Polytypism of Compounds with the General Formula Cs{Al2[TP6O20]} (T = B, Al): OD (Order-Disorder) Description, Topological Features, and DFT-Calculations
Abstract
:1. Introduction
2. Methods
3. Results
3.1. OD (Order-Disorder) Relationships
- Nonpolar L2n type consists of aluminum and oxygen atoms on the borders of a thin slab with the layer symmetry pcam [Pc(a)m or P21/c (2/a) 21/m].
3.2. Topological Features
3.3. Ion Migration Path
3.4. DFT Calculations
4. Discussion
- The first one corresponds to a layer with the symmetry P2(2)21 consisting of tetrahedral chains. The tetrahedral layer in Cs{Al2[TP6O20]} and tetrahedral pseudolayer in Rb{M2[TP6O20] are formed by the same FBU and demonstrate the symmetrical relationship (Figure 8) indicating the possible OD-character as was previously shown for compounds with tetrameric [57] and pentameric [20] borophosphate FBUs, as well as for the silicate layers [58,59];
- The second one consists of an octahedral layer with the symmetry P21(2)21 similar to that observed in Cs{Al2[TP6O20]} (T = Al, B) (the layer group P21221 is a subgroup of the layer group Pcam). To date, there are no other polytypes of this type of framework, however they may be found later.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vinodkumar, P.; Panda, S.; Jaiganesh, G.; Padhi, R.K.; Madhusoodanan, U.; Panigrahi, B.S. SrBPO5: Ce3+, Dy3+—A cold white-light emitting phosphor. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 253, 119560. [Google Scholar] [CrossRef]
- He, X.; Hu, D.; Yang, G.; Adamietz, F.; Rodriguez, V.; Dussauze, M.; Fargues, A.; Fargin, E.; Cardinal, T. Microstructured SHG patterns on Sm2O3-doped borophosphate niobium glasses by laser-induced thermal poling. Ceram. Int. 2021, 47, 10123–10129. [Google Scholar] [CrossRef]
- Joseph, P.A.J.; Maheshvaran, K.; Rayappan, I.A. Structural and optical studies on Dy3+ ions doped alkali lead borophosphate glasses for white light applications. J. Non. Cryst. Solids 2021, 557, 120652. [Google Scholar] [CrossRef]
- Xiang, J.; Fang, Z.; Yang, D.; Zheng, Y.; Zhu, J. Optimizational orange emitting behavior of Li2Na1-BP2O8:xPr solid solutions under an short-wave ultraviolet irradiation. Scr. Mater. 2020, 187, 82–87. [Google Scholar] [CrossRef]
- Zhao, D.; Cheng, W.-D.; Zhang, H.; Huang, S.-P.; Xie, Z.; Zhang, W.-L.; Yang, S.-L. KMBP2O8 (M = Sr, Ba): A New Kind of Noncentrosymmetry Borophosphate with the Three-Dimensional Diamond-like Framework. Inorg. Chem. 2009, 48, 6623–6629. [Google Scholar] [CrossRef]
- Magistris, A.; Chiodelli, G.; Duclot, M. Silver borophosphate glasses: Ion transport, thermal stability and electrochemical behaviour. Solid State Ion. 1983, 9–10, 611–615. [Google Scholar] [CrossRef]
- Mouyane, M.; Jumas, J.-C.; Olivier-Fourcade, J.; Cassaignon, S.; Jordy, C.; Lippens, P.-E. One-pot synthesis of tin-borophosphate-carbon composites as anode materials for Li-ion batteries. J. Solid State Chem. 2016, 233, 52–57. [Google Scholar] [CrossRef]
- Yaghoobnejad Asl, H.; Stanley, P.; Ghosh, K.; Choudhury, A. Iron Borophosphate as a Potential Cathode for Lithium- and Sodium-Ion Batteries. Chem. Mater. 2015, 27, 7058–7069. [Google Scholar] [CrossRef]
- Shenouda, A.Y.; Liu, H.K. Electrochemical behaviour of tin borophosphate negative electrodes for energy storage systems. J. Power Sources 2008, 185, 1386–1391. [Google Scholar] [CrossRef]
