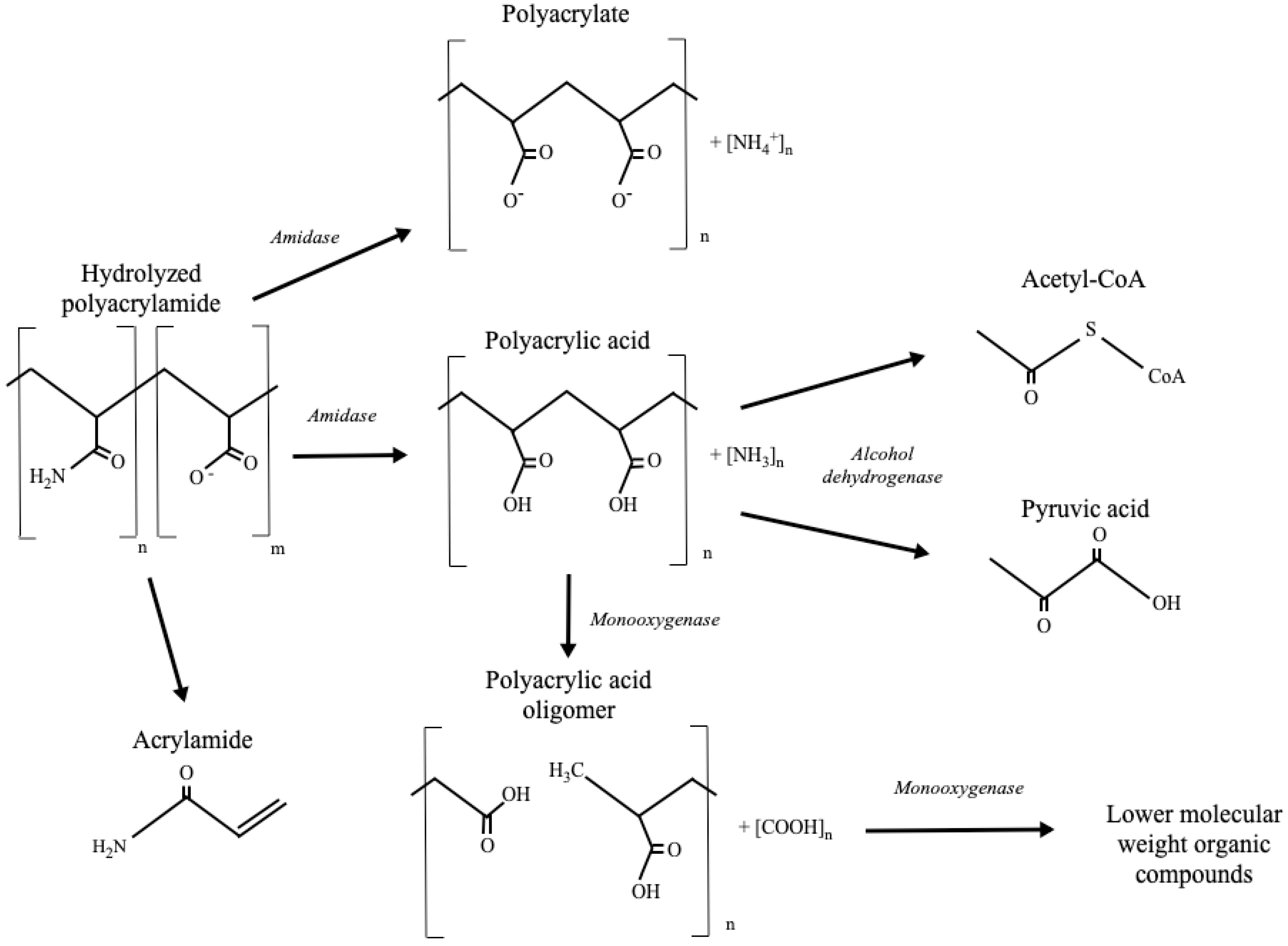
Abstract
Oil sands surface mining in Alberta has generated over a billion cubic metres of waste, known as tailings, consisting of sands, silts, clays, and process-affected water that contains toxic organic compounds and chemical constituents. All of these tailings will eventually be reclaimed and integrated into one of two types of mine closure landforms: end pit lakes (EPLs) or terrestrial landforms with a wetland feature. In EPLs, tailings deposits are capped with several metres of water while in terrestrial landforms, tailings are capped with solid materials, such as sand or overburden. Because tailings landforms are relatively new, past research has heavily focused on the geotechnical and biogeochemical characteristics of tailings in temporary storage ponds, referred to as tailings ponds. As such, the geochemical stability of tailings landforms remains largely unknown. This review discusses five mechanisms of geochemical change expected in tailings landforms: consolidation, chemical mass loading via pore water fluxes, biogeochemical cycling, polymer degradation, and surface water and groundwater interactions. Key considerations and knowledge gaps with regard to the long-term geochemical stability of tailings landforms are identified, including salt fluxes and subsequent water quality, bioremediation and biogenic greenhouse gas emissions, and the biogeochemical implications of various tailings treatment methods meant to improve geotechnical properties of tailings, such as flocculant (polyacrylamide) and coagulant (gypsum) addition.
1. Introduction to Oil Sands Tailings
Alberta, Canada is home to the third largest oil reserve in the world [1]. Most of Alberta’s oil is unconventional oil because it is trapped within oil sands and cannot be extracted using the natural pressure differential created by drilling an oil well. Oil sands consist of sand, silt, clay, water, and a heavy oil referred to as bitumen. Surface mining (ex situ recovery) is used to extract oil sands reserves with less than 75 m of overburden, while deeper reserves must be extracted using in situ recovery methods (not discussed here) [1,2]. Oil sands exist beneath approximately 142,200 km2 of land in Alberta and are situated in three distinct regions: Peace River, Athabasca, and Cold Lake [1]. The Athabasca region, situated in northeastern Alberta, is the only region in Alberta in which oil sands ore can be extracted through surface mining. Surface mining is advantageous where possible because it results in significantly greater bitumen recovery than in situ recovery methods [3]. However, surface mining also requires more land disturbance and results in large volumes of waste, known as tailings, which consist of sand, silt, clay, oil sands process-affected water (OSPW), and unrecovered bitumen. Surface mining in the Athabasca oil sands has disturbed approximately 895 km2 of land and created over 1.3 billion m3 of tailings [1,4]. Tailings are temporarily stored above ground in dams referred to as tailings ponds but eventually must be reclaimed and integrated into mine closure landforms.
Alberta’s oil sands operate under a zero-effluent discharge policy [5]. Approximately 80 to 85% of OSPW generated during surface mining can be recovered and recycled back into the extraction process [6]. The remainder of the OSPW cannot be recovered because it is trapped between mineral grains in the tailings and in its place, fresh water from the Athabasca River is used in the extraction process. The water holding capacity of tailings contributes to a number of tailings management and reclamation challenges, such as physical and geochemical stability, tailings impoundment volumes, and land use requirements. Some of the other key environmental issues associated with oil sands tailings include OSPW toxicity [7,8,9], biogenic greenhouse gas emissions, namely methane (CH4) and carbon dioxide (CO2) [10,11,12,13,14,15], and potential seepage of OSPW from tailings into underlying clay till and sand channel sediments [16]. Tailings accumulation and land use has prompted concerns from stakeholders over the permanency of tailings ponds, while toxicity and potential contamination of the surrounding environment from OSPW has raised questions over whether tailings reclamation can be achieved [17]. To date, there have been limited reclamation certificates issued for oil sands sites and none in relation to tailings. A lack of certified, reclaimed land is a representation of the time it takes for tailings to be reclaimed, and the limited knowledge that currently exists for their reclamation.
The geochemical stability of tailings is intricately connected with tailings management and reclamation and is key to the long-term success of tailings landforms. Tailings geochemistry varies with each oil sands ore deposit and operator. Currently, there are four oil sands companies operating surface mines in Alberta: Suncor Energy Inc. (Suncor), Syncrude Canada Ltd. (Syncrude), Canadian Natural Upgrading Ltd. (CNUL) and Canadian Natural Resources Ltd. (CNRL) (CNUL and CNRL are collectively referred to as Canadian Natural), and Imperial Oil Resources Ltd. (Imperial), listed in order from oldest to newest surface mining operations. Each operator uses different tailings treatment methods and generates tailings with unique geochemical characteristics. This variability presents additional challenges to operators and regulators in predicting and assessing the long-term geochemical stability of tailings in closure landforms. Because tailings landforms are a relatively recent development, research to date has heavily focused on the biogeochemistry and geotechnical stability of tailings in tailings ponds. As such there are significant knowledge gaps surrounding the long-term geochemical stability of tailings in closure landforms. This review focuses on five mechanisms of geochemical change that are expected to have the greatest impact on the geochemical stability of tailings in closure landforms: consolidation, chemical mass loading via pore water fluxes, biogeochemical cycling, polymer degradation, and surface water and groundwater interactions. This review identifies key considerations and knowledge gaps surrounding the geochemical stability of tailings in closure landforms to direct future research and development, reduce the long-term environmental impacts of mine closure practices, and moderate the economic and environmental liabilities associated with oil sands tailings reclamation.
1.1. Tailings Generation
Bitumen is extracted from surface mined oil sands ore using the Clark Hot Water Process (HWP) patented by Dr. Karl Clark in 1929 [2]. The process has since been improved to achieve a recovery of greater than 90% from high grade ore containing > 10 wt% bitumen [18]. In HWP, caustic soda (sodium hydroxide, NaOH) and hot water are mixed with crushed oil sands ore, allowing bitumen to froth and float to the surface of primary and secondary separation vessels while solids settle to the bottom [19]. NaOH addition promotes bitumen recovery by increasing the pH of the crushed ore mixture and causing organic acids that are naturally present in bitumen to become water-soluble surfactants [2,20]. This effectively reduces surface and interfacial tensions in the mixture and causes the ore structure to disintegrate. However, while NaOH addition is beneficial for bitumen recovery, it also produces tailings that are difficult to process. Because of the reduced interfacial tensions, NaOH addition produces water-in-bitumen emulsions and dispersion of clays [2]. The water-in-bitumen emulsions must undergo further treatment (froth treatment) to separate the bitumen. The organic acids released from bitumen during the HWP are called naphthenic acid fraction compounds (NAFCs), which are a broad family of polar organic carboxylic acids, some of which are acutely and chronically toxic to a number of aquatic organisms [9,21,22,23,24,25].
While the bitumen froth undergoes froth treatment with a diluent to reduce bitumen viscosity and enhance separation, the remainder of the slurry is treated in separation vessels to further separate the solids and recover any residual bitumen [2,18]. This results in three main tailings streams: coarse tailings (approximately 55 wt% solids), fine tailings (6–10 wt% solids), and froth treatment tailings [2,3]. All three streams are made up of varying amounts of sands, fines, and OSPW, which contains dissolved salts, NAFCs, petroleum hydrocarbons, and unrecovered bitumen [26,27]. Coarse tailings are collected from the bottom of the primary separation cell and as such coarse tailings solids consist largely of sand (>44 µm). Conversely, fine tailings consist mostly of fines (<44 µm) and have a sand to fines ratio (SFR) of less than 1. The fines fraction of tailings is made up of silt and clay minerals, predominately kaolinite, illite, and quartz [3]. The fine tailings stream is treated using a thickener and polymeric flocculant prior to deposition in the case of the Imperial’s Kearl mine and CNUL’s Jackpine and Muskeg River mines to create a thickened tailings stream. Froth treatment tailings contain diluent, most commonly naphtha, comprised mainly of aliphatic hydrocarbons C5 to C16 and monoaromatic hydrocarbons (BTEX, benzene, toluene, ethylbenzene and xylenes), though light paraffinic diluents, consisting mainly of C5 to C6 alkanes, may also be used [3,28,29]. Paraffinic solvents have been primarily used at CNUL’s Jackpine Mine and Muskeg River Mine, Imperial’s Kearl mine, and Fort Hills operated by Suncor [30,31,32]. Froth treatment tailings may be referred to as Naphtha Recovery Unit tailings or Tailings Solvent Recovery Unit tailings depending on whether the froth treatment process is naphthenic or paraffinic. The produced streams (coarse tailings, fine tailings, thickened tailings, and froth treatment tailings) may or may not be combined, depending on the operator.
After being deposited in tailings ponds, tailings sand is quick to settle and segregate from the rest of the tailings [2,3]. The sand forms a beach that can be used to build containment dykes to retain the remainder of the tailings. After a few years of settling, the remainder of the tailings develop a loose card-house structure of clay particles surrounded by water with a solids content of roughly 30 wt% and will remain in this state for decades as they slowly dewater and consolidate. This stable slurry structure, historically referred to as mature fine tailings (MFT), is due to the low flocculation and consolidation characteristics of the clay water slurry and the high clay content of the tailings.
Because of the stability of the MFT, the inventory of tailings being stored in tailings ponds has been steadily increasing year over year. As of 2016, tailings ponds in northern Alberta cover a surface area of 257 km2 [5]. As such, many operators are treating tailings with physical and/or chemical amendments to enhance dewatering and consolidation and increase bearing capacity and shear strength of the tailings. Treated tailings may be deposited (back) in tailings ponds or in terrestrial dedicated disposal areas (DDAs) to undergo further dewatering. Moreover, the Government of Alberta implemented the Lower Athabasca Region Tailings Management Framework for the Mineable Athabasca Oil Sands in 2015 [33] and a regulation under the Tailings Management Framework, Directive 085: Fluid Tailings Management for Oil Sands Mining Projects, in 2017 [34] that require that fluid tailings (often used synonymously with fluid fine tailings (FFT)) be progressively treated and reclaimed throughout the life of a project and that all fluid tailings be ready to reclaim within 10 years of the end-of-mine-life of a project. The Tailings Management Framework defines fluid tailings as tailings with more than 5 wt% solids and an undrained shear strength of less than 5 kPa [34].
There are a number of terms that may be used to describe different types of fine-grained oil sands tailings. FFT refers to fine grained tailings with a solids content greater than 2 wt% but less than that of the liquid limit (which is the boundary between liquid and solid behavior) (see Figure 1) [35]. MFT is a term for FFT with an SFR of less than 0.3 and a solids content greater than 30 wt% [35]. MFT often have substantial microbial populations because of the length of time this material has been stored in tailings ponds. Thin fine tailings refer to FFT with a solids content between 15 and 30 wt% and a low SFR like that of MFT (<0.3) [35]. Fresh tailings may be used to describe tailings coming directly off of a primary separation vessel or other process line and are expected to have lower microbial activity. Flotation tailings are ‘fresh’ tailings collected from the bottom of flotation cells used in secondary separation of middlings and typically have an SFR between 0.8 and 1.5 (which is higher than that of FFT) and contain the majority of the fine-grained solids. Whole tailings refer to the combined tailings streams from flotation cells and primary separation vessels. The term FFT is often used as a catch-all term for fine grained tailings where the solids content and microbial populations are not specified. Therefore, FFT will be used hereinafter in place of fluid tailings, fine tailings, MFT, and thin fine tailings, unless it is important to differentiate between these types of tailings.
Figure 1.
Untreated fluid fine tailings (FFT) (A) and thickened tailings (B).
1.2. Tailings Treatment and Anticipated Landforms
There are a number of tailings management and reclamation strategies being investigated and implemented by oil sands operators. This review includes the most current and/or relevant future tailings management and reclamation practices. While there are many different tailings treatment technologies, these technologies will typically result in one of two tailings landforms: (i) end pit lakes (EPLs); and (ii) capped soft deposits (terrestrial landform with a wetland feature). A summary of the treatment technologies used by each oil sands operator, as well as the associated deposition method and anticipated landform, is provided in Table 1 and described in the following subsections.
A relatively new technology for the oil sands industry involving tailings filtration may also be implemented in the future and could result in dry stack tailings deposits. Dry stacking, in which coarse and/or fine grained tailings are compacted to form an unsaturated, dense, and stable stack, requires tailings with a solids content of at least 70 wt%, which can be difficult and costly to achieve with high fines content tailings [36,37]. Recently, a dry stack deposit was constructed at Syncrude by mixing FFT and Clearwater Shale to achieve a solids content of 72 wt% [38]. While this technology may be promising, it has yet to be widely adopted in the oil sands industry and has not been the focus of geochemical research. Because of the limited research available, dry stack tailings will not be a focus of this review. However, the geochemical stability of these landforms is a knowledge gap that should be addressed if this technology is adopted as an oil sands tailings management and reclamation strategy in the future.

Table 1.
Summary of oil sands fluid fine tailings treatment methods used by Canadian Natural Resources Ltd. (CNRL), Canadian Natural Upgrading Ltd. (CNUL), Imperial, Suncor, and Syncrude, as well as the associated deposition method and anticipated landform.
Table 1.
Summary of oil sands fluid fine tailings treatment methods used by Canadian Natural Resources Ltd. (CNRL), Canadian Natural Upgrading Ltd. (CNUL), Imperial, Suncor, and Syncrude, as well as the associated deposition method and anticipated landform.
| Fluid Fine Tailings (FFT) Treatment Method 1 | Operator | Deposition Method | Anticipated Landform | References |
|---|---|---|---|---|
| Untreated FFT | Syncrude Mildred Lake (Base Mine Lake (BML)) | Deep in-pit disposal | End pit lake | [39] |
| Permanent Aquatic Storage Structure (PASS) (FFT + coagulant + in-line flocculation) | Suncor Base Plant (Upper Pit Lake (UPL) and Lake Miwasin) 3 | [40] | ||
| Thickened tailings (TT) (FFT + mechanical thickener + flocculant) | Imperial Kearl | Deep in-pit disposal or thin lifts | Terrestrial deposit with wetland 3 | [4,31,41,42] |
| Fort Hills operated by Suncor | ||||
| CNUL Muskeg River Mine and Jackpine Mine | ||||
| Thickened tailings (TT) (FFT + in-line flocculation) | Imperial Kearl | Deep in-pit disposal or thin lifts | [4,31,39,40,43] | |
| Suncor - Tailings Reduction Operation (TRO) 4 | ||||
| Syncrude Aurora North and Mildred Lake | ||||
| Centrifugation (FFT + flocculant/coagulant 2 + centrifuge) | Syncrude Mildred Lake | Deep in-pit disposal or thin lifts | [39,42] | |
| CNUL Jackpine Mine | ||||
| Composite tailings (CT) (FFT + coarse tailings sand + coagulant 2) | Syncrude Aurora North and Mildred Lake | In-pit disposal | [39,41,43] | |
| CNUL Muskeg River Mine | ||||
| Non-segregating tailings (NST) (TT + coarse tailings sand + coagulant) | CNRL Horizon - NST or enhanced NST (eNST) 5 | In-pit disposal | [44] |
1 Gypsum is typically used as the coagulant, though alum is used for PASS and CO2 is used in NST; polyacrylamide (PAM) is the flocculant; 2 Coagulant may be gypsum or Flue gas desulphurization (FGD) solids (a mix of calcium sulphite, residual lime, petroleum coke, and gypsum); 3 Fort Hills operated by Suncor also plans to adopt PASS technology in the future [45]; 4 TRO: (FFT + in-line flocculation + thin-lift drying); 5 NST: (FFT + coarse tailings sand + CO2); eNST: (FFT + coarse tailings sand + CO2 + in-line flocculation).
1.2.1. End Pit Lakes
EPLs are engineered water bodies that contain oil sands by-product materials stored below grade in decommissioned open pits [46]. In general, there are expected to be two main types of EPLs in oil sands mine closure landscapes: (i) EPLs with tailings storage; and (ii) EPLs without tailings storage [47]. EPLs with tailings storage will generally be comprised of thick deposits (10–80 m) of treated or untreated tailings capped with fresh water (3–10 m). Oil sands by-product materials that may be stored in these EPLs include FFT, coarse tailings sand, lean oil sands, overburden, petroleum coke, and OSPW. Because of the low strength (0.01 to 1 kPa) and low wet density (1250 kg/m3) of untreated FFT, water capping is the only possible capping and closure option for these tailings [48,49]. However, water capping untreated FFT is more challenging than capping denser, treated tailings and increases the potential for particle resuspension in the water cap [48]. EPLs without tailings storage are simpler, especially from a design and management point of view, as they will consist of decommissioned open pits that have been allowed to fill with surface runoff and groundwater. Both types of EPLs will likely receive significant quantities of tailings seepage water from the reclaimed watersheds and saline groundwater from bedrock.
