Molecular and Carbon Isotopic Variation during Canister Degassing of Terrestrial Shale: A Case Study from Xiahuayuan Formation in the Xuanhua Basin, North China
Abstract
:1. Introduction
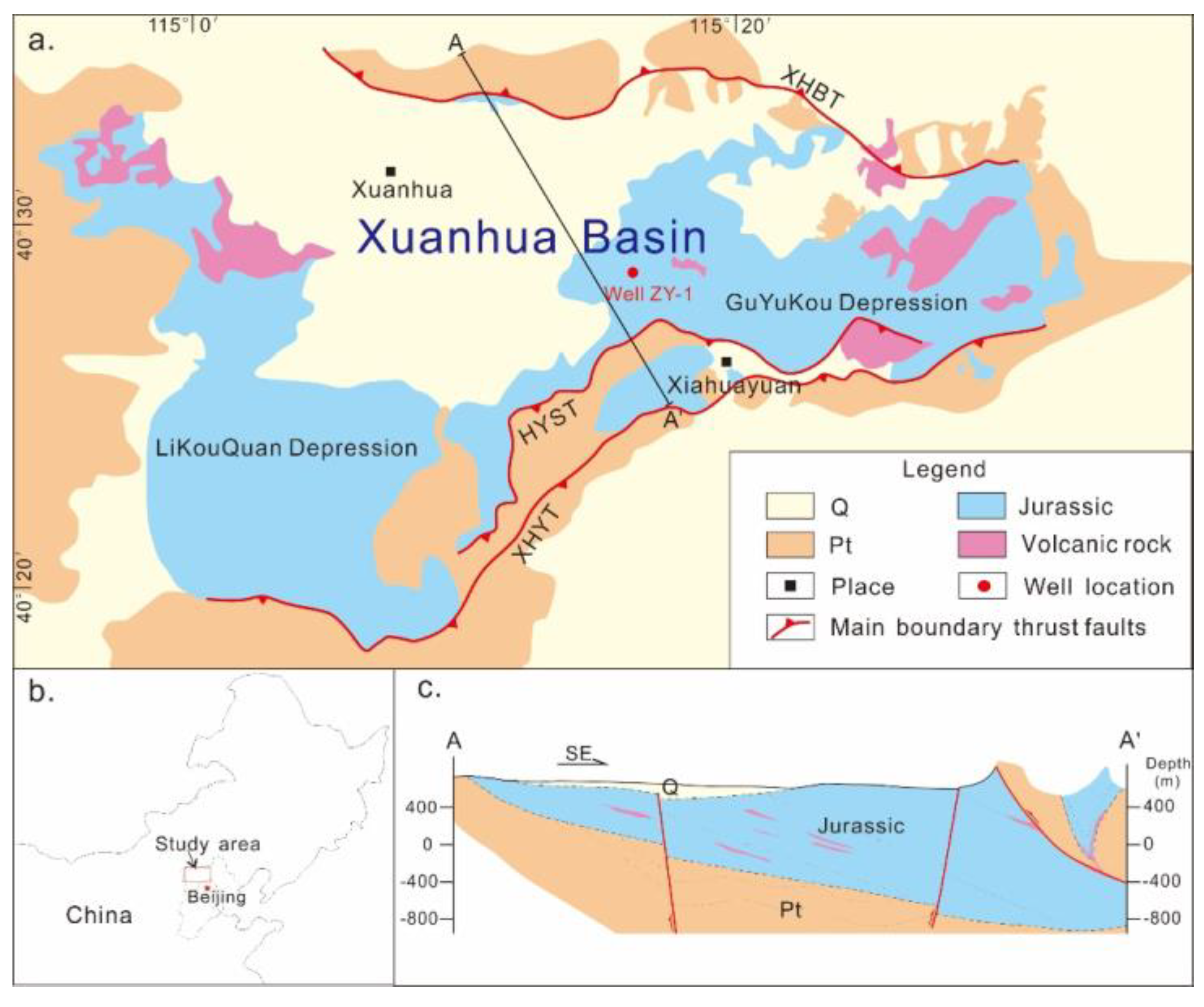
2. Geological Setting
3. Samples and Methods
3.1. Samples
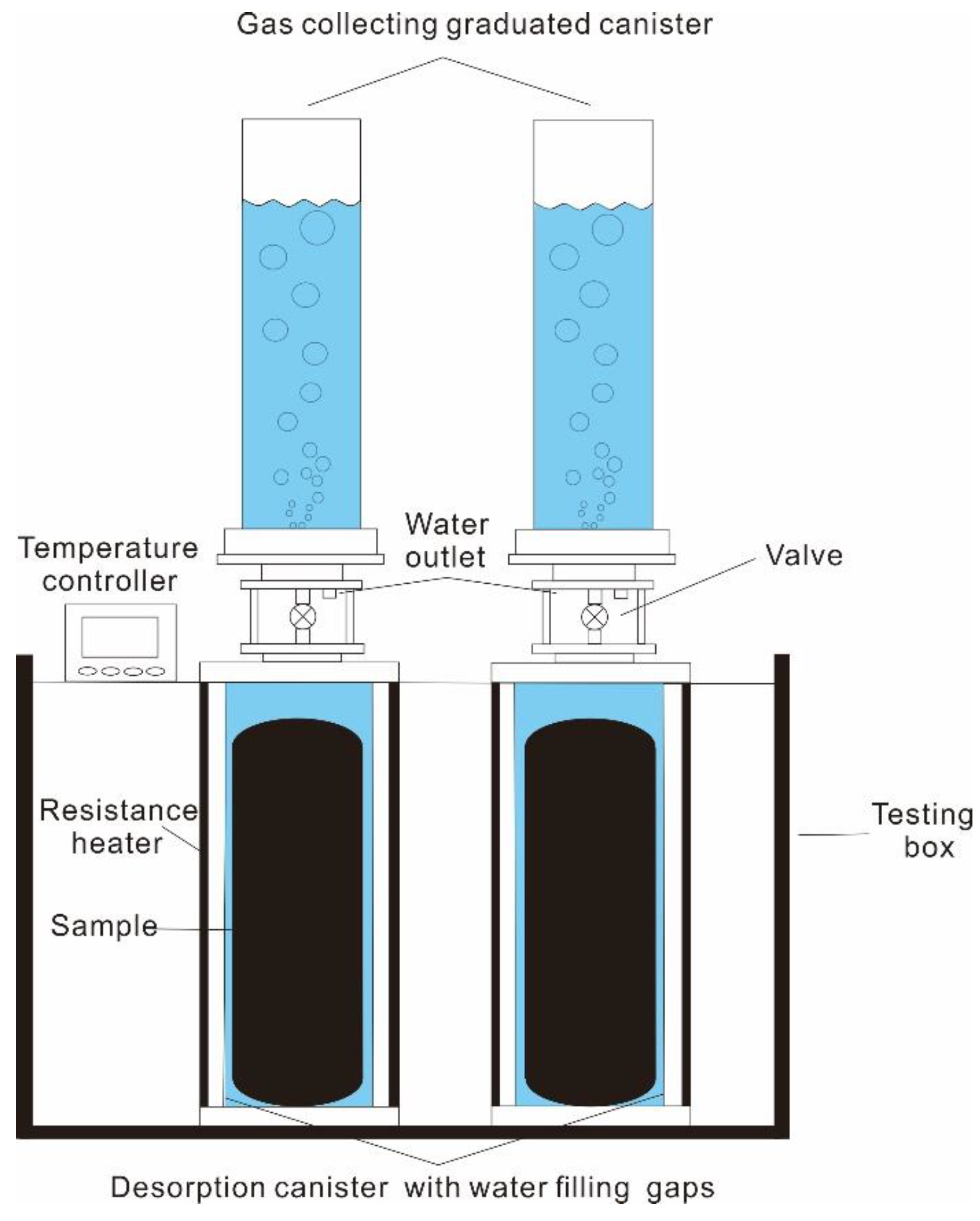
3.2. Field Degassing Experiment
3.3. Gas Geochemical Analysis
3.4. TOC Content Analysis
3.5. Petrophysical Parameter Measurement
3.6. Low-Pressure CO2 Adsorption
4. Results
4.1. Molecular Composition of Shale Gas
4.2. Stable Carbon Isotopes
4.3. Total Organic Carbon (TOC) and Petrophysical Parameters
4.4. Micropore Characteristics
5. Discussion
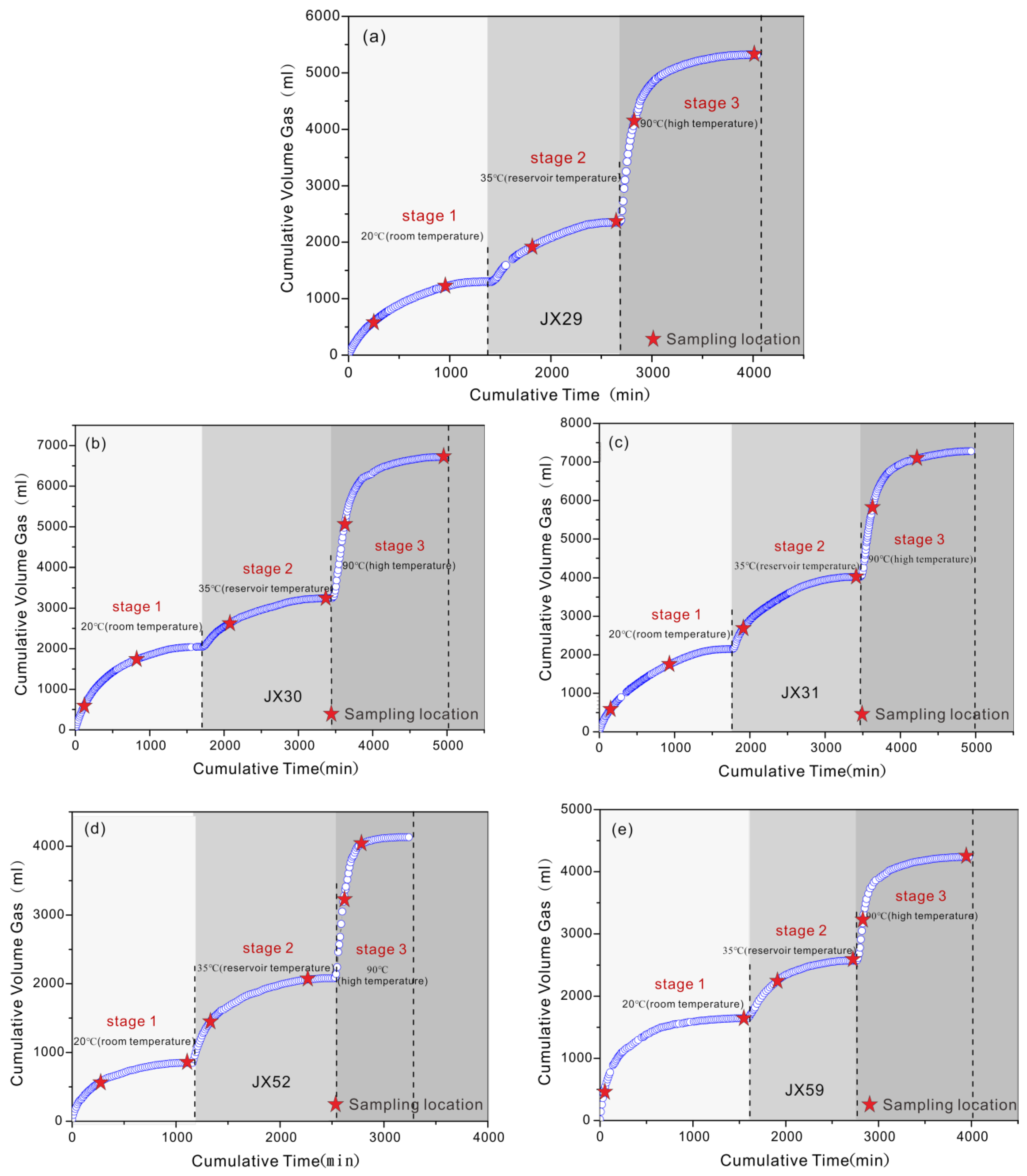
5.1. Gas Component Variation during Canister Degassing
5.2. Carbon Isotope Variation during Canister Degassing
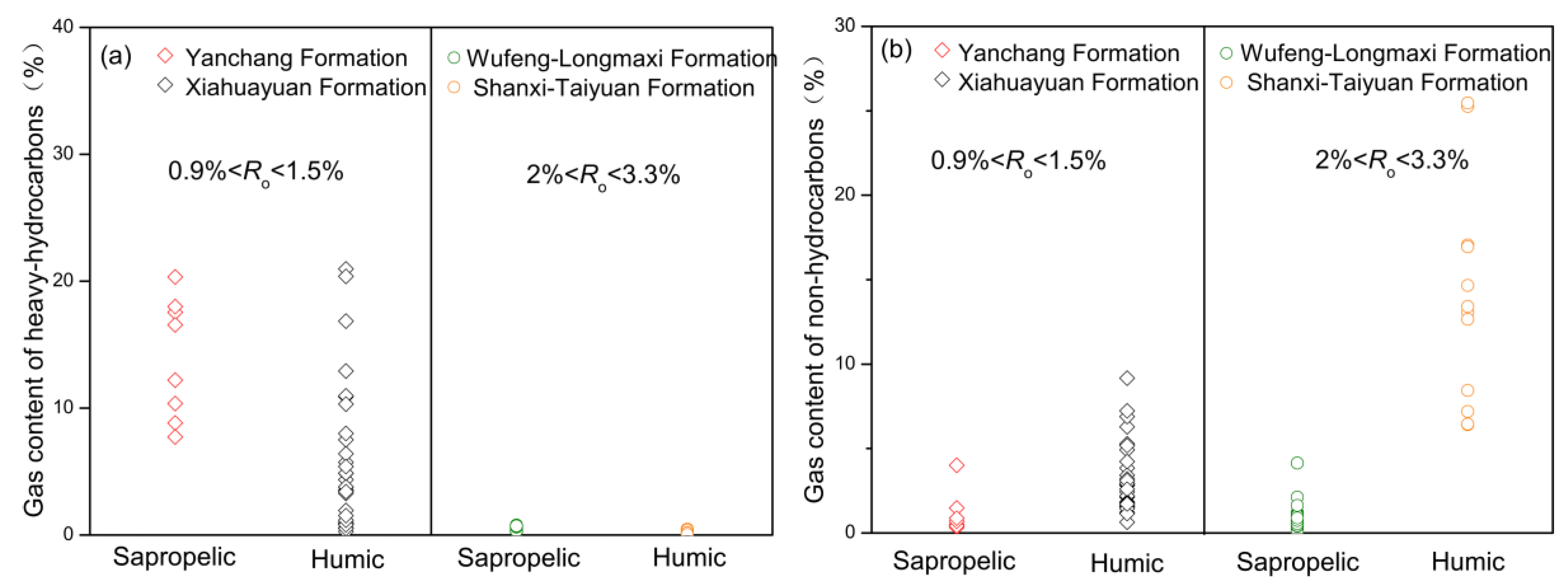
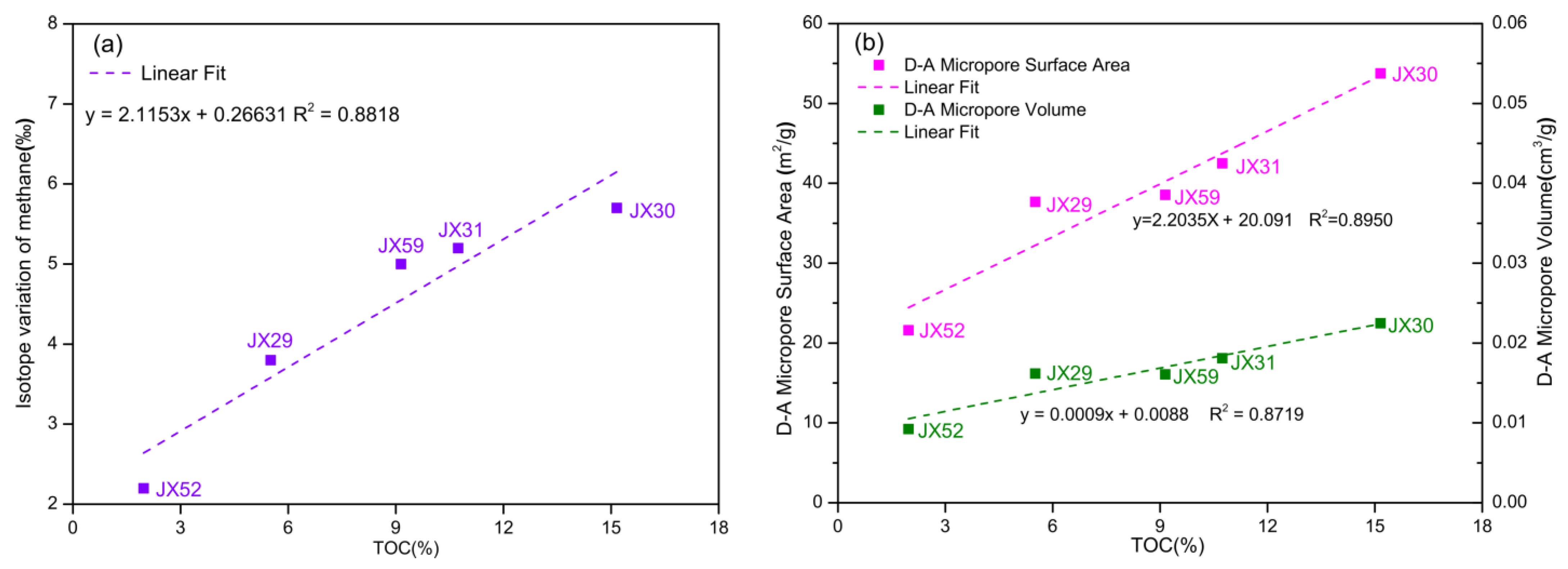
5.3. Influence of Petrophysics on Carbon Isotope Variation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoell, M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim. Cosmochim. Acta 1980, 44, 649–661. [Google Scholar] [CrossRef]
- Berner, U.; Faber, E. Maturity related mixing model for methane, ethane and propane, based on carbon isotopes. Org. Geochem. 1988, 13, 67–72. [Google Scholar] [CrossRef]
- Dai, J.X.; Li, J.; Luo, X.; Zhang, W.Z.; Hu, G.Y.; Ma, C.H.; Guo, J.M.; Ge, S.G. Alkane carbon isotopic composition and gas source in giant gas fields of Ordos Basin. Acta Pet. Sin. 2005, 26, 18–26, (In Chinese with English abstract). [Google Scholar]
- Dong, Z.; Zhang, J.C.; Tang, X.; Liu, G.X.; Dang, W.; Liu, Y.; Tao, J.; Su, Z.X. Origin and diffusion of the over-mature transitional natural gas in multiple lithologic reservoirs: A case study of Carboniferous-Permian strata in the southeastern margin of Ordos Basin. Int. J. Coal Geol. 2020, 219, 103380. [Google Scholar] [CrossRef]
- Gunter, B.D.; Gleason, J.D. Isotope fractionation during gas chromatographic separations. J. Chromatogr. Sci. 1971, 9, 191–192. [Google Scholar] [CrossRef]
- Smith, J.W.; Rigby, D.; Gould, K.W.; Hart, G.; Hargraves, A.J. An isotopic study of hydrocarbon generation processes. Org. Geochem. 1985, 8, 341–347. [Google Scholar] [CrossRef]
- Krooss, B.M. Experimental investigation of the molecular migration of C1-C6 hydrocarbons: Kinetics of hydrocarbon release from source rocks. Org. Geochem. 1988, 13, 513–523. [Google Scholar] [CrossRef]
- Zhang, M.J.; Tang, Q.Y.; Cao, C.H.; Lv, Z.G.; Zhang, T.W.; Zhang, D.K.; Li, Z.P.; Du, L. Molecular and carbon isotopic variation in 3.5 years shale gas production from Longmaxi Formation in Sichuan Basin, China. Mar. Pet. Geol. 2018, 89, 27–37. [Google Scholar] [CrossRef]
