Laboratory Investigations Coupled to VIR/Dawn Observations to Quantify the Large Concentrations of Organic Matter on Ceres
Abstract
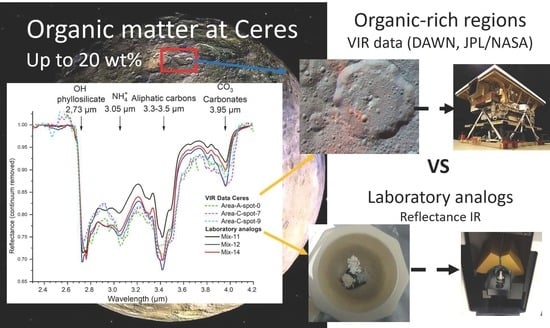
:1. Introduction
2. Methods and Analyses
2.1. VIR/Dawn Spectra and Region of Interest
2.2. Reflectance Spectroscopy
2.3. Endmembers for Ceres Analogues
2.3.1. Mg-Phyllosilicate
2.3.2. Carbonate
2.3.3. Ammonium-Phyllosilicate
2.3.4. Darkening Agent
2.3.5. Aliphatic Carbons
2.4. Mixture Preparation and Analysis
3. Results and Discussion on the Laboratory Mixtures
3.1. Reflectance of the Mixtures
3.2. Composition and Quantification Compared to Ceres Spectra
3.3. Darkening Agent and Grains Sizes
4. Implications for the Composition of Ceres
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivkin, A.S.; Volquardsen, E.L.; Clark, B.E. The Surface Composition of Ceres: Discovery of Carbonates and Iron-Rich Clays. Icarus 2006, 185, 563–567. [Google Scholar] [CrossRef]
- Takir, D.; Emery, J.P. Outer Main Belt Asteroids: Identification and Distribution of Four 3-Μm Spectral Groups. Icarus 2012, 219, 641–654. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Coradini, A.; Ammannito, E.; Filacchione, G.; Capria, M.T.; Fonte, S.; Magni, G.; Barbis, A.; Bini, A.; Dami, M.; et al. The VIR Spectrometer. Space Sci. Rev. 2011, 163, 329–369. [Google Scholar] [CrossRef]
- Ammannito, E.; DeSanctis, M.C.; Ciarniello, M.; Frigeri, A.; Carrozzo, F.G.; Combe, J.-P.; Ehlmann, B.L.; Marchi, S.; McSween, H.Y.; Raponi, A.; et al. Distribution of Phyllosilicates on the Surface of Ceres. Science 2016, 353, aaf4279. [Google Scholar] [CrossRef]
- Carrozzo, F.G.; Sanctis, M.C.D.; Raponi, A.; Ammannito, E.; Castillo-Rogez, J.; Ehlmann, B.L.; Marchi, S.; Stein, N.; Ciarniello, M.; Tosi, F.; et al. Nature, Formation, and Distribution of Carbonates on Ceres. Sci. Adv. 2018, 4, e1701645. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis, M.C.; Raponi, A.; Ammannito, E.; Ciarniello, M.; Toplis, M.J.; McSween, H.Y.; Castillo-Rogez, J.C.; Ehlmann, B.L.; Carrozzo, F.G.; Marchi, S.; et al. Bright Carbonate Deposits as Evidence of Aqueous Alteration on (1) Ceres. Nature 2016, 536, 54–57. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Ammannito, E.; Raponi, A.; Marchi, S.; McCord, T.B.; McSween, H.Y.; Capaccioni, F.; Capria, M.T.; Carrozzo, F.G.; Ciarniello, M.; et al. Ammoniated Phyllosilicates with a Likely Outer Solar System Origin on (1) Ceres. Nature 2015, 528, 241–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sanctis, M.C.; Ammannito, E.; Raponi, A.; Frigeri, A.; Ferrari, M.; Carrozzo, F.G.; Ciarniello, M.; Formisano, M.; Rousseau, B.; Tosi, F.; et al. Fresh Emplacement of Hydrated Sodium Chloride on Ceres from Ascending Salty Fluids. Nat. Astron. 2020, 4, 786–793. [Google Scholar] [CrossRef]
- Raponi, A.; De Sanctis, M.C.; Carrozzo, F.G.; Ciarniello, M.; Castillo-Rogez, J.C.; Ammannito, E.; Frigeri, A.; Longobardo, A.; Palomba, E.; Tosi, F.; et al. Mineralogy of Occator Crater on Ceres and Insight into Its Evolution from the Properties of Carbonates, Phyllosilicates, and Chlorides. Icarus 2019, 320, 83–96. [Google Scholar] [CrossRef]