The end goal is for EPLs to develop into self-sustaining aquatic ecosystems that will receive surface and groundwater inputs from the surrounding land and discharge water to downstream environments [46]. Theoretically, EPLs are a suitable tailings management practice and mine closure strategy because the water cap serves as habitat for an aquatic ecosystem, meanwhile the tailings can naturally dewater over time (in the case of EPLs with tailings storage). There are currently 23 EPLs planned for northern Alberta, with the intention that these will become permanent features in reclaimed mine closure landscapes [50]. However, Alberta Energy Regulator has not approved EPLs as a tailings reclamation technology yet as the technology needs further demonstration, assessment, and research before it can be approved [4]. Currently, one 800 ha full-scale demonstration EPL has been developed in northern Alberta, Base Mine Lake (BML). BML was established in 2013 and is operated by Syncrude [50]. Between 1994 and 2012, approximately 186 Mm3 of untreated FFT was deposited in BML [27] and it currently consists of approximately 45 m of FFT covered with 9 m of water. Syncrude also has a demonstration scale EPL known as the Syncrude Demonstration Pond [50]. Suncor is also researching aquatic reclamation strategies and is currently implementing a commercial scale permanent aquatic storage structure (PASS) (which will eventually become Upper Pit Lake (UPL)) as well as an 18 ha demonstration scale PASS, known as Lake Miwasin. PASS is a tailings treatment method in which tailings are treated with a coagulant (alum) and flocculant (polyacrylamide, PAM, via in-line flocculation) and deposited in decommissioned mined-out pits for aquatic closure [4,45,50]. Fort Hills operated by Suncor also plans to implement PASS in the future [45].
While operators view EPLs as technically feasible, environmentally acceptable, and economically attractive, major concerns include the safety and stability of EPLs for public use and impacts on vegetation, fish, and wildlife [46]. Further, Indigenous communities are concerned over the ability of the end land use to allow for traditional activities [46]. Stakeholder opinions, contaminant remediation, and the fate of toxic compounds will be critical in EPLs becoming a self-sustaining ecosystem and a recognized closure landscape for tailings.
1.2.2. Capped Soft Deposits
To reclaim tailings and construct terrestrial closure landforms on the deposits, a capping layer made up of solid materials, ranging from half a meter to several meters of coarse sand tailings, coke, or overburden, can be placed over soft tailings deposits [35,36,48]. Capping of soft deposits (i) increases the surface load to accelerate dewatering and shorten consolidation time; (ii) creates suitable closure landforms that can account for surface water drainage patterns; and (iii) provides a suitable subsoil for surface vegetation [35]. Soft tailings have a higher strength than fluid tailings and as such, may be capped with solid materials (instead of water) [49]. Soft tailings deposits, suitable for capping with solids materials, can include both fines-dominated tailings (thickened tailings (TT) and centrifuged tailings) and fines-enriched sand tailings (composite tailings (CT) and non-segregating tailings (NST)) [35].
TT technology uses a high-rate mechanical thickener (or in-line thickening) and the addition of a chemical flocculant, commonly PAM, to promote rapid settling and sedimentation of suspended fines in tailings [37]. In-line thickening involves adding flocculant directly to tailings in the pipeline, with or without additional static mixers. TT with mechanical thickening as currently implemented is primarily treatment of flotation tailings, though Imperial’s Kearl also has a provision to feed FFT into their thickener. In-line thickening as currently implemented is either secondary treatment of a thickener underflow (Kearl) or treatment of FFT. TT using mechanical thickening is being used at Imperial’s Kearl, Fort Hills operated by Suncor, and CNUL’s Muskeg River and Jackpine mines [4,31,41,42]. In-line thickening is being used at Imperial’s Kearl, Suncor Base Plant, and Syncrude Aurora North and Mildred Lake mines [4,31,39,40,43]. TT that has undergone in-line thickening is commonly referred to as in-line thickened tailings (ILTT). TT technology typically produces tailings with a solids content of between 35 and 50 wt% [48]. Figure 1 provides a visual comparison of untreated FFT and TT.
After mechanical thickening or in-line thickening, TT may be deposited in deep (>10 m depth) in-pit disposal areas or in thin lifts [35,48]. Flocculant addition is primarily responsible for the initial dewatering that occurs in deep in-pit deposits though over time, self-weight consolidation also contributes to dewatering. Environmental effects (evaporation and freeze/thaw cycles) play a minimal role in dewatering tailings in deep deposits except to develop a surface crust once filling is complete [35]. Syncrude is currently investigating a tailings treatment and disposal method, referred to as Accelerated Dewatering, at their Mildred Lake mine in which ILTT is placed in thick lifts [39]. In thin-lift deposition (also called Atmospheric Fines Drying), TT is deposited in thin layers over a large area and allowed to dry in-place to form a solid deposit [4,35,51]. Initially, water drains from the deposit by gravity and capillary action however, over the course of several weeks, environmental effects (namely evaporation and freeze/thaw cycles) enable additional dewatering. After a layer is dry, another thin layer is placed over top and the dewatering process is repeated. Atmospheric Fines Drying of TT is currently being used by Suncor’s Base Plant (referred to as Tailings Reduction Operation (TRO)) and at both CNUL mines (Muskeg River and Jackpine) [40,41,42].
Another commercially used tailings treatment method is centrifugation, in which FFT is treated with a coagulant (usually gypsum) or flocculant and is separated into water and solids streams using centrifuges [35,37,52]. The released water is recycled back to the plant and the sediment, the ‘centrifuge cake’, which is roughly 45 to 55 wt% solids, is deposited in either thin lifts or deep in-pit disposal areas and capped [37,48]. Centrifuge tailings typically have a lower peak undrained shear strength (<1 kPa) and lower wet density (1400 kg/m3) than TT (<5 kPa; 1650 kg/m3) [48]. Because of their low strength, centrifuged tailings deposits cannot be capped with sand. Centrifugation is being used at Syncrude’s Mildred Lake mine and CNUL’s Jackpine Mine [39,42]. Syncrude uses either gypsum or flue gas desulphurization (FGD) solids, which are a mix of calcium sulphite, residual lime, petroleum coke, and gypsum, as the coagulant. Centrifugation is very capital intensive which is why it is not more widely used.
Combined management of FFT and beach sand produces fines-enriched sand tailings: CT or NST. CT is a mixture of FFT, coarse tailings sand, and a coagulant (often gypsum) and has a solids content of approximately 60 wt% and a wet density of 2000 kg/m3 [37,53]. The intent of CT is to capture fines within the voids between sand particles to produce non-segregating tailings. The target SFR for CT is typically 4 [48]. CT is then discharged into a containment area and becomes a rapidly consolidating, soft, cappable deposit [35,37]. CT is currently used at Syncrude’s Aurora North and Mildred Lake mines and CNUL’s Muskeg River Mine [39,41,43]. Similar to centrifugation, Syncrude uses either gypsum or FGD solids as the coagulant. NST is similar to CT except instead of FFT, TT is used. CNRL’s Horizon adds CO2 to their NST as the coagulant to accelerate dewatering and may also use a process referred to as enhanced NST (eNST) which involves NST and in-line flocculation [44]. Implementation of CT and NST technologies can be challenging due to the amount of sand required and issues with sand and fines segregating upon deposition. However, unlike TT and centrifuged tailings, large post-reclamation settlements extending over several decades are not expected for capped CT and NST deposits [48]. Consolidation (dewatering) of fines-enriched sand deposits (CT and NST) is predicted to be achieved within a decade, whereas consolidation of fines dominated tailings (TT, centrifuged tailings, and untreated FFT) is expected to occur over centuries [36].
After deposition, tailings deposits will consolidate and may also develop a surface crust through environmental effects (in the case of fines-dominated deposits with drainage) [35]. Once the soft tailings deposit reaches a strength of approximately 10 kPa it can be capped, depending on the capping method [49]. Capping enhances consolidation of the soft deposits, which must reach a strength of 25 to 75 kPa before they are trafficable for mobile equipment [35]. Once consolidation has advanced sufficiently, contouring and the placement of surface soil and vegetation can commence. The closure landform targeted for all of these capped soft deposits is a terrestrial deposit with a wetland [4]. Some amount of residual subsidence is anticipated for tailings deposits, particularly for soft fines-dominated deposits in deep in-pit disposal areas, however if the subsidence is substantial, a water capped deposit may be more appropriate [35]. Sandhill Fen is a sand-capped CT deposit that was reclaimed (though it does not have reclamation certification) to a 52 ha wetland beginning in 2012, consisting of 35 ha of upland and a 17 ha fen [48,54,55]. The Sandhill Fen is part of a research watershed that is being continuously monitored.
1.3. Tailings Water Chemistry and Mineralogy
Table 2 and Table 3 present the latest available (2019) surface water chemistry data for tailings deposits with ponded surface water at all oil sands mines operating in northern Alberta, with the exception of CNUL’s Jackpine Mine and Muskeg River Mine for which data were not available, compared to that of the Athabasca River. Table 2 presents surface water chemistry data for Imperial, CNRL, and Suncor tailings deposits and Table 3 presents similar data for Syncrude tailings deposits and the Athabasca River. Two Athabasca River monitoring stations were chosen for this comparison; the Fort McMurray station and the Old Fort Station which are upstream and downstream, respectively, of Alberta’s oil sands mining area [56]. The tailings deposits in Table 2 and Table 3 include tailings ponds, temporary DDAs, deep in-pit deposits, and EPLs. While Table 2 and Table 3 contain data on the surface water chemistry of tailings deposits, tailings pore water chemistry generally falls within the ranges seen in Table 2 and Table 3 though it may vary with depth (age) of the deposits [16,26,39,43,48,57,58,59,60,61]. The one exception to this is BML surface water, which is diluted with fresh water and as such, tailings pore water in BML FFT is more concentrated and in some cases contains twice the concentration of the chemical constituents and organics listed in Table 3 [39].
Tailings water typically has a circumneutral pH, and elevated sodium (Na+), chloride (Cl−), sulfate (SO42−), and bicarbonate (HCO3−) concentrations relative to the nearby Athabasca River. Conductivity measurements were eight to 18 times higher in surface water in tailings deposits than in the Athabasca River. In some instances, Cl− concentrations were nearly 200 times greater in tailings surface water than in the Athabasca River. The high salinity in tailings deposits is a result of caustic soda addition during bitumen extraction, highly saline oil sands ore in the McMurray Formation, and the zero-discharge policy in which OSPW is continuously recycled back into the extraction process resulting in a build-up of ions [62,63]. Tailings water chemistry will vary depending on the ore deposit, the operator, the age of the tailings (as organics may be degraded over time and salt accumulates in recycle water over time), and any chemical amendments. Amendments such as alum, gypsum, and FGD can contribute to higher concentrations of SO42−, such as in Suncor’s DDA3/PASS (615 mg/L), Syncrude’s Aurora East Pits (AEPs) (605 mg/L), and Syncrude’s deep cake deposit (850 mg/L). Suncor’s Mine Dump 9 has the highest SO42− (1100 mg/L), Cl− (890 mg/L), and Na+ (1240 mg/L) concentrations of all the tailings deposits listed in Table 2 and Table 3. This is presumably a result of the FFT being desiccated prior to co-disposal, resulting in an accumulation of salts. Overall, tailings treatment methods will influence the pH, salinity, ion concentrations, buffering capacity, and toxicity of pore water [64], which will ultimately impact vegetation establishment and fauna survival. As such, tailings water chemistry will likely be a major challenge for reclamation.

Table 2.
Year 2019 surface water chemistry for Imperial, Canadian Natural Resources Ltd. (CNRL), and Suncor oil sands tailings deposits with ponded surface water in Alberta. Tailings deposits include tailings ponds, temporary dedicated disposal areas (DDAs), deep in-pit deposits, and EPLs. Table 3 contains 2019 surface water chemistry for Syncrude tailings deposits and surface water chemistry from the nearby Athabasca River (provided for comparison).
Table 2.
Year 2019 surface water chemistry for Imperial, Canadian Natural Resources Ltd. (CNRL), and Suncor oil sands tailings deposits with ponded surface water in Alberta. Tailings deposits include tailings ponds, temporary dedicated disposal areas (DDAs), deep in-pit deposits, and EPLs. Table 3 contains 2019 surface water chemistry for Syncrude tailings deposits and surface water chemistry from the nearby Athabasca River (provided for comparison).
| Company | Imperial | CNRL | Operated by Suncor | Suncor | ||||
|---|---|---|---|---|---|---|---|---|
| Tailings Deposit | Kearl West External Tailings Area (WETA) [31] | Horizon External Tailings Facility (ETF)/DDA1 [44] | Fort Hills Out of Pit Tailings Area (OPTA) [45] | Pond 2/3 [40] | Ponds: Average of 7 1 [40] | DDA1 [40] | Mine Dump 9 [40] | DDA3 2 [40] |
| Type of Tailings Deposited 3 | FFT, FTT, CST | FFT, NST/eNST, FTT, CST | FFT, FTT, CST, TT | FTT | FFT, Historical CT | TRO | Co-Disposal: TRO from DDA1 and Overburden | PASS |
| Conductivity (µS/cm) | 4043 | 1738 | 3800 4 | 3342 4 | 4100 4 | 5800 4 | 4100 4 | |
| pH | 8.1 | 8.14 | 7.9 | 8.1 | 8.4 | 8.5 | 8.6 | 8.1 |
| Carbonate (mg/L) | <1 | 0 | 11 | 25 | 21 | 25 | 1 | |
| Bicarbonate (mg/L) | 300 | 1326 | 524 | 951 | 743 | 884 | 570 | 685 |
| Chloride (mg/L) | 24 | 767 | 114 | 638 | 533 | 731 | 890 | 594 |
| Sulfate (mg/L) | 270 | 364 | 215 | 251 | 249 | 1100 | 615 | |
| Calcium (mg/L) | 33.7 | 15.2 | 20.5 | 12 | 23 | 15 | 26 | 28 |
| Magnesium (mg/L) | 14 | 11.6 | 7.6 | 10 | 14 | 11 | 18 | 14 |
| Sodium (mg/L) | 193 | 947 | 331 | 730 | 649 | 842 | 1240 | 858 |
| Potassium (mg/L) | 11 | 20.1 | 15.2 | 13 | 13 | 12 | 13 | 16 |
| Total suspended solids (mg/L) | 61.3 | 260 | 1588 | 211 | 8942 | 5400 | 4 | 13 |
| Total dissolved solids (mg/L) | 716 | 2631 | 1235 | 2003 | 1861 | 2320 | 3600 | 2469 |
| Naphthenic acid fraction compounds (mg/L) 5 | ||||||||
1 Average of 7 Suncor Ponds: 1A, 5, 6, 7, 8B, STP, TFT; 2 DDA3 will become Upper Pit Lake; 3 Tailings acronyms: fluid fine tailings (FFT); froth treatment tailings (FTT); coarse sand tailings (CST); thickened tailings (TT); composite tailings (CT); non-segregating tailings/enhanced non-segregating tailings (NST/eNST); Tailings Reduction Operation (TRO); Permanent Aquatic Storage Structure (PASS); 4 Conductivity reported in S/cm but the authors believe this was an error. The conductivity measurements have been corrected and reported as μS/cm in this paper; 5 Determined using Fourier-transform Infrared Spectrometry, presumably following the procedure outlined in Ripmeester and Duford [65]. Note. Empty cell indicates measurement not provided.

Table 3.
Year 2019 surface water chemistry for Syncrude oil sands tailings deposits with ponded surface water in Alberta. Tailings deposits include tailings ponds, temporary dedicated disposal areas (DDAs), deep in-pit deposits, and EPLs. Surface water chemistry from the nearby Athabasca River is provided for comparison. Table 2 contains 2019 surface water chemistry for Imperial, Canadian Natural Resources Ltd. (CNRL), and Suncor oil sands tailings deposits.
Table 3.
Year 2019 surface water chemistry for Syncrude oil sands tailings deposits with ponded surface water in Alberta. Tailings deposits include tailings ponds, temporary dedicated disposal areas (DDAs), deep in-pit deposits, and EPLs. Surface water chemistry from the nearby Athabasca River is provided for comparison. Table 2 contains 2019 surface water chemistry for Imperial, Canadian Natural Resources Ltd. (CNRL), and Suncor oil sands tailings deposits.
| Company | Syncrude | Athabasca River | ||||||
|---|---|---|---|---|---|---|---|---|
| Tailings Deposit | Aurora Settling Basin [43] | Aurora East Pits (AEPs): Average of 2 1 [43] | Mildred Lake Settling Basin (MLSB) [39] | Ponds: Average of 3 2 [39] | BML [39] | Deep Cake Deposit [39] | Fort McMurray Station: Average Monthly 2019 Data 3 [66] | Old Fort Station: Average Monthly 2019 Data [66] |
| Type of Tailings Deposited 4 | FFT, CST | FFT, CT, CST | FFT, FTT, CST | FFT, CT, CST | FFT | Centrifuge Cake | N/A | N/A |
| Conductivity (µS/cm) | 3400 | 3600 | 3200 | 4167 | 2600 | 4400 | 332 | 320 |
| pH | 8.26 | 8.30 | 7.96 | 8.48 | 8.48 | 8.51 | 7.98 | 7.78 |
| Carbonate (mg/L) | 10 | 5 | 10 | 21 | 16 | 16 | ||
| Bicarbonate (mg/L) | 670 | 675 | 680 | 933 | 740 | 750 | 168 | 150 |
| Chloride (mg/L) | 440 | 430 | 410 | 593 | 390 | 490 | 4.6 | 15.1 |
| Sulfate (mg/L) | 550 | 605 | 435 | 452 | 160 | 850 | 34.4 | 27.9 |
| Calcium (mg/L) | 44 | 54 | 22 | 9 | 27 | 30 | 42.2 | 37.8 |
| Magnesium (mg/L) | 25 | 27 | 12 | 7 | 13 | 26 | 11.3 | 10.3 |
| Sodium (mg/L) | 725 | 725 | 685 | 950 | 580 | 850 | 13.7 | 18.2 |
| Potassium (mg/L) | 26 | 28 | 12 | 12 | 9.5 | 15 | 2.3 | 1.8 |
| Total suspended solids (mg/L) | 300 | 300 | 350 | 30 | ||||
| Total dissolved solids (mg/L) | 2300 | 2400 | 1900 | 2750 | 1700 | 2600 | 191.8 | 184.2 |
| Naphthenic acid fraction compounds (mg/L) 5 | 55 | 49 | 31 | |||||
1 Average of 2 Syncrude Ponds: AEPN, AEPS; 2 Average of 3 Syncrude Ponds: SWSS, SWIP, NMSP; 3 Monthly data for November 2019 not available; 4 Tailings acronyms: fluid fine tailings (FFT); froth treatment tailings (FTT); composite tailings (CT); coarse sand tailings (CST); not applicable (N/A); 5 Determined using Fourier-transform Infrared Spectrometry, presumably following the procedure outlined in Ripmeester and Duford [65]. Note. Empty cell indicates measurement not provided.
Table 4 presents typical ranges of bitumen, solids, and fines content for untreated FFT. These parameters will vary depending on the ore deposit, processing techniques, and the depth from which samples are collected in tailings ponds. The solids content and SFR of FFT generally increase with depth (age) within tailings ponds due to consolidation and segregation of coarser (heavier) and finer particles [39,43,67]. For example, at a depth of 4 m in Syncrude’s Mildred Lake Settling Basin (MLSB), FFT has a solids content of 23.1 wt% and an SFR of 0 [39]. However, FFT at depth of 37 m within MLSB has had years to consolidate and as such has a solids content of 78.1 wt% and an SFR of 1.07. Clay sized (2 µm) minerals in oil sands tailings are typically dominated by quartz, kaolinite, and illite-smectite [3,68]. Other components may include illite, kaolinite-smectite, and chlorite, as well as trace amounts of pyrite (FeS2(s)), siderite (FeCO3(s)), rutile, anatase, lepidocrocite, and marcasite [3,68]. Kaminsky et al. [68] examined the distribution of clay minerals in tailings process streams and found that 30 ± 4 wt% of the 0.2–2 µm fraction of tailings solids consisted of non-clay components. Geochemical analysis of BML FFT showed a dominance of quartz, illite, kaolinite, and chlorite with small amounts of siderite, pyrite, and iron(II) sulfide (FeS(s)) [27].

Table 4.
Typical range of bitumen, solids, and fines content in untreated oil sands fluid fine tailings.
