Metal Accumulation and Tolerance in Artemisia indica var. maximowiczii (Nakai) H. Hara. and Fallopia sachalinensis (F.Schmidt) Ronse Decr., a Naturally Growing Plant Species at Mine Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Vegetation Survey
2.2. Sampling and Analysis of Root-Zone Soil and Plant Tissues and Calculations of Translocation Factor and Bioconcentration Factor
2.3. Analysis of Phenolic Compounds in Roots of A. indica var. maximowiczii
2.4. Statistical Analysis
3. Results
3.1. The Vegetation Survey
3.2. pH and Total Element Concentrations in Root-Zone Soil
3.3. Element Concentrations in the Target Plant Tissues
3.4. Translocation Factors (TFs) and Bioconcentration Factors (BCFs)
3.5. Phenolic Compounds in the Roots of A. indica var. maximowiczii
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Marques, A.P.; Rangel, A.O.; Castro, P.M. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.J.M. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ernst, W.H.O.; Verkleij, J.A.C.; Schat, H. Metal tolerance in plants. Acta Bot. Neerl. 1992, 41, 229–248. [Google Scholar] [CrossRef]
- Larcher, W. Physiological plant ecology. In Ökophysiologie der Pflanzen, 6th ed.; Huber-Sannwald, E., Translator; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Anjum, N.A.; Tuteja, N. Amelioration of cadmium stress in crop plants by nutrients management: Morphological, physiological and biochemical aspects. Plant Stress 2011, 5, 1–23. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Morishita, T.; Boratynski, J.K. Accumulation of cadmium and other metals in organs of plants growing around metal smelters in Japan. Soil Sci. Plant Nutr. 1992, 38, 781–785. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Liang, S.; Yi, L.; Xu, B.; Cao, J.; Guo, Y.; Zhou, Y. Heavy metal accumulation and phytostabilization potential of dominant plant species growing on manganese mine tailings. Front. Environ. Sci. Eng. 2014, 8, 394–404. [Google Scholar] [CrossRef]
- Baryla, A.; Carrier, P.; Franck, F.; Coulomb, C.; Sahut, C.; Havaux, M. Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: Causes and consequences for photosynthesis and growth. Planta 2001, 212, 696–709. [Google Scholar] [CrossRef]
- Hagemeyer, J. Ecophysiology of plant growth under heavy metal stress. In Heavy Metal Stress in Plants, 2nd ed.; Prasad, M.N.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 201–222. [Google Scholar]
