Preparation and Characterization of Acid-Activated Bentonite with Binary Acid Solution and Its Use in Decreasing Electrical Conductivity of Tap Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Properties of Raw and Acid-Activated Bentonite
2.3. Changes in the Electrical Conductivity With Adsorption Treatment
3. Results and Discussion
3.1. Characteristics of Raw and Acid-Activated Bentonite
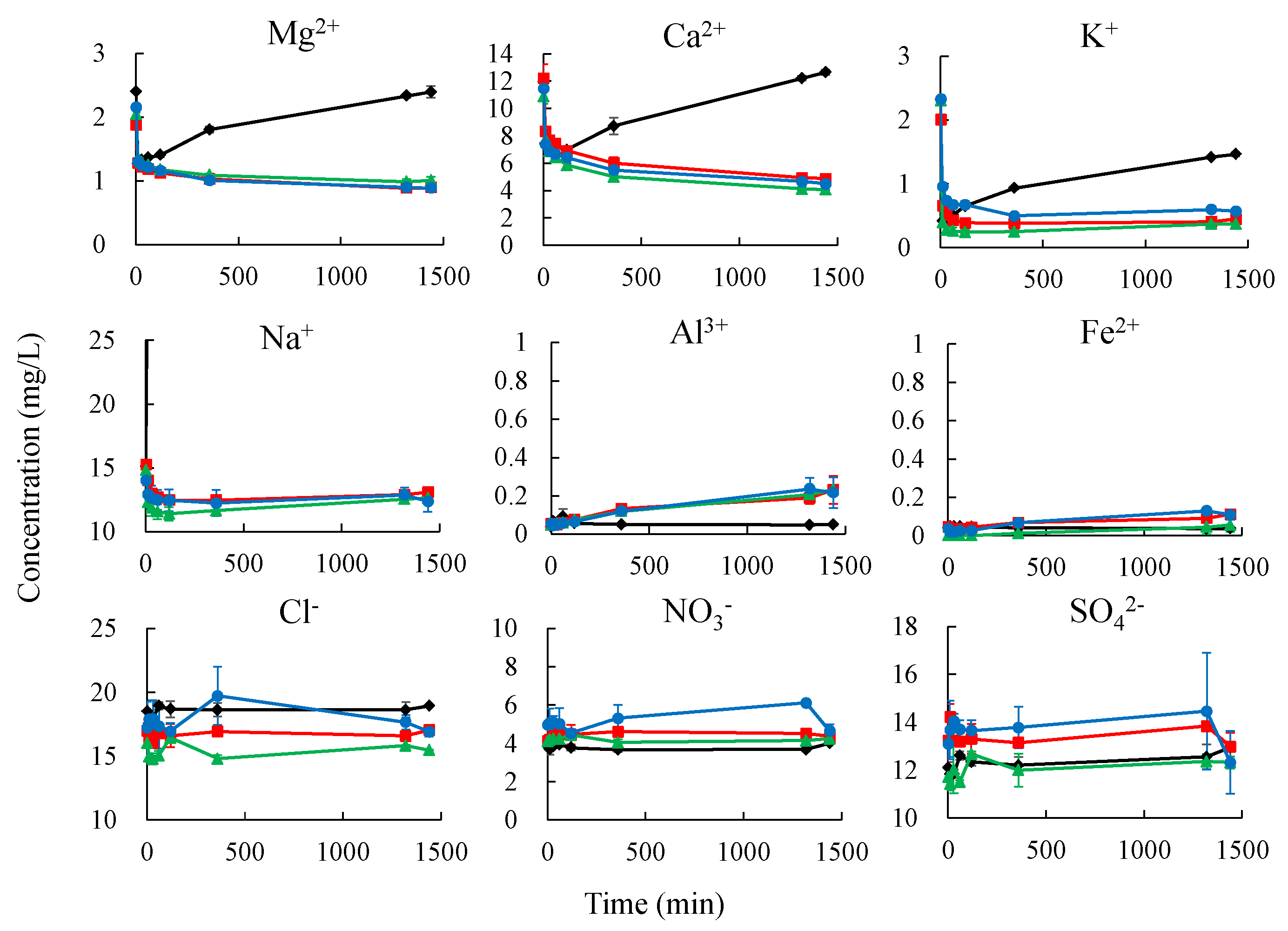
3.2. Changes in the Electrical Conductivity Using 5-SN, 5-NP, and 5-PS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maher, I.; Sarhan, A.A.D.; Barzani, M.M.; Hamdi, M. Increasing the productivity of the wire-cut electrical discharge machine associated with sustainable production. J. Clean. Prod. 2015, 108, 247–255. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Singh, T.P.; Sethi, B.L. Surface modification by electrical discharge machining: A review. Mater. Process. Technol. 2009, 209, 3675–3687. [Google Scholar] [CrossRef]
- Chen, S.L.; Yan, B.H.; Huang, F.Y. Influence of kerosene and distilled water as dielectrics on the electric discharge machining characteristics of Ti-6Al-4V. J. Mater. Process. Technol. 1999, 87, 107–111. [Google Scholar] [CrossRef]