Froth treatment tailings solids consist largely of quartz and kaolinite and typically contain the highest concentrations of heavy minerals [71]. Titanium bearing minerals identified in froth treatment solids include rutile, anatase, brookite, and ilmenite. In addition, quartz and other silicates are often intergrown with FeS(s), titanium oxides, iron carbonates, iron-titanium oxides, and other rare earth element oxides [71]. Minerals in the froth treatment tailings often contain impurities such as manganese, iron, calcium, silicon, and aluminum, with iron being the most common contaminant in minerals in which it is not a fundamental component, such as rutile [71]. The unique mineralogy of froth treatment tailings presents additional biogeochemical challenges when reclaiming these tailings, as discussed in Section 2.3.7.
2. Mechanisms of Geochemical Change Expected for Tailings Landforms
2.1. Consolidation
Consolidation of FFT refers to the decrease in pore pressure that occurs as water escapes the tailings matrix, and the subsequent increase in effective stress. During consolidation, the volume of tailings decreases in accordance with the volume of water expressed. Thus, tailings consolidation also corresponds with an increase in density and solids content. Self-weight consolidation of tailings has been used to naturally dewater tailings for over 50 years, however this process can be slow depending on the properties of the tailings and requires large areas to contain the tailings and expressed pore water [37]. Because of the unique properties of oil sands tailings, their consolidation behavior can only be described using Gibson’s finite strain theory which accounts for the large volume, hydraulic conductivity, and compressibility changes tailings undergo during consolidation [72,73,74]. FFT has low hydraulic conductivity, typically between 1 × 10−6 and 1 × 10−9 m/s, and low compressibility and as such self-weight consolidation of this material can take decades [37]. Tailings consolidation is closely tied to geochemistry as tailings dewatering contributes to chemical mass loading in overlying surface water and contaminant transport to nearby surface water and groundwater.
2.1.1. Consolidation Behavior of Different Types of Tailings
Consolidation behavior is different for untreated and treated tailings and will vary depending on material properties of the tailings including solids content, fines content, and SFR. Generally, tailings with a higher initial solids content consolidate at a faster rate [69]. SFR is also indicative of consolidation behavior as sand can act as an internal surcharge on FFT. Therefore, mixing sand and FFT will result in a mixture that undergoes self-weight consolidation faster and to a greater extent than FFT alone [69,75]. CT, which typically has a solids content of 60 wt% and an SFR of 4, has a higher hydraulic conductivity and lower compressibility than untreated FFT and will therefore consolidate at a faster rate. Untreated FFT typically has a low solids content (~30 wt%) and low SFR (~0.1) and is very slow to consolidate [67,69,76]. For example, Jeeravipoolvarn et al. [69] analyzed self-weight consolidation in three 10 m standpipes filled with tailings and found that untreated FFT (30.6 wt% solids, 89.0 dry wt% fines, ~45 dry wt% clays, 0.12 SFR) strained more than 30% over 25 years and the solids content increased to 41.8 wt%, but there was little to no effective stress generation in the tailings. The lack of strength generation in untreated FFT even after large volume changes has implications for closure of these tailings, as trafficability (bearing capacity) and capping options are limited [69]. As such, most operators are using physical and/or chemical amendments to improve the geotechnical properties of their tailings for closure.
Wilson et al. [77] used large strain consolidation tests to compare consolidation behavior in six tailings samples: five TT samples (49.0–55.0 wt% solids; 51–67 dry wt% fines) and one untreated FFT sample (46.1 wt% solids; 96 dry wt% fines). In all cases, the treatment (flocculant addition and thickening) of the tailings increased hydraulic conductivity of the tailings by at least half an order of magnitude, which in turn results in faster consolidation. Further, Sorta et al. [78] reported that an FFT-sand mixture (20 dry wt% fines, 10 dry wt% clay) had an order of magnitude higher hydraulic conductivity and a lower compressibility than TT (50–60 dry wt% fines, 26–31 dry wt% clay), indicating improved consolidation behavior for sand-treated tailings over TT.
2.1.2. Long-Term Consolidation and Settlement in Tailings Landforms
McKenna et al. [36] conducted long-term consolidation modeling for various types of FFT: untreated FFT, dried FFT (70 wt% solids), dried FFT (80 wt% solids), TT (1:1 SFR), CT (4:1 SFR), and centrifuged FFT (50 wt% solids). McKenna et al. [36] predicted that 10 years after filling a 40 m deposit, centrifuged FFT and dried (80 wt%) FFT would undergo between 0 to 2 m of settlement; CT, dried FFT (70 wt%), and TT would undergo between 2 and 4 m of settlement; and untreated FFT would undergo greater than 6 m of settlement. Further, untreated FFT and centrifuged FFT were predicted to experience the largest ultimate settlement (>10 m) over several hundred years, whereas consolidation of CT was achieved within a decade [36,48]. Capping deposits will only increase this settlement over time, with a thicker cap resulting in greater settlement and thus greater water release [48]. Settlements of this scale over time would have huge implications for the long-term management and maintenance of the capping layer and the expressed water. Consolidation is expected to have the largest impact on deep in-pit deposits that are targeted for terrestrial reclamation with a wetland and depending on the tailings treatment, large settlements in these deposits are likely. Settlement greater than 2 m in terrestrial tailings deposits will result in the generation of a lake as opposed to the targeted shallow-water wetland area which would drastically alter the intended design and purpose of these landforms [48]. Consolidation of tailings deposits is expected to occur over decades to centuries, depending on the tailings deposited, and as such, will influence the geochemical stability of tailings landforms for many years.
2.2. Chemical Mass Loading via Pore Water Fluxes
Tailings dewatering, through consolidation and/or environmental effects such as evaporation or freeze/thaw cycles, coincides with vertical chemical fluxes of the organic compounds and chemical constituents in tailings pore water. While treating tailings generally increases hydraulic conductivity and consolidation rates, it is also likely to increase advective chemical fluxes in the short term. These chemical fluxes can have negative impacts on water quality in the case of wet reclamation methods (EPLs or wetlands) and may lead to salt accumulation or cover salinization in dry reclamation scenarios. The following subsections are divided into wet and dry reclamation scenarios to highlight the impact of chemical fluxes in different types of tailings landforms.
2.2.1. Wet Reclamation Scenarios
Recent BML studies by Dompierre and Barbour [79] and Dompierre et al. [80] identified two key processes by which chemical constituents in tailings pore water may move from FFT into the overlying water cap: (i) advection and dispersion driven by upward pore water flow as tailings dewater and consolidate; and (ii) FFT disturbance due to fluid movement in the water cover (for example, internal waves), which would cause rapid, but intermittent mass loading to the water cap. Dompierre and Barbour [79] and Dompierre et al. [80] determined that an advection-dispersion (from FFT dewatering) mass transport regime is dominant in BML, with intermittent disturbance near the FFT-water interface causing additional mass transport of pore water constituents. Using a mass balance of BML water, Dompierre et al. [80] estimated the advective vertical pore water flux to be 0.002 m3/m2/d, equivalent to 0.73 m of FFT settlement per year. Mass balance calculations and numerical modeling confirmed that BML is currently undergoing self-weight consolidation, which is releasing pore water and causing chemical mass loading to the water cap [80]. In an earlier study conducted one year after BML was commissioned, advective vertical mass transport was estimated to be 0.004 m3/m2/d [79], suggesting that consolidation rates are decreasing over time and/or that disturbances at the FFT-water interface are decreasing due to the greater depth of the water cap, thus reducing chemical mass loading [80]. While chemical mass loading into BML water cap is primarily due to advection, over time as consolidation rates decrease, chemical mass loading into the water cap is expected to occur predominantly as a result of diffusion [80].
Chemical pore water constituents in BML FFT include high concentrations of Na+ (977 mg/L), Cl− (633 mg/L), and HCO3− (1885 mg/L) as well as lesser amounts of calcium (Ca2+), magnesium (Mg2+), potassium, and ammonia [39]. The presence and concentrations of these constituents in BML pore water indicates that pore water release (advective transport) will likely contribute these constituents to the water cap for many years, thus influencing BML water quality in the long term [27]. White and Liber [81] identified Na+, Cl−, and HCO3− as the ions making up the majority of BML’s surface water salinity, comprising 92% of the total osmolarity, which is consistent with the dominant ions in BML FFT [27,39]. These ions present the highest toxicological risk to sensitive freshwater aquatic organisms [81,82]. While the acute toxicity of OSPW has been attributed to NAFCs, chronic toxicity is due to a combination of NAFCs and salts, which may have an additive effect on fish toxicity [50,83,84]. White and Liber [82] demonstrated that there was reduced survival of the freshwater invertebrate Chironomus dilutus in 2014 BML surface water. The water quality in BML appears to have improved over time though, as no adverse effects were observed after exposure of Chironomus dilutus to 2015 BML surface water [82]. However, because BML salinity is likely to persist for decades, BML is expected to be capable of supporting only salt-tolerant aquatic organisms with reduced diversity [82]. Additionally, BML FFT contains organic constituents such as residual bitumen, NAFCs (65 mg/L), and polycyclic aromatic hydrocarbons which are also being released into BML surface water [39,47]. This is a significant environmental concern as NAFCs and polycyclic aromatic hydrocarbons are both bioavailable and toxic to aquatic organisms and may hinder the development of an aquatic ecosystem in BML [24,85,86].
In addition to chemical mass loading, pore water expression from FFT may be contributing to BML water cap turbidity which can impact light penetration and thereby water quality, aquatic habitat, and aerobic biodegradation [47]. Other processes that may impact total suspended solids concentrations in BML include particle settling, wind waves, convection, and biogenic gas ebullition from FFT [87]. Field data have shown that surface water turbidity in BML after lake turnover can reach as high as 308 NTU (nephelometric turbidity units) [88]. Studies have found that even an increase in turbidity from 10 to 50 NTU can have a rapid, negative effect on aquatic metabolism [89,90]. Conversely, if processes that contribute to physical water mixing, and thereby turbidity, are limited, dissolved oxygen will be consumed and potentially lead to hypoxia in EPL water caps (discussed further in Section 2.3.1). Depositing chemically amended tailings in EPLs (such as PASS treated tailings which have been amended with alum) may improve the stability of the FFT-water interface and mitigate turbidity issues while also increasing the volume of tailings that can be stored in an EPL [48]. However, treated tailings may increase chemical mass loading to the water cap initially, as treated tailings have higher initial hydraulic conductivities than untreated tailings and therefore may generate larger chemical fluxes in the short term.
Syncrude CT was deposited in a pilot wetland, the Sandhill Fen, consisting of approximately 40 m of CT, a 10 m intermediary layer of sand tailings, and a surface wetland constructed of peat and clay till [91]. The major ions in near-surface water in Syncrude’s Sandhill Wetland are HCO3−, SO42−, Cl−, Na+, Ca2+, and Mg2+ [92], which is consistent with the chemicals elevated in OSPW. Biagi et al. [55] found that during the first years of operation (2013–2015), concentrations of SO42−, Cl−, Na+, Ca2+, and Mg2+ increased annually in the wetland. The fen was designed with an underdrain system and a fresh water supply to limit salinization which kept conductivity levels low throughout 2013 (<1000 µS/cm). In 2014 and 2015, conductivity increased to >1000 µS/cm and >2000 µS/cm, respectively, due to reduced water management practices during these years. Using stable isotopes, Biagi et al. [55] were able to show that these ions primarily stemmed from upward fluxes of pore water from the underlying CT deposit.
Sodium is the dominant cation in the Sandhill Wetland and has increased by roughly 48 mg/L each year since 2015 [92]. Vessey et al. [93] demonstrated that the peat and clay till cover on the Sandhill Fen has limited and short-lived Na+ attenuation capacity that is controlled by cation exchange. This suggests that elevated Na+ concentrations will likely persist in the wetland and soil cover which has implications for water quality and vegetation growth. SO42− and HCO3− are the dominant anions in the Sandhill Wetland [92], though elevated SO42− may constrain the production of methane and methylmercury, which bioaccumulates and poses a threat to aquatic organisms, wildlife, and humans [94,95]. Saline pore water migration is apparent in the peat and clay till cover of the Sandhill Fen [93]. Ion concentrations were fairly uniform with depth but varied across six sampling locations, indicating that more than one source is contributing to soil pore water within the Sandhill Fen [93]. Soil pore water in some locations was dominated by the Na-Cl type pore water released from the upland tailings sand and underlying CT, while soil pore water in other locations was influenced by the Ca-Mg-SO4 type water from Mildred Lake Reservoir. As anion and cation concentrations and conductivity measurements have been increasing annually since 2015, the water chemistry in the Sandhill Wetland is becoming increasingly similar to that of natural saline fens which exhibit elevated conductivity and high sodicity [92]. The impacts of pore water salinity in tailings should be understood and incorporated into landform design, as chemical fluxes are likely to contribute salinity to wetlands, water caps, and/or cover material in landforms for many years.
From a water chemistry standpoint, the Sandhill Fen is indicative of a saline fen, whereas vegetation surveys have concluded the site will consist of a variety of wetland types [92,96]. After a three-year study of vegetation communities at the Sandhill Fen, Vitt et al. [96] determined that the wetland was not on a trajectory to a peat-forming fen but was representative of the early stages of a marsh or riparian community. At the time of the study, approximately 40% of the fen was occupied by desirable fen species, whereas 48% was dominated by upland and weedy species, and the remainder was characterized by marsh species [96]. These differences in vegetation coincided with variations in salinity concentrations and water levels throughout the fen. Similarly, Biagi et al. [97] found that marsh-like conditions had developed in much of the fen, due in part to a high water table and salinity throughout the fen. As the CT deposit continues to consolidate and the fen establishes hydrologic connections with the surrounding watershed, water levels, water chemistry, and vegetation within the fen will continue to be impacted, all of which will influence the type of wetland(s) that develop at the Sandhill Fen. Peatlands will require centuries to accumulate enough peat to reach pre-disturbance conditions [98], suggesting that peatlands will be replaced by marshes and ponds within initial tailings reclamation landscapes [99]. Further, due to elevated ion concentrations, the reclaimed landforms will likely be considerably different from pre-disturbance conditions [100]. Though peatland restoration will likely not be achieved in the near future, mineral wetlands such as marshes are still important features of the boreal forest which encompasses Alberta’s oil sands.
2.2.2. Dry Reclamation Scenarios
Roshani et al. [101] conducted laboratory experiments to examine geochemical changes in two 25 cm thin-lift deposits of FFT under atmospheric drying conditions. Initially FFT pore water contained high concentrations of Na+ (617 ppm) and lesser amounts of Ca2+, Mg2+, iron, and aluminum. However, water content decreased in the lifts due to evaporation, which resulted in a significant reduction in pore water conductivity and increased ion concentrations in the solid phase. Salt precipitation (mostly Na) in the FFT voids increased with time and resulted in decreased evaporation over time by reducing vapour diffusion which may have implications for long-term dewatering of thin lifts [101]. Hydraulic conductivity of the FFT also decreased over time as the pores rearranged into a compact, aggregated fabric due to evaporation, suction development, and salt precipitation [101]. Similarly, Cilia [102] found that self-weight consolidation and seasonal freeze/thaw cycles in six field lysimeters containing centrifuged FFT resulted in an upward advective flux of pore water which, in conjunction with evaporation, led to salt accumulation near the tailings surface. Advection-dispersion was thought to be the dominant mass transport process initially, but over time as consolidation decreases mass transport should occur primarily through diffusion. Cilia [102] also investigated the effects of a 1 m cover layer on salt accumulation and found that under saturated conditions, the presence of a cover reduced, but did not eliminate, salt migration to the surface. Under unsaturated lysimeter conditions, salt accumulation in the cover layer was minimal [102]. Studies have also found that increasing the thickness of a cover can reduce salt migration and/or salt accumulation in cover soils [103,104].
A modeling study by Dobchuk et al. [105] on tailings sand topped with 45 cm of cover soil, a scenario applicable to uplands in terrestrial tailings landforms, found that the advective water flux across the tailings sand-soil interface was downward if the water table was deeper than 3 m below the interface. Under these conditions, the risk of cover salinization from upward advective movement of saline tailings pore water was low (1 in 50 years) [105]. Water tables shallower than 2.5 m had a greater risk of cover salinization while a water table deeper than 3.5 m virtually removed the risk. Further evaluation is necessary to better understand the potential for salt accumulation in the rooting zone of cover soils under dry reclamation scenarios, as this will have implications for capping and vegetation options for these deposits. Studies have found variability in the response of vegetation to tailings and OSPW, particularly regarding salinity. Conifer species, such as Picea glauca (white spruce) and Pinus banksiana (jack pine) exhibited high sodium concentration in their needles when exposed to CT water [106] and NST with Na+ concentrations of ~175 mg/L [107], leading to leaf necrosis and high mortality. Though 175 mg/L is below the Alberta Tier 1 Groundwater Remediation Guideline of 200 mg/L for Na+, concentrations of this magnitude may impact vegetation [108]. However, shrub species, such as Cornus stolonifera (red osier dogwood), Sheperdia canadensis (buffalo berry), and Populus deltoids x Populus balsamifera (northwest hybrid poplar) exhibited high tolerance of Suncor CT water with Na+ concentrations ranging from 14–1440 mg/L [63,106]. Further, CT amended with topsoil or peat showed successful establishment of Elymus angustus (altai wildrye) and Elymus trachycaulum (slender wheatgrass) [109]. Understanding salt migration and vegetation tolerance will determine if tailings landforms can support species native to the boreal forest.
2.3. Biogeochemical Cycling
Oil sands tailings, especially sand-dominated tailings such as CT and coarse sand tailings, are nutrient limited, with generally low amounts of phosphorous and nitrogen available for microbial metabolism [29,110]. Carbon sources in tailings include residual bitumen, aliphatic and aromatic hydrocarbons, and carboxylic acids, whereas possible nitrogen sources may include PAM (see Section 2.4), N2 fixation, recalcitrant organic nitrogen, and/or nitrogen cycling [30,111]. However, the extent to which these sources or mechanisms of generating nitrogen are available or occur in situ remains unknown. Despite nutrient limitations, diverse and active microbial communities in FFT contribute to the cycling of nitrogen, iron, sulfur, and carbon in tailings [29,58,112]. Microbes in FFT are thought to contribute to a diverse microbial community in CT, resulting in biogeochemical cycling in these deposits as well [113]. Redox conditions and the availability of terminal electron acceptors (e.g., O2, NO3−, Fe(III), SO42−, CO2) within tailings will dictate the biogeochemical cycling that occurs within a particular redox zone. As such the following subsections on biogeochemical cycling are organized based on redox conditions.
