Self-heating in large quantities; may catch fire |
| Tungsten Powder (<1.0 µm) | Flammable solid 1 | Flammable solid |
| Tungsten Powder (1.0–1.5 µm) | Flammable solid 2 | Flammable solid |
| Tungsten Powder (>1.5 µm) | Not classified | None |
| Tungsten Blue Oxide | Not classified | None |
| Tungsten Carbide | Not classified | None |
| Tungsten Disulfide | Not classified | None |
| Tungsten Trioxide | Not classified | None |
| Tungsten Tailings Type | Deposits | Major Tungsten Minerals | Tailings Grade, WO3% | Reprocessing Methods | Reprocessing Results | Reference |
|---|---|---|---|---|---|---|
| High-intensity magnetic separation tungsten ore slime | Rajasthan (India) | Wolframite | 2.87 and 5.30 | Polymeric dispersant with magnetic separation | Wolframite was enriched from tungsten slimes to 5.4–11% WO3 concentrates. The grade of tungsten concentrates was increased to 10% when dispersant is applied | [76] |
| Fine tungsten tailings | Dajishan (China) | Wolframite | 0.45 | Flotation | 30.18% WO3 concentrates with an 80% recovery rate from very fine wolframite slime | [77] |
| Historical mine tailings and current plant slimes tailings | Panasqueira (Portugal) | Wolframite, most of the particles below 25 µm | 0.1 | Flotation, magnetic separation, and gravity concentration | A three-stage gravity separation combined with intermediate sulfide flotation produced tungsten concentrates with 50–55% WO3 | [45] |
| Tin mine tailings | Potosi Mine tin processing plant (Bolivia) | Wolframite | 0.64 | Chlorination segregation, flotation, high-intensity magnetic separation, and gravity separation | 60.22% WO3 concentrate with 64.26% recovery rate; 25.04% copper concentrate with 83.19% recovery; and 40.11% tin concentrate with 65.59% recovery | [78] |
| Old tailing dumps | Kolar and Hutti goldfields (India) | Scheelite | 0.2 | Tabling, flotation, and magnetic separation | 65% WO3 concentrate from a feed of tungsten tailings | [79] |
| Old molybdenum mine tailings | Tyrnyauz processing plant (Russia) | Scheelite | 0.05 | Flotation | 54–55% WO3 concentrate with 61.91–62.08% recovery rate from wolframite-molybdenum sand tailings | [80,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. https://doi.org/10.3390/min11070701
Han Z, Golev A, Edraki M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals. 2021; 11(7):701. https://doi.org/10.3390/min11070701
Chicago/Turabian StyleHan, Zhengdong, Artem Golev, and Mansour Edraki. 2021. "A Review of Tungsten Resources and Potential Extraction from Mine Waste" Minerals 11, no. 7: 701. https://doi.org/10.3390/min11070701
APA StyleHan, Z., Golev, A., & Edraki, M. (2021). A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals, 11(7), 701. https://doi.org/10.3390/min11070701






