A Review of the Phyllosilicates in Gale Crater as Detected by the CheMin Instrument on the Mars Science Laboratory, Curiosity Rover
Abstract
:1. Introduction
2. Background
2.1. Orbital Phyllosilicate Detections on Mars
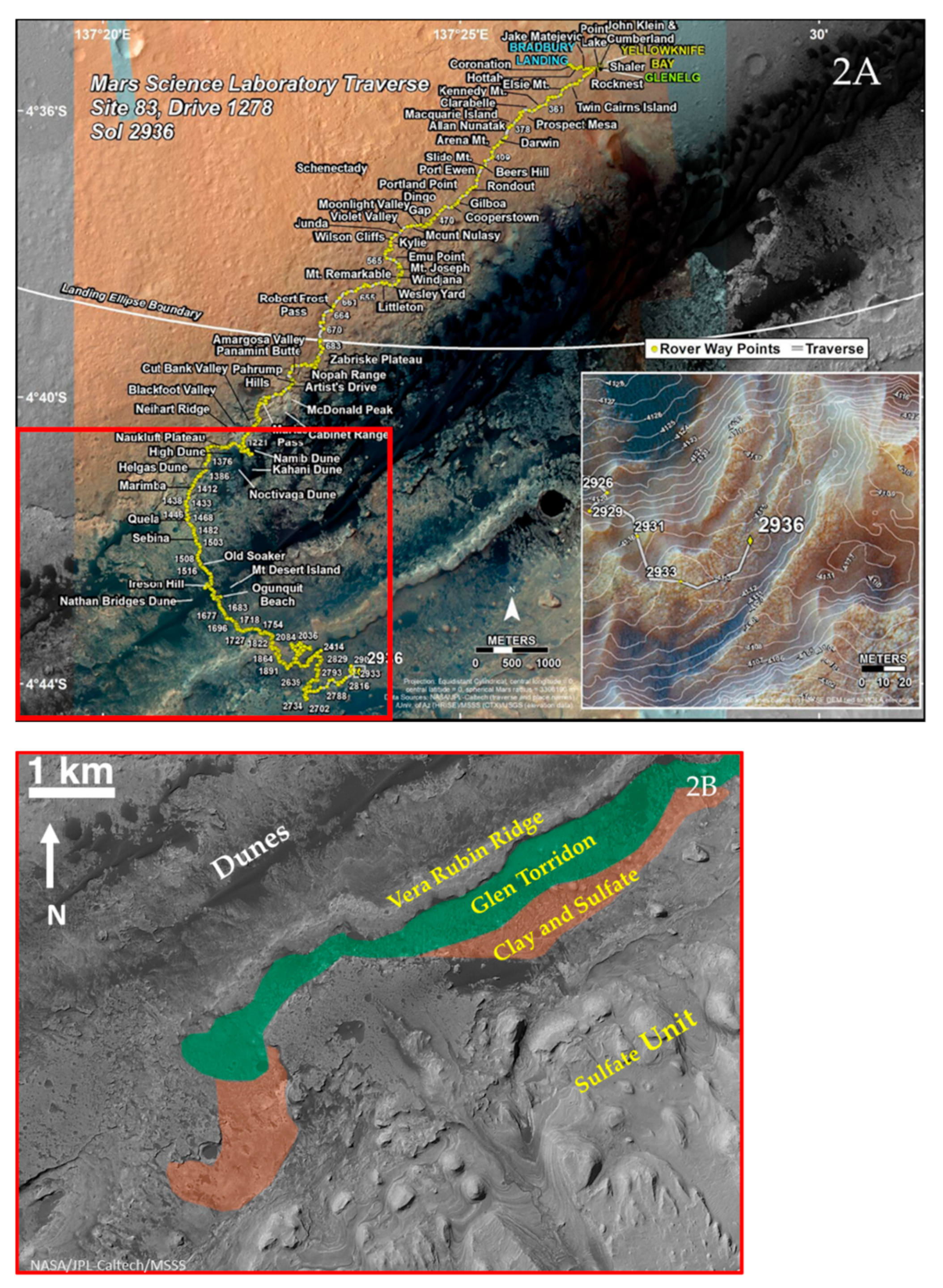
2.2. Stratigraphy from Curiosity’s Traverse

2.3. Habitability and Microbial Organisms
3. Materials and Methods
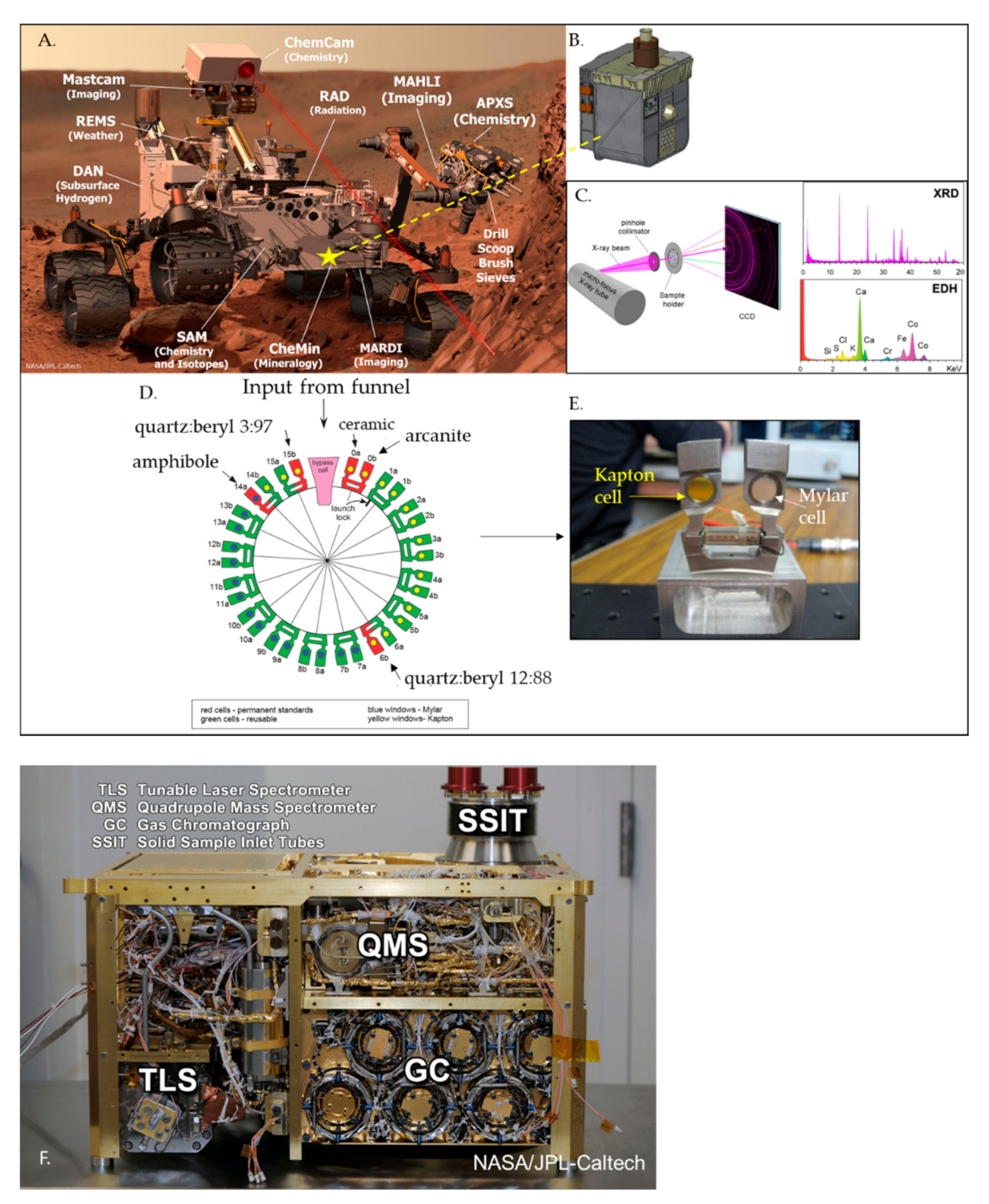
Phyllosilicate Detection Payload Instruments
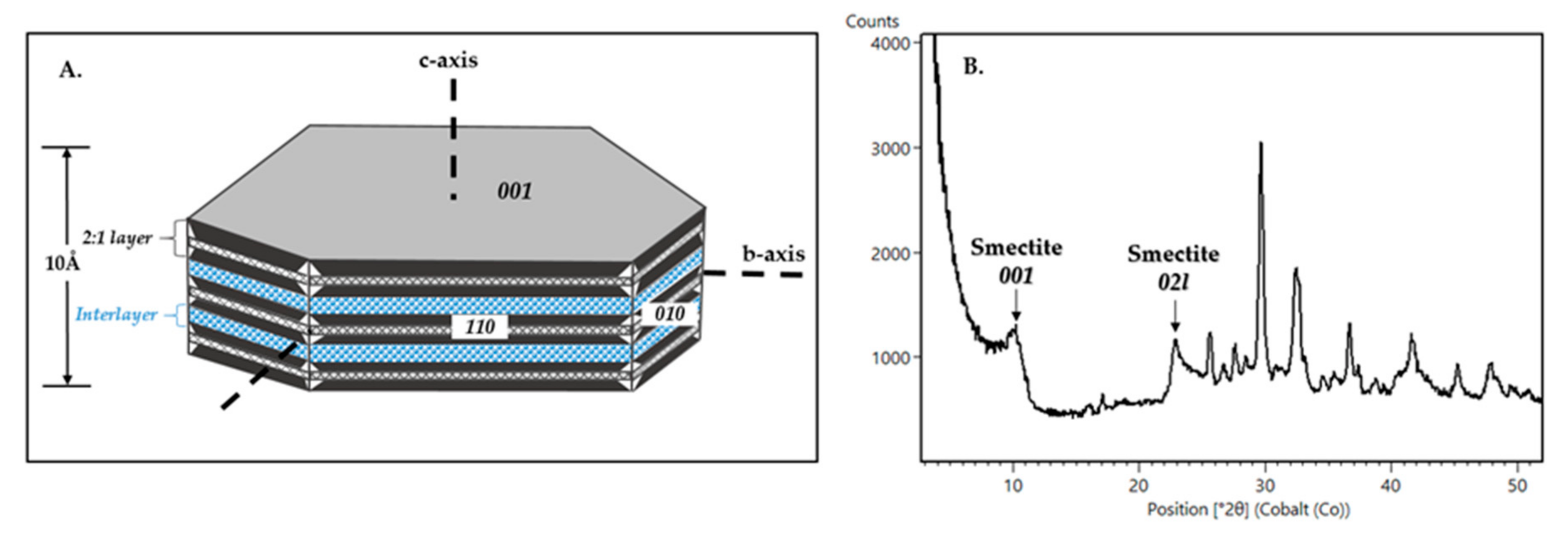
4. Limitations on Phyllosilicate Detection
5. Review of Phyllosilicate Detections by Curiosity
5.1. Phyllosilicate Results Grouped by Stratigraphy
5.1.1. Yellowknife Bay and Kimberly Formations in the Bradbury Group
5.1.2. Murray Formation in the Mount Sharp Group
5.1.3. Stimson Formation in the Siccar Point Group (Big Sky (BS), Greenhorn (GH), Lubango (LB), Okoruso (OK), Edinburgh (EB))
6. Discussion and Interpretations
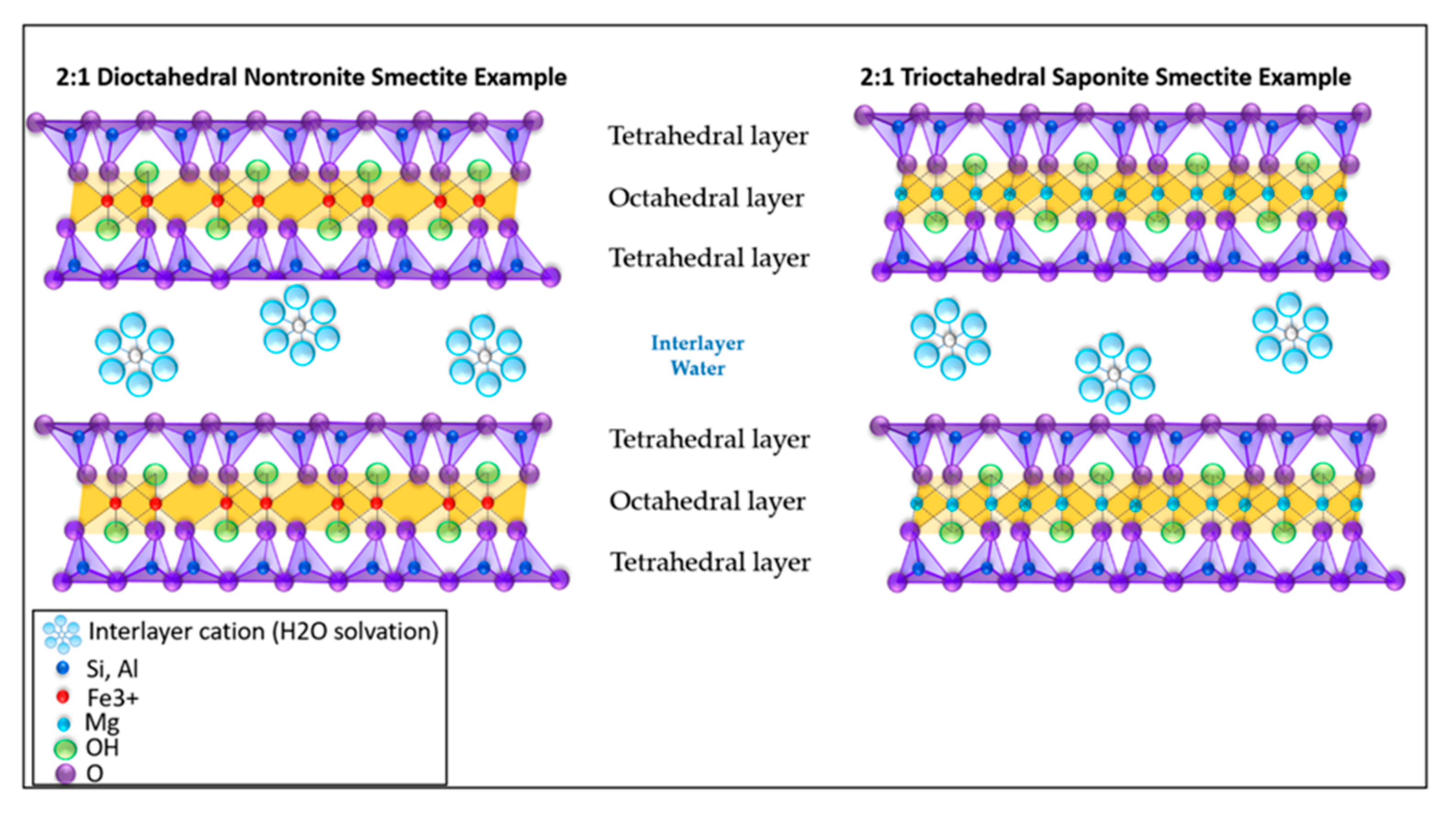
6.1. Smectitic Samples in Gale Crater
6.2. Non-Smectitic Phyllosilicate Samples in Gale Crater
6.3. Phyllosilicates, Formation and Organic Preservation
6.4. Global Perspective and Implications for Sample Return and Future Planetary Missions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Thomson, B.J.; Bridges, N.T.; Milliken, R.; Baldridge, A.; Hook, S.J.; Crowley, J.K.; Marion, G.K.; de Souza Filho, C.E.; Brown, A.J.; Weitz, C.M. Constraints on the origin and evolution of the layered mound in Gale Crater, Mars using Mars Reconnaissance Orbiter data. Icarus 2011, 214, 413–432. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Crisp, J.; Vasavada, A.R.; Anderson, R.C.; Baker, C.J.; Barry, R.; Blake, D.F.; Conrad, P.; Edgett, K.S.; Ferdowski, B. Mars Science Laboratory mission and science investigation. Space Sci. Rev. 2012, 170, 5–56. [Google Scholar] [CrossRef] [Green Version]
- Milliken, R.; Grotzinger, J.; Thomson, B. Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef] [Green Version]
- Grotzinger, J.P. Habitability, Taphonomy, and the Search for Organic Carbon on Mars: Introduction to Special Issue. Science 2014, 343, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Grotzinger, J.P.; Gupta, S.; Malin, M.C.; Rubin, D.M.; Schieber, J.; Siebach, K.; Sumner, D.Y.; Stack, K.M.; Vasavada, A.R.; Arvidson, R.E.; et al. Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science 2015, 350, aac7575. [Google Scholar] [CrossRef] [PubMed]
- Stack, K.M.; Grotzinger, J.P.; Lamb, M.P.; Gupta, S.; Rubin, D.M.; Kah, L.C.; Edgar, L.A.; Fey, D.M.; Hurowitz, J.A.; McBride, M. Evidence for plunging river plume deposits in the Pahrump Hills member of the Murray formation, Gale crater, Mars. Sedimentology 2019, 66, 1768–1802. [Google Scholar] [CrossRef] [Green Version]
- Fedo, C.; Grotzinger, J.; Gupta, S.; Banham, S.; Bennett, K.; Edgar, L.; Fox, V.; Fraeman, A.; House, C.; Lewis, K. Evidence for persistent, water-rich, lacustrine deposition preserved in the Murray Formation, Gale crater: A depositional system suitable for sustained habitability. In Proceedings of the LPI Contributions, Ninth International Conference on Mars, Pasadena, CA, USA, 22–25 July 2019; Volume 2089, p. 6308. [Google Scholar]
- Rivera-Hernández, F.; Sumner, D.Y.; Mangold, N.; Banham, S.G.; Edgett, K.S.; Fedo, C.M.; Gupta, S.; Gwizd, S.; Heydari, E.; Maurice, S. Grain size variations in the murray formation: Stratigraphic evidence for changing depositional environments in Gale crater, mars. J. Geophys. Res. Planets 2020, 125. [Google Scholar] [CrossRef] [Green Version]
- Edgar, L.A.; Fedo, C.M.; Gupta, S.; Banham, S.; Fraeman, A.A.; Grotzinger, J.P.; Stack, K.; Stein, N.; Bennett, K.; Rivera-Hernández, F. A lacustrine paleoenvironment recorded at Vera Rubin Ridge, Gale crater: Overview of the sedimentology and stratigraphy observed by the Mars Science Laboratory Curiosity Rover. J. Geophys. Res. Planets 2020, 125. [Google Scholar] [CrossRef] [Green Version]
- Vaniman, D.; Bish, D.; Ming, D.; Bristow, T.; Morris, R.; Blake, D.; Chipera, S.; Morrison, S.; Treiman, A.; Rampe, E. Mineralogy of a mudstone at Yellowknife Bay, Gale crater, Mars. Science 2014, 343. [Google Scholar] [CrossRef]
- Bristow, T.F.; Rampe, E.B.; Achilles, C.N.; Blake, D.F.; Chipera, S.J.; Craig, P.; Crisp, J.A.; Des Marais, D.J.; Downs, R.T.; Gellert, R.; et al. Clay mineral diversity and abundance in sedimentary rocks of Gale crater, Mars. Sci. Adv. 2018, 4, eaar3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, M.; Bristow, T.; Rampe, E.; Grotzinger, J.; Fox, V.; Bennett, K.; Bryk, A.; Yen, A.; Vasavada, A.; Vaniman, D. The Mineralogy and Sedimentary History of the Glen Torridon Region, Gale Crater, Mars. In Proceedings of the 52nd Lunar and Planetary Science Conference, held virtually, 15–19 March 2021; p. 1519. [Google Scholar]
- Ehlmann, B.L.; Mustard, J.F.; Fassett, C.I.; Schon, S.C.; Head, J.W., III; Des Marais, D.J.; Grant, J.A.; Murchie, S.L. Clay minerals in delta deposits and organic preservation potential on Mars. Nat. Geosci. 2008, 1, 355–358. [Google Scholar] [CrossRef]
- Summons, R.E.; Amend, J.P.; Bish, D.; Buick, R.; Cody, G.D.; Des Marais, D.J.; Dromart, G.; Eigenbrode, J.L.; Knoll, A.H.; Sumner, D.Y. Preservation of martian organic and environmental records: Final report of the Mars Biosignature Working Group. Astrobiology 2011, 11, 157–181. [Google Scholar] [CrossRef]