- Castillo-Rogez, J.C.; Neveu, M.; McSween, H.Y.; Fu, R.R.; Toplis, M.J.; Prettyman, T. Insights into Ceres’s Evolution from Surface Composition. Meteorit. Planet. Sci. 2018, 53, 1820–1843. [Google Scholar] [CrossRef]
- Russell, C.T.; Raymond, C.A.; Ammannito, E.; Buczkowski, D.L.; De Sanctis, M.C.D.; Hiesinger, H.; Jaumann, R.; Konopliv, A.S.; McSween, H.Y.; Nathues, A.; et al. Dawn Arrives at Ceres: Exploration of a Small, Volatile-Rich World. Science 2016, 353, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Rogez, J.C.; Neveu, M.; Scully, J.E.C.; House, C.H.; Quick, L.C.; Bouquet, A.; Miller, K.; Bland, M.; De Sanctis, M.C.; Ermakov, A.; et al. Ceres: Astrobiological Target and Possible Ocean World. Astrobiology 2020, 20, 269–291. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, M.C.; Mitri, G.; Castillo-Rogez, J.; House, C.H.; Marchi, S.; Raymond, C.A.; Sekine, Y. Relict Ocean Worlds: Ceres. Space Sci. Rev. 2020, 216, 60. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Ammannito, E.; McSween, H.Y.; Raponi, A.; Marchi, S.; Capaccioni, F.; Capria, M.T.; Carrozzo, F.G.; Ciarniello, M.; Fonte, S.; et al. Localized Aliphatic Organic Material on the Surface of Ceres. Science 2017, 355, 719–722. [Google Scholar] [CrossRef]
- De Sanctis, M.C.; Vinogradoff, V.; Raponi, A.; Ammannito, E.; Ciarniello, M.; Carrozzo, F.G.; De Angelis, S.; Raymond, C.A.; Russell, C.T. Characteristics of Organic Matter on Ceres from VIR/Dawn High Spatial Resolution Spectra. Mon. Not. R. Astron. Soc. 2019, 482, 2407–2421. [Google Scholar] [CrossRef]
- Raponi, A.; De Sanctis, M.C.; Giacomo Carrozzo, F.; Ciarniello, M.; Rousseau, B.; Ferrari, M.; Ammannito, E.; De Angelis, S.; Vinogradoff, V.; Castillo-Rogez, J.C.; et al. Organic Material on Ceres: Insights from Visible and Infrared Space Observations. Life 2021, 11, 9. [Google Scholar] [CrossRef]
- Prettyman, T.H.; Yamashita, N.; Toplis, M.J.; McSween, H.Y.; Schörghofer, N.; Marchi, S.; Feldman, W.C.; Castillo-Rogez, J.; Forni, O.; Lawrence, D.J.; et al. Extensive Water Ice within Ceres’ Aqueously Altered Regolith: Evidence from Nuclear Spectroscopy. Science 2017, 355, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Pearson, V.K.; Sephton, M.A.; Franchi, I.A.; Gibson, J.M.; Gilmour, I. Carbon and Nitrogen in Carbonaceous Chondrites: Elemental Abundances and Stable Isotopic Compositions. Meteorit. Planet. Sci. 2006, 41, 1899–1918. [Google Scholar] [CrossRef]
- McSween, H.Y.; Emery, J.P.; Rivkin, A.S.; Toplis, M.J.C.; Castillo-Rogez, J.; Prettyman, T.H.; De Sanctis, M.C.; Pieters, C.M.; Raymond, C.A.; Russell, C.T. Carbonaceous Chondrites as Analogs for the Composition and Alteration of Ceres. Meteorit. Planet. Sci. 2017, 1–12. [Google Scholar] [CrossRef]
- Pieters, C.M.; Nathues, A.; Thangiam, G.; Hoffmann, M.; Platz, T.; De Sanctis, M.C.; Ammannito, E.; Tosi, F.; Zambon, F.; Pasckert, J.H.; et al. Geologic Constraints on the Origin of Red Organic-Rich Material on Ceres. Meteorit. Planet. Sci. 2018, 53, 1983–1998. [Google Scholar] [CrossRef]