- Shvanskaya, L.; Yakubovich, O.; Krikunova, P.; Ovchenkov, E.; Vasiliev, A. Chain caesium borophosphates with B:P ratio 1:2: Synthesis, structure relationships and low-temperature thermodynamic properties. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Yakubovich, O.V.; Shvanskaya, L.V.; Kiriukhina, G.V.; Volkov, A.S.; Dimitrova, O.V.; Ovchenkov, E.A.; Tsirlin, A.A.; Shakin, A.A.; Volkova, O.S.; Vasiliev, A.N. Crystal structure and spin-trimer magnetism of Rb2.3(H2O)0.8Mn3[B4P6O24(O,OH)2]. Dalt. Trans. 2017, 46, 2957–2965. [Google Scholar] [CrossRef] [Green Version]
- Shvanskaya, L.; Yakubovich, O.; Melchakova, L.; Ivanova, A.; Vasiliev, A. Crystal chemistry and physical properties of the A2M3 (H2O)2[B4P6O24(OH)2] (A = Cs, Rb; M = Ni, Cu, (Ni, Fe)) borophosphate family. Dalt. Trans. 2019, 48, 8835–8842. [Google Scholar] [CrossRef] [PubMed]
- Scheide, M.R.; Peterle, M.M.; Saba, S.; Neto, J.S.S.; Lenz, G.F.; Cezar, R.D.; Felix, J.F.; Botteselle, G.V.; Schneider, R.; Rafique, J.; et al. Borophosphate glass as an active media for CuO nanoparticle growth: An efficient catalyst for selenylation of oxadiazoles and application in redox reactions. Sci. Rep. 2020, 10, 15233. [Google Scholar] [CrossRef] [PubMed]
- Matzkeit, Y.H.; Tornquist, B.L.; Manarin, F.; Botteselle, G.V.; Rafique, J.; Saba, S.; Braga, A.L.; Felix, J.F.; Schneider, R. Borophosphate glasses: Synthesis, characterization and application as catalyst for bis(indolyl)methanes synthesis under greener conditions. J. Non. Cryst. Solids 2018, 498, 153–159. [Google Scholar] [CrossRef]
- Wang, B.; Lu, W.-X.; Huang, Z.-Q.; Chen, W.-J.; Xie, J.-L.; Pan, D.-S.; Zhou, L.-L.; Song, J.-L. Amorphous N-Doped Cobalt Borophosphate Nanoparticles as Robust and Durable Electrocatalyst for Water Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 13981–13988. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Dimitrova, O.V. Fe2.5[BP2O7(OH)2][PO3(OH)][PO3(O0.5OH0.5)] · H2O, a new phosphate-borophosphate with a microporous structure. Crystallogr. Rep. 2015, 60, 361–366. [Google Scholar] [CrossRef]
- Yang, M.; Yan, P.; Xu, F.; Ma, J.; Welz-Biermann, U. Role of boron-containing ionic liquid in the synthesis of manganese borophosphate with extra-large 16-ring pore openings. Microporous Mesoporous Mater. 2012, 147, 73–78. [Google Scholar] [CrossRef]
- Kang, Q.-Y.; Song, Q.; Li, S.-Y.; Liu, Z.-H. Thermodynamic properties of microporous materials for two borophosphates, K[ZnBP2O8] and NH4[ZnBP2O8]. J. Chem. Thermodyn. 2014, 69, 43–47. [Google Scholar] [CrossRef]
- Yang, T.; Li, G.; Ju, J.; Liao, F.; Xiong, M.; Lin, J. A series of borate-rich metalloborophosphates Na2[MIIB3P2O11(OH)] 0.67H2O (MII=Mg, Mn, Fe, Co, Ni, Cu, Zn): Synthesis, structure and magnetic susceptibility. J. Solid State Chem. 2006, 179, 2534–2540. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Yamnova, N.A.; Borovikova, E.Y.; Stefanovich, S.Y.; Volkov, A.S.; Deyneko, D.V.; Dimitrova, O.V.; Hixon, A.E.; Krivovichev, S.V. Topological features of borophosphates with mixed frameworks. Synthesis, crystal structure of Li3{Al2[BP4O16]}∙2H2O, and comparative crystal chemistry. J. Struct. Chem. 2020, 61. [Google Scholar] [CrossRef]