- Freissinet, C.; Glavin, D.; Mahaffy, P.R.; Miller, K.; Eigenbrode, J.; Summons, R.; Brunner, A.; Buch, A.; Szopa, C.; Archer, P., Jr. Organic molecules in the Sheepbed mudstone, Gale crater, Mars. J. Geophys. Res. Planets 2015, 120, 495–514. [Google Scholar] [CrossRef] [Green Version]
- Eigenbrode, J.L.; Summons, R.E.; Steele, A.; Freissinet, C.; Millan, M.; Navarro-González, R.; Sutter, B.; McAdam, A.C.; Franz, H.B.; Glavin, D.P. Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars. Science 2018, 360, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- Gil-Lozano, C.; Fairén, A.G.; Muñoz-Iglesias, V.; Fernández-Sampedro, M.; Prieto-Ballesteros, O.; Gago-Duport, L.; Losa-Adams, E.; Carrizo, D.; Bishop, J.L.; Fornaro, T.; et al. Constraining the preservation of organic compounds in Mars analog nontronites after exposure to acid in alkaline fluids. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Loizeau, D.; McKeown, N.K.; Saper, L.; Dyar, M.D.; De Marais, D.J.; Parente, M.; Murchie, S.L. What the ancient phyllosilicates at Mawrth Vallis can tell us about possible habitability on early Mars. Planet. Space Sci. 2013, 86, 130–149. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Langevin, Y.; Gendrin, A.; Gondet, B.; Poulet, F.; Berthé, M.; Soufflot, A.; Arvidson, R.; Mangold, N.; Mustard, J.; et al. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 2005, 307, 1576–1581. [Google Scholar] [CrossRef] [Green Version]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.; Ehlmann, B.; Milliken, R.; Grant, J.A.; Bibring, J.-P.; Poulet, F.; Bishop, J.; Dobrea, E.N. Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the Martian surface. Annu. Rev. Earth Planet. Sci. 2014, 42. [Google Scholar] [CrossRef] [Green Version]
- Wray, J.J.; Murchie, S.L.; Squyres, S.W.; Seelos, F.P.; Tornabene, L.L. Diverse aqueous environments on ancient Mars revealed in the southern highlands. Geology 2009, 37, 1043–1046. [Google Scholar] [CrossRef]
- Bristow, T.F.; Bish, D.L.; Vaniman, D.T.; Morris, R.V.; Blake, D.F.; Grotzinger, J.P.; Rampe, E.B.; Crisp, J.A.; Achilles, C.N.; Ming, D.W.; et al. The origin and implications of clay minerals from Yellowknife Bay, Gale crater, Mars. Am. Miner. 2015, 100, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Poulet, F.; Bibring, J.-P.; Mustard, J.F.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.E.; Gondet, B.; Gomez, C. Phyllosilicates on Mars and implications for early Martian climate. Nature 2005, 438, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Poulet, F.; Bibring, J.P.; Murchie, S. Detection of hydrated silicates in crustal outcrops in the northern plains of Mars. Science 2010, 328, 1682–1686. [Google Scholar] [CrossRef]
- Blake, D.; Vaniman, D.; Achilles, C.; Anderson, R.; Bish, D.; Bristow, T.; Chen, C.; Chipera, S.; Crisp, J.; Des Marais, D. Characterization and calibration of the CheMin mineralogical instrument on Mars Science Laboratory. Space Sci. Rev. 2012, 170, 341–399. [Google Scholar] [CrossRef] [Green Version]
- Ming, D.W.; Gellert, R.; Morris, R.V.; Arvidson, R.E.; Brueckner, J.; Clark, B.C.; Cohen, B.A.; d’Uston, C.; Economou, T.; Fleischer, I.; et al. Geochemical properties of rocks and soils in Gusev crater, Mars: Results of the Alpha Particle X-ray Spectrometer from Cumberland Ridge to Home Plate. JGR Planets 2008, 113, E12. [Google Scholar] [CrossRef]
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA nomenclature and CMS nomenclature committees. Clays Clay Miner. 2005, 43, 255–256. [Google Scholar] [CrossRef]
- Weaver, C.E. Clays, Muds and Shales; Elsevier: New York, NY, USA, 1989; p. 810. [Google Scholar]
- Chamley, H. Clay Mineralogy; Springer: Berlin, Germany, 1989; p. 623. [Google Scholar]
- Bristow, T.F.; Milliken, R.E. Terrestrial perspective on authigenic clay mineral production in ancient martian lakes. Clays Clay Miner. 2011, 59, 339–358. [Google Scholar] [CrossRef]
- Faure, G. Principles and Applications of Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997; p. 625. [Google Scholar]
- Meunier, A. Clays; Springer: Berlin, Germany, 2005; p. 472. [Google Scholar]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.-P.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of Mars. Nature 2011, 479, 53–60. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. JGR Planets 2013, 118, 831–858. [Google Scholar] [CrossRef]
- Hicks, L.J.; Bridges, J.C.; Gurman, S.J. Ferric saponite and serpentine in the nakhlite martian meteorites. GCA 2014, 136, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Laird, D.A. Layer Charge Influences on the Hydration of Expandable 2:1 Phyllosilicates. Clays Clay Miner. 1999, 47, 630–636. [Google Scholar] [CrossRef]
- Hedges, J.I.; Keil, R.G. Sedimentary organic matter preservation: An assessment and speculative synthesis. Mar. Chem. 1995, 49, 81–115. [Google Scholar] [CrossRef]
- Mayer, L.M. Relationships between mineral surfaces and organic carbon concentrations in soils and sediments. Chem. Geol. 1994, 114, 347–363. [Google Scholar] [CrossRef]
- Keil, R.G.; Mayer, L.M. Mineral matrices and organic matter. Org. Geochem. 2014, 12, 337. [Google Scholar]
- Murchie, S.L.; Mustard, J.F.; Ehlmann, B.L.; Milliken, R.E.; Bishop, J.L.; McKeown, N.K.; Noe Dobrea, E.Z.; Seelos, F.P.; Buczkowski, D.L.; Wiseman, S.M.; et al. A synthesis of Martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. JGR Planets 2009, 114, E2. [Google Scholar] [CrossRef]