- Marchi, S.; Raponi, A.; Prettyman, T.H.; Sanctis, M.C.D.; Castillo-Rogez, J.; Raymond, C.A.; Ammannito, E.; Bowling, T.; Ciarniello, M.; Kaplan, H.; et al. An Aqueously Altered Carbon-Rich Ceres. Nat. Astron. 2019, 3, 140–145. [Google Scholar] [CrossRef]
- Bowling, T.J.; Johnson, B.C.; Marchi, S.; De Sanctis, M.C.; Castillo-Rogez, J.C.; Raymond, C.A. An Endogenic Origin of Cerean Organics. Earth Planet. Sci. Lett. 2020, 534, 116069. [Google Scholar] [CrossRef]
- Ciarniello, M.; Sanctis, M.C.D.; Ammannito, E.; Raponi, A.; Longobardo, A.; Palomba, E.; Carrozzo, F.G.; Tosi, F.; Li, J.-Y.; Schröder, S.E.; et al. Spectrophotometric Properties of Dwarf Planet Ceres from the VIR Spectrometer on Board the Dawn Mission. Astron. Astrophys. 2017, 598, A130. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; De Angelis, S.; De Sanctis, M.C.; Ammannito, E.; Stefani, S.; Piccioni, G. Reflectance Spectroscopy of Ammonium-Bearing Phyllosilicates. Icarus 2019, 321, 522–530. [Google Scholar] [CrossRef]
- Frondini, F.; Zucchini, A.; Comodi, P. Water-Rock Interactions and Trace Elements Distribution in Dolomite Aquifers: The Sassolungo and Sella Systems (Northern Italy). Geochem. J. 2014, 48, 231–246. [Google Scholar] [CrossRef] [Green Version]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High Molecular Diversity of Extraterrestrial Organic Matter in Murchison Meteorite Revealed 40 Years after Its Fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, C.M.O.; Cody, G.D.; De Gregorio, B.T.; Nittler, L.R.; Stroud, R.M. The Nature, Origin and Modification of Insoluble Organic Matter in Chondrites, the Major Source of Earth’s C and N. Geochemistry 2017, 77, 227–256. [Google Scholar] [CrossRef]
- Danger, G.; Ruf, A.; Maillard, J.; Hertzog, J.; Vinogradoff, V.; Schmitt-Kopplin, P.; Afonso, C.; Carrasco, N.; Schmitz-Afonso, I.; d’Hendecourt, L.L.S.; et al. Unprecedented Molecular Diversity Revealed in Meteoritic Insoluble Organic Matter: The Paris Meteorite’s Case. Planet. Sci. J. 2020, 1, 55. [Google Scholar] [CrossRef]
- Moroz, L.V.; Arnold, G.; Korochantsev, A.V.; Wäsch, R. Natural Solid Bitumens as Possible Analogs for Cometary and Asteroid Organics. Icarus 1998, 134, 253–268. [Google Scholar] [CrossRef]
- Rotundi, A.; Rietmeijer, F.J.M. Carbon in Meteoroids: Wild 2 Dust Analyses, IDPs and Cometary Dust Analogues. In Advances in Meteoroid and Meteor Science; Trigo-Rodríguez, J.M., Rietmeijer, F.J.M., Llorca, J., Janches, D., Eds.; Springer: New York, NY, USA, 2008; pp. 473–483. ISBN 978-0-387-78419-9. [Google Scholar]
- Angelis, S.D.; Ferrari, M.; Sanctis, M.C.D.; Ammannito, E.; Raponi, A.; Ciarniello, M. High-Temperature VIS-IR Spectroscopy of NH4-Phyllosilicates. J. Geophys. Res. Planets 2021, 126, e2020JE006696. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ehlmann, B.L.; Sanctis, M.C.D.; Lapôtre, M.G.A.; Usui, T.; Stein, N.T.; Prettyman, T.H.; Raponi, A.; Ciarniello, M. A Probabilistic Approach to Determination of Ceres’ Average Surface Composition from Dawn Visible-Infrared Mapping Spectrometer and Gamma Ray and Neutron Detector Data. J. Geophys. Res. Planets 2020, 125, e2020JE006606. [Google Scholar] [CrossRef]