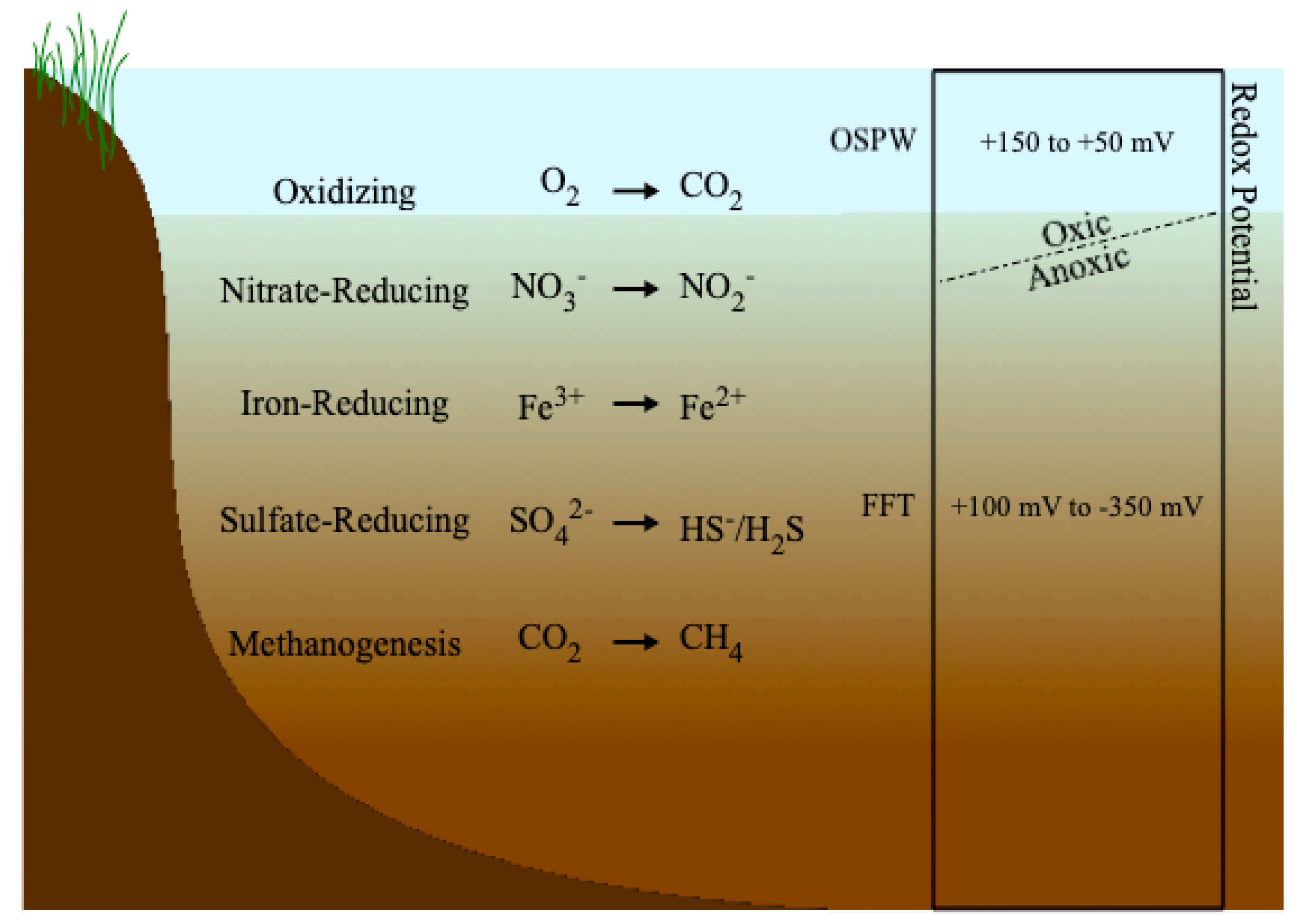
Redox potentials measured by Dompierre et al. [27] in BML indicated anoxic conditions throughout the BML FFT, starting at the FFT-water interface, with Eh values ranging from <−250 to −100 mV. Though the redox potential of the BML water cap was not measured in that study, oxic conditions near the water surface have been reported in other studies [114,115]. Studies of CT deposits have also indicated reducing conditions throughout the tailings, with oxic conditions present only in overlying ponded water [91,112]. Figure 2 illustrates the redox profile typically found within tailings deposits. At the FFT-water interface the redox conditions transition from oxic to anoxic, with the environment becoming more reducing as depth increases. Anoxic conditions with prevalent microbial activity below the FFT-water interface have been observed in oil sands tailings ponds [58,116,117], terrestrial deposits [91,112], and BML [27].
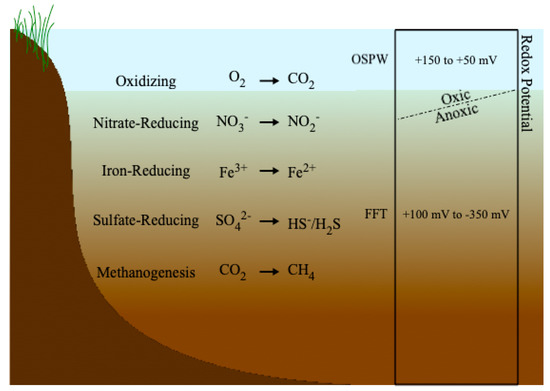
Figure 2.
Redox profile for tailings landforms and redox potential ranges for cap water and tailings deposits.
2.3.1. Oxic Conditions
Oxic conditions in tailings ponds, deposits, and landforms exist when there is free molecular oxygen (O2) present. Tailings ponds surface water often reaches anoxic conditions at a depth of roughly 1 m below the water surface, though they typically have a shallower water cap than BML [117]. Risacher et al. [114] found that BML had oxygen throughout the 10 m water cap during the summer season, reaching as high as 85% saturation near the surface, although oxygen concentrations decreased with depth and were at low levels near the FFT-water interface (<5% saturation). During ice cover, oxygen levels in BML have been found to decrease steadily, and Lawrence et al. [87] reported oxygen levels reaching 0% saturation near the BML surface right before ice-off in 2013/2014. However, seasonal lake turnovers in the spring and fall can help to increase oxygen levels in the BML water cap [115].
Oxygen levels within the BML water cap are impacted by carbon, sulfur, and nitrogen cycling [114,115]. Despite inputs of organic carbon from the underlying FFT, the BML water cap may be limited in labile organic matter, potentially a result of inputs of less aerobically biodegradable carbon [118] and turbidity reducing the phototrophic production of biodegradable organic matter. This may lead to hotspots for microbial methanotrophy and nitrification in the water cap [118]. Aqueous CH4 concentrations (generated in the underlying FFT) decreased moving upwards from the FFT-water interface to BML surface due to aerobic methanotrophy [114,118,119]. In addition to CH4, ammonium (NH4+) and hydrogen sulfide species (ΣH2S) and have been identified as important oxygen consuming constituents (OCCs) in the BML water cap or near the FFT-water interface and their biogeochemical cycling will likely impact BML oxygen concentrations for decades [114,115,118]. As such, physical mixing processes will be important in maintaining oxic conditions throughout the BML water cap [87,115].
Bioremediation can occur in tailings deposits through aerobic and anaerobic biodegradation of hydrocarbons and NAFCs. Biodegradation of hydrocarbons and NAFCs in tailings ponds has been studied extensively [10,12,13,14,22,58,60,116,120,121,122,123,124,125,126,127]. A number of studies have demonstrated aerobic biodegradation of hydrocarbons [122,124,127] and NAFCs [22,120,128] in oil sands tailings by bacteria. Rochman et al. [124] measured aerobic oxidation of benzene and naphthalene in OSPW collected from the top 10 cm of a Syncrude tailings pond and reported oxidation rates of 4.3 µmol/L/d and 21.4 µmol/L/d for benzene and naphthalene, respectively. Studies have also demonstrated that passive treatment of OSPW under oxic conditions can reduce concentrations of NAFCs and thus acute toxicity of OSPW in several months, though chronic toxicity persists for a longer period [50,129]. Additional studies have found algae [130,131] and fungi [132] capable of biodegrading NAFCs. Aerobic biodegradation likely occurs in tailings ponds in the uppermost layer of surface water as oxygen may be present due to wind and wave action during the summer, though aerobic degradation rates would slow in the winter [29]. Similarly, aerobic biodegradation may occur in tailings landforms, particularly in the uppermost layer of EPL water caps and in wetlands, and in aerated tailings deposits, which would improve water and seepage quality over time. However, anaerobic biodegradation is more likely to occur in tailings because the saturated, fine grained nature of tailings makes it difficult for oxygen to penetrate [133,134,135]. Terrestrial tailings landscapes that predominately consist of sand will have substantially more oxygen penetration and diffusion and thus would be more likely to experience oxic conditions.
2.3.2. Nitrate-Reducing Conditions
Information on nitrogen cycling in FFT is limited. While facultative nitrate (NO3−) reducing bacteria are abundant in tailings [29], nitrite (NO2−) and nitrate concentrations are generally below detection [60,117]. This finding may suggest that nitrate and nitrate reduction are not important microbial processes in FFT. However, NH4+ was recently identified as an important OCC in the BML water cap through nitrification [114]. It is thus important to note the potential for nitrate-reducing conditions within the tailings redox profile (Figure 2). NAFC biodegradation has been demonstrated under a number of anoxic conditions in the laboratory, with nitrate-reducing conditions resulting in the most extensive NAFC degradation [136,137]. As such, Foght et al. [29] concluded that anaerobic NAFC degradation is unlikely to be substantial in tailings deposits as nitrate concentrations and nitrate reduction is typically low in tailings.
2.3.3. Iron-Reducing Conditions
Iron-reducing bacteria, such as Rhodoferax Ferrireducens, can influence solid phase chemistry of clay minerals containing insoluble ferric (FeIII) oxides and can potentially impact water quality by allowing ferrous (FeII) cations, Fe2+, to react with reduced sulfur species to precipitate FeS(s) [58,138]. Dompierre et al. [27] reported slightly elevated concentrations of dissolved Fe directly below the FFT-water interface in BML, which was attributed to Fe2+ (because Fe3+ is relatively insoluble at a pH > 5) and therefore iron reduction. Sulfate reduction was also evident in this same zone below the FFT-water interface in BML. Similarly, Stasik et al. [117] reported synchronous iron and sulfur cycling 1 to 4 m below the FFT-water interface of an active tailings pond and that reduced sulfide species, primarily HS−, were precipitating to form FeS(s). A study of Syncrude’s Kingfisher CT deposit found that the presence of FeIII minerals increased with depth (and age) throughout the 34 m depth that was sampled [112]. The uppermost portion of the CT was iron reducing, with higher concentrations of FeII, lower temperatures, and lower total organic carbon concentrations. FeII concentrations in pore water ranged from 1.2 to 38.5 µM [112], which is higher than that of typical oil sands tailings (<0.09 to 10.9 µM) [39,43,58]. Iron reduction was limited to the top 8 m of the CT deposit. Warren et al. [112] suggested that iron reduction was limited to the upper portion of the CT due to deeper (and older) mineral FeIII substrates and total organic carbon being too recalcitrant or nutrient limiting for iron-reducing activity.
Iron reduction and subsequent secondary mineral precipitation, specifically FeS(s), have been linked to dewatering in BML [27] and will be discussed further in Section 2.3.6.
2.3.4. Sulfate-Reducing Conditions
Sulfate reduction, in which sulfate acts as the electron acceptor, may dominate in sulfate-amended FFT, which includes centrifuged tailings, CT, and PASS treated tailings. Syncrude and CNUL are currently the only operators actively using gypsum and/or FGD solids in tailings treatment [39,41,43]. Though the use of CT at Suncor has been discontinued, these deposits will still be incorporated into their closure plan and may provide conditions that encourage sulfate-reducing bacteria (SRB). For example, Bordenave et al. [139] reported an average sulfate reduction rate of 10 mmol sulfate reduced/m3 tailings/day in Suncor Pond 6, which contains CT, and Ramos-Padrón et al. [140] reported active sulfate reducing zones 2 to 15 m below the FFT-water interface in Pond 6.
SRB that have been identified in oil sands tailings include Desulfoivibrio, Desulfatibacillum, Desulfarculacea, Desulfosarcina, Desulfuromonas, Desulfobacca, Desulfoglaeba, and Desulfobulbaceae [58,112,138]. SRB can use low molecular weight fatty acids and possibly labile hydrocarbons as their carbon source [116,141]. Sulfate reduction is typically constrained to a narrow depth range below the FFT-water interface in tailings ponds due to decreasing numbers of SRB with depth [58,117]. Stasik et al. [117] found that sulfate reduction was highest in the anoxic zone of a tailings pond 1 to 3 m below the FFT-water interface. Similarly, both Dompierre et al. [27] and Syncrude [39] have reported zones of low SO42− below the FFT-water interface in BML. While SRB may comprise a large portion of the bacteria in FFT, there are few SRB present in water caps as water caps are generally oxic [58,140].
SRB present in FFT can produce toxic hydrogen sulfide (H2S) gas through the degradation of a variety of organic compounds, including hydrocarbons such as BTEX, and thus the environmental fate of H2S in tailings is of importance [60]. Under oxic conditions, ΣH2S should quickly oxidize, while under anoxic conditions, ΣH2S should precipitate as metal sulfides resulting in heavy metal immobilization and sedimentation within FFT. Field and laboratory studies have shown that sulfide mineral precipitation helps to reduce concentrations of Fe in pore water [117,142]. Oxidation has been found to rapidly remove ΣH2S in BML and as such ΣH2S is an OCC near the FFT-water interface, potentially leading to acidification [114]. Small et al. [30] reported no detectable gaseous H2S emissions from the 19 tailings ponds that they evaluated, which they attributed to either sulfur cycling within the ponds or limitations of the gas sampling equipment.
Reid and Warren [91] evaluated the biogeochemistry of three zones within Syncrude’s Sandhill Fen: the 40 m of CT deposit, the 10 m intermediary layer of sand tailings, and the wetland. Oxygen was present only in the ponded water in the wetland, while anoxic conditions existed at the wetland-sand interface and continued down through the sand cap and CT. Sulfur cycling due to the presence of gypsum in the CT generated ΣH2S in all three zones, however, the highest concentrations of aqueous and gaseous H2S were found in the sand cap. The sand layer acted as a mixing zone wherein sulfur rich porewater from the dewatering CT mixed with labile organic carbon from the developing wetland and stimulated microbial generation of H2S [91].
A biogeochemical study of Syncrude’s Kingfisher CT deposit by Warren et al. [112] found the 34 m of depth that was sampled was highly reducing. An extensive SRB zone (22–34 m) was found in the deposit, characterized by higher temperatures, higher total organic carbon concentrations, and highly reducing environments [112]. An average of 70% of the SO42− originally present in the CT (due to gypsum amendments) was lost, though the uppermost layer of CT had lost less than 20% of SO42− and the lowest depth (32–34 m) had lost almost 100% of SO42−. ΣH2S (>300 µM) was detected in pore water throughout the SRB zone. While the shallow (2–4 m) zone of the CT deposit was primarily an iron reducing zone, an abundance of Clostridium, which is a thiosulfate and/or S0 reducer, was found within the iron reducing zone and the transition zone (between 6–8 m), which contained the highest microbial diversity, suggesting sulfur cycling was also occurring in the surface layers of the deposit. Warren et al. [112] found that microbial diversity and abundance in the Syncrude CT deposit was low relative to the original tailings ponds, suggesting that CT is less hospitable for microbial communities. However, the microbial communities present in the CT were highly sulfur active. Interestingly, DNA sequencing of microbial communities in the deposit revealed that known SRB made up only 2% of the total bacterial community, though up to 90% of the Operational Taxonomic Units were considered to be capable of sulfur metabolism. This indicates that enigmatic microbial communities containing relatively unknown microbial taxa may have a substantial impact on biogeochemical cycling in tailings landforms. Further, sulfur cycling is likely to be an important biogeochemical process in landforms containing CT (and other sulfate amended tailings) and may have a substantial impact on CT reclamation efforts.
2.3.5. Methanogenesis
Methanogenesis is the dominant microbial process occurring in FFT and is a major mechanism of hydrocarbon degradation [11,12,13,14,60]. Residual diluent, commonly naphtha, in the tailings is the main substrate that sustains methanogenesis in FFT [13]. Hydrogenotrophic and acetoclastic methanogens, namely Methanosaeta spp., as well as other methanogenic archaea and a consortium of syntrophic bacteria such as Syntrophus spp., are primarily responsible for methanogenic activity in tailings [14,30,58]. Siddique et al. [12,13,14] found that under methanogenic conditions in the laboratory, n-alkanes (short and long chain) and monoaromatics (BTEX) can be degraded (and both of which are found in the residual diluent naphtha). It was later found that iso- and cyclo-alkanes can also be metabolized in tailings under methanogenic conditions following the depletion of n-alkanes, and that both naphtha and paraffinic diluents can be degraded [121,125,126,141,143]. While hydrocarbon degradation is beneficial in tailings deposits in that it reduces acute diluent toxicity, methanogenic biodegradation also contributes substantially to greenhouse gas emissions of CO2 and CH4 [30].
Methanogenic degradation of hydrocarbons has also been observed in tailings ponds. The concentration of light hydrocarbons, such as BTEX and naphtha, typically decreases with tailings depth (age) as these compounds are preferentially degraded over more recalcitrant compounds [58,123]. Tailings can take years to develop competent communities of methanogens, with 15-year lag periods reported for Suncor’s Pond 1 and Syncrude’s MLSB [10,113]. As such, the microbial activity and hydrocarbon degradation in tailings deposits will vary for deposits that consist of legacy tailings (tailings that are currently in tailings ponds) versus fresh tailings [29]. Microbial activity will also vary for different types of tailings and different treatment methods. For example, froth treatment tailings have been amended with diluent and therefore are more likely to trigger methanogenesis. Syncrude’s MLSB and Suncor’s Pond 2/3 both contain froth treatment tailings and are responsible for the highest CH4 production, 26.3 t/ha/year and 9.5 t/ha/year, respectively, of all tailings deposits based on 2011 and 2012 data compiled by Burkus et al. [15]. Tailings deposits that contain CT or a higher percentage of coarse sand tailings are expected to have lower CH4 emissions [30].
Similar to tailings ponds, microbial activity has generated greenhouse gas emissions of CO2 and CH4 from BML. Clark et al. [144] used eddy covariance to measure CH4 and CO2 fluxes from BML and found that fluxes were highest in 2014, the year following the fresh water capping, with median fluxes of 2.0 t/ha/year and 20.2 t/ha/year for CH4 and CO2, respectively. Carbon fluxes from BML have since decreased, with mean fluxes of 0.9 t/ha/year and 3.2 t/ha/year for CH4 and CO2, respectively, for the last three years of the Clark et al. [144] study (2017 to 2019). CH4(aq) consumption by aerobic methanotrophy has been noted in BML water cap which may contribute to lower CH4 emissions, though it also contributes to oxygen depletion [114]. Biogenic gas ebullition in BML as a result of microbial degradation of hydrocarbons causes not only greenhouse gas emissions, but also generates turbidity in the water cap as the bubbles migrate up to the FFT-water interface. Further, residual bitumen in the FFT may coat these bubbles resulting in bitumen floating on the lake surface, dissolved in the lake water, or absorbed onto sediments and/or tailings surrounding the water cap, thereby hindering reclamation efforts [47]. The greenhouse gas potential of each EPL will be different and will relate to the amount of unrecovered hydrocarbons remaining in the tailings at the time of deposition [47]. It is not known yet how the greenhouse gas emissions from EPLs will compare to those of terrestrial reclamation methods. Subaqueous or deep tailings deposits may be more likely to develop anoxic conditions and generate biogenic greenhouse gases, depending on other competing biogeochemical cycling processes [30]. Over time, terrestrial landforms that incorporate peat may develop into carbon sinks, as is the case for the lowland within Syncrude’s Sandhill Fen, though the midland and upland areas are still carbon sources [145]. CH4 fluxes from the Sandhill Fen were low for the years 2013 to 2015, with a median flux of 0.04 t/ha/year, possibly a result of SRB outcompeting methanogens given the abundance of electron acceptors for SRB [146].
The relationship between SRB and methanogens is of interest, as some researchers have suggested that SRB have a competitive advantage over methanogens [10,140] while others believe the two act synergistically under anoxic conditions, particularly when there are numerous carbon sources or the substrates are used non-competitively [29,60,116,117,138]. Bordenave et al. [139] found that locations within Suncor Pond 6 (which contains CT) with the highest rates of methanogenesis (80 mmol CH4/m3 tailings/day) had the lowest rates of sulfate reduction, suggesting that methanogenesis occurs when sulfate reduction is lowest. If SRB have an advantage over methanogens, sulfate addition, such as in CT, centrifuge tailings, and PASS, may help reduce greenhouse gas emissions from tailings, producing less CH4 but a higher CO2:CH4 ratio [15,29]. For example, Burkus et al. [15] noted that Suncor CT ponds produced less CH4 than Syncrude ponds, though this may be because Suncor deposits all their froth treatment tailings (a substantial source of carbon) in Pond 2/3, while multiple Syncrude ponds contain froth treatment tailings. These competing and mutualistic relationships between microbial communities and the impact of chemical tailings amendments on microbial activity should be evaluated further to better predict biogeochemical cycling in FFT.
Microbial activity in tailings landforms is expected to impact long-term stability of these deposits and reclamation efforts by degrading contaminants, transforming minerals, contributing to consolidation, and generating greenhouse gas emissions for years to come [29]. However, the rate and extent to which bioremediation will influence EPL and wetland water quality is unknown [147].
2.3.6. Bioconsolidation
The biogenic production of CO2 and CH4 through methanogenic degradation of hydrocarbons in tailings can contribute to tailings dewatering and consolidation [11,59,148]. Firstly, the ebullition of these gases can create transient physical channels through which pore water can escape [59]. The ebullition of bubbles may be dominated by CH4 (rather than CO2) because of its poor solubility in water. Research has also shown that CH4 bubbles moving across the FFT-water interface can increase fluxes of salts moving into the water cap [149]. Second, biogenic gases promote tailings dewatering by altering the pore water chemistry. Siddique et al. [59] found that the dissolution of biogenic CO2 lowered the pH of MFT, resulting in the dissolution of carbonate minerals, which released divalent cations (Ca2+ and Mg2+) and increased the concentration of HCO3−. These higher ion concentrations increased the ionic strength of the pore water, decreasing the thickness of the diffuse double layer surrounding the clay particles thereby promoting clay flocculation. SRB also contributes to biogenic CO2 production and could therefore, theoretically, aid in tailings bioconsolidation.