- Bishop, J.L.; Pieters, C.M.; Edwards, J.O. Infrared spectroscopic analyses on the nature of water in montmorillonite. Clays Clay Miner. 1994, 42.6, 702–716. [Google Scholar] [CrossRef]
- Clark, R.N.; Swayze, G.A.; Singer, R.B.; Pollack, J.B. High-resolution reflectance spectra of Mars in the 2.3-μm region: Evidence for the mineral scapolite. JGR Solid Earth 1990, 95, 14463–14480. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F. Orbital identification of clays and carbonates in Gusev crater. Icarus 2012, 219, 250–253. [Google Scholar] [CrossRef]
- Fox, V.; Arvidson, R.; Guinness, E.; McLennan, S.; Catalano, J.; Murchie, S.; Powell, K. Smectite deposits in Marathon Valley, Endeavour Crater, Mars, identified using CRISM hyperspectral reflectance data. Geophys. Res. Lett. 2016, 43, 4885–4892. [Google Scholar] [CrossRef] [Green Version]
- Quantin-Nataf, C.; Carter, J.; Mandon, L.; Thollot, P.; Balme, M.; Volat, M.; Pan, L.; Loizeau, D.; Millot, C.; Breton, S. Oxia Planum: The Landing Site for the ExoMars “Rosalind Franklin” Rover Mission: Geological Context and Prelanding Interpretation. Astrobiology 2021, 21, 345–366. [Google Scholar] [CrossRef]
- Williams, R.M.E.; Grotzinger, J.P.; Dietrich, W.E.; Gupta, S.; Sumner, D.Y.; Wiens, R.C.; Mangold, N.C.; Malin, M.C.; Edgett, K.S.; Maurice, S.; et al. Martian fluvial conglomerates at Gale crater. Science 2013, 340, 1068–1072. [Google Scholar] [CrossRef]
- Edgar, L.A.; Gupta, S.; Rubin, D.M.; Lewis, K.W.; Kocurek, G.A.; Anderson, R.B.; Bell, J.F., III; Dromart, G.; Edgett, K.S.; Grotzinger, J.P.; et al. Shaler: In situ analysis of a fluvial sedimentary deposit on Mars. Sedimentology 2018, 65, 96–122. [Google Scholar] [CrossRef] [Green Version]
- Fedo, C.; Grotzinger, J.; Gupta, S.; Fraeman, A.; Edgar, L.; Edgett, K.; Stein, N.; Rivera-Hernandez, F.; Lewis, K.; Stack, K. Sedimentology and stratigraphy of the Murray formation, Gale crater, Mars. In Proceedings of the 49th Lunar Planetary Science Conference, The Woodlands, TX, USA, 19–23 March 2018; p. 2083. [Google Scholar]
- Rivera-Hernández, F.; Sumner, D.Y.; Mangold, N.; Stack, K.M.; Forni, O.; Newsom, H.; Williams, A.; Nachon, M.; l’Haridon, J.; Gasnault, O. Using ChemCam LIBS data to constrain grain size in rocks on Mars: Proof of concept and application to rocks at Yellowknife Bay and Pahrump Hills, Gale crater. Icarus 2019, 321, 82–98. [Google Scholar] [CrossRef]
- Stein, N.; Grotzinger, J.; Schieber, J.; Mangold, N.; Hallet, B.; Newsom, H.; Stack, K.; Berger, J.; Thompson, J.; Siebach, K. Desiccation cracks provide evidence of lake drying on Mars, Sutton Island member, Murray formation, Gale Crater. Geology 2018, 46, 515–518. [Google Scholar] [CrossRef]
- Gwizd, S.; Fedo, C.; Grotzinger, J.; Edgett, K.; Gupta, S.; Stack, K.; Banham, S.; Edgar, L.; Sumner, D. Toward a Greater Understanding of Cross-Stratified Facies in the Hartmann’s Valley Member of the Murray Formation, Gale Crater, Mars. LPI Contrib. 2019, 2089, 6183. [Google Scholar]
- Banham, S.G.; Gupta, S.; Rubin, D.M.; Watkins, J.A.; Sumner, D.Y.; Edgett, K.S.; Grotzinger, J.P.; Lewis, K.P.; Edgar, L.A.; Stack-Morgan, K.M. Ancient Martian aeolian processes and palaeomorphology reconstructed from the Stimson formation on the lower slope of Aeolis Mons, Gale crater, Mars. Sedimentology 2018, 65, 993–1042. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.S.; Gupta, S.; Treiman, A.H.; Stack, K.M.; Calef, F.; Edgar, L.A.; Grotzinger, J.; Lanza, N.; Le Deit, L.; Lasue, J.; et al. Geologic overview of the Mars Science Laboratory rover mission at the Kimberley, Gale crater, Mars. JGR Planets 2017, 122, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, S.; Shibuya, T. Thermodynamic Constrains on Smectite and Iron Oxide Formation at Gale Crater, Mars: Insights into Potential Free Energy from Aerobic Fe Oxidation in Lake Water-Groundwater Mixing Zone. Minerals 2021, 4, 341. [Google Scholar] [CrossRef]
- Price, A.; Pearson, V.K.; Schwenzer, S.P.; Miot, J.; Olsson-Francis, K. Nitrate-dependent iron oxidation; a potential Mars metabolism. Front. Microbiol. 2018, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Tung, H.C.; Bramall, N.E.; Price, P.B. Microbial origin of excess methane in glacial ice and implications for life on Mars. Proc. Natl. Acad. Sci. USA 2005, 102, 18292018296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macey, M.C.; Fox-Powell, M.; Ramkissoon, N.K.; Stephens, B.P.; Barton, T.; Schwenzer, S.P.; Pearson, V.K.; Cousins, C.R.; Olsson-Francis, K. The identification of sulfide oxidation as a potential metabolism driving primary production on late Noachian Mars. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- King, G.M. Carbon monoxide as a metabolic energy source for extremely halophilic microbes: Implications for microbial activity in Mars regolith. Proc. Natl. Acad. Sci. USA 2015, 112, 4465–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edward, K.J.; Bach, W.; McCollum, T.M.; Rogers, D.R. Neutrophilic iron-oxidizing bacteria in the ocean: Their habitats, diversity, and roles in mineral deposition, rock alteration, and biomass production in the deep-sea. Geomicrobiol. J. 2004, 21, 393–404. [Google Scholar] [CrossRef]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-oxidizing bacteria: An environmental and genomic perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Popa, P.; Smith, A.R.; Popa, R.; Jane Boone, J.; Fisk, M. Olivine-respiring bacteria isolated from the rock-ice interface in a lava-tube cave, a Mars analog environment. Astrobiology 2012, 12, 9–18. [Google Scholar] [CrossRef]