- Ma, Y.; Zhong, N.N.; Yao, L.P.; Huang, H.P.; Larter, S.; Jiao, W.W. Shale gas desorption behavior and carbon isotopic variations of gases from canister desorption of two sets of gas shales in south China. Mar. Pet. Geol. 2020, 113, 104127. [Google Scholar] [CrossRef]
- Colombo, U.; Gazzarrini, F.; Sironi, G.; Gonfiantini, R.; Tongiorgi, E. Carbon isotope composition of individual hydrocarbons from Italian natural gases. Nature 1965, 205, 1303–1304. [Google Scholar] [CrossRef]
- Hoşgörmez, H.; Yalçın, M.N.; Cramer, B.; Gerling, P.; Mann, U. Isotopic and molecular composition of coal-bed gas in the Amasra region (Zonguldak basin-western Black Sea). Org. Geochem. 2002, 33, 1429–1439. [Google Scholar] [CrossRef]
- Richter, F.M.; Mendybaev, R.A.; Christensen, J.N.; Hutcheon, I.D.; Williams, R.W.; Sturchio, N.C.; Beloso, A.D., Jr. Kinetic isotopic fractionation during diffusion of ionic species in water. Geochim. Cosmochim. Acta 2006, 70, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.K.; Golding, S.D.; Baublys, K.A.; Esterle, J.S. Stable isotopic and molecular composition of desorbed coal seam gases from the Walloon Subgroup, eastern Surat Basin, Australia. Int. J. Coal Geol. 2014, 122, 21–36. [Google Scholar] [CrossRef]
- Gould, K.W.; Hargraves, A.J.; Smith, J.W. Variation in the composition of seam gases issuing from coal. Bull. Proc. Aust. Inst. Min. Metall. 1987, 292, 69–73. [Google Scholar]
- Strąpoć, D.; Schimmelmann, A.; Mastalerz, M. Carbon isotopic fractionation of CH4 and CO2 during canister desorption of coal. Org. Geochem. 2006, 37, 152–164. [Google Scholar] [CrossRef]
- Strąpoć, D.; Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Hasenmueller, N.R. Geochemical constraints on the origin and volume of gas in the New Albany Shale (Devonian-Mississippian), eastern Illinois Basin. AAPG Bull. 2010, 94, 1713–1740. [Google Scholar] [CrossRef]
- Niemann, M. Stable Isotope Systematics of Coalbed Methane. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2006. [Google Scholar]
- Leythaeuser, D.; Schaefer, R.G.; Yükler, A. Diffusion of light hydrocarbons through near-surface rocks. Nature 1980, 284, 522–525. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Pernaton, E. Isotopically light methane in natural gas: Bacterial imprint or diffusive fractionation? Chem. Geol. 1997, 142, 193–200. [Google Scholar] [CrossRef]
- Zhang, T.; Krooss, B.M. Experimental investigation on the carbon isotope fractionation of methane during gas migration by diffusion through sedimentary rocks at elevated temperature and pressure. Geochim. Cosmochim. Acta 2001, 65, 2723–2742. [Google Scholar] [CrossRef]