- Abbas, N.M.; Solomon, D.G.; Fuad Bagari, M. A review on current research trends in electrical discharge machining (EDM). Int. J. Mach. Tools Manuf. 2007, 47, 1214–1228. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Du, R.; Zhang, J.H.; Zhang, Q. An investigation of ultrasonic-assisted electrical discharge machining in gas. Int. J. Mach. Tools Manuf. 2006, 46, 1582–1588. [Google Scholar] [CrossRef]
- Leão, F.N.; Pashby, I.R. A review on the use of environmentally-friendly dielectric fluids in electrical discharge machining. Mater. Process. Technol. 2004, 149, 341–346. [Google Scholar] [CrossRef]
- Nagahashi, E.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Characteristics of raw and acid-activated bentonite and its application for improving electrical conductivity of tap water. Chem. Pharm. Bull. 2021, 68, 92–98. [Google Scholar] [CrossRef]
- Önal, M.; Sarıkaya, Y. Preparation and characterization of acid-activated bentonite powders. Powder Technol. 2007, 172, 14–18. [Google Scholar] [CrossRef]
- Rožić, L.; Novaković, T.; Petrović, S. Modeling and optimization process parameters of acid activation of bentonite by response surface methodology. Appl. Clay Sci. 2010, 48, 154–158. [Google Scholar] [CrossRef]
- Noyan, H.; Önal, M.; Sarıkaya, Y. The effect of sulphuric acid activation on the crystallinity, surface area, surface acidity, and bleaching power of a bentonite. Food Chem. 2007, 105, 156–163. [Google Scholar] [CrossRef]
- Pawar, R.R.; Bajaj, H.C.; Lee, S.M. Activated bentonite as a low-cost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: Batch and column studies. J. Ind. Eng. Chem. 2016, 34, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Ere, E.; Afsin, B. Removal of basic dye using raw and acid activated bentonite samples. J. Hazard. Mater. 2009, 166, 830–835. [Google Scholar]
- Hajjaji, M.; El Arfaoui, H. Adsorption of methylene blue and zinc ions on raw and acid-activated bentonite from Morocco. Appl. Clay Sci. 2009, 46, 418–421. [Google Scholar] [CrossRef]
- Doulia, D.; Leodopoulos, C.; Gimouhopoulos, K.; Rigas, F. Adsorption of humic acid on acid-activated Greek bentonite. J. Colloid Interf. Sci. 2009, 340, 131–141. [Google Scholar] [CrossRef]