- Tosca, N.J.; Ahmed, I.A.; Tutolo, B.M.; Ashpitel, A.; Hurowitz, J.A. Magnetite authigenesis and the warming of early Mars. Nat. Geosci. 2018, 11, 635–639. [Google Scholar] [CrossRef]
- Canfield, D.E.; Kristensen, E.; Thamdrup, B. Aquatic Geomicrobiology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakman, R.; Smith, E. The emergence and early evolution of biological carbon-fixation. PLoS Comput. Biol. 2012, 8, e1002455. [Google Scholar] [CrossRef] [Green Version]
- Rampe, E.B.; Blake, D.F.; Bristow, T.F.; Ming, D.W.; Vaniman, D.T.; Morris, R.V.; Achilles, C.N.; Chipera, S.J.; Morrison, S.M.; Tu, V.M.; et al. Mineralogy and geochemistry of sedimentary rocks and eolian sediments in Gale crater, Mars: A review after six Earth years of exploration with Curiosity. Geochemistry 2020, 80, 125605. [Google Scholar] [CrossRef]
- Fraeman, A.A.; Edgar, L.A.; Rampe, E.B.; Thompson, L.M.; Frydenvang, J.; Fedo, C.M.; Catalano, J.G.; Dietrich, W.E.; Gabriel, T.S.; Vasavada, A.R.; et al. Evidence for a diagenetic origin of Vera Rubin ridge, Gale crater, Mars: Summary and synthesis of Curiosity’s exploration campaign. J. Geophys. Res. Planets 2020, 125, e2020JE006527. [Google Scholar] [CrossRef] [PubMed]
- Dera, P.; Zhuravlev, K.; Prakapenka, V.; Rivers, M.L.; Finkelstein, G.J.; Grubor-Urosevic, O.; Tschauner, O.; Clark, M.; Downs, R.T. High pressure single-crystal micro X-ray diffraction analysis with GSE_ADA/RSV software. High Press. Res. 2013, 33, 466–484. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Bish, D.L.; Post, J.E. (Eds.) Modern Powder Diffraction; De Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Morrison, S.M.; Downs, R.T.; Blake, D.F.; Vaniman, D.T.; Ming, D.W.; Hazen, R.M.; Treiman, A.H.; Achilles, C.N.; Yen, A.S.; Morris, R.V.; et al. Crystal chemistry of martian minerals from Bradbury Landing through Naukluft Plateau, Gale crater, Mars. Am. Min. J. Earth Planet. Mater. 2018, 103, 857–871. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1997; p. 382. [Google Scholar]
- Morris, R.V.; Ming, D.; Blake, D.; Vaniman, D.; Bish, D.; Chipera, S.; Downs, R.; Gellert, R.; Treiman, A.; Yen, A. The Amorphous Component in Martian Basaltic Soil in Global Perspective from MSL and MER Missions. In Proceedings of the 44th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2013. [Google Scholar]
- Chipera, S.J.; Bish, D.L. FULLPAT: A full-pattern quantitative analysis program for X-ray powder diffraction using measured and calculated patterns. J. Appl. Crystallogr. 2002, 35, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Chipera, S.J.; Bish, D.L. Fitting full X-ray diffraction patterns for quantitative analysis: A method for readily quantifying crystalline and disordered phases. AMPC 2013, 3, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Leshin, L.; Mahaffy, P.; Webster, C.; Cabane, M.; Coll, P.; Conrad, P.; Archer, P.; Atreya, S.; Brunner, A.; Buch, A. Volatile, isotope, and organic analysis of martian fines with the Mars Curiosity rover. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D., Jr.; Atreya, S.K.; Brinckerhoff, W.B. Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J. Geophys. Res. Planets 2013, 118, 1955–1973. [Google Scholar] [CrossRef]
- Sutter, B.; Mcadam, A.C.; Mahaffy, P.R.; Ming, D.W.; Edgett, K.E.; Rampe, E.B.; Eigenbrode, J.L.; Franz, H.B.; Freissinet, C.; Grotzinger, J.P. Evolved gas analyses of sedimentary rocks and eolian sediment in Gale Crater, Mars: Results of the Curiosity rover’s sample analysis at Mars instrument from Yellowknife Bay to the Namib Dune. J. Geophys. Res. Planets 2017, 122, 2574–2609. [Google Scholar] [CrossRef] [Green Version]
- Bish, D.; Duffy, C. Thermogravimetric analysis of minerals. Therm. Anal. Clay Sci. 1990, 3, 96–157. [Google Scholar]
- Maurice, S.; Wiens, R.; Saccoccio, M.; Barraclough, B.; Gasnault, O.; Forni, O.; Mangold, N.; Baratoux, D.; Bender, S.; Berger, G. The ChemCam instrument suite on the Mars Science Laboratory (MSL) rover: Science objectives and mast unit description. Space Sci. Rev. 2012, 170, 95–166. [Google Scholar] [CrossRef]
- Wiens, R.C.; Maurice, S.; Barraclough, B.; Saccoccio, M.; Barkley, W.C.; Bell, J.F.; Bender, S.; Bernardin, J.; Blaney, D.; Blank, J. The ChemCam instrument suite on the Mars Science Laboratory (MSL) rover: Body unit and combined system tests. Space Sci. Rev. 2012, 170, 167–227. [Google Scholar] [CrossRef]
- Clegg, S.M.; Wiens, R.C.; Anderson, R.; Forni, O.; Frydenvang, J.; Lasue, J.; Cousin, A.; Payre, V.; Boucher, T.; Dyar, M.D. Recalibration of the Mars Science Laboratory ChemCam instrument with an expanded geochemical database. Spectrochim. Acta Part. B At. Spectrosc. 2017, 129, 64–85. [Google Scholar] [CrossRef]
- Meslin, P.-Y.; Gasnault, O.; Forni, O.; Schröder, S.; Cousin, A.; Berger, G.; Clegg, S.; Lasue, J.; Maurice, S.; Sautter, V. Soil diversity and hydration as observed by ChemCam at Gale Crater, Mars. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Mouélic, S.; Gasnault, O.; Herkenhoff, K.; Bridges, N.; Langevin, Y.; Mangold, N.; Maurice, S.; Wiens, R.; Pinet, P.; Newsom, H. The ChemCam Remote Micro-Imager at Gale crater: Review of the first year of operations on Mars. Icarus 2015, 249, 93–107. [Google Scholar] [CrossRef]