- Schloemer, S.; Krooss, B.M. Molecular transport of methane, ethane and nitrogen and the influence of diffusion on the chemical and isotopic composition of natural gas accumulations. Geofluids 2004, 4, 81–108. [Google Scholar] [CrossRef]
- Xia, X.; Tang, Y. Isotope fractionation of methane during natural gas flow with coupled diffusion and adsorption/desorption. Geochim. Cosmochim. Acta 2012, 77, 489–503. [Google Scholar]
- Fuex, A.N. Experimental evidence against an appreciable isotopic fractionation of methane during migration. Phys. Chem. Earth 1980, 12, 725–732. [Google Scholar] [CrossRef]
- Bacsik, Z.; Lopes, J.N.C.; Gomes, M.F.C.; Jancso, G.; Mink, J.; Padua, A.A.H. Solubility isotope effects in aqueous solutions of methane. J. Chem. Phys. 2002, 116, 10816–10824. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Larson, T.E.; Smyth, R.C. Carbon isotope effects of methane transport through Anahuac Shale—A core gas study. J. Geochem. Explor. 2015, 148, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Rahn, T.; Eiler, J.M. Experimental constrains on the fractionation of 13C/12C and 18O/16O ratios due to adsorption of CO2 on mineral substrates at conditions relevant to the surface of Mars. Geochim. Cosmochim. Acta 2001, 65, 839–846. [Google Scholar] [CrossRef]
- Su, X.B.; Chen, R.; Lin, X.Y.; Guo, S.Q. Adsorption characteristic curves of 13CH4 and 12CH4 on coal and their application. J. China. Coal Soc. 2007, 32, 539–543, (In Chinese with English abstract). [Google Scholar]
- Wang, X.F.; Li, X.F.; Wang, X.Z.; Shi, B.G.; Luo, X.R.; Zhang, L.X.; Lei, Y.H.; Jiang, C.F.; Meng, Q. Carbon isotopic fractionation by desorption of shale gases. Mar. Pet. Geol. 2015, 60, 79–86. [Google Scholar] [CrossRef]
- Li, W.B.; Lu, S.F.; Li, J.Q.; Wei, Y.B.; Feng, W.J.; Zhang, P.F.; Song, Z.J. Geochemical modeling of carbon isotope fractionation during methane transport in tight sedimentary rocks. Chem. Geol. 2021, 566, 120033. [Google Scholar] [CrossRef]
- Krooss, B.M. Diffusion of C1 to C5 hydrocarbons in water-saturated sedimentary rocks. Erdöl Kohle-Erdgas 1986, 39, 399–402. [Google Scholar]
- Li, J.; Liu, Z.L.; Li, Z.S.; Hu, G.Y.; Yan, Q.T.; Shan, X.Q.; Ma, C.H.; Wang, C.Y. Experiment investigation on the carbon isotope and composition fractionation of methane during gas migration by diffusion. Nat. Gas Geosci. 2003, 14, 463–468. (In Chinese) [Google Scholar]
- Zumberge, J.E.; Ferworn, K.A.; Curtis, J.B. Gas character anomalies found in highly productive shale gas wells. Geochim. Cosmochim. Acta 2009, 73, A1539. [Google Scholar]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lewis, R.; Hemingway, J.; Grau, J.; Rylander, E.; Pirie, I. Determination of formation organic carbon content using a new neutron-induced gamma ray spectroscopy service that directly measures carbon. Soc. Petrol. Eng. 2013, 230, 265–269. [Google Scholar]