- Bendou, S.; Amrani, M. Effect of Hydrochloric Acid on the Structural of Sodic-Bentonite Clay. J. Min. Mat. Character. Eng. 2014, 2, 404–413. [Google Scholar] [CrossRef]
- Falaras, P.; Kovanis, I.; Lezou, F.; Seiragakis, G. Cottonseed oil bleaching by acid-activated montmorillonite. Clay Miner. 1999, 34, 221–232. [Google Scholar] [CrossRef]
- Diaz, F.C.; Sanctos, P.S. Studies on the acid activation of Brazilian smectitic clays. Química Nova 2001, 24, 345–353. [Google Scholar]
- Pesquera, C.; Gonzalez, F.; Benito, I.; Blanco, C.; Mendioroz, S.; Pajares, J. Passivation of a montmo- rillonite by the silica created in acid activation. J. Mater. Chem. 1992, 2, 907–912. [Google Scholar] [CrossRef]
- Temuujin, J.; Jadamobaa, T.; Burmaa, G.; Erdenechimeg, S.; Amarsanaa, J.; Mackenzie, K.J.D. Characterisation of acid activated montmorillonite clay from Tuulant (Mongolia). Ceram. Int. 2004, 30, 251–255. [Google Scholar] [CrossRef]
- Murray, H.H. Applied clay mineralogy today and tomorrow. Clay Miner. 1999, 34, 39–49. [Google Scholar] [CrossRef]