- Kaplan, H.H.; Milliken, R.E.; Alexander, C.M.O. New Constraints on the Abundance and Composition of Organic Matter on Ceres. Geophys. Res. Lett. 2018, 45, 5274–5282. [Google Scholar] [CrossRef] [Green Version]
- Cloutis, E.A. Quantitative Characterization of Coal Properties Using Bidirectional Diffuse Reflectance Spectroscopy. Fuel 2003, 82, 2239–2254. [Google Scholar] [CrossRef]
- Rousseau, B.; Érard, S.; Beck, P.; Quirico, É.; Schmitt, B.; Brissaud, O.; Montes-Hernandez, G.; Capaccioni, F.; Filacchione, G.; Bockelée-Morvan, D.; et al. Laboratory Simulations of the Vis-NIR Spectra of Comet 67P Using Sub-Μm Sized Cosmochemical Analogues. Icarus 2018, 306, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Campins, H.; Hargrove, K.; Pinilla-Alonso, N.; Howell, E.S.; Kelley, M.S.; Licandro, J.; Mothe-Diniz, T.; Fernandez, Y.; Ziffer, J. Water Ice and Organics on the Surface of the Asteroid 24 Themis. Nature 2010, 464, 1320–1321. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, A.S.; Emery, J.P. Detection of Ice and Organics on an Asteroidal Surface. Nature 2010, 464, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- Licandro, J.; Campins, H.; Kelley, M.; Hargrove, K.; Pinilla-Alonso, N.; Cruikshank, D.; Rivkin, A.S.; Emery, J. (65) Cybele: Detection of Small Silicate Grains, Water-Ice, and Organics. Astron. Astrophys. 2011, 525, A34. [Google Scholar] [CrossRef] [Green Version]
- Brearley, A.J. The Action of Water. In Meteorites and the Early Solar System II; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 584–624. [Google Scholar]
- King, A.J.; Schofield, P.F.; Howard, K.T.; Russell, S.S. Modal Mineralogy of CI and CI-like Chondrites by X-ray Diffraction. Geochim. Cosmochim. Acta 2015, 165, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Raponi, A.; Carrozzo, F.G.; Zambon, F.; De Sanctis, M.C.; Ciarniello, M.; Frigeri, A.; Ammannito, E.; Tosi, F.; Combe, J.-P.; Longobardo, A.; et al. Mineralogical Mapping of Coniraya Quadrangle of the Dwarf Planet Ceres. Icarus 2017. [Google Scholar] [CrossRef]
- Quirico, E.; Moroz, L.V.; Schmitt, B.; Arnold, G.; Faure, M.; Beck, P.; Bonal, L.; Ciarniello, M.; Capaccioni, F.; Filacchione, G.; et al. Refractory and Semi-Volatile Organics at the Surface of Comet 67P/Churyumov-Gerasimenko: Insights from the VIRTIS/Rosetta Imaging Spectrometer. Icarus 2016, 272, 32–47. [Google Scholar] [CrossRef] [Green Version]
- Cloutis, E.A.; Gaffey, M.J.; Smith, D.G.W.; Lambert, R.S.J. Reflectance Spectra of Mafic Silicate-Opaque Assemblages with Applications to Meteorite Spectra. Icarus 1990, 84, 315–333. [Google Scholar] [CrossRef]
- Moroz, L.; Baratta, G.; Strazzulla, G.; Starukhina, L.; Dotto, E.; Barucci, M.A.; Arnold, G.; Distefano, E. Optical Alteration of Complex Organics Induced by Ion Irradiation:: 1. Laboratory Experiments Suggest Unusual Space Weathering Trend. Icarus 2004, 170, 214–228. [Google Scholar] [CrossRef]