- Ewald, B.; Huang, Y.-X.; Kniep, R. Structural Chemistry of Borophosphates, Metalloborophosphates, and Related Compounds. Z. Anorg. Allg. Chem. 2007, 633, 1517–1540. [Google Scholar] [CrossRef]
- Gurbanova, O.A.; Belokoneva, E.L. Comparative crystal chemical analysis of borophosphates and borosilicates. Crystallogr. Rep. 2007, 52, 624–633. [Google Scholar] [CrossRef]
- Li, M.; Verena-Mudring, A. New Developments in the Synthesis, Structure, and Applications of Borophosphates and Metalloborophosphates. Cryst. Growth Des. 2016, 16, 2441–2458. [Google Scholar] [CrossRef]
- Yakubovich, O.; Steele, I.; Massa, W. Genetic aspects of borophosphate crystal chemistry. Z. Krist. Cryst. Mater. 2013, 228. [Google Scholar] [CrossRef]
- Shvanskaya, L.V.; Yakubovich, O.V.; Belik, V.I. New type of borophosphate anionic radical in the crystal structure of CsAl2BP6O20. Crystallogr. Rep. 2016, 61, 786–795. [Google Scholar] [CrossRef]
- Lesage, J.; Guesdon, A.; Raveau, B. Two aluminotriphosphates with closely related intersecting tunnel structures involving tetrahedral “AlP” chains and layers: AAl3(P3O10)2, A=Rb, Cs. J. Solid State Chem. 2005, 178, 1212–1220. [Google Scholar] [CrossRef]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials; Oxford University Press: Oxford, UK, 2008; ISBN 9780191712111. [Google Scholar]
- Dornberger-Schiff, K. Grundzüge einer Theorie der OD-Strukturen aus Schichten. Abh. Dtsch. Akad. Wiss. Berlin. Kl. Chem. Geol. Biol. 1964, 3, 1–107. [Google Scholar]
- Dornberger-Schiff, K. Lehrgang Über OD-Strukturen; Akademie-Verlag: Berlin, Germany, 1966. [Google Scholar]
- Dornberger-Schiff, K.; Grell-Niemann, H. On the theory of order–disorder (OD) structures. Acta Crystallogr. 1961, 14, 167–177. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K.; Grell, H. Geometrical properties of MDO polytypes and procedures for their derivation. II. OD families containing OD layers of M > 1 kinds and their MDO polytypes. Acta Crystallogr. Sect. A 1982, 38, 491–498. [Google Scholar] [CrossRef]
- Grell, H. How to choose OD layers. Acta Crystallogr. Sect. A Found. Crystallogr. 1984, 40, 95–99. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: Recommended terminology. Cryst. Eng. Comm. 2010, 12, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Krivovichev, S.V. Structural and topological complexity of zeolites: An information-theoretic analysis. Microporous Mesoporous Mater. 2013, 171, 223–229. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Blatov, V.A.; Ilyushin, G.D.; Blatova, O.A.; Anurova, N.A.; Ivanov-Schits, A.K.; Dem’yanets, L.N. Analysis of migration paths in fast-ion conductors with Voronoi–Dirichlet partition. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 1010–1018. [Google Scholar] [CrossRef]
- Anurova, N.A.; Blatov, V.A.; Ilyushin, G.D.; Blatova, O.A.; Ivanov-Schits, A.K.; Dem’yanets, L.N. Migration maps of Li+ cations in oxygen-containing compounds. Solid State Ion. 2008, 179, 2248–2254. [Google Scholar] [CrossRef]
- Eremin, R.A.; Kabanova, N.A.; Morkhova, Y.A.; Golov, A.A.; Blatov, V.A. High-throughput search for potential potassium ion conductors: A combination of geometrical-topological and density functional theory approaches. Solid State Ion. 2018, 326, 188–199. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Vienna Ab-initio Simulation Package (VASP), V.5.4.4. Available online: www.vasp.at (accessed on 25 June 2021).