- Eren, E. Removal of copper ions by modified Unye clay, Turkey. J. Hazard. Mater. 2008, 159, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Advan. Colloid Interf. Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef] [Green Version]
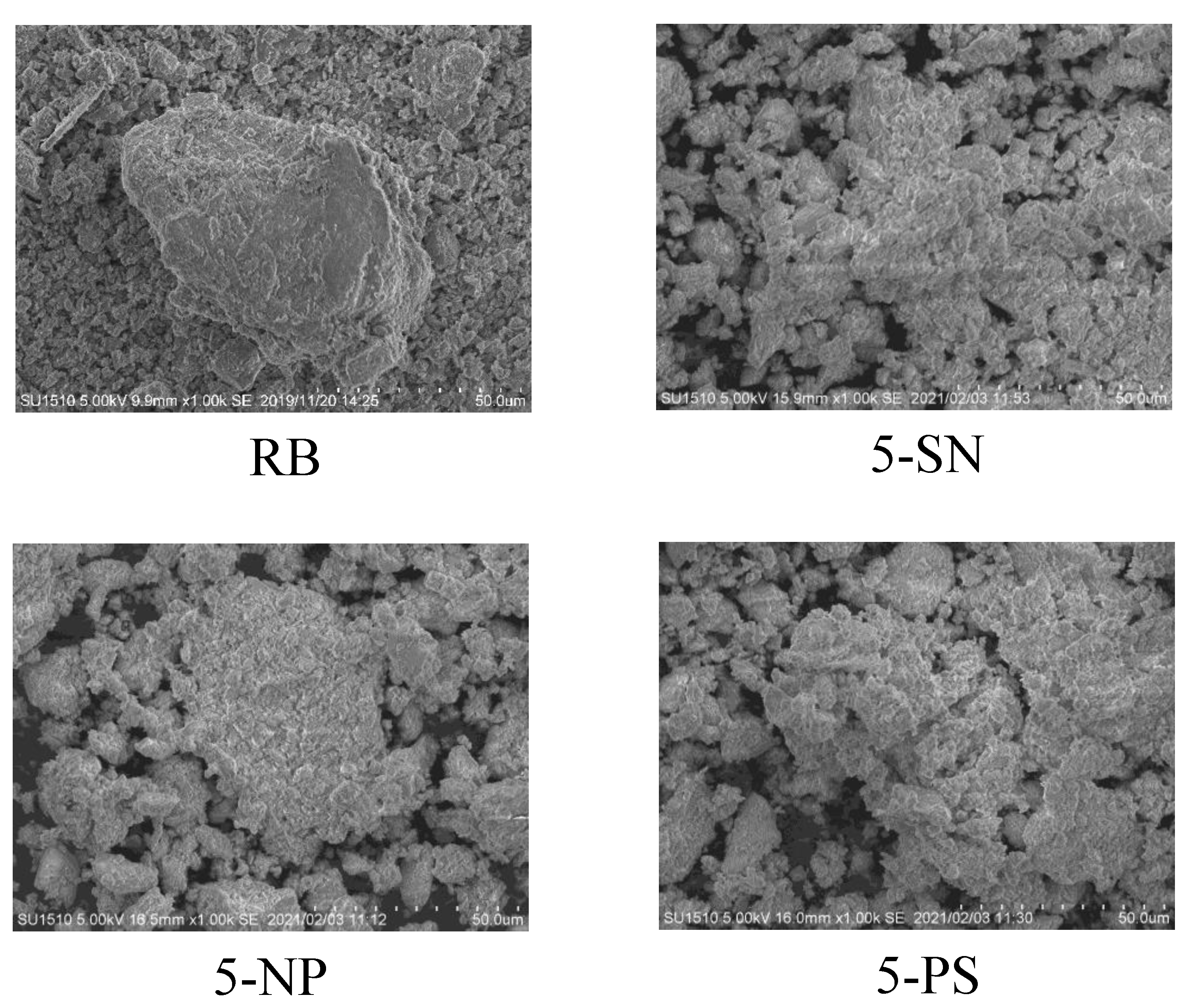
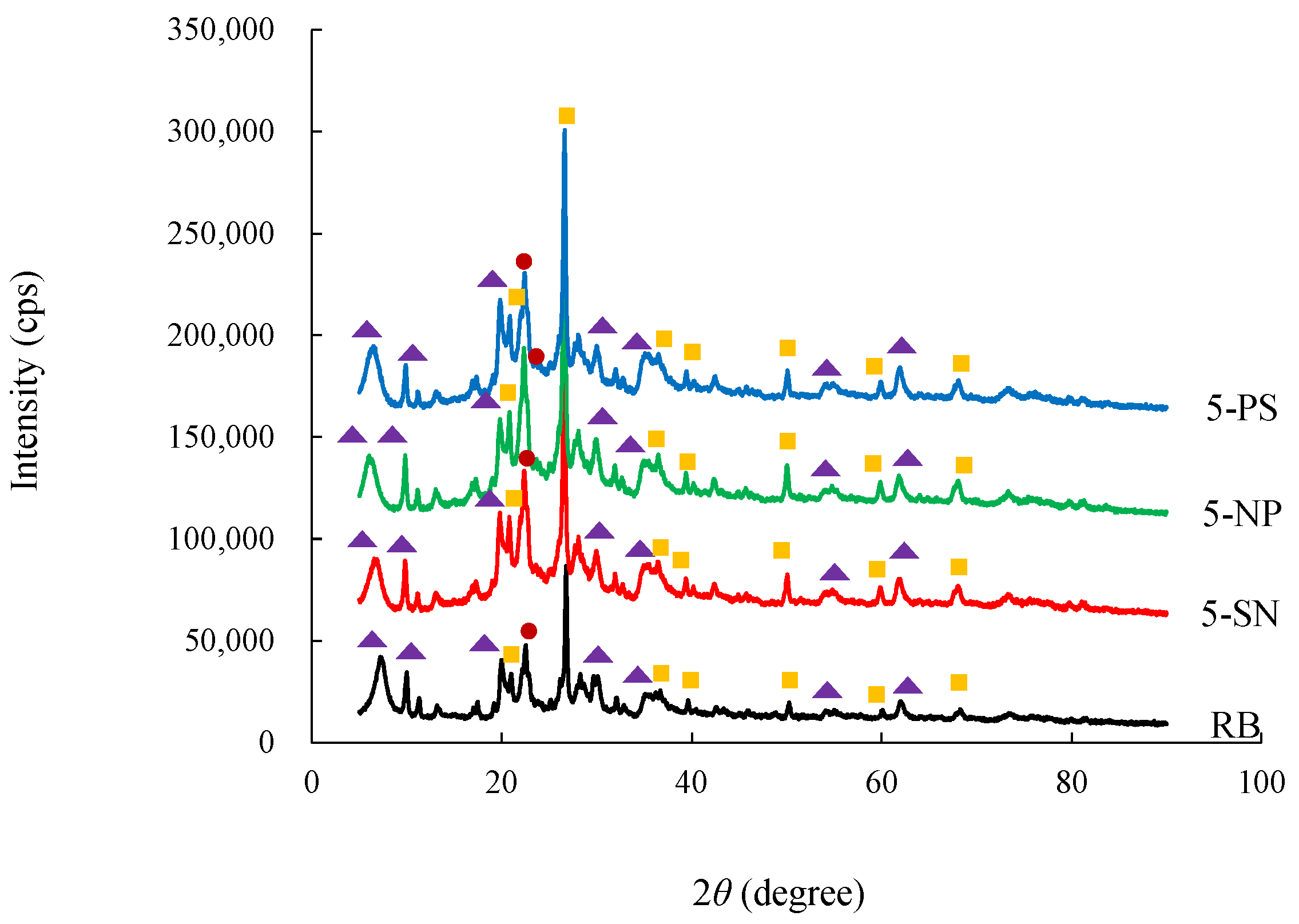
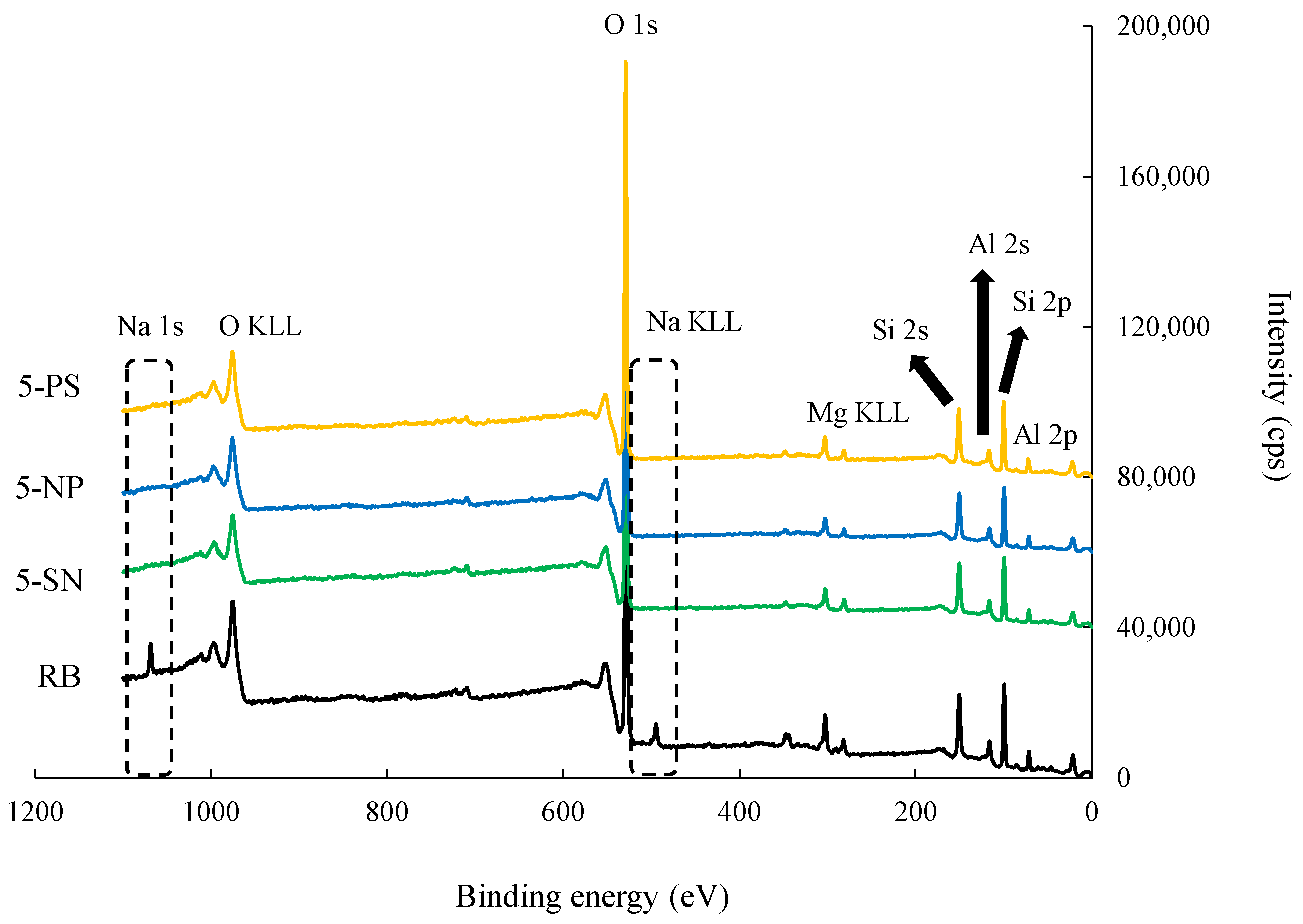


| Samples | RB | 5-SN | 5-NP | 5-PS | |
|---|---|---|---|---|---|
| CEC (mmol/g) | 100.1 | 70.4 | 35.0 | 53.1 | |
| Specific surface area (m2/g) | 29.0 | 38.0 | 30.1 | 51.1 | |
| Pore volume (µL/g) | d ≤ 20 (Å) | 0.70 | 0.73 | N.D. | 0.73 |
| 20 < d ≤ 500 (Å) | 63.8 | 47.9 | 64.5 | 59.8 | |
| d ≥ 500 (Å) | 17.8 | 17.5 | 19.3 | 5.9 | |
| Samples | Time (h) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.17 | 0.5 | 1 | 2 | 6 | 22 | 24 | |
| RB | 7.5 | 8.0 | 8.1 | 8.1 | 8.1 | 8.1 | 7.9 | 7.9 |
| 5-RB | 7.9 | 7.4 | 7.3 | 7.3 | 7.2 | 7.2 | 7.1 | 7.2 |
| 5-SN | 7.7 | 7.3 | 7.3 | 7.1 | 6.8 | 6.8 | 6.3 | 6.1 |