- Bustin, A.; Bustin, R. Total gas-in-place, gas composition and reservoir properties of coal of the Mannville coal measures, Central Alberta. Int. J. Coal Geol. 2016, 153, 127–143. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, J.C.; Chang, M.H.; Wang, S.; Chen, L.; Yu, H.C. Gas-generation potential of shales in small and medium-sized basins: A case study from the Xuanhua Basin, North China. Aust. J. Earth Sci. 2020, 67, 411–424. [Google Scholar] [CrossRef]
- Song, H.L. Basic characteristics and dynamics of Yanshan intraplate orogenic belt. Earth Sci. Front. 1999, 6, 309–310. [Google Scholar]
- Cope, T.D.; Shultz, M.R.; Graham, S.A. Detrital record of Mesozoic shortening in the Yanshan belt, NE China: Testing structural interpretations with basin analysis. Basin Res. 2007, 2, 1–20. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Liu, X.M.; Wang, Y.; Liu, X.W. Rapid exhumation of basement rocks along the northern margin of the North China craton in the early Jurassic: Evidence from the Xiabancheng Basin, Yanshan Tectonic Belt. Basin Res. 2012, 24, 544–558. [Google Scholar] [CrossRef]
- Liu, S.F.; Su, S.; Zhang, G.W. Early Mesozoic basin development in Noth China: Indications of cratonic deformation. J. Asian Earth Sci. 2013, 62, 221–236. [Google Scholar] [CrossRef]
- Liu, S.F.; Lin, C.F.; Liu, X.B.; Zhuang, Q.T. Syn-tectonic sedimentation and its linkage to fold-thrusting in the region of Zhangjiakou, North Hebei, China. Sci. China Earth Sci. 2018, 61, 49–78. [Google Scholar] [CrossRef]
- Zorin, Y.A.; Belichenko, V.G.; Turutanov, E.K.; Mazukabzov, A.M.; Sklyarov, E.V.; Mordvinova, V.V. The East Siberia Transect. Int. Geol. Rev. 1995, 37, 154–175. [Google Scholar] [CrossRef]
- Liu, S.F.; Li, Z.; Zhang, J.F. Mesozoic basin evolution and tectonic mechanism in Yanshan, China. Sci. China Ser. D Earth Sci. 2004, 47, 24–38. [Google Scholar] [CrossRef]
- Zhang, J.C.; Tang, Y.; Tang, X.; Song, Z.L.; Zhai, D.J.; Xue, H. Adsorbed Gas Content Measuring Instrument and Its Testing Method. U.S. Patent 8578754, 12 November 2013. [Google Scholar]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Diamond, W.P.; Schatzel, S.J. Measuring the gas content of coal: A review. Int. J. Coal Geol. 1998, 35, 311–331. [Google Scholar] [CrossRef]
- Dang, W.; Zhang, J.C.; Tang, X.; Wei, X.L.; Li, Z.M.; Wang, C.H.; Chen, Q.; Liu, C. Investigation of gas content of organic-rich shale: A case study from Lower Permian shale in southern North China Basin, central China. Geosci. Front. 2018, 9, 559–575. [Google Scholar] [CrossRef]