- Frydenvang, J.; Mangold, N.; Wiens, R.C.; Fraeman, A.A.; Edgar, L.A.; Fedo, C.M.; L’Haridon, J.; Bedford, C.C.; Gupta, S.; Grotzinger, J.P. The chemostratigraphy of the Murray formation and role of diagenesis at Vera Rubin ridge in Gale crater, Mars, as observed by the ChemCam instrument. J. Geophys. Res. Planets 2020, 125, e2019JE006320. [Google Scholar] [CrossRef]
- Bedford, C.; Bridges, J.; Schwenzer, S.; Wiens, R.C.; Rampe, E.; Frydenvang, J.; Gasda, P.J. Alteration trends and geochemical source region characteristics preserved in the fluviolacustrine sedimentary record of Gale crater, Mars. GCA 2019, 246, 234–266. [Google Scholar] [CrossRef]
- Mangold, N.; Dehouck, E.; Fedo, C.; Forni, O.; Achilles, C.; Bristow, T.; Downs, R.; Frydenvang, J.; Gasnault, O.; L’haridon, J. Chemical alteration of fine-grained sedimentary rocks at Gale crater. Icarus 2019, 321, 619–631. [Google Scholar] [CrossRef]
- Le Deit, L.; Mangold, N.; Forni, O.; Cousin, A.; Lasue, J.; Schröder, S.; Wiens, R.C.; Sumner, D.; Fabre, C.; Stack, K.M. The potassic sedimentary rocks in Gale Crater, Mars, as seen by ChemCam on board Curiosity. J. Geophys. Res. Planets 2016, 121, 784–804. [Google Scholar] [CrossRef] [Green Version]
- Mangold, N.; Forni, O.; Dromart, G.; Stack, K.; Wiens, R.C.; Gasnault, O.; Sumner, D.Y.; Nachon, M.; Meslin, P.Y.; Anderson, R.B. Chemical variations in Yellowknife Bay formation sedimentary rocks analyzed by ChemCam on board the Curiosity rover on Mars. J. Geophys. Res. Planets 2015, 120, 452–482. [Google Scholar] [CrossRef]
- Anderson, S.; Ehlmann, B.; Morris, R.; McLennan, S.; Boucher, T.; Dyar, M.; McInroy, R.; Delapp, D.; Wiens, R.; Frydenvang, J. Expanded Compositional Database for ChemCam Quantitative Calibration. RB. In Proceedings of the 8th International Conference on Mars, Pasadena, CA, USA, 14–18 July 2014. [Google Scholar]
- Mangold, J.E.; Park, C.M.; Liljestrand, H.M.; Katz, L.E. Surface complexation modeling of Hg (II) adsorption at the goethite/water interface using the Charge Distribution Multi-Site Complexation (CD-MUSIC) model. J. Colloid Interface Sci. 2014, 418, 147–161. [Google Scholar] [CrossRef] [PubMed]
- McLennan, S.M.; Anderson, R.; Bell, J.; Bridges, J.C.; Calef, F.; Campbell, J.L.; Clark, B.; Clegg, S.; Conrad, P.; Cousin, A. Elemental geochemistry of sedimentary rocks at Yellowknife Bay, Gale crater, Mars. Science 2014, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollila, A.M.; Newsom, H.E.; Clark, B., III.; Wiens, R.C.; Cousin, A.; Blank, J.G.; Mangold, N.; Sautter, V.; Maurice, S.; Clegg, S.M. Trace element geochemistry (Li, Ba, Sr, and Rb) using Curiosity’s ChemCam: Early results for Gale crater from Bradbury landing site to Rocknest. J. Geophys. Res. Planets 2014, 119, 255–285. [Google Scholar] [CrossRef] [Green Version]
- Gellert, R.; Yen, A.S. Elemental Analyses of Mars from Rovers Using the Alpha-Particle X-Ray Spectrometer. Remote Compos. Anal. Tech. Underst. Spectrosc. 2019, 555–572. [Google Scholar] [CrossRef]
- Gellert, R.; Campbell, J.L.; King, P.L.; Leshin, L.A.; Lugmair, G.W.; Spray, J.G.; Squyres, S.W.; Yen, A.S. The alpha-particle-x-ray-spectrometer (apxs) for the mars science laboratory (msl) rover mission. In Proceedings of the 40th Lunar and Planetary Science Conference, LPI Contribution, Woodlands, TX, USA, 23–27 March 2009; Volume 1468, p. 2364. [Google Scholar]
- Achilles, C.N.; Rampe, E.B.; Downs, R.T.; Bristow, T.F.; Ming, D.W.; Morris, R.V.; Vaniman, D.T.; Blake, D.F.; McAdam, A.C.; Sutter, B. Evidence for multiple diagenetic episodes in ancient fluvial-lacustrine sedimentary rocks in Gale crater, Mars. JGR Planets 2020, 125, e2019JE006295. [Google Scholar] [CrossRef]
- Vaniman, D.T.; Martinex, G.M.; Rampe, E.B.; Bristow, T.F.; Blake, D.F.; Yen, A.S.; Ming, D.W.; Rapin, W.; Meslin, P.Y.; Morookian, J.M.; et al. Gypsum, basanite, and anhydrite at Gale crater, Mars. Am. Min. J. Earth Planet. Mater. 2018, 103, 1011–1020. [Google Scholar]
- Blake, D.F.; Morris, R.V.; Kocurek, G.; Morrison, S.M.; Downs, R.T.; Bish, D.; Ming, D.W.; Edgett, K.S.; Rubin, D.; Goetz, W.; et al. Curiosity at Gale crater, Mars: Characterization and analysis of the Rocknest sand shadow. Science 2013, 341, 6153. [Google Scholar] [CrossRef] [Green Version]
- Treiman, A.H.; Bish, D.L.; Vaniman, D.T.; Chipera, S.J.; Blake, D.F.; Ming, D.W.; Morris, R.V.; Bristow, T.F.; Morrison, S.M.; Baker, M.B. Mineralogy, provenance, and diagenesis of a potassic basaltic sandstone on Mars: CheMin X-ray diffraction of the Windjana sample (Kimberley area, Gale Crater). J. Geophys. Res. Planets 2016, 121, 75–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampe, E.; Ming, D.; Blake, D.; Bristow, T.; Chipera, S.; Grotzinger, J.; Morris, R.; Morrison, S.; Vaniman, D.; Yen, A. Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale crater, Mars. EPSL 2017, 471, 172–185. [Google Scholar] [CrossRef]
- Morris, R.V.; Vaniman, D.T.; Blake, D.F.; Gellert, R.; Chipera, S.J.; Rampe, E.B.; Ming, D.W.; Morrison, S.M.; Downs, R.T.; Treiman, A.H.; et al. Silicic volcanism on Mars evidenced by tridymite in high-SiO2 sedimentary rock at Gale crater. Proc. Natl. Acad. Sci. USA 2016, 113, 7071–7076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.V.; Bristow, T.F.; Rampe, E.B.; Yen, A.S.; Vaniman, D.T.; Tu, V.; Thorpe, M.T.; Peretyazhko, T.S.; Morrison, S.M.; Ming, D.W.; et al. Mineralogy and formation processes for the Vera Rubin Ridge at Gale crater, Mars from CheMin and XRD analyses. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 18–22 March 2019. [Google Scholar]