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dornberger-Schiff, K. On the nomenclature of the 80 plane groups in three dimensions. Acta Crystallogr. 1959, 12, 173. [Google Scholar] [CrossRef]
- Burns, P.C.; Grice, J.D.; Hawthorne, F.C. Borate minerals. I. Polyhedral clusters and foundamental building block. Can. Mineral. 1995, 33, 1131–1151. [Google Scholar]
- Grell, H.; Dornberger-Schiff, K. Symbols for OD groupoid families referring to OD structures (polytypes) consisting of more than one kind of layer. Acta Crystallogr. Sect. A 1982, 38, 49–54. [Google Scholar] [CrossRef]
- Ďurovič, S. Desymmetrization of OD Structures. Krist. Tech. 1979, 14, 1047–1053. [Google Scholar] [CrossRef]
- Merlino, S. EMU Notes in Mineralogy. Vol. 1. Modular Aspects of Minerals; Merlino, S., Ed.; Eötvös University Press: Budapest, Hungary, 1997. [Google Scholar]
- Rocha, J.; Lin, Z. Microporous Mixed Octahedral-Pentahedral-Tetrahedral Framework Silicates. Rev. Mineral. Geochem. 2005, 57, 173–201. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K. Crystal chemistry, properties and synthesis of microporous silicates containing transition elements. Russ. Chem. Rev. 2004, 73, 205–223. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V. Heterosilicates with Tetrahedral-Octahedral Frameworks: Mineralogical and Crystal-Chemical Aspects. Rev. Mineral. Geochem. 2005, 57, 105–143. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 25 June 2021).
- Voronkov, A.A.; Ilyukhin, V.V.; Belov, N.V. Crystal chemistry of mixed frameworks—Principles of their formation. Kristallografiya 1975, 20, 556–566. [Google Scholar]
- Sandomirskiy, P.A.; Belov, N.V. Crystal Chemistry of Mixed Anionic Radicals; Nauka: Moscow, Russia, 1984. [Google Scholar]
- Ilyushin, G.D.; Blatov, V.A. Crystal chemistry of zirconosilicates and their analogs: Topological classification of MT frameworks and suprapolyhedral invariants. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 198–218. [Google Scholar] [CrossRef] [Green Version]