- Ma, Y.; Zhong, N.N.; Li, D.H.; Pan, Z.J.; Cheng, L.J.; Liu, K.Y. Organic matter/clay mineral intergranular pores in the lower Cambrian Lujiaping shale in the north-eastern part of the upper Yangtze area, China: A possible microscopic mechanism for gas preservation. Int. J. Coal Geol. 2015, 137, 38–54. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Dai, J.X.; Zhu, G.Y.; Zhang, S.C.; Zhang, D.J.; Su, J.; Tao, X.W.; Liao, F.R.; Wu, W.; Gong, D.Y.; et al. Stable hydrogen and carbon isotopic ratios of coal-derived and oil-derived gases: A case study in the Tarim basin, NW China. Int. J. Coal Geol. 2013, 116–117, 302–313. [Google Scholar] [CrossRef]
- Jenden, P.D.; Titley, P.A.; Worden, R.H. Enrichment of nitrogen and 13C of methane in natural gases from the Khuff Formation, Saudi Arabia, caused by thermochemical sulfate reduction. Org. Geochem. 2015, 82, 54–68. [Google Scholar] [CrossRef]
- Determination of Total Organic Carbon in Sedimentary Rock; GB/T 19145-2003; Standardization Administration of the People’s Republic of China: Shenzhen, China, 2003. (In Chinese)
- Xu, H.; Tang, D.Z.; Zhao, J.L.; Li, S. A precise measurement method for shale porosity with low-field nuclear magnetic resonance: A case study of the Carboniferous-Permian strata in the Linxing area, eastern Ordos Basin, China. Fuel 2015, 143, 47–54. [Google Scholar] [CrossRef]
- Measurement of Helium Porosity and Pulse Decay Permeability of Shale; GB/T 34533-2017; Standardization Administration of the People’s Republic of China: Shenzhen, China, 2017. (In Chinese)
- Chinese Energy Industry Standard. Shale Scanning and Imagination Method Using X-ray Computed Tomography; NB/T 10122-2018; Standardization Administration of the People’s Republic of China: Shenzhen, China, 2018. (In Chinese)
- Cao, Z.; Jiang, H.; Zeng, J.H.; Saibi, H.; Lu, T.Z.; Xie, X.M.; Zhang, Y.C.; Zhou, G.G.; Wu, K.Y.; Guo, J.R. Nanoscale liquid hydrocarbon adsorption on clay minerals: A molecular dynamics simulation of shale oils. Chem. Eng. J. 2021, 420, 127578. [Google Scholar] [CrossRef]
- Cheng, A.L.; Huang, W.L. Selective adsorption of hydrocarbon gases on clays and organic matter. Org. Geochem. 2004, 35, 413–423. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.C.; Tang, X. Predicting the proportion of free and adsorbed gas by isotopic geochemical data: A case study from lower Permian shale in the Southern North China Basin (SNCB). Int. J. Coal Geol. 2016, 156, 25–35. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Gong, L.; Qu, Y.; Zhang, K.; Zhang, G.; Wang, S. The tight oil potential of the Lucaogou Formation from the southern Junggar Basin, China. Fuel 2018, 234, 858–871. [Google Scholar] [CrossRef]