- Yen, A.S.; Ming, D.W.; Vaniman, D.T.; Gellert, R.; Blake, D.F.; Morris, R.V.; Morrison, S.M.; Bristow, T.F.; Chipera, S.J.; Edgett, K.S.; et al. Multiple stages of aqueous alteration along fractures in mudstone and sandstone strata in Gale Crater, Mars. EPSL 2017, 471, 186–198. [Google Scholar] [CrossRef]
- Rampe, E.B.; Lapotre, M.G.A.; Bristow, T.F.; Arvidson, R.E.; Morris, R.V.; Achilles, C.N.; Weitz, C.; Blake, D.F.; Ming, D.W.; Morrison, S.M.; et al. Sand mineralogy within the Bagnold Dunes, Gale crater, as observed in situ and from orbit. Geophys. Res. Lett. 2018, 45, 9488–9497. [Google Scholar] [CrossRef]
- Bristow, T.F.; Grotzinger, J.P.; Rampe, E.B.; Cuadros, J.; Chipera, S.J.; Downs, G.; Fedo, C.M.; Frydenvang, J.; McAdam, A.C.; Morris, R.V.; et al. Brine Driven Destruction of Clay Minerals in Gale Crater Mars. Science 2021. [Google Scholar] [CrossRef]
- Léveillé, R.J.; Bridges, J.; Wiens, R.C.; Mangold, N.; Cousin, A.; Lanza, N.; Forni, O.; Ollila, A.; Grotzinger, J.; Clegg, S.; et al. Chemistry of fracture-filling raised ridges in Yellowknife Bay, Gale Crater: Window into past aqueous activity and habitability on Mars. J. Geophys. Res. Planets 2014, 119, 2398–2415. [Google Scholar] [CrossRef]
- Siebach, K.; Grotzinger, J.; Kah, L.; Stack, K.; Malin, M.; Léveillé, R.; Sumner, D. Subaqueous shrinkage cracks in the Sheepbed mudstone: Implications for early fluid diagenesis, Gale crater, Mars. J. Geophys. Res. Planets 2014, 119, 1597–1613. [Google Scholar] [CrossRef] [Green Version]
- Stack, K.; Grotzinger, J.; Kah, L.; Schmidt, M.; Mangold, N.; Edgett, K.; Sumner, D.; Siebach, K.; Nachon, M.; Lee, R. Diagenetic origin of nodules in the Sheepbed member, Yellowknife Bay formation, Gale crater, Mars. J. Geophys. Res. Planets 2014, 119, 1637–1664. [Google Scholar] [CrossRef]
- Treiman, A.H.; Morris, R.V.; Agresti, D.G.; Graff, T.G.; Achilles, C.N.; Rampe, E.B.; Bristow, T.F.; Ming, D.W.; Blake, D.F.; Vaniman, D.T.; et al. Ferrian saponite from the Santa Monica Mountains (California, USA, Earth): Characterization as an analog for clay minerals on Mars with application to Yellowknife Bay in Gale Crater. Am. Min. 2014, 99, 2234–2250. [Google Scholar] [CrossRef]
- Ming, D.W.; Archer, P.; Glavin, D.; Eigenbrode, J.; Franz, H.; Sutter, B.; Brunner, A.; Stern, J.; Freissinet, C.; McAdam, A. Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science 2014, 343. [Google Scholar] [CrossRef]
- Fedo, C.; Grotzinger, J.; Gupta, S.; Stein, N.; Watkins, J.; Banham, S.; Edgett, K.; Minitti, M.; Schieber, J.; Siebach, K. Facies analysis and basin architecture of the upper part of the Murray formation, Gale Crater, Mars. In Proceedings of the 48th Lunar and Planetary Science Conference, Houston, TX, USA, 20–24 March 2017; p. 1689. [Google Scholar]
- Gwizd, S.; Fedo, C.; Grotzinger, J.; Edgett, K.; Rivera-Hernandez, F.; Stein, N. Depositional history of the Hartmann’s valley member, Murray formation, Gale crater, Mars. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 19–23 March 2018; p. 2150. [Google Scholar]
- Stein, N.; Ehlmann, B.; Ammannito, E.; Palomba, E.; Desanctis, M.; Jaumann, R.; Nathues, A.; Raymond, C.; Hiesinger, H.; Schenk, P. Characteristics, formation, and evolution of Faculae (bright spots) on Ceres. In Proceedings of the 48th Lunar and Planetary Science Conference, Houston, TX, USA, 20–24 March 2017. [Google Scholar]
- Craig, P.; Ming, D.; Rampe, E. Sulfate Formation From Acid-Weathered Phyllosilicates: Implications for the Aqueous History of Mars. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 17–21 March 2014. [Google Scholar]
- Fraeman, A.; Ehlmann, B.; Arvidson, R.; Edwards, C.; Grotzinger, J.; Milliken, R.; Quinn, D.; Rice, M. The stratigraphy and evolution of lower Mount Sharp from spectral, morphological, and thermophysical orbital data sets. J. Geophys. Res. Planets 2016, 121, 1713–1736. [Google Scholar] [CrossRef]
- Carter, J.; Gondet, B.; Langevin, Y. MSL Homing in on a large smectite clay deposit: An orbital perspective. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 21–25 March 2016; p. 1899. [Google Scholar]
- Heller-Kallai, L.; Rozenson, L. Dehydroxylation of dioctahedral phyllosilicates. Clays Clay Miner. 1980, 28, 355–368. [Google Scholar] [CrossRef]
- Derkowski, A.; Bristow, T.F. On the problems of total specific surface area and cation exchange capacity measurements in organic-rich sedimentary rocks. Clays Clay Miner. 2012, 60, 348–362. [Google Scholar] [CrossRef]
- Cuadros, J.; Afsin, B.; Jadubansa, P.; Ardakani, M.; Ascaso, C.; Wierzchos, J.; Adams, J. Pathways of volcanic glass alteration in laboratory experiments through inorganic and microbially-mediated processes. Clay Miner. 2013, 48, 423–445. [Google Scholar] [CrossRef]
- Rampe, E.B.; Bristow, T.F.; Morris, R.V.; Morrison, S.M.; Achilles, C.N.; Ming, D.W.; Vaniman, D.T.; Blake, D.F.; Tu, V.M.; Chipera, S.J.; et al. Mineralogy of Vera Rubin Ridge From the Mars Science Laboratory CheMin Instrument. J. Geophys. Res. Planets 2020, 125, e2019JE006306. [Google Scholar] [CrossRef]
- Fraeman, A.; Arvidson, R.; Catalano, J.; Grotzinger, J.; Morris, R.; Murchie, S.; Stack, K.; Humm, D.; McGovern, J.; Seelos, F. A hematite-bearing layer in Gale Crater, Mars: Mapping and implications for past aqueous conditions. Geology 2013, 41, 1103–1106. [Google Scholar] [CrossRef]