- Ghosh, M.; Singh, S.P. A comparative study of cadmium phytoextraction by accumulator and weed species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ichihara, Y. The role of catechin and epicatechin in chemical defense against damping-off fungi of current-year Fagus crenata seedlings in natural forest. For. Pathol. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Asami, T. Harmful Metal Pollution of Soils in Japan; AGNE Gijutsu Center: Tokyo, Japan, 2001; p. 21. (In Japanese) [Google Scholar]
- Hutchinson, G.E. Aluminum in soils, plants, and animals. Soil Sci. 1945, 60, 29–40. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Mine vegetation in Europe. In Heavy Metal Tolerance in Plants: Evolutionary Aspects; Shaw, A.J., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 21–38. [Google Scholar]
- Peng, K.; Li, X.; Luo, C.; Shen, Z. Vegetation composition and heavy metal uptake by wild plants at three contaminated sites in Xiangxi area, China. J. Environ. Sci. Health Part A 2006, 41, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, T. Phytosociological research in copper mine vegetation, Japan. J. Humanit. Nat. Sci. 1974, 38, 177–226. (In Japanese) [Google Scholar]
- Hiroi, T. Vegetation in area around heavy metal mines and smelters. J. Humanit. Nat. Sci. 1980, 55, 63–98. (In Japanese) [Google Scholar]
- Haruma, T.; Yamaji, K.; Masuya, H.; Hanyu, K. Root endophytic Chaetomium cupreum promotes plant growth and detoxifies aluminum in Miscanthus sinensis Andersson growing at the acidic mine site. Plant Species Biol. 2018, 33, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Bidar, G.; Garçon, G.; Pruvot, C.; Dewaele, D.; Cazier, F.; Douay, F.; Shirali, P. Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environ. Pollut. 2007, 147, 546–553. [Google Scholar] [CrossRef]
- Seo, K.W.; Son, Y.; Rhoades, C.C.; Noh, N.J.; Koo, J.W.; Kim, J.G. Seedling growth and heavy metal accumulation of candidate woody species for revegetating Korean mine spoils. Restor. Ecol. 2008, 16, 702–712. [Google Scholar] [CrossRef]
- Ghassemzadeh, F.; Yousefzadeh, H.; Arbab-Zavar, M.H. Removing arsenic and antimony by Phragmites australis: Rhizofiltration technology. J. Appl. Sci. 2008, 8, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Kim, J.G.; Lim, S.K.; Lee, S.H.; Yoon, Y.M.; Lee, C.H.; Jeong, C.Y. Evaluation of heavy metal pollution and plant survey around inactive and abandoned mining areas for phytoremediation of heavy metal contaminated soils. Korean J. Environ. Agric. 1999, 18, 28–34, (In Korean with English Abstract). [Google Scholar]