- Hunt, J.M. Generation of gas and oil from coal and other terrestrial organic matter. Org. Geochem. 1991, 37, 673–680. [Google Scholar] [CrossRef]
- Cui, X.J.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Du, X.D.; Gu, M.; Hou, Z.K.; Liu, Z.J.; Wu, T.F. Experimental study on kinetics of adsorption of CO2 and CH4 in gas-bearing shale reservoirs. Energy Fuels 2019, 34, 12587–12600. [Google Scholar] [CrossRef]
- Dai, J.X.; Zou, C.N.; Dong, D.Z.; Ni, Y.Y.; Wu, W.; Gong, D.Y.; Wang, Y.M.; Huang, S.P.; Huang, J.L.; Fang, C.C.; et al. Geochemical characteristics of marine and terrestrial shale gas in China. Mar. Pet. Geol. 2016, 76, 444–463. [Google Scholar] [CrossRef]
- Stainforth, J.G. Practical kinetic modeling of petroleum generation and expulsion. Mar. Pet. Geol. 2009, 26, 552–572. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Shi, B.Q.; Fang, C.B. The isotopic compositions of molecular nitrogen: Implications on their origins in natural gas accumulations. Chem. Geol. 2000, 164, 321–330. [Google Scholar] [CrossRef]
- Whiticar, M.J. Stable isotope geochemistry of coals, humic kerogens and related natural gases. Int. J. Coal Geol. 1996, 32, 191–215. [Google Scholar] [CrossRef]
- Chanton, J.P. The effect of gas transport on the isotope signature of methane in wetlands. Org. Geochem. 2005, 36, 753–768. [Google Scholar] [CrossRef]
- Luo, S.Y.; Chen, X.H.; Liu, A.; Li, H.; Sun, C. Characteristics and geological significance of canister desorption gas from the Lower Cambrian Shuijingtuo Formation shale in Yichang area, Middle Yangtze region. Acta Petrol. Sin. 2019, 40, 941–955, (In Chinese with English abstract). [Google Scholar]
- Dai, J.X.; Gong, D.Y.; Ni, Y.Y.; Huang, S.P.; Wu, W. Stable carbon isotopes of coal-derived gases sourced from the Mesozoic coal measures in China. Org. Geochem. 2014, 74, 123–142. [Google Scholar] [CrossRef]
- Dai, J.X.; Zou, C.N.; Liao, S.M.; Dong, D.Z.; Ni, Y.Y.; Huang, W.H.; Gong, D.Y.; Huang, S.P.; Hu, G.Y. Geochemistry of the extremely high thermal maturity Longmaxi shale gas, southern Sichuan basin. Org. Geochem. 2014, 74, 3–12. [Google Scholar] [CrossRef]
- Bernard, B.B.; Brooks, J.M.; Sackett, W.M. Light hydrocarbons in recent Texas continental shelf and slope sediments. J. Geophys. 1978, 83, 4053–4061. [Google Scholar] [CrossRef] [Green Version]
- Whiticar, M.J. Carbon and hydrogen isotope systematic of microbial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Niemann, M.; Whiticar, M. Stable isotope systematics of coalbed gas during desorption and production. Geosciences 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.J.; Kang, Y.L.; Zhang, T.S.; You, L.J.; Li, X.C.; Chen, Z.X.; Wu, K.L.; Yang, B. Methane diffusion in shales with multiple pore sizes at supercritical conditions. Chem. Eng. J. 2018, 334, 1455–1465. [Google Scholar] [CrossRef]