- Jacob, S.R.; Wellington, D.F.; Bell, J.F., III; Achilles, C.; Fraeman, A.A.; Horgan, B.; Johnson, J.R.; Maurice, S.; Peters, G.; Rampe, E.B.; et al. Spectral, compositional, and physical properties of the upper Murray formation and Vera Rubin ridge, Gale Crater, Mars. J. Geophys. Res. Planets 2020, 125, e2019JE006290. [Google Scholar] [CrossRef]
- Berger, J.A.; Gellert, R.; Boyd, N.I.; King, P.L.; McCraig, M.A.; O’Connell-Cooper, C.D.; Schmidt, M.E.; Spray, J.G.; Thompson, L.M.; VanBommel, S.J.; et al. Elemental composition and chemical evolution of geologic materials in Gale Crater, Mars: APXS results from Bradbury landing to the Vera Rubin ridge. J. Geophys. Res. Planets 2020, 125, 12. [Google Scholar] [CrossRef]
- McAdam, A.C.; Sutter, B.; Archer, P.D.; Franz, H.B.; Wong, G.M.; Lewis, J.M.; Eigenbrode, J.L.; Stern, J.C.; Knudson, C.A.; Clark, J.V. Constraints on the Mineralogy and Geochemistry of Vera Rubin Ridge, Gale Crater, Mars, From Mars Science Laboratory Sample Analysis at Mars Evolved Gas Analyses. J. Geophys. Res. Planets 2020, 125, e2019JE006309. [Google Scholar] [CrossRef]
- Horgan, B.H.; Anderson, R.B.; Dromart, G.; Amador, E.S.; Rice, M.S. The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars. Icarus 2020, 339, 113526. [Google Scholar] [CrossRef]
- Sheppard, R.Y.; Milliken, R.E.; Parente, M.E.; Itoh, Y. Updated perspectives and hypotheses on the mineralogy of lower Mt. Sharp, Mars, as seen from orbit. J. Geophys. Res. 2020, 126, e2020JE006372. [Google Scholar]
- Fox, V.; Jacob, S.; Maurice, S.; Rampe, E.; Rice, M.; Seeger, C.; Wiens, R. Diagenesis in the Glen Torridon Region of Gale Crater, Mars Using VNIR Spectral Data From the Curiosity Rover. In Proceedings of the Lunar and Planetary Science Conference, LPI Contribution, Houston, TX, USA, 15–19 March 2021; Volume 2548, p. 1502. [Google Scholar]
- Bedford, C.C.; Schwenzer, S.P.; Bridges, J.C.; Banham, S.; Wiens, R.C.; Gasnault, O.; Rampe, E.B.; Frydenvang, J.; Gasda, P.J. Geochemical variation in the Stimson formation of Gale crater: Provenance, mineral sorting, and a comparison with modern Martian dunes. Icarus 2020, 341, 113622. [Google Scholar] [CrossRef]
- Banham, S.; Gupta, S.; Bryk, A.; Rubin, D.M.; Edgett, K.S.; Dietrich, W.; Caravaca, G.; Edgar, L.A.; Vasavada, A. Reconstructing Martian environmental change across a major unconformity at Gale crater: Sedimentology of the Stimson formation at the Greenheugh pediment. In Proceedings of the AGU Fall Meeting, Online, 1–17 December 2020. [Google Scholar]
- Banham, S.; Sanjeev, G.; Rubin, D.M.; Edgett, K.S.; Barnes, R.; Van Beek, J.; Watkins, J.A.; Edgar, L.A.; Fedo, C.M.; Williams, R.M.; et al. A Rock Record of Complex Aeolian Bedforms in a Hesperian Desert Landscape: The Stimson Formation as Exposed in the Murray Buttes, Gale Crater, Mars. J. Geophys. Res. Planets 2021, 126, e2020JE006554. [Google Scholar] [CrossRef]
- Gasda, P.J.; Haldeman, E.B.; Wiens, R.C.; Rapin, W.; Bristow, T.F.; Bridges, J.C.; Schwenzer, S.P.; Clark, B.; Herkenhoff, K.; Frydenvang, J. In situ detection of boron by ChemCam on Mars. Geophys. Res. Lett. 2017, 44, 8739–8748. [Google Scholar] [CrossRef]
- Hurowitz, J.A.; Grotzinger, J.P.; Fischer, W.W.; McLennan, S.M.; Milliken, R.E.; Stein, N.; Vasavada, A.R.; Blake, D.; Dehouck, E.; Eigenbrode, J.L. Redox stratification of an ancient lake in Gale crater, Mars. Science 2017, 356. [Google Scholar] [CrossRef] [Green Version]
- Che, C.; Glotch, T.D.; Bish, D.L.; Michalski, J.R.; Xu, W. Spectroscopic study of the dehydration and/or dehydroxylation of phyllosilicate and zeolite minerals. JGR Planets 2011, 116. [Google Scholar] [CrossRef]
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F.; et al. Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 2006, 312, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Vicente, M.A.; Razzaghe, M.; Robert, M. Formation of aluminium hydroxy vermiculite (intergrade) and smectite from mica under acidic conditions. Clay Miner. 1977, 12, 101–112. [Google Scholar] [CrossRef]
- Sutter, B.; Archer, P.D.; Ming, D.W.; Niles, P.B.; Eigenbrode, J.L.; Franz, H.; Glavin, D.P.; McAdam, A.C. The investigation of chlorates as a possible source of oxygen and chlorine detected by the sample analysis at Mars (SAM) instrument in Gale Crater, Mars. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 17–21 March 2014. [Google Scholar]
- Peretyazhko, T.; Sutter, B.; Morris, R.; Agresti, D.; Le, L.; Ming, D. Fe/Mg smectite formation under acidic conditions on early Mars. GCA 2016, 173, 37–49. [Google Scholar] [CrossRef]
- Peretyazhko, T.; Niles, P.; Sutter, B.; Morris, R.; Agresti, D.; Le, L.; Ming, D. Smectite formation in the presence of sulfuric acid: Implications for acidic smectite formation on early Mars. GCA 2018, 220, 248–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gainey, S.T.; Hausrath, E.M.; Adcock, C.T.; Tschauner, O.; Hurowitz, J.A.; Ehlmann, B.L.; Xiao, Y.; Bartlett, C.L. Clay mineral formation under oxidized conditions and implications for paleoenvironments and organic preservation on Mars. Nat. Commun. 2017, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.C.; Schwenzer, S.P. The Nakhlite Hydrothermal Brine. EPSL 2012, 359, 117–123. [Google Scholar] [CrossRef]