- Porębska, G.; Ostrowska, A. Heavy metal accumulation in wild plants: Implications for phytoremediation. Pol. J. Environ. Stud. 1999, 8, 433–442. [Google Scholar]
- Alirzayeva, E.G.; Shirvani, T.S.; Alverdiyeva, S.M.; Shukurov, E.S.; Öztürk, L.; Ali-zade, V.M.; Çakmak, İ. Heavy metal accumulation in Artemisia and foliaceous lichen species from the Azerbaijan flora. For. Snow Landsc. Res. 2006, 80, 339–348. [Google Scholar]
- Alirzayeva, E.; Neumann, G.; Horst, W.; Allahverdiyeva, Y.; Specht, A.; Alizade, V. Multiple mechanisms of heavy metal tolerance are differentially expressed in ecotypes of Artemisia fragrans. Environ. Pollut. 2017, 220, 1024–1035. [Google Scholar] [CrossRef]
- Nishizono, H.; Kubota, K.; Suzuki, S.; Ishii, F. Accumulation of heavy metals in cell walls of Polygonum cuspidatum roots from metalliferous habitats. Plant Cell Physiol. 1989, 30, 595–598. [Google Scholar] [CrossRef]
- Sołtysiak, J.; Berchová-Bímová, K.; Vach, M.; Brej, T. Heavy metals content in the Fallopia genus in Central European Cities—study from Wroclaw and Prague. Acta Bot. Sil. 2011, 7, 209–218. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Yang, H.; Lee, D.Y.; Jeon, M.; Suh, Y.; Sung, S.H. Determination of five active compounds in Artemisia princeps and A. capillaris based on UPLC-DAD and discrimination of two species with multivariate analysis. Arch. Pharmacal. Res. 2014, 37, 617–625. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, T.; Li, W.; Nagata, K.; Higai, K.; Feng, F.; Wang, J.; Cheng, M.; Koike, K. Identification of caffeoylquinic acid derivatives as natural protein tyrosine phosphatase 1B inhibitors from Artemisia princeps. Bioorg. Med. Chem. Lett. 2018, 28, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Iizuka, Y.; Kurihara, M.; Onuki, M.; Kubo, M.; Matsue, M. Manual of the Slope Revegetation Method for Conservation of Regional Ecosystem; Technical Note of National Institute for Land and Infrastructure Management No. 722; Landscape and Ecology Division, National Institute for Land and Infrastructure Management, Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, January 2013. (In Japanese) [Google Scholar]