- Nie, H.K.; Yang, Z.H.; Dang, W.; Chen, Q.; Li, P.; Wang, R.J. Study of shale gas release from freshly drilled core samples using a real-time canister monitoring technique: Release kinetics, influencing factors, and upscaling. Energy Fuels 2020, 34, 2916–2924. [Google Scholar] [CrossRef]
- Bustin, R.M.; Bustin, A.; Ross, D.; Chalmers, G.; Murthy, V.; Laxmi, C.; Cui, X. Shale gas opportunities and challenges. Search Discov. Artic. 2009, 40382, 20–23. [Google Scholar]
- Li, D.H.; Nie, H.K. A new method to calculate shale gas content based on gas reservoir characterization—A case study of Wells JY 1 and PY 1 in Sichuan Basin and its surrounding areas. Oil Gas Geol. 2019, 40, 1324–1332, (In Chinese with English abstract). [Google Scholar]

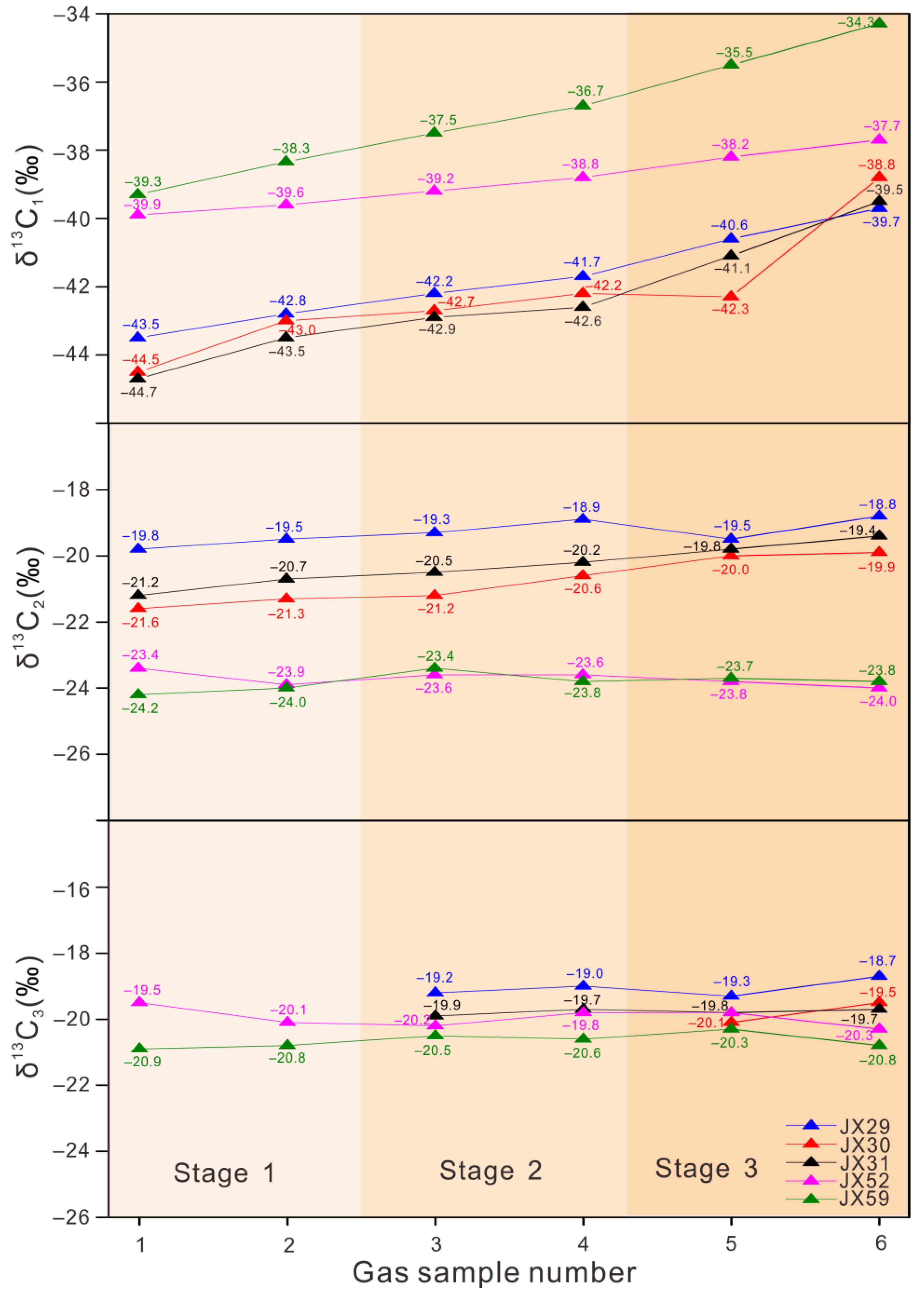
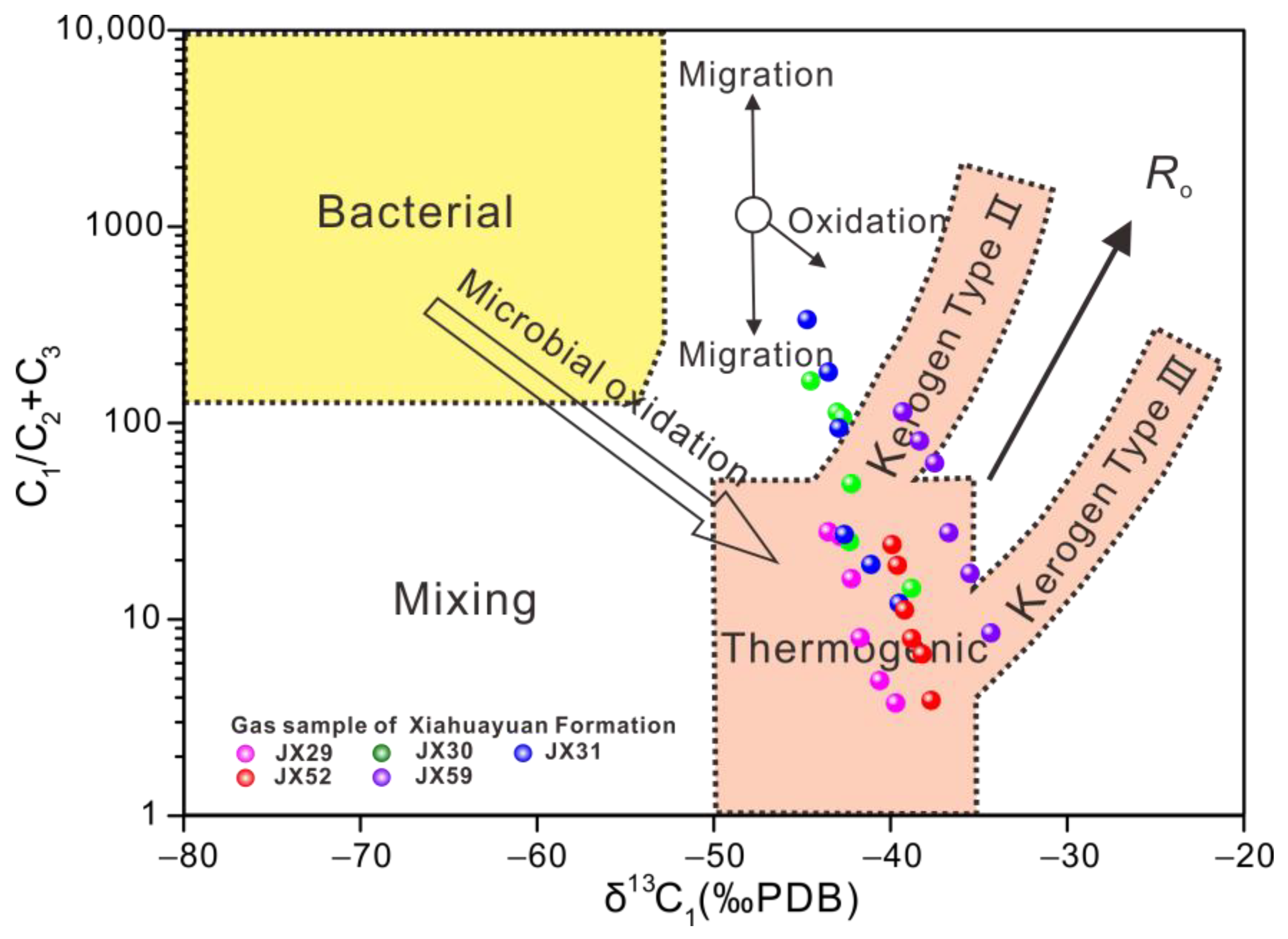
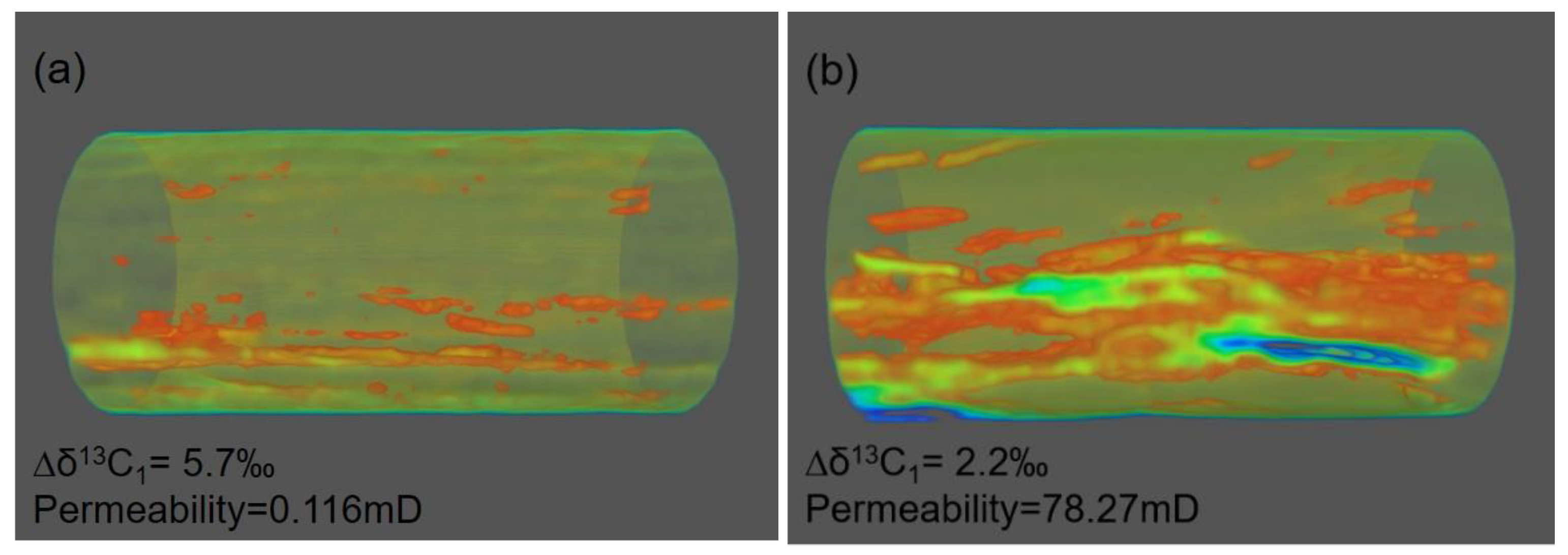
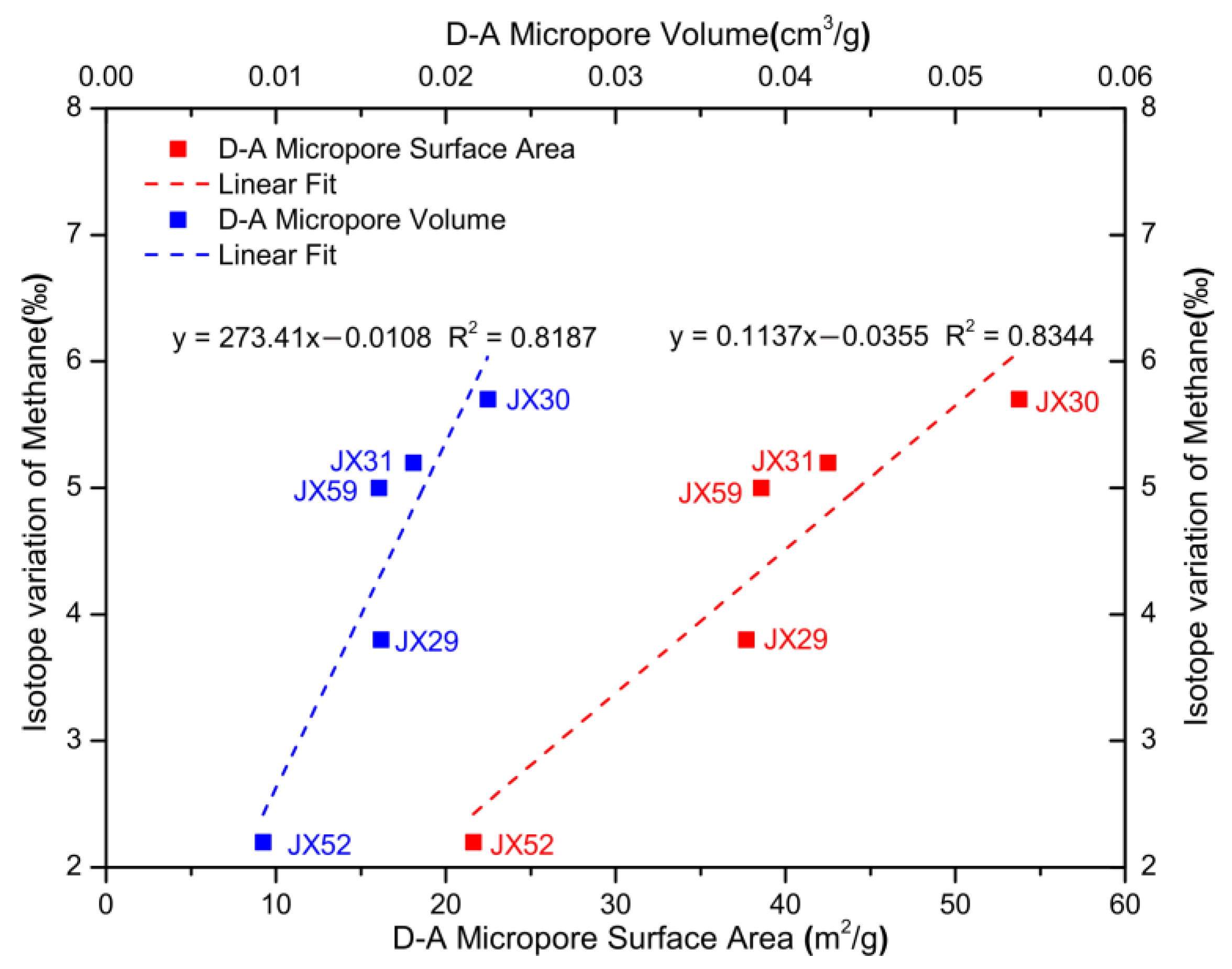
| Sample | Degassing Stage | Temperature (°C) | Gas Composition (%) | δ13C (‰) VPDB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H6 | CO2 | N2 | CH4 | C2H6 | C3H6 | |||
| JX29-1 | 1 | 20 | 91.43 | 3.21 | 0.08 | 2.36 | 2.92 | −43.5 | −19.8 | n.d. |
| JX29-2 | 1 | 20 | 93.64 | 3.46 | 0.09 | 1.79 | 1.03 | −42.8 | −19.5 | n.d. |
| JX29-3 | 2 | 35 | 92.16 | 5.19 | 0.53 | 1.44 | 0.67 | −42.2 | −19.3 | −19.2 |
| JX29-4 | 2 | 35 | 87.52 | 7.52 | 3.38 | 1.02 | 0.56 | −41.7 | −18.9 | −19.0 |
| JX29-5 | 3 | 90 | 81.94 | 10.37 | 6.48 | 0.76 | 0.45 | −40.6 | −19.5 | −19.3 |
| JX29-6 | 3 | 90 | 78.39 | 13.98 | 6.99 | 0.32 | 0.33 | −39.7 | −18.8 | −18.7 |
| JX30-1 | 1 | 20 | 93.15 | 0.57 | n.d. | 2.65 | 3.63 | −44.5 | −21.6 | n.d. |
| JX30-2 | 1 | 20 | 93.90 | 0.83 | n.d. | 1.91 | 3.36 | −43.0 | −21.3 | n.d. |
| JX30-3 | 2 | 35 | 95.26 | 0.89 | n.d. | 1.55 | 2.30 | −42.7 | −21.2 | n.d. |
| JX30-4 | 2 | 35 | 95.17 | 1.94 | n.d. | 0.69 | 2.21 | −42.2 | −20.6 | n.d. |
| JX30-5 | 3 | 90 | 93.80 | 2.71 | 1.06 | 0.66 | 1.78 | −42.3 | −20.0 | −20.1 |
| JX30-6 | 3 | 90 | 91.97 | 4.20 | 2.21 | 0.40 | 1.22 | −38.8 | −19.9 | −19.5 |
| JX31-1 | 1 | 20 | 90.56 | 0.27 | n.d. | 4.22 | 4.95 | −44.7 | −21.2 | n.d. |
| JX31-2 | 1 | 20 | 92.6 | 0.51 | n.d. | 3.74 | 3.14 | −43.5 | −20.7 | n.d. |
| JX31-3 | 2 | 35 | 94.08 | 0.78 | 0.22 | 2.52 | 2.41 | −42.9 | −20.5 | −19.9 |
| JX31-4 | 2 | 35 | 93.14 | 2.47 | 0.97 | 1.64 | 1.78 | −42.6 | −20.2 | −19.7 |
| JX31-5 | 3 | 90 | 92.22 | 3.52 | 1.33 | 1.21 | 1.73 | −41.1 | −19.8 | −19.8 |
| JX31-6 | 3 | 90 | 90.31 | 4.63 | 2.87 | 0.87 | 1.32 | −39.5 | −19.4 | −19.7 |
| JX52-1 | 1 | 20 | 91.03 | 3.26 | 0.54 | 2.47 | 2.7 | −39.9 | −23.4 | −19.5 |