In a companion paper, Siddique et al. [148] suggest an additional pathway by which microbial metabolism accelerates tailings dewatering. They found that an anaerobic microbial community comprised of bacteria and archaea reduced FeIII minerals in MFT (ferrihydrite and goethite) to amorphous FeII minerals (such as FeS(s) and possibly green rust (mixed-valence FeII-FeIII hydroxides, carbonates and/or sulfates)). Siddique et al. [148] proposed a pathway in which Clostridiales and Synergistaceae bacteria ferment organic carbon in MFT to produce fatty acids, alcohols, CO2, and H2 as substrates for SRB (such as Desulfobulbaceae) and methanogens. Because fermenters do not completely oxidize fermentable substrates to CO2 when FeIII is the sole electron acceptor, they divert a portion of the electrons from fermentation to FeIII, thus reducing it to FeII. The resulting amorphous FeII minerals entrapped, coated, and masked negative clay surfaces. This reduces the repulsive forces between the clay particles, increasing consolidation.
Bioconsolidation may be an important mode of consolidation in EPLs and fines-dominated deposits that have active anaerobic microbial communities and slow rates of self-weight consolidation. Dompierre et al. [27] found that FFT in BML likely underwent microbially enhanced dewatering through carbonate mineral dissolution and subsequent ion exchange reactions, as suggested by Siddique et al. [59,148]. Fifteen BML FFT samples had pH values ranging from 6.6 and 8.3, with an average of 7.4. The pH in BML FFT decreased by approximately 0.5 pH units from the FFT-water interface to a depth of 10 m below the FFT-water interface, consistent with biogenic CO2 dissolution [27]. Pore water in BML was near saturation for calcite, dolomite, and siderite but X-ray diffraction indicated that the dissolution of carbonate minerals had largely depleted calcite and dolomite minerals in the tailings at the time of sampling in 2013. Thus, bioconsolidation through carbonate mineral dissolution is likely to decrease over time in tailings deposits. Further, the relative importance of bioconsolidation processes in dewatering tailings deep within a tailings deposit is unclear as the presence of substrates and microbial activity deep within these deposits remains largely unknown. Penner and Foght [58] noted a general decrease in Most Probable Number values, which indicate the viable number of methanogen and sulfate-reducing prokaryotes, with depth (and therefore age) in two tailings ponds. They attributed this to limited replenishment of substrates deep within tailing deposits.
2.3.7. Acid Rock Drainage
Acid rock drainage refers to the chemical and/or biological oxidation of sulfide minerals, commonly pyrite, in the presence of water and oxygen (at least initially). This sulfide mineral oxidation leads to acid generation and metal(loid) release [150]. Of the three tailings streams, froth treatment tailings contain the highest proportions of pyrite, siderite, and titanium oxides [68,150]. Because froth treatment tailings are enriched with sulfide minerals and any carbonate minerals present typically contain iron (such as siderite) and likely have insufficient neutralization capacity, oxidative weathering of these tailings could generate acidic drainage and elevated concentrations of metal(loids) in tailings pore water [150]. Metal(loids) such as arsenic, copper, cobalt, manganese, nickel, and zinc, are often associated with sulfides such as pyrite, and iron, manganese, nickel, lead, strontium and zinc are associated with carbonates [150]. Froth treatment TT samples have been found to generate acidic leachate (pH < 2) within 50 days of exposure to irrigation and airflow in the laboratory [151]. Acidic leachate from tailings undergoing evaporative drying would likely contain high concentrations of heavy metals [152].
The potential for acid drainage from froth treatment tailings will likely limit reclamation options for these tailings; upland reclamation scenarios may not be suitable given the potential for oxidation of the pyrite or other sulfide bearing minerals. Further, thin lift drying of froth treatment tailings could generate acid rock drainage as it exposes the tailings to atmospheric oxygen and water (rain and snow), unless the dried layers are quickly covered with fresh layers of wet tailings [29]. Limiting oxygen diffusion through water saturation and/or water capping would reduce the potential for sulfide mineral oxidation in tailings.
2.4. Polymer Degradation
PAM is a high molecular weight, water-soluble polymer and the most commonly used flocculant for oil sands mining operations, though it also has a number of water and wastewater treatment applications [153]. Numerous laboratory studies have been conducted on additional polymers or copolymers, such as polypropylene oxide (PPO), poly (ethyleneoxide methyl ether methacrylate) (PEOMA), and poly (vinylbenzyl trimethylammonium chloride) (poly (VBTMAC)), that can be utilized for tailings dewatering [154,155,156,157], though to date they have not been adopted by the oil sands industry. As such, this polymer degradation section will focus solely on PAM and its various forms. PAM is available in a variety of forms with varying charges (anionic, cationic, or non-ionic), charge densities, and molecular weights, though the primary monomer of all these different types of PAM is acrylamide [158,159]. The most frequently used form of PAM in tailings treatment is HPAM, where a portion of the acrylamide monomers are replaced with acrylate, making the polymer slightly anionic. A medium charge density (22–30%) for HPAM is ideal for oil sands tailings, as it promotes the repulsion of the charged segments, increasing the polymers chain length and bridging capacity [153,160,161]. Having a charge density that is too high may interfere with the adsorption of HPAM to negatively charged clay particles in tailings [153,162]. Studies often do not distinguish between PAM and HPAM, and thus in the following discussion, the term PAM will be used to collectively refer to PAM in all of its various forms, unless it is important to differentiate between the degradation of PAM and HPAM.
Despite being the most commonly used flocculant for oil sands tailings treatment, little is known about the biogeochemical implications and environmental fate of PAM, including how it affects water chemistry, biogenic gas concentrations, and hydrocarbon biodegradation. In addition to impacting biogeochemical cycling, degradation of PAM could potentially alter geotechnical properties of the tailings. PAM addition improves dewatering and consolidation and likely reduces EPL water cap turbidity, but little is known about how or if the degradation of PAM after tailings deposition could reverse the benefits of PAM addition and thereby impact the long-term geotechnical performance of deposits. Based on previous studies investigating the degradation of PAM in various environments, the predicted degradation processes for tailings include biological, photolytic, and mechanical. Additional degradation processes, including thermal and chemical degradation, may occur but are less likely.
2.4.1. Biodegradation
It is expected that biodegradation will be the primary mechanism of PAM degradation in oil sands tailings deposits. In comparison to water and wastewater treatment applications of PAM, biodegradation of PAM in oil sands tailings is more likely, given the higher doses used (often 10 times higher) and the lack of other available nutrients in tailings. Studies have demonstrated the microbial degradation of PAM in both oxic [163,164,165,166,167,168,169] and anoxic [159,167,170,171,172] environments. PAM biodegradation is thought to occur primarily as a result of microbial degradation of the main carbon chain backbone or hydrolysis of the amide nitrogen [163,165,173,174]. Kay-Shoemake et al. [163] found that aerobic microorganisms can produce extracellular amidases that remove the amide group from PAM, which generates NH4+ that can be used as a nitrogen source (see also Figure 3). Further, Haveroen et al. [159] found that nitrogen derived from PAM stimulated methanogenesis in Syncrude TT, Syncrude FFT, and domestic sewage sludge. In addition to methanogenesis, PAM may also serve as a nitrogen source and stimulate SRB [175].
Figure 3.
Potential biodegradation pathways of partially hydrolyzed polyacrylamide in oil sands tailings.
In contrast to the findings by Haveroen et al. [159], Collins et al. [111] found that PAM did not serve as a nitrogen source for methanogenesis in Shell Albian Sands (now CNUL) MFT. Instead, methanogenic activity was determined to be a result of nitrogen fixation. The contradictory findings of Haveroen et al. [159] and Collins et al. [111] may be due to: (i) differences in tailings microbial communities because each study used tailings from different operators; (ii) different carbon amendments; (iii) different PAM concentrations (CNUL’s PAM doses are much lower than that of other operators); and (iv) the fact that Haveroen et al. [159] performed serial transfers in order to dilute fixed nitrogen [111]. The discrepancy in the results of Havereon et al. [159] and Collins et al. [111] suggest that the extent to which PAM will biodegrade will vary between tailings deposits and operators.
The large size of the polymer (molecular weight > 106 Da) and a lack of enzymes capable of depolymerizing the polymer make it is much more difficult for microorganisms to use PAM as the sole carbon source [111,159,163]. Conditions where PAM was the only available carbon source resulted in a decrease in higher molecular weight compounds, suggesting cleavage of the polymer backbone [167,172]. However, biodegradation of PAM is expected to occur in TT and other PAM-amended tailings primarily due to PAM serving as a nitrogen source. Methanogens or SRB are most likely to benefit from PAM addition due to their predicted dominance in anoxic environments of terrestrial and subaqueous tailings deposits. The potential increase in biogenic greenhouse gas emissions from PAM degradation in tailings should be evaluated for both EPLs and terrestrial landforms.
Little is known about the extent of PAM biodegradation in tailings and therefore, the generation of PAM biodegradation products and their fate in tailings deposits. Potential PAM and HPAM biodegradation products and intermediates may include polyacrylate [159,163] or polyacrylic acid [164,166,168] following hydrolysis of the amide group, as well as pyruvic acid [171], acetyl-coenzyme A (CoA) [168,171], and acrylamide monomer, a neurotoxin [170], though to date there is limited research that shows PAM biodegradation to acrylamide monomer. These products may be further deaminated or metabolized to carboxylic acids and lower molecular weight organic compounds [164,168,170,171]. Based on previous biodegradation studies for PAM and HPAM, possible mechanisms for HPAM biodegradation and their respective enzymes are presented in Figure 3. Further study is needed to determine which microbial communities are responsible for PAM degradation. However, there are some bacteria that have been found to have a strong correlation with PAM biodegradation, including Bacteroidetes, Firmicutes, Proteobacteria, Spirochaetes, Synergistetes, and Thermotogae phyla, and Bacillus, Trichococcus, Brooklawnia, Pseudomonas, and Methanosaeta genera [164,165,168,171].
The predicted biodegradation products for HPAM and PAM are similar, though the negatively charged carboxylic group of HPAM may inhibit interactions of the polymer backbone with the negatively charged bacteria cell wall, shifting priority to hydrolysis of the amide group [164,168]. PAM may also be a more sufficient nitrogen source due to its higher percentage of nitrogen compared to HPAM. Although hydrolysis of the amide group during PAM biodegradation has been observed in a number of studies [163,166,176], complete biodegradation of PAM has not been observed in oil sands treated tailings. It is hypothesized that after hydrolysis of the amide group, the resulting carbon backbone structure, polyacrylate, is recalcitrant to further biodegradation. In general, PAM biodegradation pathways and the impacts of PAM on the biodegradation of other organics present in treated tailings, including petroleum hydrocarbons and NAFCs, are not well understood.
2.4.2. Photolytic Degradation
Photolytic degradation of PAM is possible during thin-lift deposition of TT, though this has not been evaluated to date. Photodegradation of PAM may also occur in EPLs or wetlands depending on the depth of light penetration or if PAM enters the water. In the presence of oxygen, light exposure can lead to chain scission by generating free radicals (often hydroxyl radicals) that produce carbon-centered polymer radicals [177,178]. Woodrow et al. [178] found that under natural and simulated sunlight and in the presence of Fe3+ and acid/neutral pH conditions, acrylamide monomer was rapidly released from PAM. This could occur under conditions in which froth treatment TT or TT with a high concentration of sulfide minerals are generating acidic leachate. However, under alkaline conditions, photoreduction of Fe3+ was reduced and PAM was relatively stable [178].
2.4.3. Mechanical Degradation
Mechanical degradation of PAM is possible through shear flow and direct mechanical loads. Industrial applications such as high volume hydraulic fracturing and enhanced oil recovery utilize PAM at high pressure and flow velocities, enhancing the mechanical stress on the polymer chain and subsequently deteriorating the physical structure of PAM [179,180]. Xiong et al. [180] assessed degradation under fluid strain conditions similar to those experienced under high volume hydraulic fracturing. High shear rates of ∼107 s−1 resulted in the degradation of high molecular weight PAM, with a decrease in molecular weight from 107 to 105 Da. Though there was an impact on the polymer chain, the degraded PAM remained chemically similar to the undegraded parent molecule, and minimal degradation occurred at lower molecular weights. At lower shear rates of 500 s−1, HPAM degradation in sandstone cores was below 20% [181].
Mechanical degradation of PAM through shear flow likely occurs in tailings operations during transport in pumps and pipelines and deposition in a pond or DDA. Neelakantan et al. [182] noted that shear degradation of PAM-treated tailings caused aggregate breakdown and restructuring which subsequently changed the rheological behavior of the tailings. Further, the presence of ions can enhance mechanical degradation of anionic PAM [181,183]. Ions interact with polymer functional groups, resulting in weakening of the main chain and acceleration of polymer degradation [183]. Being that tailings are often highly saline, this has the potential to influence mechanical degradation of PAM especially with anionic PAM that readily interacts with cations. It is predicted that mechanical degradation results in bond scission and the formation of free radical species [179]. Such radical species are then subject to combination reactions to form the original polymer, disproportionation reactions to produce two polymer species, or reactions with oxygen to produce peroxy radicals [179]. It is unclear how, if at all, mechanical degradation of PAM during tailings transport and deposition will impact its biodegradation.
2.4.4. Other Degradation Mechanisms
Thermal degradation of PAM is also possible given that fresh tailings may be deposited at temperatures of up to 60 °C [184]. Chemical transformation of PAM can occur at elevated temperatures, wherein the amide group thermally hydrolyzes to a carboxylate group [185]. Uranta et al. [185] found that after 30 days at elevated temperatures of 50, 70, and 90 °C, the hydrolysis level of PAM was 53, 65, and 75%, respectively, under highly saline (brine; total dissolved salts of 43 g/L) conditions. Even without brine, PAM degraded as a result of elevated temperatures in deionized water. However, temperatures in tailings ponds and BML range from approximately 10 to 20 °C depending on the depth and time of year [27,58,140], and as such, thermal degradation of PAM is not expected to be a significant component of PAM degradation in water capped tailings deposits.
Chemical degradation involves the breakdown of a substance, implying that the previously mentioned processes are all forms of PAM chemical degradation. Aside from these, other conditions that may lead to chemical degradation of PAM in tailings are limited. Under acidic conditions, PAM is subject to hydrolysis through the nucleophilic addition of water to the protonated amide group, which produces polyacrylate and NH4+ [179]. Following hydrolysis of the amide group, cleavage of the polymer backbone is also possible under acidic conditions [186]. In addition to hydrolysis, chain scission can occur in the presence of free radicals, resulting in the formation of radical and non-radical polymer fragments [187]. Hydroxyl and peroxyl radicals generated from the autoxidation of Fe2+ resulted in the chemical degradation of PAM [188]. Without dissolved oxygen though PAM degradation is insignificant in the presence of Fe2+ [189]. As such, PAM in TT may only be susceptible to chemical degradation at sites with acid rock drainage.
2.5. Surface Water and Groundwater Interactions
Tailings landforms will receive surface water and groundwater inputs from surrounding land and will discharge to downstream environments. As such, they will be hydrologically connected to the reclaimed mine and surrounding environments, including the Athabasca River. Surface water inflow and outflow and groundwater recharge and discharge from tailings landforms will vary seasonally with evapotranspiration and precipitation and will impact the water balance (and water levels) in these landforms [47,190]. Biotic and abiotic effects, such as wet/dry and freeze/thaw cycles and plant root development, can alter the physical properties of cover soils on terrestrial deposits which will also influence surface water flow through the deposits and groundwater recharge [191]. Over time, lateral and vertical hydrological flow through and contaminant fluxes from tailings deposits will change as the landforms undergo progressive reclamation [192].
2.5.1. Seepage and Contaminant Transport
As FFT in EPLs undergoes self-weight consolidation, primarily vertical fluxes of expressed pore water are expected given the relatively low hydraulic conductivity of the underlying formations [193]. However, there are a number of permeable strata in the Athabasca oil sands region through which tailings seepage can reach other groundwater units or surface water bodies. Bottom seepage from EPLs will depend largely on the underlying geology which will be different for each tailings deposits [193,194]. Even under a single deposit that covers a large area there will be spatial heterogeneity in hydraulic and geochemical properties. Modeling conducted by Kabwe et al. [193], using a finite strain consolidation model, indicates that FFT will tend to seal the bottom of EPLs and will eventually reach a very low hydraulic conductivity of 10−10 m/s. However, because of the large area of an EPL and the high water pressure at the base of EPLs, there will still likely be a considerable amount of seepage into the underlying permeable strata, though this will decrease as the tailings consolidate and hydraulic conductivity decreases. Kabwe et al. [193] estimated that after 25 years, a 25 to 100 m thick deposit would have a bottom seepage flux of approximately 0.02 m/m2/year. Over a 10 km2 area this equates to a seepage rate of 200,000 m3/year. Thus, both the quantity and quality of seepage flux out of the bottom of EPLs will need to be continuously monitored for many years after an EPL is developed, as consolidation and seepage will likely continue for decades [193].
In terrestrial landforms, uplands, primarily made of tailings sand, will be hydrologically connected to lowlands. Tailings sand contains precipitated salts from tailings pore water which are highly leachable because the sand consists of quartz which has low specific surface area and sorption site density [195,196]. Thus, flushing of the salts in tailings sand (which has a high hydraulic conductivity relative to FFT) will occur as the groundwater from uplands feeds low lying wetlands. This can lead to wetland salinization and salt accumulation depending on the surface water and groundwater flow out of the lowlands. Suncor’s 3 ha Nikanotee Fen Watershed contains an upland aquifer, made of 2 to 3 m of coarse tailings sand capped with fine grained cover soil, which supplies groundwater to a downgradient groundwater-fed peat fen with a petroleum coke underdrain [191,197]. Both the upland and fen are underlain by an impermeable geosynthetic liner to isolate them from the regional groundwater system. Flushing and dilution of the tailings sand (which has an average hydraulic conductivity of 4 × 10−6 m/s) in the upland has been attributed to recharge from surface overland flow, precipitation, and spatial differences in cover soil thickness and cover soil weathering [191,197]. These processes result in large-scale transport of Na+, ranging from 2 to 13 tonnes of Na+ per year, from the upland to the fen [197]. Over a four-year period, Kessel et al. [197] found that Na+ concentrations in the tailings sand decreased from 232 to 196 mg/L while Na+ concentrations in the fen rooting zone increased from 87 to 200 mg/L. Simhayov et al. [195] further estimated that there is a large amount of leachable Na (27 t), Ca (14 t), S (37.3 t), and Mg (8.8 t) in the tailings sand, petroleum coke, and peat system, the majority of which originate from the tailings sand. As such, a high influx of ions to the fen is still expected and evaporation may lead to salt accumulation at the fen surface [195]. As vegetation develops in the upland cover soil, root water uptake should maintain the capillary barrier between the soil and underlying sand, which is expected to reduce groundwater recharge and thereby flushing of tailings sand [191].
Without pumping and a natural surface water outlet, vertical fluxes of saline pore water from underlying CT dominate the Sandhill Fen wetland water balance as outward groundwater fluxes are estimated to be an order of magnitude less [97,190,192]. In contrast to the Nikanotee Fen, the Sandhill Wetland does not have a naturally flowing outlet, which has resulted in a high water table and high year-over-year increases in salinity, relative to the Nikanotee Fen [97]. Over time, the Sandhill Fen wetland is expected to be more heavily influenced by groundwater and surface water interactions as it becomes linked to the surrounding mine closure landscape, which is expected to reduce salinization of the wetland [55,97,190].