- Skulteti, A.; Kereszturi, A.; Szabo, M.; Kereszty, Z.; Cipriani, F. Mid-Infrared Spectroscopic Investigation of Meteorites and Perspectives for Thermal Infrared Observations at the Binary Asteroid Didymos. Planet. Space Sci. 2020, 184, 104855. [Google Scholar] [CrossRef]
- Hapke, B. Space Weathering from Mercury to the Asteroid Belt. J. Geophys. Res. Planets 2001, 106, 10039–10073. [Google Scholar] [CrossRef]
- Shkuratov, Y.; Starukhina, L.; Hoffmann, H.; Arnold, G. A Model of Spectral Albedo of Particulate Surfaces: Implications for Optical Properties of the Moon. Icarus 1999, 137, 235–246. [Google Scholar] [CrossRef]
- Chapman, C.R. Space Weathering of Asteroid Surfaces. Annu. Rev. Earth Planet. Sci. 2004, 32, 539–567. [Google Scholar] [CrossRef] [Green Version]
- Bousige, C.; Ghimbeu, C.M.; Vix-Guterl, C.; Pomerantz, A.E.; Suleimenova, A.; Vaughan, G.; Garbarino, G.; Feygenson, M.; Wildgruber, C.; Ulm, F.-J.; et al. Realistic Molecular Model of Kerogen’s Nanostructure. Nat. Mater. 2016, 15, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Lantz, C.; Brunetto, R.; Barucci, M.A.; Dartois, E.; Duprat, J.; Engrand, C.; Godard, M.; Ledu, D.; Quirico, E. Ion Irradiation of the Murchison Meteorite: Visible to Mid-Infrared Spectroscopic Results. Astron. Astrophys. 2015, 577, A41. [Google Scholar] [CrossRef] [Green Version]
- Godard, M.; Féraud, G.; Chabot, M.; Carpentier, Y.; Pino, T.; Brunetto, R.; Duprat, J.; Engrand, C.; Bréchignac, P.; d’Hendecourt, L.; et al. Ion Irradiation of Carbonaceous Interstellar Analogues—Effects of Cosmic Rays on the 3.4 Μm Interstellar Absorption Band. Astron. Astrophys. 2011, 529, A146. [Google Scholar] [CrossRef] [Green Version]
- Lagaly, G.; Ogawa, M.; Dékány, I. Chapter 10.3—Clay Mineral–Organic Interactions. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505. [Google Scholar]
- Vinogradoff, V.; Le Guillou, C.; Bernard, S.; Viennet, J.C.; Jaber, M.; Remusat, L. Influence of Phyllosilicates on the Hydrothermal Alteration of Organic Matter in Asteroids: Experimental Perspectives. Geochim. Cosmochim. Acta 2020, 269, 150–166. [Google Scholar] [CrossRef]
- Poch, O.; Jaber, M.; Stalport, F.; Nowak, S.; Georgelin, T.; Lambert, J.-F.; Szopa, C.; Coll, P. Effect of Nontronite Smectite Clay on the Chemical Evolution of Several Organic Molecules under Simulated Martian Surface Ultraviolet Radiation Conditions. Astrobiology 2015, 15, 221–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, R.; Patel, M.; Cuadros, J.; Martins, Z. Influence of Mineralogy on the Preservation of Amino Acids under Simulated Mars Conditions. Icarus 2016, 277, 342–353. [Google Scholar] [CrossRef]
- Zeilnhofer, M.F.; Barlow, N.G. The Characterization and Distribution of Polygonal Impact Craters on Ceres and Their Implications for the Cerean Crust. Icarus 2021, 114586. [Google Scholar] [CrossRef]
- Hargitai, H.; Kereszturi, Á. (Eds.) Encyclopedia of Planetary Landforms; Springer: New York, NY, USA, 2015; ISBN 978-1-4614-3133-6. [Google Scholar]