- Lesage, J.; Guesdon, A.; Raveau, B. RbGa3 (P3O10)2: A new gallium phosphate isotypic with RbAl3(P3O10)2. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2005, 61, i44–i46. [Google Scholar] [CrossRef] [Green Version]
- Ruchkina, E.A.; Belokoneva, E.L. Structural features of lead iron borophosphates of alkali metals as analyzed in terms of topologically similar structural blocks. Russ. J. Inorg. Chem. 2003, 48, 1969–1978. [Google Scholar]
- Topnikova, A.; Belokoneva, E.; Dimitrova, O.; Volkov, A.; Deyneko, D. Rb1.66Cs1.34Tb[Si5.43Ge0.57O15]·H2O, a New Member of the OD-Family of Natural and Synthetic Layered Silicates: Topology-Symmetry Analysis and Structure Prediction. Minerals 2021, 11, 395. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Reutova, O.V.; Dimitrova, O.V.; Volkov, A.S. Germanosilicate Cs2In2[(Si2.1Ge0.9)2O15](OH)2 H2O with a New Corrugated Tetrahedral Layer: Topological Symmetry-Based Prediction of Anionic Radicals. Crystallogr. Rep. 2020, 65, 566–572. [Google Scholar] [CrossRef]




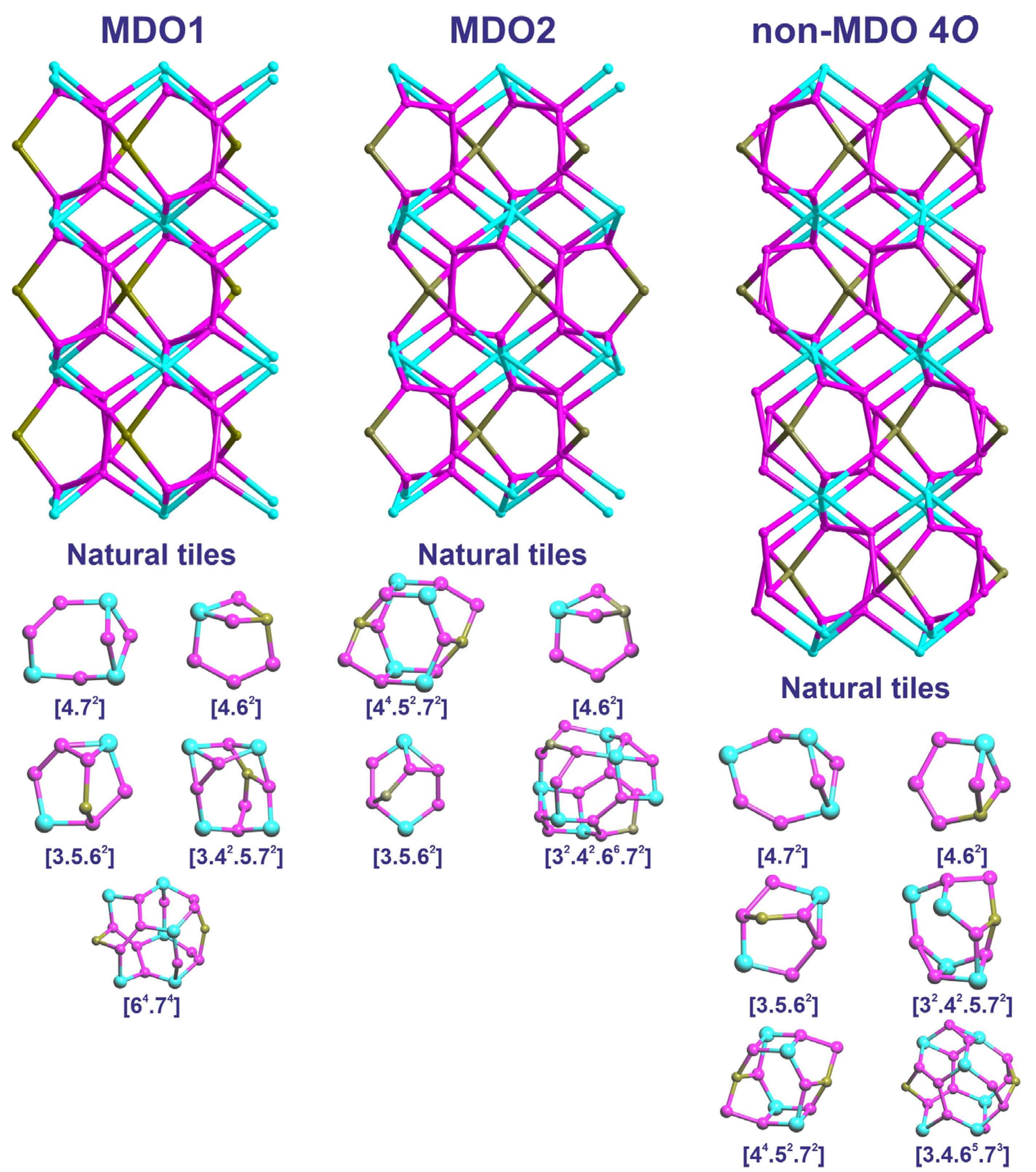



| Polytype | Natural Tiles | |||||
|---|---|---|---|---|---|---|
| MDO1 | [4.62]2 | [3.5.62]2 | [64.74] | [3.42.5.72]2 | [4.72]2 | |
| MDO2 | [4.62]2 | [3.5.62]2 | [44.52.72] | [32.42.66.72] | ||
| non-MDO 4O | [4.62]4 | [3.5.62]4 | [44.52.72] | [3.42.5.72]2 | [4.72]2 | [3.4.65.73]2 |
| Polytype | Natural Tiles | |||||
|---|---|---|---|---|---|---|
| Li+ | Na+ | Ag+ | K+ | Rb+ | Cs+ | |
| MDO1 | 3D | 2D | 2D | – | – | – |
| MDO2 | 3D | 1D | 1D | – | – | – |
| non-MDO 4O | 3D | 2D | 2D | – | – | – |
| Parameter | MDO1 Polytype | MDO2 Polytype | Non-MDO 4O Polytype | |||
|---|---|---|---|---|---|---|
| T = B | T = Al | T = B | T = Al | T = B | T = Al | |
| Unit cell parameters (Å), a, b, c | n.d. | 12.170, 13.301, 10.005 | n.d. | n.d. | 11.815, 26.630, 10.042 | n.d. |
| Volume (Å3) | n.d. | 1619.46 | n.d. | n.d. | 3159.55 | n.d. |