Contaminant transport studies have investigated the fate of the dominant ions in OSPW, Na+, Cl−, SO42−, and HCO3−, in surficial sediments in the Athabasca region. Holden et al. [16] found that the ingress of OSPW led to dissolution of calcium and magnesium sulfate salts in the sediment, further elevating concentrations of SO42− in the pore water. In the presence of glacial clay till, Na+ can exchange with sediment-bound Ca2+ and Mg2+, resulting in an increase in the concentration of these divalent ions in the water and precipitation of the calcium-magnesium carbonate mineral phase (alkalinity precipitation) [16,194]. Abolfazlzadehdoshanbehbazari et al. [194] also noted the uptake of molybdenum and release of manganese, barium, and strontium from clay till. Cl− is conservative and will remain in solution [16]. However, in the absence of glacial till, Na+ adsorption and attenuation may not occur, and thus both Na+ and Cl− will remain in solution and SO42− concentrations will be elevated from sulfate salt dissolution. These observations of inorganic solute transport in surficial sediments in the Athabasca regime have implications for pore water seepage from surficial tailings deposits and may impact neighbouring aquatic environments.
Groundwater transport of organic compounds in OSPW has also been investigated, with some studies suggesting that NAFCs and polycyclic aromatic hydrocarbons will partition to the solid phase, depending on the fraction of organic carbon in the solid material [198,199,200]. Conversely, Oiffer et al. [201] reported that attenuation of NAFCs in the subsurface adjacent to an oil sands tailings pond was not significant over a period of 20 years, suggesting little to no sorption [202,203] or anaerobic biodegradation of NAFCs occurred [201]. Similarly, Bowman et al. [204] found no significant changes in the abundances of NAFCs in four wells in the Sandhill Fen sand cap and CT layers over a period of one year, with the exception of one well, though these changes were attributed to pore water dilution. Thus, the extent to which NAFCs will be transported through the subsurface will likely depend on site specific conditions, including the presence of oxygen and the fraction of organic carbon in soils.
2.5.2. Desalination via Fresh Water Flushing
Over time, fresh water inflows may desalinate tailings deposits which could cause the loose card-house structure of clay particles within FFT to collapse when strained [47,48,205]. This would cause pore pressures to increase such that effective stresses in the deposit approach zero and the deposit becomes mobile and behaves like a thick fluid [47]. This would impact the integrity and performance of tailings deposits, depending on their location and configuration. Though desalination of FFT would likely take decades to occur, the potential mobility of these deposits after fresh water flushing should be assessed.
3. Long-Term Geochemical Behavior of Tailings Landforms—Implications and Knowledge Gaps
Table 5
summarizes the key considerations and knowledge gaps surrounding the geochemical stability of anticipated oil sands tailings landforms. Research to date has largely focused on untreated FFT, such as in tailings ponds and BML, and CT, in various deposits including Syncrude’s Sandhill Fen. Other tailings treatment methods outlined in
Table 1
are more recent technologies or have not been investigated as thoroughly. While previous research has identified salinity, methanogenesis, and sulfate reduction/sulfur cycling as key biogeochemical considerations for tailings landforms, there are still many unknowns, particularly with respect to the biogeochemical implications of tailings treatment methods that have not been as extensively studied.

Table 5.
Key considerations and knowledge gaps surrounding the geochemical stability of oil sands tailings landforms.
Consolidation of tailings deposits and subsequent subsidence in cover systems in terrestrial landforms has the potential to drastically alter the landform and may result in the unintended generation of large wetlands or saline lakes that disrupt and contaminate surface drainage pathways and disturb established vegetation [48]. EPLs will not have a substantial cap surcharge and as such they may retain more tailings pore water and will release water at a slower rate than a more heavily capped deposit [48]. Further, because EPLs have a planned water cap, expressed pore water from consolidation is more likely to be diluted, whereas the unanticipated generation of large wetlands in terrestrial landforms would result in highly saline water bodies that contain higher concentrations of chemical constituents and organics compared to an EPL water cap. The type of tailings treatment will also impact consolidation and therefore landform progression.
Vertical chemical fluxes are likely under all reclamation scenarios, as tailings undergo dewatering through consolidation and/or environmental effects. As tailings deposits dewater over time and the hydraulic conductivities of tailings deposits decrease, advective pore water fluxes will also decrease over time, which may reduce chemical mass loading to overlying cover soils, wetlands, and water caps. However, given the salinity in BML, the Sandhill Wetland, and the Nikanotee Fen, salinity in tailings landforms should be anticipated and therefore incorporated into reclamation plans.
Biogeochemical cycling within tailings deposits will be highly dependent on treatment methods. CT deposits and centrifuge tailings may undergo substantial sulfur cycling due to gypsum addition and may produce aqueous and gaseous H2S, as Reid and Warren [91] have found in Syncrude’s Sandhill Fen and Warren et al. [112] reported in another Syncrude CT deposit. As such, the environmental fate of H2S in these deposits should be evaluated. To date, there is limited research on Suncor’s novel PASS treatment technology, which involves both PAM and alum addition. Because of the alum addition, sulfur cycling may also become important for PASS treated tailings. Further, the environmental fate of aluminum, which is known to bioaccumulate and be toxic to aquatic organisms [206,207], in PASS treated tailings should be evaluated. In addition to treatment methods, biogeochemical cycling in tailings landforms will vary based on the type of tailings (for example, FFT versus froth treatment tailings), landform design, the presence or absence of oxygen, tailings age, and ore deposit, as each of these factors will influence electron acceptors, microbial communities, and available substrates. Deposits incorporating froth treatment tailings or tailings with large amounts of sulfide minerals may need to be water capped or fully saturated with water to limit the potential for acid generation and metal(loid) release. Further, microbial activity in tailings landforms will contribute to bioremediation of organic compounds (and greenhouse gas emissions) and bioconsolidation, though the extent to which these processes will occur in different tailings landforms is not well understood.
The long-term implications of polymer addition and subsequent degradation in tailings deposits containing PAM, including TT and PASS, remain largely unknown. PAM biodegradation could impact biogeochemical cycling in tailings and thus the environmental fate of PAM and its biodegradation by-products in tailings deposits is a substantial knowledge gap that needs to be thoroughly investigated. PAM degradation also has the potential to reverse or alter the geotechnical benefits that come with polymer addition, though it is unknown at what stage, if any, PAM degradation impacts geotechnical properties of tailings.
Surface water and groundwater interactions will influence water levels, contaminant concentrations, and water and mass transport within tailings deposits and throughout the entire reclaimed landscape. As mine closure landscapes develop, tailings landforms may also receive substantial amounts of tailings seepage water from surrounding reclaimed watersheds [47,48]. EPLs and terrestrial landforms with a wetland feature will need to have inflows of sufficient quantity and quality to avoid excessive water level drawdowns and to prevent salinization, and at the same time have outflows and discharges of sufficient water quality so as not to contaminate downstream ecosystems [48]. Given the potential for seepage and contaminant transport, surface water and groundwater monitoring around tailings landforms and throughout the entire reclaimed landscape will likely have to continue for decades after mine closure.
4. Conclusions
While much research has been conducted to evaluate oil sands tailings management and reclamation options, uncertainty still exists around the geochemical stability of tailings deposits and final landforms. This review has identified a number of key considerations and knowledge gaps surrounding the geochemical stability of tailings landforms that warrant further investigation. These include: (i) the implications and duration of consolidation of tailings in landforms, and in particular settlement and water release in terrestrial landforms; (ii) chemical fluxes and subsequent water quality in EPLs, wetlands, cover soils, and seepage; (iii) the extent to which bioremediation and biogenic greenhouse gas emissions will occur in EPLs and terrestrial landforms; and (iv) the environmental fate and biogeochemical implications of chemical tailings amendments including gypsum, alum, and PAM. Understanding these biogeochemical processes and the biogeochemical implications of various tailings treatment methods will help reduce the potential long-term environmental impacts and moderate the environmental and economic liabilities associated with oil sands tailings reclamation.
Funding
This review received no external funding.
Acknowledgments
H. Cossey is grateful for financial support from the Vanier-Banting Secretariat throughout her Ph.D. program.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AEP, Aurora East Pit; BML, Base Mine Lake; BTEX, benzene, toluene, ethylbenzene, and xylenes; Ca2+, calcium; CH4, methane; Cl−, chloride; CNRL, Canadian Natural Resources Ltd.; CNUL, Canadian Natural Upgrading Ltd.; CO2, carbon dioxide; CoA, coenzyme A; CT, composite tailings; DDA, dedicated disposal area; eNST, enhanced non-segregating tailings; EPL, end pit lake; FeII, ferrous iron; FeIII, ferric iron; FeS(s), iron(II) sulfide; FGD, flue gas desulphurization; FFT, fluid fine tailings; HCO3−, bicarbonate; H2S, hydrogen sulphide; HPAM, hydrolyzed polyacrylamide; HWP, Hot Water Process; ILTT, in-line thickened tailings; MFT, mature fine tailings; Mg2+, magnesium; MLSB, Mildred Lake Settling Basin; Na+, sodium; NAFC, naphthenic acid fraction compounds; NaOH, sodium hydroxide; NH4+, ammonium; NO3−, nitrate; NO2−, nitrite, NST, non-segregating tailings; NTU, nephelometric turbidity units; OSPW, oil sands process-affected water; PAM, polyacrylamide; PASS, Permanent Aquatic Storage Structure; OCC, oxygen consuming constituent; SFR, sand to fines ratio; SO42−, sulfate; SRB, sulfate reducing bacteria; TRO, Tailings Reduction Operation; TT, thickened tailings.
References
- Government of Alberta. Oil Sands: Facts and Stats. 2021. Available online: https://www.alberta.ca/oil-sands-facts-and-statistics.aspx (accessed on 19 May 2021).
- Chalaturnyk, R.J.; Scott, J.D.; Özüm, B. Management of oil sands tailings. Pet. Sci. Technol. 2002, 20, 1025–1046. [Google Scholar] [CrossRef]
- Kasperski, K.L.; Mikula, R.J. Waste streams of mined oil sands: Characteristics and remediation. Elements 2011, 7, 387–392. [Google Scholar] [CrossRef]
- Alberta Energy Regulator (AER). State of Fluid Tailings Management for Mineable Oil Sands, 2019; Alberta Energy Regulator: Calgary, AB, Canada, 2020; Available online: https://static.aer.ca/prd/2020-09/2019-State-Fluid-Tailings-Management-Mineable-OilSands.pdf (accessed on 14 January 2021).
- Government of Alberta. Oil Sands Information Portal. 2021. Available online: http://osip.alberta.ca/map/ (accessed on 19 May 2021).
- Canada’s Oil Sands Innovation Alliance (COSIA). Water Performance Goals. Available online: https://staging.cosia.ca/performance-goals/water#water-perf-miningn.d. (accessed on 12 June 2021).
- Headley, J.V.; McMartin, D.W. A review of the occurrence and fate of naphthenic acids in aquatic environments. J. Environ. Sci. Health A 2004, 39, 1989–2010. [Google Scholar] [CrossRef] [PubMed]
- Marentette, J.R.; Frank, R.A.; Bartlett, A.J.; Gillis, P.L.; Hewitt, L.M.; Peru, K.M.; Headley, J.V.; Brunswick, P.; Shang, D.; Parrott, J.L. Toxicity of naphthenic acid fraction components extracted from fresh and aged oil sands process-affected waters, and commercial naphthenic acid mixtures, to fathead minnow (Pimephales promelas) embryos. Aquat. Toxicol. 2015, 164, 108–117. [Google Scholar] [CrossRef]
- Morandi, G.D.; Wiseman, S.B.; Pereira, A.; Mankidy, R.; Gault, I.G.M.; Martin, J.W.; Giesy, J.P. Effects-directed analysis of dissolved organic compounds in oil sands process-affected water. Environ. Sci. Technol. 2015, 49, 12395–12404. [Google Scholar] [CrossRef]
- Holowenko, F.M.; MacKinnon, M.D.; Fedorak, P.M. Methanogens and sulfate-reducing bacteria in oil sands fine tailings waste. Can. J. Microbiol. 2000, 46, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, P.M.; Coy, D.L.; Dudas, M.J.; Simpson, M.J.; Renneberg, A.J.; MacKinnon, M.D. Microbially-mediated fugitive gas production from oil sands tailings and increased tailings densification rates. J. Environ. Eng. Sci. 2003, 2, 199–211. [Google Scholar] [CrossRef]
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of short-chain n-alkanes in oil sands tailings under methanogenic conditions. Environ. Sci. Technol. 2006, 40, 5459–5464. [Google Scholar] [CrossRef]
- Siddique, T.; Fedorak, P.M.; Mackinnon, M.D.; Foght, J.M. Metabolism of BTEX and naphtha compounds to methane in oil sands tailings. Environ. Sci. Technol. 2007, 41, 2350–2356. [Google Scholar] [CrossRef]
- Siddique, T.; Penner, T.; Semple, K.; Foght, J.M. Anaerobic biodegradation of longer-chain n-alkanes coupled to methane production in oil sands tailings. Environ. Sci. Technol. 2011, 45, 5892–5899. [Google Scholar] [CrossRef]
- Burkus, Z.; Wheler, J.; Pletcher, S. GHG Emissions from oil sands tailings ponds: Overview and modelling based on fermentable substrates. In Part I: Review of Tailings Pond Facts and Practices; Alberta Environment and Sustainable Resource Development: Edmonton, AB, Canada, 2014; Available online: https://open.alberta.ca/dataset/9a7c1413-f02b-4b88-a277-ec571ae884f3/resource/f87efbea-5739-4dfa-993e-8ad854417c31/download/2014-ghg-emissions-from-oil-sands-tailings-ponds-overview-and-modelling-based-on-fermentable-sub.pdf (accessed on 14 May 2021).
- Holden, A.A.; Donahue, R.B.; Ulrich, A.C. Geochemical interactions between process-affected water from oil sands tailings ponds and North Alberta surficial sediments. J. Contam. Hydrol. 2011, 119, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.K.; Forrest, D. Oil Sands Mining Reclamation Challenge Dialogue—Report and Appendices. In Oil Sands Research and Information Network; University of Alberta, School of Energy and the Environment: Edmonton, AB, Canada, 2010. [Google Scholar]
- Chow, D.L.; Nasr, T.N.; Chow, R.S.; Sawatzky, R.P. Recovery techniques for Canada’s heavy oil and bitumen resources. J. Can. Petrol. Technol. 2008, 47, 12–17. [Google Scholar]
- Clark, K.A.; Pasternack, D.S. Hot water separation of bitumen from Alberta bituminous sand. Ind. Eng. Chem. 1932, 24, 1410–1416. [Google Scholar] [CrossRef]
- Bakhtiari, M.T. Development of a Tailings Management Simulation and Technology Evaluation Tool. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2015. [Google Scholar]
- Headley, J.V.; Peru, K.M.; Barrow, M.P. Advances in mass spectrometric characterization of naphthenic acids fraction compounds in oil sands environmental samples and crude oil—A review. Mass Spectrom. Rev. 2016, 35, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.C.; Mackinnon, M.D.; Fedorak, P.M. Naphthenic acids in Athabasca oil sands tailings waters are less biodegradable than commercial naphthenic acids. Environ. Sci. Technol. 2005, 39, 8388–8394. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, R.J.; Frank, R.A.; Oakes, K.D.; Servos, M.R.; Young, R.F.; Fedorak, P.M.; MacKinnon, M.D.; Solomon, K.R.; Dixon, D.G.; Van Der Kraak, G. Fathead minnow (Pimephales promelas) reproduction is impaired in aged oil sands process-affected waters. Aquat. Toxicol. 2011, 101, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.D.; Ulrich, A.C. Oil sands naphthenic acids: A review of properties, measurement, and treatment. Chemosphere 2015, 127, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, P.J.; Tam, K.C. Water treatment technologies for the remediation of naphthenic acids in oil sands process-affected water. Chem. Eng. J. 2015, 279, 696–714. [Google Scholar] [CrossRef]
- Allen, E.W. Process water treatment in Canada’s oil sands industry: II. A review of emerging technologies. J. Environ. Eng. Sci. 2008, 7, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Dompierre, K.A.; Lindsay, M.B.J.; Cruz-Hernández, P.; Halferdahl, G.M. Initial geochemical characteristics of fluid fine tailings in an oil sands end pit lake. Sci. Total Environ. 2016, 556, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Liu, Q. Froth treatment in Athabasca oil sands bitumen recovery process: A review. Energy Fuels 2013, 27, 7199–7207. [Google Scholar] [CrossRef]
- Foght, J.M.; Gieg, L.M.; Siddique, T. The microbiology of oil sands tailings: Past, present, future. FEMS Microbiol. Ecol. 2017, 93, fix034. [Google Scholar] [CrossRef] [Green Version]
- Small, C.C.; Cho, S.; Hashisho, Z.; Ulrich, A.C. Emissions from oil sands tailings ponds: Review of tailings pond parameters and emission estimates. J. Petrol. Sci. Eng. 2015, 127, 490–501. [Google Scholar] [CrossRef]
- Imperial Oil Resources Ltd. (Imperial). Kearl Oil Sands Mine: Fluid Tailings Management Report; Imperial Oil Resources Ltd.: Calgary, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Suncor Energy Inc. (Suncor). Mining Technologies. 2021. Available online: https://sustainability.suncor.com/en/innovation/mining-technologies (accessed on 23 May 2021).
- Government of Alberta. Lower Athabasca Region Tailings Management Framework for the Mineable Athabasca Oil Sands; Government of Alberta: Edmonton, AB, Canada, 2015. Available online: https://open.alberta.ca/dataset/962bc8f4-3924-46ce-baf8-d6b7a26467ae/resource/7c49eb63-751b-49fd-b746-87d5edee3131/download/2015-larp-tailingsmgtathabascaoilsands.pdf (accessed on 23 April 2021).
- Alberta Energy Regulator (AER). Directive 085: Fluid Tailings Management for Oil Sands Mining Projects; Alberta Energy Regulator: Calgary, AB, Canada, 2017; Available online: https://static.aer.ca/prd/2020-07/Directive085_0.pdf (accessed on 23 April 2021).
- Oil Sands Tailings Consortium (OSTC) and Canada’s Oil Sands Innovation Alliance (COSIA). Technical Guide for Fluid Fine Tailings Management; OSTC and COSIA: Calgary, AB, Canada, 2012. [Google Scholar]
- McKenna, G.; Mooder, B.; Burton, B.; Jamieson, A. Shear Strength and Density of Oil Sands Fine Tailings for Reclamation of a Boreal Forest Landscape. In Proceedings of the 5th International Oil Sands Tailings Conference, Lake Louise, AB, Canada, 4–7 December 2016. [Google Scholar]
- BGC Engineering Inc. Oil Sands Tailings Technology Review; Oil Sands Research and Information Network (OSRIN) Report No. TR-1, OSRIN; School of Energy and the Environment, University of Alberta: Edmonton, AB, Canada, 2010. [Google Scholar]
- Burden, R.; Wilson, G.W. Simulating deposition of Clearwater shale—FFT blends using a novel consolidation test. In Proceedings of the Canada’s Oil Sands Innovation Alliance (COSIA) Innovation Summit, Calgary, AB, Canada, 7–8 June 2018. [Google Scholar]
- Syncrude Canada Ltd. (Syncrude). 2019 Mildred Lake Tailings Management Report; Syncrude Canada Ltd.: Fort McMurray, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Suncor Energy Inc. (Suncor). 2019 Base Plant Fluid Tailings Management Report; Suncor Energy Inc.: Calgary, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Canadian Natural Upgrading Ltd. (CNUL). Canadian Natural Muskeg River Mine Fluid Tailings Management Report; Canadian Natural Upgrading Ltd.: Fort McMurray, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Canadian Natural Upgrading Ltd. (CNUL). Canadian Natural Jackpine Mine Fluid Tailings Management Report; Canadian Natural Upgrading Ltd.: Fort McMurray, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Syncrude Canada Ltd. (Syncrude). 2019 Aurora North Tailings Management Report; Syncrude Canada Ltd.: Fort McMurray, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Canadian Natural Resources Limited (CNRL). Canadian Natural Horizon: 2019 Horizon Tailings Management Report; Canadian Natural Resources Limited: Fort McMurray, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Suncor Energy Operating Inc. (Suncor). 2019 Fort Hills Fluid Tailings Management Report; Suncor Energy Operating Inc.: Calgary, AB, Canada, 2020; Available online: https://www.aer.ca/providing-information/by-topic/tailings (accessed on 23 April 2021).
- Westcott, F.; Watson, L. End pit Lakes Technical Guidance Document; Clearwater Environmental Consultants for Cumulative Effects; Project 2005-61; Management Association End Pit Lakes Subgroup: Calgary, AB, Canada, 2007; Available online: https://www.ceaa.gc.ca/050/documents_staticpost/cearref_37519/44851/t.pdf (accessed on 14 May 2020).