- Shi, X.; Castillo-Rogez, J.; Hsieh, H.; Hui, H.; Ip, W.-H.; Lei, H.; Li, J.-Y.; Tosi, F.; Zhou, L.; Agarwal, J.; et al. GAUSS—A Sample Return Mission to Ceres. arXiv 2020, arXiv:190807731. [Google Scholar]
- Castillo-Rogez, J.; Scully, J.; Neveu, M.; Wyrick, D.; Thangjam, G.; Rivkin, A.; Sori, M.; Vinogradoff, V.; Miller, K.; Ermakov, A.; et al. Science Motivations for the Future Exploration of Ceres. Bull. Am. Astron. Soc. 2021, 53, 303. [Google Scholar] [CrossRef]








| Compound | Grain Size (Before Grinding) | Density (g/cm3) | Carbon (wt.%) |
|---|---|---|---|
| Antigorite | <50 µm | 2.9 | / |
| Dolomite | <36µm | 2.85 | / |
| NH4-montmorillonite a | <36 µm | 2.25 | / |
| Amorphous carbon | <50 µm | 2 | 100 |
| Magnetite | <50 µm | 5.2 | / |
| DECS-19 b | <250 µm | 1.5–2 | 85.6 |
| DECS-16 b | <250 µm | 1.5–2 | 69.6 |
| Mixtures N° | Quantity (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Coals | Serpentine | Mt + NH4+ | Dolomite | Magnetite | Amorphous Carbon | ||
| Name | Carbon (wt.%) | ||||||
| mix-10 | DECS-19 | 7.8 | 16.8 | 8.3 | 16.8 | 39.0 | 0.0 |
| DECS-16 | 11.4 | ||||||
| mix-11 | DECS-19 | 8.5 | 19.0 | 14.2 | 19.0 | 28.4 | 0.0 |
| DECS-16 | 10.9 | ||||||
| mix-12 | DECS-19 | 7.7 | 17.3 | 13.0 | 26.0 | 26.0 | 0.0 |
| DECS-16 | 9.9 | ||||||
| mix-13 | DECS-19 | 10.4 | 22.3 | 11.2 | 22.3 | 0.0 | 18.6 |
| DECS-16 | 15.2 | ||||||
| mix-14 | DECS-19 | 9.2 | 13.0 | 16.4 | 19.7 | 28.2 | 0.0 |
| DECS-16 | 13.4 | ||||||
| mix-15 | DECS-19 | 7.8 | 16.8 | 8.3 | 16.8 | 0.0 | 39.0 |
| DECS-16 | 11.4 | ||||||
| mix-16 | DECS-19 | 1.4 | 3.0 | 1.5 | 3.0 | 89.2 | 0.0 |
| DECS-16 | 2 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradoff, V.; Poggiali, G.; Raponi, A.; Ciarniello, M.; De Angelis, S.; Ferrari, M.; Castillo-Rogez, J.C.; Brucato, J.; De Sanctis, M.-C. Laboratory Investigations Coupled to VIR/Dawn Observations to Quantify the Large Concentrations of Organic Matter on Ceres. Minerals 2021, 11, 719. https://doi.org/10.3390/min11070719
Vinogradoff V, Poggiali G, Raponi A, Ciarniello M, De Angelis S, Ferrari M, Castillo-Rogez JC, Brucato J, De Sanctis M-C. Laboratory Investigations Coupled to VIR/Dawn Observations to Quantify the Large Concentrations of Organic Matter on Ceres. Minerals. 2021; 11(7):719. https://doi.org/10.3390/min11070719
Chicago/Turabian StyleVinogradoff, Vassilissa, Giovanni Poggiali, Andrea Raponi, Mauro Ciarniello, Simone De Angelis, Marco Ferrari, Julie C Castillo-Rogez, John Brucato, and Maria-Cristina De Sanctis. 2021. "Laboratory Investigations Coupled to VIR/Dawn Observations to Quantify the Large Concentrations of Organic Matter on Ceres" Minerals 11, no. 7: 719. https://doi.org/10.3390/min11070719
APA StyleVinogradoff, V., Poggiali, G., Raponi, A., Ciarniello, M., De Angelis, S., Ferrari, M., Castillo-Rogez, J. C., Brucato, J., & De Sanctis, M.-C. (2021). Laboratory Investigations Coupled to VIR/Dawn Observations to Quantify the Large Concentrations of Organic Matter on Ceres. Minerals, 11(7), 719. https://doi.org/10.3390/min11070719