- Yuasa, Y.; Sawata, K.; Murai, H.; Inoue, K. Vegetational changes in revegetated open-cut mining lands of former Matsuo sulfur mine, Iwate prefecture. Jpn. J. Soil Sci. Plant Nutr. 1995, 66, 646–654, (In Japanese with English Abstract). [Google Scholar]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, M.J.; Zhang, X.T.; Zhang, H.B.; Sha, T.; Zhao, Z.W. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci. Total Environ. 2011, 409, 1069–1074. [Google Scholar] [CrossRef]
- Yamaji, K.; Watanabe, Y.; Masuya, H.; Shigeto, A.; Yui, H.; Haruma, T. Root fungal endophytes enhance heavy-metal stress tolerance of Clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS ONE 2016, 11, e0169089. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Cao, L. Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere 2017, 168, 1100–1106. [Google Scholar] [CrossRef]

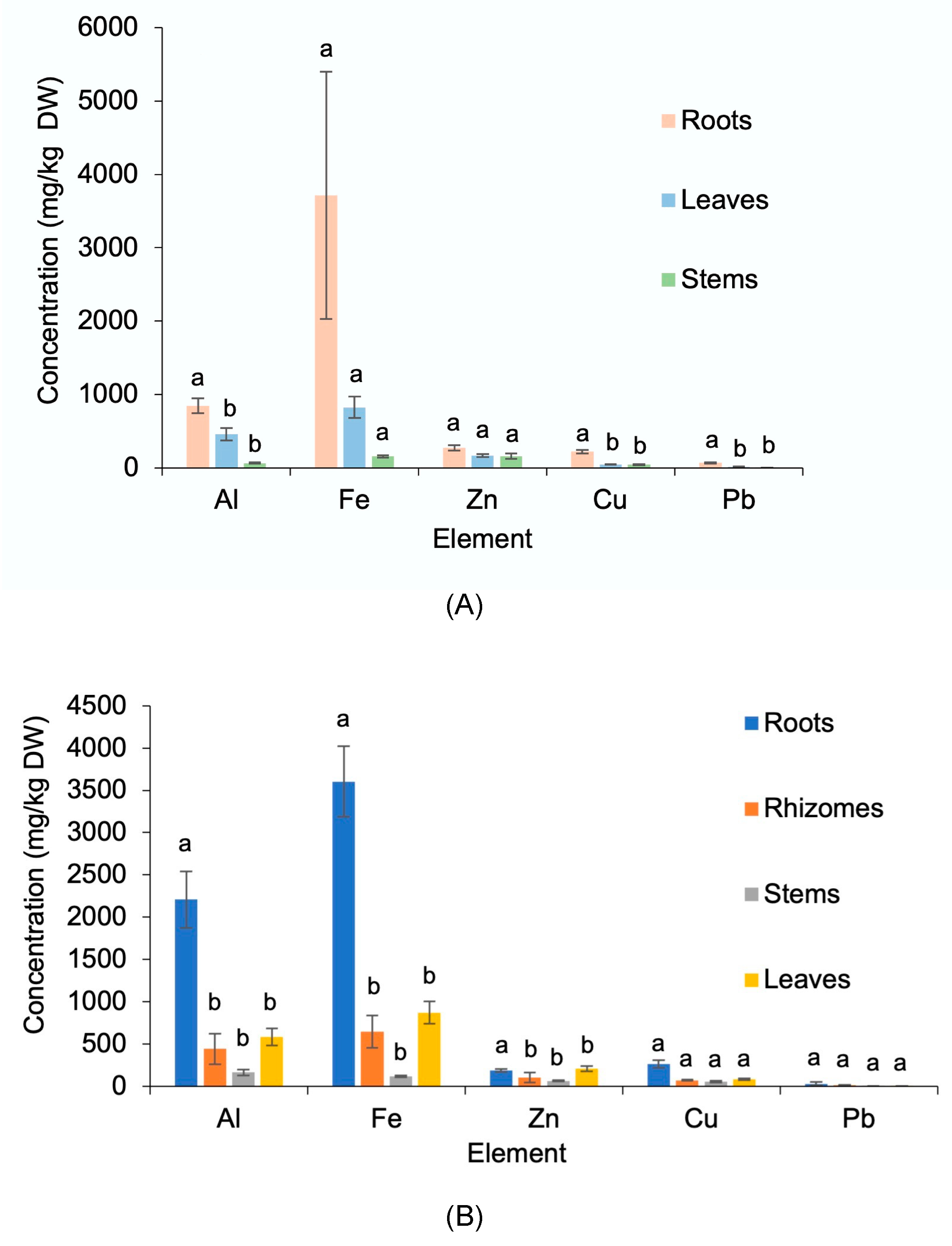
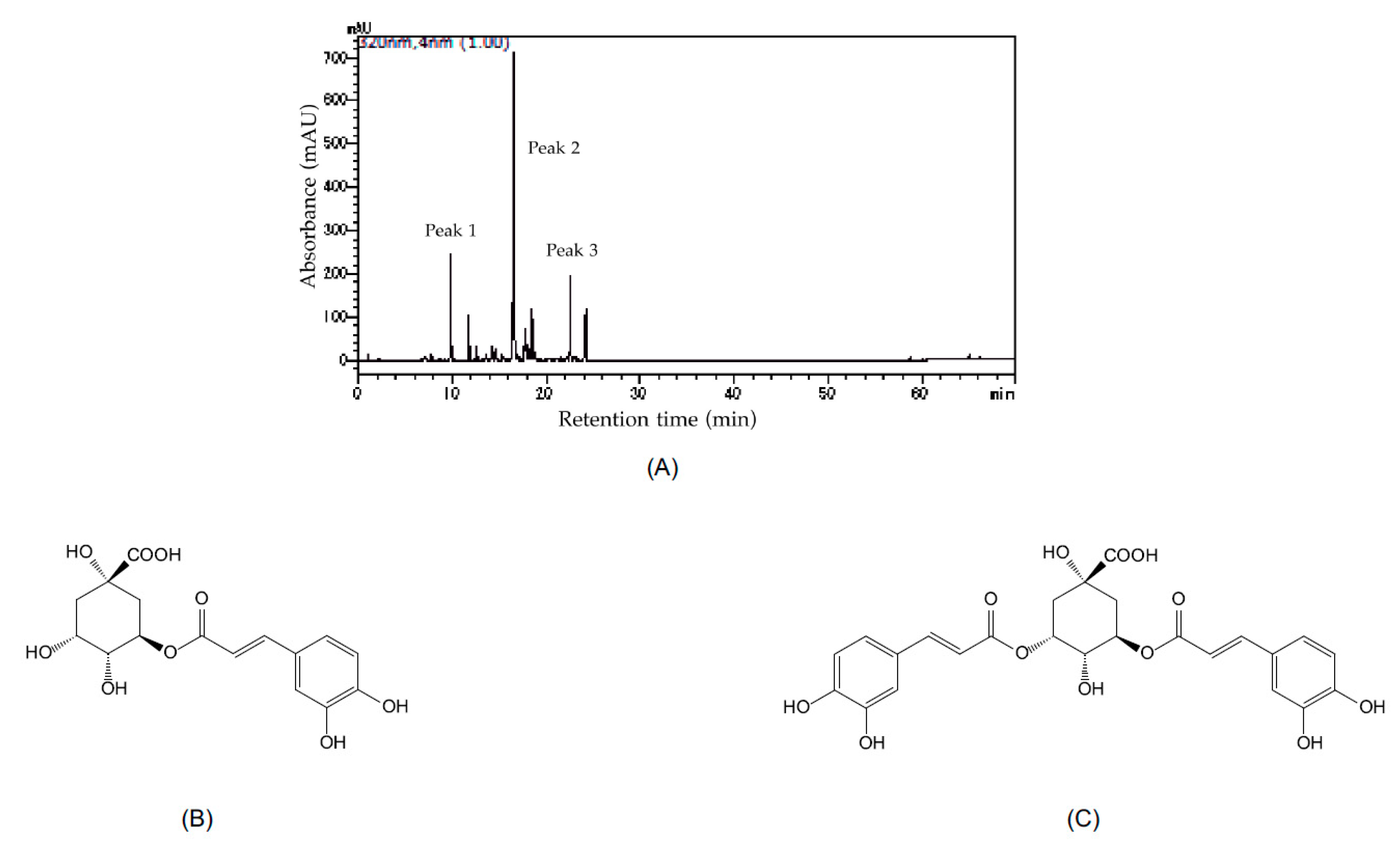
| Plant Species 1 | Average Cover Rate (%) |
| Miscanthus sinensis | 22.5 |
| Trifolium repens | 20.7 |
| Fallopia sachalinensis | 6.0 |
| Robinia pseudoacacia | 5.2 |
| Euonymus spp. | 4.4 |
| Artemisia indica var. maximowiczii | 3.9 |
| Quercus crispula | 1.8 |
| Pinus densiflora | 1.5 |
| Salix spp. | 1.2 |
| Plantago asiatica | 1.0 |
| Plant Species 2 | Average Cover Rate (%) |
| Pinus densiflora | 20.0 |
| Miscanthus sinensis | 14.3 |
| Euonymus spp. | 7.1 |
| Fallopia sachalinensis | 3.3 |
| Weigela hortensis | 2.5 |
| Salix chaenomeloides | 2.5 |
| Populus tremula var. sieboldii | 1.9 |
| Toxicodendron trichocarpum | 1.3 |
| Acer rufinerve | 0.4 |
| Tripterospermum japonicum | 0.4 |
| Trifolium repens | 0.4 |
| Plant Species 3 | Average Cover Rate (%) |
| Phragmites australis | 10.0 |
| Fallopia sachalinensis | 7.1 |
| Miscanthus sinensis | 5.3 |
| Artemisia indica var. maximowiczii | 4.7 |
| Trifolium repens | 3.4 |
| Typha latifolia | 2.8 |
| Equisetum arvense | 2.5 |
| Carex spp. | 1.7 |
| Eragrostis ferruginea | 0.5 |
| Salix spp. | 0.3 |
| Elements | Root-Zone Soil | Unpolluted Soil in Japan * | General Soil ** | |
|---|---|---|---|---|
| A. indica var. maximowiczii | F. sachalinensis | |||
| Fe | 30,616.1 ± 904.0 | 46,640.9 ± 11,356.1 | – | 40,000 |
| Al | 8699.5 ± 564.7 | 8490.1 ± 862.5 | – | 70,000 |
| Zn | 226.4 ± 15.1 | 624.3 ± 165.7 | 60 | 90 |
| Cu | 390.5 ± 21.1 | 1291.5 ± 263.2 | 19 | 30 |
| Pb | 209.6 ± 31.8 | 1367.5 ± 523.2 | 17 | 30 |
| Elements | Stems/Roots | Leaves/Roots | ||
|---|---|---|---|---|
| A. indica var. maximowiczii | F. sachalinensis | A. indica var. maximowiczii | F. sachalinensis | |