- Charette, T.; Castendyk, D.; Hrynyshyn, J.; Küpper, A.; McKenna, G.; Mooder, B. End Pit Lakes Guidance Document 2012; Cumulative Effects Management Association: Fort McMurray, AB, Canada, 2010. [Google Scholar]
- Sawatsky, L.; Hyndman, A.; McKenna, G.; Vandenberg, J. Fluid Fine Tailings Processes: Disposal, Capping, and Closure Alternatives. In Proceedings of the 6th International Oil Sands Tailings Conference, Edmonton, AB, Canada, 9–12 December 2018. [Google Scholar]
- McKenna, G. Landscape design for mine reclamation. In Proceedings of the Tailings and Mine Waste ’09, Banff, AB, Canada, 1–4 November 2009; Available online: https://sites.google.com/a/ualberta.ca/tmw-2009/ (accessed on 14 May 2020).
- Canada’s Oil Sands Innovation Alliance (COSIA). Pit Lakes: A Surface Mining Perspective; Canada’s Oil Sands Innovation Alliance: Calgary, AB, Canada, 2021; Available online: https://cosia.ca/sites/default/files/attachments/Park%20-%20COSIA%20-%20Pit%20Lakes%20-%20Final.pdf (accessed on 11 May 2021).
- McKenna, G.; Dawson, R. Oil Sands Tailings Technology Deployment Roadmap: Project Report—Volume 2; component 1 results; BGC Engineering Inc. and Norwest Corporation for Alberta Innovates—Energy and Environmental Solutions: Calgary, AB, Canada, 2012; Available online: https://www.cosia.ca/uploads/documents/id10/Tailings%20Roadmap%20Volume%202%20June%202012.pdf (accessed on 14 January 2021).
- Syncrude Canada Ltd. (Syncrude). Centrifuge Tails. 2020. Available online: https://www.syncrude.ca/environment/tailings-management/tailings-reclamation/centrifuged-tails/ (accessed on 14 May 2020).
- Syncrude Canada Ltd. (Syncrude). Composite Tails. 2020. Available online: https://www.syncrude.ca/environment/tailings-management/tailings-reclamation/composite-tails/ (accessed on 14 May 2020).
- Syncrude Canada Ltd. (Syncrude). Sandhill fen Recognized for Environmental Excellence. 2014. Available online: https://www.syncrude.ca/2014/05/13/sandhill-fen-recognized-for-environmental-excellence/ (accessed on 19 January 2021).
- Biagi, K.M.; Oswald, C.J.; Nicholls, E.M.; Carey, S.K. Increases in salinity following a shift in hydrologic regime in a constructed wetland watershed in a post-mining oil sands landscape. Sci. Total Environ. 2019, 653, 1445–1457. [Google Scholar] [CrossRef]
- Gibson, J.J.; Fennell, J.; Birks, S.J.; Yi, Y.; Moncur, M.C.; Hansen, B.; Jasechko, S. Evidence of discharging saline formation water to the Athabasca River in the oil sands mining region, northern Alberta. Can. J. Earth Sci. 2013, 50, 1244–1257. [Google Scholar] [CrossRef]
- Kaminsky, H.; Omotoso, O. Variability in fluid fine tailings. In Proceedings of the 5th International Oil Sands Tailings Conference, Lake Louise, AB, Canada, 4–7 December 2016. [Google Scholar]
- Penner, T.J.; Foght, J.M. Mature fine tailings from oil sands processing harbour diverse methanogenic communities. Can. J. Microbiol. 2010, 56, 459–470. [Google Scholar] [CrossRef]
- Siddique, T.; Kuznetsov, P.; Kuznetsova, A.; Arkell, N.; Young, R.; Li, C.; Guigard, S.; Underwood, E.; Foght, J.M. Microbially-accelerated consolidation of oil sands tailings. Pathway I: Changes in porewater chemistry. Front. Microbiol. 2014, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Stasik, S.; Wendt-Potthoff, K. Interaction of microbial sulphate reduction and methanogenesis in oil sands tailings ponds. Chemosphere 2014, 103, 59–66. [Google Scholar] [CrossRef]
- Willis, C.E.; St. Louis, V.L.; Kirk, J.L.; St. Pierre, K.A.; Dodge, C. Tailings ponds of the Athabasca oil sands region, Alberta, Canada, are likely not significant sources of total mercury and methylmercury to nearby ground and surface waters. Sci. Total Environ. 2019, 647, 1604–1610. [Google Scholar] [CrossRef]
- Cowie, B.R.; James, B.; Mayer, B. Distribution of total dissolved solids in McMurray Formation water in the Athabasca oil sands region, Alberta, Canada: Implications for regional hydrogeology and resource development. AAPG Bull. 2015, 99, 77–90. [Google Scholar] [CrossRef]
- Renault, S.; Lait, C.; Zwiazek, J.; MacKinnon, M. Effect of high salinity tailings waters produced from gypsum treatment of oil sands tailings on plants of the boreal forest. Environ. Pollut. 1998, 102, 177–184. [Google Scholar] [CrossRef]
- MacKinnon, M.D.; Matthews, J.G.; Shaw, W.H.; Cuddy, R.G. Water Quality Issues Associated With Composite Tailings (CT) Technology for Managing Oil Sands Tailings. Int. J. Min. Reclam. Environ. 2001, 15, 235–256. [Google Scholar] [CrossRef]
- Ripmeester, M.J.; Duford, D.A. Method for routine “naphthenic acids fraction compounds’ determination in oil sands process-affected water by liquid-liquid extraction in dichloromethane and Fourier-Transform Infrared Spectroscopy. Chemosphere 2019, 233, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Government of Alberta. Long Term River Station Data. 2021. Available online: http://environment.alberta.ca/apps/EdwReportViewer/LongTermRiverStation.aspx (accessed on 14 May 2021).
- Jeeravipoolvarn, S.; Chalaturnyk, R.J.; Scott, J.D. Consolidation Modeling of Oil Sands Fine Tailings: History Matching. In Proceedings of the 61st Canadian Geotechnical Conference, Edmonton, AB, Canada, 22–24 September 2008. [Google Scholar]
- Kaminsky, H.A.W.; Etsell, T.H.; Ivey, D.G.; Omotoso, O. Distribution of clay minerals in the process streams produced by the extraction of bitumen from Athabasca oil sands. Can. J. Chem. Eng. 2009, 87, 85–93. [Google Scholar] [CrossRef]
- Jeeravipoolvarn, S.; Scott, J.D.; Chalaturnyk, R.J. 10 m standpipe tests on oil sands tailings: Long-term experimental results and prediction. Can. Geotech. J. 2009, 46, 875–888. [Google Scholar] [CrossRef]
- Mikula, R.J.; Munoz, V.A.; Omotoso, O. Centrifugation options for production of dry stackable tailings in surface mined oil sands tailings management. J. Can. Petrol. Technol. 2009, 48, 19–23. [Google Scholar] [CrossRef]
- Kaminsky, H.A.W.; Etsell, T.H.; Ivey, D.G.; Omotoso, O. Characterization of heavy minerals in the Athabasca oil sands. Miner. Eng. 2008, 21, 264–271. [Google Scholar] [CrossRef]
- Gibson, R.E.; England, G.L.; Hussey, M.J.L. The theory of one-dimensional consolidation of saturated clays I, finite non-linear consolidation of thin homogeneous layers. Géotechnique 1967, 17, 261–273. [Google Scholar] [CrossRef]
- Jeeravipoolvarn, S.; Scott, J.D.; Chalaturnyk, R.J. Multi-dimensional Finite Strain Consolidation Theory: Modeling Study. In Proceedings of the 61st Canadian Geotechnical Conference, Edmonton, AB, Canada, 22–24 September 2008. [Google Scholar]
- Suthaker, N.N.; Scott, J.D. Large Scale Consolidation Testing of Oil Sand Fine Tails. In Proceedings of the 1st International Congress on Environmental Geotechnics, Edmonton, AB, Canada, 10–15 July 1994. [Google Scholar]
- Sorta, A.; Beier, N.; Kabwe, L.K.; Scott, J.D.; Wilson, G.W. The Case for Using Fines Void Ration. In Proceedings of the 68th Canadian Geotechnical Conference, Quebec City, QC, Canada, 21–23 September 2015. [Google Scholar]
- Beier, N.A. Role of Sodium Hydroxide in Bitumen Extraction: Production of Natural Surfactants and Slime Coating. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2015. [Google Scholar]
- Wilson, G.W.; Kabwe, L.K.; Beier, N.A.; Scott, J.D. Effect of various treatments on consolidation of oil sands fluid fine tailing. Can. Geotech. J. 2017, 55, 1059–1066. [Google Scholar] [CrossRef]
- Sorta, A.R.; Sego, D.C.; Wilson, W. Physical modelling of oil sands tailings consolidation. Int. J. Phys. Model. Geotech. 2016, 16, 47–64. [Google Scholar] [CrossRef]
- Dompierre, K.A.; Barbour, S.L. Characterization of physical mass transport through oil sands fluid fine tailings in an end pit lake: A multi-tracer study. J. Contam. Hydrol. 2016, 189, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Dompierre, K.A.; Barbour, S.L.; North, R.L.; Carey, S.K.; Lindsay, M.B.J. Chemical mass transport between fluid fine tailings and the overlying water cover of an oil sands end pit lake. Water Resour. Res. 2017, 53, 4725–4740. [Google Scholar] [CrossRef]
- White, K.B.; Liber, K. Early chemical and toxicological risk characterization of inorganic constituents in surface water from the Canadian oil sands first large-scale end pit lake. Chemosphere 2018, 211, 745–757. [Google Scholar] [CrossRef] [PubMed]
- White, K.B.; Liber, K. Chronic toxicity of surface water from a Canadian oil sands end pit lake to the freshwater invertebrates Chironomus dilutus and Ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 2020, 78, 439–450. [Google Scholar] [PubMed]
- Leung, S.S.; MacKinnon, M.D.; Smith, R.E.H. The ecological effects of naphthenic acids and salts on phytoplankton from the Athabasca oil sands region. Aquat. Toxicol. 2003, 62, 11–26. [Google Scholar]
- Nero, V.; Farwell, A.; Lee, L.E.J.; Van Meer, T.; MacKinnon, M.D.; Dixon, D.G. The effects of salinity on naphthenic acid toxicity to yellow perch: Gill and liver histopathology. Ecotoxicol. Environ. Saf. 2006, 65, 252–264. [Google Scholar] [CrossRef]
- Monaghan, J.; Richards, L.C.; Vandergrift, G.W.; Hounjet, L.J.; Stoyanov, S.R.; Gill, C.G.; Krogh, E.T. Direct mass spectrometric analysis of naphthenic acids and polycyclic aromatice hydrocarbons in waters impacted by diluted bitumen and conventional crude oil. Sci. Total Environ. 2021, 765, 144206. [Google Scholar] [CrossRef] [PubMed]
- Rundle, K.I.; Sharaf, M.S.; Stevens, D.; Kamunde, C.; van der Heuvel, M.R. Oil sands derived naphthenic acids are oxidative uncouplers and impair electron transport in isolated mitochondria. Environ. Sci. Technol. 2018, 765, 10810–10811. [Google Scholar] [CrossRef]
- Lawrence, G.A.; Tedford, E.W.; Pieters, R. Suspended solids in an end pit lake: Potential mixing mechanisms. Can. J. Civ. Eng. 2016, 43, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Tedford, E.; Halferdahl, G.; Pieters, R.; Lawrence, G.A. Temporal variations in turbidity in an oil sands pit lake. Environ. Fluid Mech. 2019, 19, 457–473. [Google Scholar]
- Shen, X.; Sun, T.; Su, M.; Dang, Z.; Yang, Z. Short-term response of aquatic ecosystem metabolism to turbidity disturbance in experimental estuarine wetlands. Ecol. Eng. 2019, 136, 55–61. [Google Scholar] [CrossRef]
- Zehrer, R.F.; Burns, C.W.; Flöder, S. Sediment resuspension, salinity and temperature affect the plankton community of a shallow coastal lake. Mar. Freshw. Res. 2015, 66, 317–328. [Google Scholar] [CrossRef]
- Reid, M.L.; Warren, L.A. S reactivity of an oil sands composite tailings deposit undergoing reclamation wetland construction. J. Environ. Manag. 2016, 166, 321–329. [Google Scholar] [CrossRef]
- Hartsock, J.A.; Piercey, J.; House, M.K.; Vitt, D.H. An evaluation of water quality at Sandhills Wetland: Implications for reclaiming wetlands above soft tailings deposits in northern Alberta, Canada. Wetl. Ecol. Manag. 2021, 29, 111–127. [Google Scholar] [CrossRef]
- Vessey, C.J.; Lindsay, M.B.J.; Barbour, S.L. Sodium transport and attenuation in soil cover materials for oil sands mine reclamation. Appl. Geochem. 2019, 100, 42–54. [Google Scholar] [CrossRef]
- Wiener, J.G.; Krabbenhoft, D.P.; Heinz, G.H.; Scheuhammer, A.M. Ecotoxicology of mercury. In Handbook of Ecotoxicology; Hoffman, D.J., Rattner, B.A., Burton, G.A., Cairns, J., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 409–463. [Google Scholar]
- Oswald, C.J.; Carey, S.K. Total and methyl mercury concentrations in sediment and water of a constructed wetland in the Athabasca Oil Sands Region. Environ. Pollut. 2016, 213, 628–637. [Google Scholar] [CrossRef]
- Vitt, D.H.; House, M.; Hartsock, J.A. Sandhill Fen, an initial trial for wetland species assembly on in-pit substrates: Lessons after three years. Botany 2016, 94, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Biagi, K.M.; Clark, M.G.; Carey, S.K. Hydrological functioning of a constructed peatland watershed in the Athabasca oil sands region: Potential trajectories and lessons learned. Ecol. Eng. 2021, 166, 106236. [Google Scholar] [CrossRef]
- Foote, L. Threshold Considerations and Wetland Reclamation in Alberta’s Mineable Oil Sands. Ecol. Soc. 2012, 17, art35. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, P.; Hrudey, S.E.; Naeth, M.A.; Plourde, A.; Therrien, R.; Van Der Kraak, G.; Xu, Z. The Royal Society of Canada Expert Panel: Environmental and Health Impacts of Canada’s Oil Sands Industry: Report; Royal Society of Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Trites, M.; Bayley, S.E. Vegetation communities in continental boreal wetlands along a salinity gradient: Implications for oil sands mining reclamation. Aquat. Bot. 2009, 91, 27–39. [Google Scholar] [CrossRef]
- Roshani, A.; Fall, M.; Kennedy, K. Microstructural, hydraulic conductivity and geochemical changes of drying mature fine oil sands tailings in column experiments. Int. J. Min. Reclam. Environ. 2018, 32, 239–253. [Google Scholar] [CrossRef]
- Cilia, C.R.C. Characterizing the Physical and Chemical Mass Transport of Dissolved Salts in Layered Oil Sands Waste Undergoing Reclamation. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2017. [Google Scholar]
- Kessler, S.; Barbour, S.L.; van Rees, K.C.J.; Dobchuk, B. Salinization of soil over saline-sodic overburden from the oil sands in Alberta. Can. J. Soil Sci. 2010, 90, 637–647. [Google Scholar] [CrossRef]
- Li, X.; Ma, B.; Drozdowski, B.; Salifu, F.; Chang, S.X. Effects of capping strategy and water balance on salt movement in oil sands reclamation soils. Water 2020, 12, 512. [Google Scholar] [CrossRef] [Green Version]
- Dobchuk, B.S.; Shurniak, R.E.; Barbour, S.L.; O’Kane, M.A.; Song, Q. Long-term monitoring and modelling of a reclaimed watershed cover on oil sands tailings. Int. J. Min. Reclam. Environ. 2013, 27, 180–201. [Google Scholar] [CrossRef]
- Renault, S.; Paton, E.; Nilsson, G.; Zwiazek, J.J.; MacKinnon, M.D. Responses of Boreal Plants to High Salinity Oil Sands Tailings Water. J. Environ. Qual. 1999, 28, 1957–1962. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Fleurial, K.; Sherr, I.; Vassov, R.; Zwiazek, J.J. Growth and physiological responses of tree seedlings to oil sands non-segregated tailings. Environ. Pollut. 2020, 259, 113945. [Google Scholar] [CrossRef] [PubMed]
- Government of Alberta. Alberta Tier 1 Soil and Groundwater Remediation Guidelines; Government of Alberta, Land Policy Branch: Edmonton, AB, Canada, 2019. Available online: https://open.alberta.ca/dataset/842becf6-dc0c-4cc7-8b29-e3f383133ddc/resource/a5cd84a6-5675-4e5b-94b8-0a36887c588b/download/albertatier1guidelines-jan10-2019.pdf (accessed on 17 June 2021).