| JX52-2 | 1 | 20 | 91.92 | 3.67 | 1.21 | 2.08 | 1.12 | −39.6 | −23.9 | −20.1 |
| JX52-3 | 2 | 35 | 89.09 | 5.89 | 2.1 | 2.25 | 0.67 | −39.2 | −23.6 | −20.2 |
| JX52-4 | 2 | 35 | 87.2 | 8.04 | 2.93 | 1.61 | 0.21 | −38.8 | −23.6 | −19.8 |
| JX52-5 | 3 | 90 | 85.63 | 9.47 | 3.45 | 1.44 | n.d. | −38.2 | −23.8 | −19.8 |
| JX52-6 | 3 | 90 | 78.48 | 15.47 | 4.91 | 1.15 | n.d. | −37.7 | −24.0 | −20.3 |
| JX59-1 | 1 | 20 | 91.96 | 0.59 | 0.22 | 3.89 | 3.35 | −39.3 | −24.2 | −20.9 |
| JX59-2 | 1 | 20 | 94.6 | 0.76 | 0.41 | 2.21 | 2.02 | −38.3 | −24.0 | −20.8 |
| JX59-3 | 2 | 35 | 95.37 | 0.99 | 0.54 | 1.78 | 1.33 | −37.5 | −23.4 | −20.5 |
| JX59-4 | 2 | 35 | 93.98 | 2.7 | 0.72 | 1.55 | 1.05 | −36.7 | −23.8 | −20.6 |
| JX59-5 | 3 | 90 | 92.82 | 3.34 | 2.05 | 1.23 | 0.57 | −35.5 | −23.7 | −20.3 |
| JX59-6 | 3 | 90 | 87.96 | 6.17 | 4.15 | 0.93 | 0.78 | −34.3 | −23.8 | −20.8 |
| Sample | Depth (m) | Desorbed Gas Content (m3/t) | TOC (wt.%) | Porosity (%) | Permeability (mD) | D-A Micropore Surface (m2/g) | D-A Micropore Volume (cm3/g) |
|---|---|---|---|---|---|---|---|
| JX29 | 1022.13 | 1.74 | 5.51 | / | / | 37.6878 | 0.016196 |
| JX30 | 1026.55 | 3.31 | 15.16 | 10.97 | 0.116 | 53.768 | 0.022476 |
| JX31 | 1028.50 | 3.03 | 10.74 | 11.5 | 5.54 | 42.5084 | 0.018089 |
| JX52 | 1117.09 | 1.95 | 1.97 | 6.81 | 78.27 | 21.6197 | 0.009241 |
| JX59 | 1120.95 | 2.84 | 9.14 | / | / | 38.5643 | 0.016072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Zhang, J.; Liu, J.; Liu, Y.; Dang, W.; Yu, H.; Cao, Z.; Wang, S.; Dong, Z. Molecular and Carbon Isotopic Variation during Canister Degassing of Terrestrial Shale: A Case Study from Xiahuayuan Formation in the Xuanhua Basin, North China. Minerals 2021, 11, 843. https://doi.org/10.3390/min11080843
Tao J, Zhang J, Liu J, Liu Y, Dang W, Yu H, Cao Z, Wang S, Dong Z. Molecular and Carbon Isotopic Variation during Canister Degassing of Terrestrial Shale: A Case Study from Xiahuayuan Formation in the Xuanhua Basin, North China. Minerals. 2021; 11(8):843. https://doi.org/10.3390/min11080843
Chicago/Turabian StyleTao, Jia, Jinchuan Zhang, Junlan Liu, Yang Liu, Wei Dang, Haicheng Yu, Zhe Cao, Sheng Wang, and Zhe Dong. 2021. "Molecular and Carbon Isotopic Variation during Canister Degassing of Terrestrial Shale: A Case Study from Xiahuayuan Formation in the Xuanhua Basin, North China" Minerals 11, no. 8: 843. https://doi.org/10.3390/min11080843
APA StyleTao, J., Zhang, J., Liu, J., Liu, Y., Dang, W., Yu, H., Cao, Z., Wang, S., & Dong, Z. (2021). Molecular and Carbon Isotopic Variation during Canister Degassing of Terrestrial Shale: A Case Study from Xiahuayuan Formation in the Xuanhua Basin, North China. Minerals, 11(8), 843. https://doi.org/10.3390/min11080843