| Al | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.56 ± 0.10 | 0.31 ± 0.08 |
| Fe | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.36 ± 0.01 | 0.26 ± 0.05 |
| Zn | 0.66 ± 0.16 * | 0.38 ± 0.07 | 0.70 ± 0.15 | 1.19 ± 0.22 |
| Cu | 0.21 ± 0.06 | 0.23 ± 0.03 | 0.21 ± 0.03 | 0.39 ± 0.10 * |
| Pb | 0.07 ± 0.00 | – | 0.19 ± 0.03 | – |
| Elements | Roots | Stems | Leaves | |||
|---|---|---|---|---|---|---|
| A. indica var. maximowiczii | F. sachalinensis | A. indica var. maximowiczii | F. sachalinensis | A. indica var. maximowiczii | F. sachalinensis | |
| Al | 0.10 ± 0.01 | 0.27 ± 0.04 * | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.01 | 0.08 ± 0.02 |
| Fe | 0.13 ± 0.06 | 0.09 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 |
| Zn | 1.26 ± 0.24 * | 0.37 ± 0.06 | 0.72 ± 0.14 | 0.13 ± 0.03 | 0.71 ± 0.06 * | 0.45 ± 0.12 |
| Cu | 0.58 ± 0.08 | 0.25 ± 0.05 | 0.11 ± 0.02 | 0.06 ± 0.02 | 0.12 ± 0.01 | 0.08 ± 0.03 |
| Pb | 0.35 ± 0.07 | – | 0.02 ± 0.00 | – | 0.06 ± 0.01 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Yamaji, K.; Haruma, T.; Yachi, M.; Doyama, K.; Tomiyama, S. Metal Accumulation and Tolerance in Artemisia indica var. maximowiczii (Nakai) H. Hara. and Fallopia sachalinensis (F.Schmidt) Ronse Decr., a Naturally Growing Plant Species at Mine Site. Minerals 2021, 11, 806. https://doi.org/10.3390/min11080806
Lu X, Yamaji K, Haruma T, Yachi M, Doyama K, Tomiyama S. Metal Accumulation and Tolerance in Artemisia indica var. maximowiczii (Nakai) H. Hara. and Fallopia sachalinensis (F.Schmidt) Ronse Decr., a Naturally Growing Plant Species at Mine Site. Minerals. 2021; 11(8):806. https://doi.org/10.3390/min11080806
Chicago/Turabian StyleLu, Xingyan, Keiko Yamaji, Toshikatsu Haruma, Mitsuki Yachi, Kohei Doyama, and Shingo Tomiyama. 2021. "Metal Accumulation and Tolerance in Artemisia indica var. maximowiczii (Nakai) H. Hara. and Fallopia sachalinensis (F.Schmidt) Ronse Decr., a Naturally Growing Plant Species at Mine Site" Minerals 11, no. 8: 806. https://doi.org/10.3390/min11080806
APA StyleLu, X., Yamaji, K., Haruma, T., Yachi, M., Doyama, K., & Tomiyama, S. (2021). Metal Accumulation and Tolerance in Artemisia indica var. maximowiczii (Nakai) H. Hara. and Fallopia sachalinensis (F.Schmidt) Ronse Decr., a Naturally Growing Plant Species at Mine Site. Minerals, 11(8), 806. https://doi.org/10.3390/min11080806