- Renault, S.; Qualizza, C.; MacKinnon, M. Suitability of altai wildrye (Elymus angustus) and slender wheatgrass (Agropyron trachycaulum) for initial reclamation of saline composite tailings of oil sands. Environ. Pollut. 2004, 128, 339–349. [Google Scholar] [CrossRef]
- Boldt-Burisch, K.; Naeth, M.A.; Schneider, U.; Schenider, B.; Hüttl, R.F. Plant growth and arbuscular mycorrhizae development in oil sands processing by-products. Sci. Total Environ. 2018, 621, 30–39. [Google Scholar] [CrossRef]
- Collins, C.E.V.; Foght, J.M.; Siddique, T. Co-occurrence of methanogenesis and N2 fixation in oil sands tailings. Sci. Total Environ. 2016, 565, 306–312. [Google Scholar] [CrossRef]
- Warren, L.A.; Kendra, K.E.; Brady, A.L.; Slater, G.F. Sulfur biogeochemistry of an oil sands composite tailings deposit. Front. Microbiol. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, P.M.; Coy, D.L.; Salloum, M.J.; Dudas, M.J. Methanogenic potential of tailings samples from oil sands extraction plants. Can. J. Microbiol. 2002, 48, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Risacher, F.F.; Morris, P.K.; Arriaga, D.; Goad, C.; Colenbrander Nelson, T.; Slater, G.F.; Warren, L.A. The interplay of methane and ammonia as key oxygen consuming constituents in early stage development of Base Mine Lake, the first demonstration oil sands pit lake. Appl. Geochem. 2018, 93, 49–59. [Google Scholar] [CrossRef]
- Arriaga, D.; Colenbrander Nelson, T.; Risacher, F.F.; Morris, P.K.; Goad, C.; Slater, G.F.; Warren, L.A. The co-importance of physical mixing and biogeochemical consumption in controlling water cap oxygen levels in Base Mine Lake. Appl. Geochem. 2019, 111, 104442. [Google Scholar] [CrossRef]
- Stasik, S.; Wendt-Potthoff, K. Vertical gradients in carbon flow and methane production in a sulfate-rich oil sands tailings pond. Water Res. 2016, 106, 223–231. [Google Scholar] [CrossRef]
- Stasik, S.; Loick, N.; Knöller, K.; Weisener, C.; Wendt-Potthoff, K. Understanding biogeochemical gradients of sulfur, iron and carbon in an oil sands tailings pond. Chem. Geol. 2014, 382, 44–53. [Google Scholar] [CrossRef]
- Mori, J.F.; Chen, L.-X.; Jessen, G.L.; Rudderham, S.G.; McBeth, J.M.; Lindsay, M.B.J.; Slater, G.F.; Banfield, J.F.; Warren, L.A. Putative mixotrophic nitrifying-denitrifying gammaproteobacterial implicated in nitrogen cycling within the ammonia/oxygen transition zone of an oil sands pit lake. Front. Microbiol. 2019, 10, 2435. [Google Scholar] [CrossRef]
- Chen, L.X.; Méheust, R.; Crits-Christoph, A.; McMahon, K.D.; Colenbrander Nelson, T.; Slater, G.F.; Warren, L.A.; Banfield, J.F. Large freshwater phages with the potential to augment aerobic methane oxidation. Nat. Microbiol. 2020, 5, 1504–1515. [Google Scholar] [CrossRef]
- Herman, D.C.; Fedorak, P.M.; Mackinnon, M.D.; Costerton, J.W. Biodegradation of naphthenic acids by microbial populations indigenous to oil sands tailings. Can. J. Microbiol. 1994, 40, 467–477. [Google Scholar] [CrossRef]
- Siddique, T.; Mohamad Shahimin, M.F.; Zamir, S.; Semple, K.; Li, C.; Foght, J.M. Long-term incubation reveals methanogenic biodegradation of C5 and C6 iso-alkanes in oil sands tailings. Environ. Sci. Technol. 2015, 49, 14732–14739. [Google Scholar] [CrossRef]
- Saidi-Mehrabad, A.; He, Z.; Tamas, I.; Sharp, C.E.; Brady, A.L.; Rochman, F.F.; Bodrossy, L.; Abell, G.C.J.; Penner, T.; Dong, X.; et al. Methanotrophic bacteria in oil sands tailings ponds of northern Alberta. ISME J. 2013, 7, 908–921. [Google Scholar] [CrossRef] [Green Version]
- Stasik, S.; Wick, L.Y.; Wendt-Potthoff, K. Anaerobic BTEX degradation in oil sands tailings ponds: Impact of labile organic carbon and sulfate-reducing bacteria. Chemosphere 2015, 138, 133–139. [Google Scholar] [CrossRef]
- Rochman, F.F.; Sheremet, A.; Tamas, I.; Saidi-Mehrabad, A.; Kim, J.J.; Dong, X.; Sensen, C.W.; Gieg, L.M.; Dunfield, P.F. Benzene and naphthalene degrading bacterial communities in an oil sands tailings pond. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Mohamad Shahimin, M.F.; Siddique, T. Sequential biodegradation of complex naphtha hydrocarbons under methanogenic conditions in two different oil sands tailings. Environ. Pollut. 2017, 221, 398–406. [Google Scholar] [CrossRef]
- Mohamad Shahimin, M.F.; Siddique, T. Methanogenic biodegradation of paraffinic solvent hydrocarbons in two different oil sands tailings. Sci. Total Environ. 2017, 583, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lee, K.; Ma, B.; Asiedu, E.; Ulrich, A.C. Indigenous microorganisms residing in oil sands tailings biodegrade residual bitumen. Chemosphere 2018, 209, 551–559. [Google Scholar] [CrossRef]
- Clemente, J.S.; MacKinnon, M.D.; Fedorak, P.M. Aerobic biodegradation of two commercial naphthenic acids preparations. Environ. Sci. Technol. 2004, 38, 1009–1016. [Google Scholar] [CrossRef]
- Toor, N.S.; Franz, E.D.; Fedorak, P.M.; MacKinnon, M.D.; Liber, K. Degradation and aquatic toxicity of naphthenic acids in oil sands process-affected waters using simulated wetlands. Chemosphere 2013, 90, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lee, K.; Ulrich, A.C. Model naphthenic acids removals by microalgae and Base Mine Lake cap water microbial inoculum. Chemosphere 2019, 234, 796–805. [Google Scholar] [CrossRef]
- Quesnel, D.M.; Bhaskar, I.M.; Gieg, L.M.; Chua, G. Naphthenic acid biodegradation by the unicellular alga Dunaliella tertiolecta. Chemosphere 2011, 84, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.M.; Asiedu, E.; Balaberda, A.; Ulrich, A.U. Oil sands process affected water sourced Trichoderma harzianum demonstrates capacity for mycoremediation of naphthenic acid fraction compounds. Chemosphere 2020, 258, 127281. [Google Scholar] [CrossRef]
- Keiluweit, M.; Fendorf, S. Texture-dependent anaerobic microsites constrain soil carbon oxidation rates. In Proceedings of the EGA General Assembly, Vienna, Austria, 17–22 April 2016. [Google Scholar]
- Neira, J.; Ortiz, M.; Morales, L.; Acevedo, E. Oxygen diffusion in soils: Understanding the factors and processes needed for modeling. Chil. J. Agric. Res. 2015, 75, 35–44. [Google Scholar] [CrossRef]
- Bornstein, J.; Hedstrom, W.E.; Scott, F.R. Oxygen Diffusion Rate Relationships under Three Soil Conditions; Life Sciences and Agriculture Experiment Station, University of Maine: Orono, ME, USA, 1980; Volume 98, pp. 1–14. [Google Scholar]
- Clothier, L.N.; Gieg, L.M. Anaerobic biodegradation of surrogate naphthenic acids. Water Res. 2016, 90, 156–166. [Google Scholar] [CrossRef]
- Gunawan, Y.; Nemati, M.; Dalai, A. Biodegradation of a surrogate naphthenic acid under denitrifying conditions. Water Res. 2014, 51, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Rudderham, S.B. Geomicrobiology and Geochemistry of Fluid Fine Tailings in an Oil Sands End Pit Lake. Master’s Thesis, Department of Geological Sciences, University of Saskatchewan, Saskatoon, SK, Canada, 2019. [Google Scholar]
- Bordenave, S.; Ramos, E.; Lin, S.; Voordouw, G.; Gieg, L.; Guo, C.; Wells, S. Use of Calcium Sulfate to Accelerate Densification while Reducing Greenhouse Gas Emissions from Oil Sands Tailings Ponds. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 16–18 June 2009. [Google Scholar]
- Ramos-Padrón, E.; Bordenave, S.; Lin, S.; Bhaskar, I.M.; Dong, X.; Sensen, C.W.; Fournier, J.; Vourdouw, G.; Gieg, L.M. Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond. Environ. Sci. Technol. 2011, 45, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.F.; Semple, K.; Foght, J. Anaerobic alkane biodegradation by cultures enriched from oil sands tailings ponds involves multiple species capable of fumarate addition. FEMS Microbiol. Ecol. 2015, 91, fiv042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Walshe, G.; Chi Fru, E.; Ciborowski, J.J.H.; Weisener, C.G. Microcosm assessment of the biogeochemical development of sulfur and oxygen in oil sands fluid fine tailings. Appl. Geochem. 2013, 37, 1–11. [Google Scholar] [CrossRef]
- Siddique, T.; Semple, K.; Li, C.; Foght, J.M. Methanogenic biodegradation of iso-alkanes and cycloalkanes during long-term incubation with oil sands tailings. Environ. Pollut. 2020, 258, 113768. [Google Scholar] [CrossRef]
- Clark, M.G.; Drewitt, G.B.; Carey, S.K. Energy and carbon fluxes from an oil sands pit lake. Sci. Total Environ. 2021, 752, 141966. [Google Scholar] [CrossRef]
- Clark, M.G.; Humphreys, E.R.; Carey, S.K. The initial three years of carbon dioxide exchange between the atmosphere and a reclaimed oil sands wetland. Ecol. Eng. 2019, 17, 667–682. [Google Scholar] [CrossRef]
- Clark, M.G.; Humphreys, E.R.; Carey, S.K. Low methane emissions from a boreal wetland constructed on oil sands mine tailings. Biogeosciences 2020, 135, 116–126. [Google Scholar] [CrossRef] [Green Version]
- McCullough, C.D.; Schultze, M.; Vandenberg, J. Realizing beneficial end uses from abandoned pit lakes. Minerals 2020, 10, 133. [Google Scholar] [CrossRef] [Green Version]
- Siddique, T.; Kuznetsov, P.; Kuznetsova, A.; Li, C.; Young, R.; Arocena, J.M.; Foght, J.M. Microbially-accelerated consolidation of oil sands tailings. Pathway II: Solid phase biogeochemistry. Front. Microbiol. 2014, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Canada’s Oil Sands Innovation Alliance (COSIA). 2019 Water Mining Research Report; Canada’s Oil Sands Innovation Alliance: Calgary, AB, Canada, 2020. [Google Scholar]
- Lindsay, M.B.J.; Vessey, C.J.; Robertson, J.M. Mineralogy and geochemistry of oil sands froth treatment tailings: Implications for acid generation and metal(loid) release. Appl. Geochem. 2019, 102, 186–196. [Google Scholar] [CrossRef]
- Kuznetsov, P.; Kuznetsova, A.; Foght, J.M.; Siddique, T. Oil sands thickened froth treatment tailings exhibit acid rock drainage potential during evaporative drying. Sci. Total Environ. 2015, 505, 1–10. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Kuznetsov, P.; Foght, J.M.; Siddique, T. Trace metal mobilization from oil sands froth treatment thickened tailings exhibiting acid rock drainage. Sci. Total Environ. 2016, 571, 699–710. [Google Scholar] [CrossRef]
- Vedoy, D.R.J.; Soares, J.B.P. Water-soluble polymers for oil sands tailing treatment: A review. Can. J. Chem. Eng. 2015, 93, 888–904. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Gumfekar, S.P.; Dixon, D.V.; Silva, M.A.; Soares, J.B.P. Enhanced dewatering of oil sands tailings by a novel water-soluble cationic polymer. Sep. Purif. Technol. 2021, 260, 118183. [Google Scholar] [CrossRef]
- Soares, J.B.P.; Motta, F.L. Using polymer reaction engineering principles to help the environment: The case of the Canadian oil sands tailings. J. Braz. Chem. Soc. 2019, 30, 426–435. [Google Scholar] [CrossRef]
- Hripko, R.; Vajihinejad, V.; Motta, F.L.; Soares, J.B.P. Enhanced flocculation of oil sands mature fine tailings using hydrophobically modified polyacrylamide copolymers. Glob. Chall. 2018, 2, 1700135. [Google Scholar] [CrossRef]
- Amoako, K.A. Geotechnical Behavior of Two Novel Polymer Treatments of Oil Sands Fine Tailings. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2020. [Google Scholar]
- Lipp, D.; Kozakiewicz, J. Acrylamide polymers. In Kirk-Othmer Encyclopedia of Chemical Technology; Kroschwitz, J.I., Ed.; Wiley: Toronto, Canada, 1991; pp. 266–287. [Google Scholar]
- Haveroen, M.E.; MacKinnon, M.D.; Fedorak, P.M. Polyacrylamide added as a nitrogen source stimulates methanogenesis in consortia from various wastewaters. Water Res. 2005, 29, 3333–3341. [Google Scholar] [CrossRef]
- Xu, Y.; Hamza, H. Thickening and disposal of oil sand tailings. Min. Eng. 2003, 55, 33–39. [Google Scholar]
- Long, J.; Li, H.; Xu, Z.; Masliyah, J.H. Role of colloidal interactions in oil sand tailings treatment. AIChE J. 2006, 52, 371–383. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef] [PubMed]
- Kay-Shoemake, J.L.; Watwood, M.E.; Lentz, R.D.; Sojka, E.R. Polyacrylamide as an organic nitrogen source for soil microorganisms with potential effects on inorganic soil nitrogen in agricultural soil. Soil Biol. Biochem. 1998, 30, 1045–1052. [Google Scholar] [CrossRef]
- Bao, M.; Chen, Q.; Li, Y.; Jiang, G. Biodegradation of partially hydrolyzed polyacrylamide by bacteria isolated from production water after polymer flooding in an oil field. J. Hazard. Mater. 2010, 184, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Lin, K.; Cai, W. Microbial degradation of polyacrylamide by aerobic granules. Environ. Technol. 2012, 33, 1049–1054. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Gao, Y.; Chen, D.; Yang, M. Cleavage of the main carbon chain backbone of high molecular weight polyacrylamide by aerobic and anaerobic biological treatment. Chemosphere 2017, 189, 277–283. [Google Scholar] [CrossRef]
- Song, T.; Li, S.; Ding, W.; Li, H.; Bao, M.; Li, Y. Biodegradation of hydrolyzed polyacrylamide by the combined expanded granular sludge bed reactor-aerobic biofilm reactor biosystem and key microorganisms involved in this bioprocess. Bioresour. Technol. 2018, 263, 153–162. [Google Scholar] [CrossRef]
- Berdugo-Clavijo, C.; Sen, A.; Seyyedi, M.; Quintero, H.; O’Neil, B.; Gieg, L.M. High temperature utilization of PAM and HPAM by microbial communities enriched from oilfield produced water and activated sludge. AMB Express 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Dai, X.; Luo, F.; Yi, J.; He, Q.; Dong, B. Biodegradation of polyacrylamide by anaerobic digestion under mesophilic condition and its performance in actual dewatered sludge system. Bioresour. Technol. 2014, 153, 55–61. [Google Scholar] [CrossRef]
- Dai, X.; Luo, F.; Zhang, D.; Dai, L.; Chen, Y.; Dong, B. Waste-activated sludge fermentation for polyacrylamide biodegradation improved by anaerobic hydrolysis and key microorganisms involved in biological polyacrylamide removal. Sci. Rep. 2015, 5, 11675. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, J.F.; Li, C.Y.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Anaerobic biodegradation of partially hydrolyzed polyacrylamide in long-term methanogenic enrichment cultures from production water of oil reservoirs. Biodegradation 2018, 29, 233–243. [Google Scholar] [CrossRef]
- Nakamiya, K.; Kinoshita, S. Isolation of polyacrylamide-degrading bacteira. J. Ferment. Bioeng. 1995, 80, 418–420. [Google Scholar] [CrossRef]
- Joshi, S.; Abed, A. Biodegradation of polyacrylamide and its derivatives. Environ. Process. 2017, 4, 463–476. [Google Scholar] [CrossRef]
- Grula, M.M.; Huang, M.; Sewell, G. Interactions of certain polyacrylamides with soil bacteria. Soil Sci. 1994, 158, 291–300. [Google Scholar] [CrossRef]
- Guezennec, A.G.; Michel, C.; Bru, K.; Touze, S.; Desroche, N.; Mnif, I.; Motelica-Heino, M. Transfer and degradation of polyacrylamide-based flocculants in hydrosystems: A review. Environ. Sci. Pollut. Res. 2015, 22, 6390–6406. [Google Scholar] [CrossRef] [Green Version]
- Xiong, B.; Dettam Loss, R.; Shields, D.; Pawlik, R.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Seiber, J.N.; Miller, G.C. Acrylamide release resulting from sunlight irradiation of aqueous polyacrylamide/iron mixtures. J. Agric. Food Chem. 2008, 56, 2773–2779. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some aspects of the properties and degradation of polyacrylamides. Chem. Rev. 2002, 102, 3067–3083. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Purswani, P.; Pawlik, T.; Samineni, L.; Karpyn, Z.T.; Zydney, A.L.; Kumar, M. Mechanical degradation of polyacrylamide at ultra high deformation rates during hydraulic fracturing. Environ. Sci. Water Res. Technol. 2020, 6, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.M.; Al-Maamari, R.S.; Al-Hashmi, A.S.; Zaitoun, A.; Al-Sharji, H. In-situ rheology and mechanical degradation of EOR polyacrylamide solutions under moderate shear rates. J. Petrol. Sci. Eng. 2014, 115, 57–65. [Google Scholar] [CrossRef]
- Neelakantan, R.; Vaezi, G.F.; Sanders, R.S. Effects of shear on the yield stress and aggregate struction of flocculant-dosed, concentrated kaolinite suspensions. Miner. Eng. 2018, 123, 95–103. [Google Scholar] [CrossRef]
- Karami, H.R.; Rahimi, M.; Ovaysi, S. Degradation of drag reducing polymers in aqueous solutions. Korean J. Chem. Eng. 2018, 35, 34–43. [Google Scholar] [CrossRef]
- MacKinnon, M.D. Development of the tailings pond at Syncrude’s oil sands plant: 1978–1987. AOSTRA J. Res. 1989, 5, 109–133. [Google Scholar]
- Uranta, K.G.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the effectiveness of polyacrylamide (PAM) application in hydrocarbon reservoirs at different operational conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Zhao, L.; Du, G.; Li, N.; Xu, K.; Yang, H. Investigation of the degradation and stability of acrylamide-based polymers in acid solution: Functional monomer modified polyacrylamide. Petroleum 2016, 2, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Gröllmann, U.; Schnabel, W. Free radical-induced oxidative degradation of polyacrylamide in aqueous solution. Polym. Degrad. Stab. 1982, 4, 203–212. [Google Scholar] [CrossRef]
- Xiong, B.; Miller, Z.; Roman-White, S.; Tasker, T.; Farina, B.; Piechowicz, B.; Burgos, W.D.; Joshi, P.; Zhu, L.; Gorski, C.A.; et al. Chemical Degradation of Polyacrylamide during Hydraulic Fracturing. Environ. Sci. Technol. 2018, 52, 327–336. [Google Scholar] [CrossRef]
- Seright, R.S.; Skjevrak, I. Effect of dissolved iron and oxygen on stability of hydrolyzed polyacrylamide polymers. SPE J. 2015, 20, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, E.M.; Carey, S.K.; Humphreys, E.R.; Clark, M.G.; Drewitt, G.B. Multi-year water balance assessment of a newly constructed wetland, Fort McMurray, Alberta. Hydrol. Process. 2016, 30, 2739–2753. [Google Scholar] [CrossRef]
- Sutton, O.F.; Price, J.S. Soil moisture dynamics modelling of a reclaimed upland in the early post-construction period. Sci. Total Environ. 2020, 718, 134628. [Google Scholar] [CrossRef]
- Spennato, H.M.; Ketcherson, S.J.; Mendoza, C.A.; Carey, S.K. Water table dynamics in a constructed wetland, Fort McMurray, Alberta. Hydrol. Process. 2018, 32, 3824–3826. [Google Scholar] [CrossRef]
- Kabwe, L.K.; Scott, J.D.; Beier, N.A.; Wilson, G.W.; Jeeravipoolvarn, S. Environmental implications of end pit lakes at oil sand mines in Alberta, Canada. Environ. Geotech. 2019, 6, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Abolfazlzadehdoshanbehbazari, M.; Birks, S.J.; Moncur, M.C.; Ulrich, A.C. Fate and transport of oil sand process-affected water into the underlying clay till: A field study. J. Contam. Hydrol. 2013, 151, 83–92. [Google Scholar] [CrossRef]
- Simhayov, R.B.; Price, J.S.; Smeaton, C.M.; Parsons, C.; Rezanezhad, F.; Van Cappellen, P. Solute pools in Nikanotee Fen watershed in the Athabasca oil sands region. Environ. Pollut. 2017, 225, 150–162. [Google Scholar] [CrossRef]
- Vaughan, D.J. Mineral surfaces: An overview. In Mineral Surfaces; Vaughan, D., Patrick, R.A.D., Eds.; Chapman & Hill: London, UK, 1995; pp. 1–16. [Google Scholar]
- Kessel, E.D.; Ketchenson, S.J.; Price, J.S. The distribution and migration of sodium from a reclaimed upland to a constructed fen peatland in a post-mined oil sands landscape. Sci. Total Environ. 2018, 630, 1553–1564. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; MacKinnon, M. Surfactants in Athabasca Oil Sands Slurry Conditioning, Flotation Recovery, and Tailings Processes; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Fennell, J.; Arciszewski, T.J. Current knowledge of seepage from oil sands tailings ponds and its environmental influence in northeastern Alberta. Sci. Total Environ. 2019, 686, 968–985. [Google Scholar] [CrossRef]
- Janfada, A.; Headley, J.V.; Peru, K.M.; Barbour, S.L. A laboratory evaluation of the sorption of oil sands naphthenic acids on organic rich soils. J. Environ. Sci. Health A 2006, 41, 985–997. [Google Scholar] [CrossRef]
- Oiffer, A.A.L.; Barker, J.F.; Gervais, F.M.; Mayer, K.U.; Ptacek, C.J.; Rudolph, D.L. A detailed field-based evaluation of naphthenic acid mobility in groundwater. J. Contam. Hydrol. 2009, 108, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Gervais, F.J.M. Fate and Transport of Naphthenic Acids in a Glacial Aquifer. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2004. [Google Scholar]
- Gervais, F.; Barker, J. Fate and Transport of Naphthenic Acids in Groundwater. In Proceedings of the 4th International Conference of Groundwater Quality: Bringing Groundwater Quality Research to the Watershed Scale, Waterloo, ON, Canada, 19–22 July 2004. [Google Scholar]
- Bowman, D.T.; Warren, L.A.; McCarry, B.E.; Slater, G.F. Profiling of individual naphthenic acids at compositie tailings reclamation fen by comprehensive two-dimensional gas chromatography-mass spectrometry. Sci. Total Environ. 2019, 649, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, M. Quick clay. In Encyclopedia of Natural Hazards; Encyclopedia of Earth Sciences Series; Bobrowsky, P.T., Ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Guida, M.; Mattei, M.; Melluso, G.; Pagano, G.; Meriç, S. Daphnia magna and Selenastrum capricornutum in evaluating the toxicity of alum and polymer used in coagulation-flocculation. Fresenius Environ. Bull. 2004, 13, 1244–1247. [Google Scholar]
- Gostomski, F. The toxicity of aluminum to aquatic species in the US. Environ. Geochem. Health 1990, 12, 51–54. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).