| Optimized unit cell parameters (Å), a, b, c | 12.0296, 13.2109, 9.9017 | 12.1698,* 13.3008, * 10.0048 * | 11.7893, 13.4876, 10.1609 | 11.9479, 13.6593, 10.3157 | 11.8248, 26.7192, 10.0423 | 12.2217, 26.9351, 10.1760 |
| Optimized volume (Å3) | 1573.60 | 1619.46 | 1615.68 | 1683.52 | 3172.86 | 3349.86 |
| Z | 4 | 4 | 8 | |||
| Energy per formula unit (eV) | –219.1885 | –218.2701 | –219.2479 | –217.7659 | –219.2780 | –218.2109 |
| FD [(M + T)/1000 Å3] | 19.76 | 19.81 | 19.01 | 22.69 | 21.49 | |
| v (atoms), framework, all | 58, 60 | 116, 120 | 232, 240 | |||
| IG (bits/atom), framework, all | 3.892, 3.974 | 3.892, 3.974 | 4.858, 4.907 | |||
| IG, total (bits/unit cell), framework, all | 225.763, 238.413 | 451.526, 476.827 | 1127.052, 1177.654 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenov, S.M.; Kuznetsov, A.N.; Antonov, A.A.; Yamnova, N.A.; Krivovichev, S.V.; Merlino, S. Polytypism of Compounds with the General Formula Cs{Al2[TP6O20]} (T = B, Al): OD (Order-Disorder) Description, Topological Features, and DFT-Calculations. Minerals 2021, 11, 708. https://doi.org/10.3390/min11070708
Aksenov SM, Kuznetsov AN, Antonov AA, Yamnova NA, Krivovichev SV, Merlino S. Polytypism of Compounds with the General Formula Cs{Al2[TP6O20]} (T = B, Al): OD (Order-Disorder) Description, Topological Features, and DFT-Calculations. Minerals. 2021; 11(7):708. https://doi.org/10.3390/min11070708
Chicago/Turabian StyleAksenov, Sergey M., Alexey N. Kuznetsov, Andrey A. Antonov, Natalia A. Yamnova, Sergey V. Krivovichev, and Stefano Merlino. 2021. "Polytypism of Compounds with the General Formula Cs{Al2[TP6O20]} (T = B, Al): OD (Order-Disorder) Description, Topological Features, and DFT-Calculations" Minerals 11, no. 7: 708. https://doi.org/10.3390/min11070708
APA StyleAksenov, S. M., Kuznetsov, A. N., Antonov, A. A., Yamnova, N. A., Krivovichev, S. V., & Merlino, S. (2021). Polytypism of Compounds with the General Formula Cs{Al2[TP6O20]} (T = B, Al): OD (Order-Disorder) Description, Topological Features, and DFT-Calculations. Minerals, 11(7), 708. https://doi.org/10.3390/min11070708