| 5-PS | 7.8 | 7.3 | 7.3 | 7.2 | 7.2 | 7.2 | 7.0 | 6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahashi, E.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Preparation and Characterization of Acid-Activated Bentonite with Binary Acid Solution and Its Use in Decreasing Electrical Conductivity of Tap Water. Minerals 2021, 11, 815. https://doi.org/10.3390/min11080815
Nagahashi E, Ogata F, Saenjum C, Nakamura T, Kawasaki N. Preparation and Characterization of Acid-Activated Bentonite with Binary Acid Solution and Its Use in Decreasing Electrical Conductivity of Tap Water. Minerals. 2021; 11(8):815. https://doi.org/10.3390/min11080815
Chicago/Turabian StyleNagahashi, Eri, Fumihiko Ogata, Chalermpong Saenjum, Takehiro Nakamura, and Naohito Kawasaki. 2021. "Preparation and Characterization of Acid-Activated Bentonite with Binary Acid Solution and Its Use in Decreasing Electrical Conductivity of Tap Water" Minerals 11, no. 8: 815. https://doi.org/10.3390/min11080815
APA StyleNagahashi, E., Ogata, F., Saenjum, C., Nakamura, T., & Kawasaki, N. (2021). Preparation and Characterization of Acid-Activated Bentonite with Binary Acid Solution and Its Use in Decreasing Electrical Conductivity of Tap Water. Minerals, 11(8), 815. https://doi.org/10.3390/min11080815