- Harder, H. The role of magnesium in the formation of smectite minerals. Chem. Geol. 1972, 10, 31–39. [Google Scholar] [CrossRef]
- Harder, H. Nontronite synthesis at low temperatures. Chem. Geol. 1976, 18, 169–180. [Google Scholar] [CrossRef]
- Decarreau, A.; Bonnin, D. Synthesis and crystallogenesis at low temperature of Fe (III)-smectites by evolution of coprecipitated gels: Experiments in partially reducing conditions. Clay Miner. 1986, 21, 861–877. [Google Scholar] [CrossRef]
- Decarreau, A.; Bonnin, D.; Badaut-Trauth, D.; Couty, R.; Kaiser, P. Synthesis and crystallogenesis of ferric smectite by evolution of Si-Fe coprecipitates in oxidizing conditions. Clay Miner. 1987, 22, 207–223. [Google Scholar] [CrossRef]
- Vogels, R.; Kerkhoffs, M.; Geus, J. Non-hydrothermal synthesis, characterisation and catalytic properties of saponite clays. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1995; pp. 1153–1161. [Google Scholar]
- Fukushi, K.; Sekine, Y.; Sakuma, H.; Morida, K.; Wordsworth, R. Semiarid climate and hyposaline lake on early Mars inferred from reconstructed water chemistry at Gale. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schieber, J.; Bish, D.; Coleman, M.; Reed, M.; Hausrath, E.M.; Cosgrove, J.; Gupta, S.; Minitti, M.E.; Edgett, K.S.; Malin, M. Encounters with an unearthly mudstone: Understanding the first mudstone found on Mars. Sedimentology 2017, 64, 311–358. [Google Scholar] [CrossRef]
- Michalopoulos, P.; Aller, R.C. Early diagenesis of biogenic silica in the Amazon delta: Alteration, authigenic clay formation, and storage. GCA 2004, 68, 1061–1085. [Google Scholar] [CrossRef]
- Cuadros, J. Clay minerals interaction with microorganisms: A review. Clay Miner. 2017, 52, 235–261. [Google Scholar] [CrossRef] [Green Version]
- Okumura, F.; Mimura, K. Gradual and stepwise pyrolyses of insoluble organic matter from the Murchison meteorite revealing chemical structure and isotopic distribution. GCA 2011, 75, 7063–7080. [Google Scholar] [CrossRef]
- Remusat, L.; Derenne, S.; Robert, F.; Knicker, H. New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic matter: A different origin than soluble? GCA 2005, 69, 3919–3932. [Google Scholar] [CrossRef]
- Damsté, J.S.S.; Eglinton, T.I.; De Leeuw, J.W.; Schenck, P.A. Organic sulphur in macromolecular sedimentary organic matter: I. Structure and origin of sulphur-containing moieties in kerogen, asphaltenes and coal as revealed by flash pyrolysis. GCA 1989, 53, 873–889. [Google Scholar]
- Baruah, B.; Khare, P. Pyrolysis of high sulfur Indian coals. Energy Fuels 2007, 21, 3346–3352. [Google Scholar] [CrossRef]
- Xu, L.; Yang, J.; Li, Y.; Liu, J. Behavior of organic sulfur model compounds in pyrolysis under coal-like environment. Fuel Process. Technol. 2004, 85, 1013–1024. [Google Scholar] [CrossRef]
- Williams, A.; Eigenbrode, J.; Millan, M.; Williams, R.; Buch, A.; Teinturier, S.; Glavin, D.; Freissinet, C.; Szopa, C.; McIntosh, O. Organic Molecules Detected with the First TMAH Wet Chemistry Experiment, Gale Crater, Mars. In Proceedings of the Lunar and Planetary Science Conference, held virtually, 15–19 March 2021; p. 1763. [Google Scholar]
- Nachon, M.; Clegg, S.; Mangold, N.; Schröder, S.; Kah, L.; Dromart, G.; Ollila, A.; Johnson, J.; Oehler, D.; Bridges, J. Calcium sulfate veins characterized by ChemCam/Curiosity at Gale crater, Mars. J. Geophys. Res. Planets 2014, 119, 1991–2016. [Google Scholar] [CrossRef] [Green Version]
- Nachon, M.; Mangold, N.; Forni, O.; Kah, L.C.; Cousin, A.; Wiens, R.C.; Anderson, R.; Blaney, D.; Blank, J.G.; Calef, F.; et al. Chemistry of diagenetic features analyzed by ChemCam at Pahrump Hills, Gale crater, Mars. Icarus 2017, 281, 121–136. [Google Scholar] [CrossRef]
- Caswell, T.E.; Milliken, R.E. Evidence for hydraulic fracturing at Gale crater, Mars: Implications for burial depth of the Yellowknife Bay formation. EPSL 2017, 468, 72–84. [Google Scholar] [CrossRef]
- Kronyak, R.; Kah, L.; Edgett, K.; VanBommel, S.; Thompson, L.; Wiens, R.; Sun, V.; Nachon, M. Mineral-filled fractures as indicators of multigenerational fluid flow in the Pahrump Hills member of the Murray formation, Gale crater, Mars. Earth Space Sci. 2019, 6, 238–265. [Google Scholar] [CrossRef]
- Frydenvang, J.; Gasda, P.J.; Hurowitz, J.A.; Grotzinger, J.P.; Wiens, R.C.; Newsom, H.E.; Edgett, K.S.; Watkins, J.; Bridges, J.C.; Maurice, S. Diagenetic silica enrichment and late-stage groundwater activity in Gale crater, Mars. Geophys. Res. Lett. 2017, 44, 4716–4724. [Google Scholar] [CrossRef] [Green Version]
- Fox, V.; Bennett, K.; Arvidson, R.; Ehlmann, B.; Stack, K.; Dehouck, E.; Grotzinger, J.; Bristow, R.; Salvatore, M.; Catalano, J. Martian clay minerals from orbit to the surface: MSL and MER rover investigations of CRISM smectite detections. LPI Contrib. 2019, 2089, 6372. [Google Scholar]
- Chipera, S.J.; Bish, D.L. Thermodynamic Modeling of Natural Zeolite Stability; LA-UR-97-2160; CONF-9706169; Los Alamos National Lab.: Los Alamos, NM, USA, 1997. [Google Scholar]
- Blake, D.F.; Hazen, R.M.; Morrison, S.M.; Bristow, T.S.; Sarrazin, P.; Zacny, K.; Rampe, E.B.; Downs, R.T.; Yen, A.; Ming, D.W.; et al. In-Situ Crystallographic Investigations of Solar System Objects in the next Decade. White Paper. 2020. Available online: https://www.geo.arizona.edu/xtal/group/pdf/01617915314072.pdf (accessed on 23 July 2021).



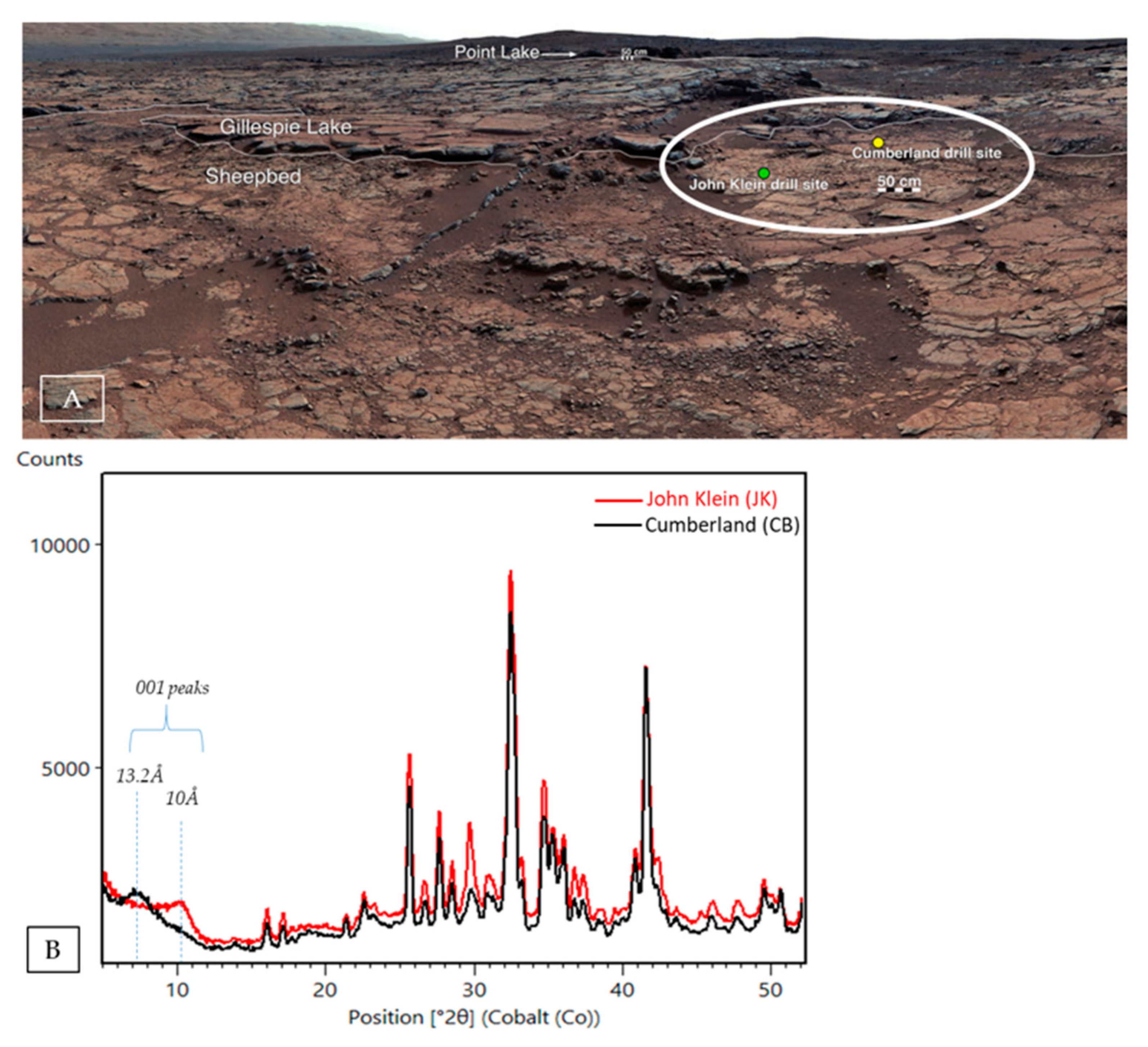
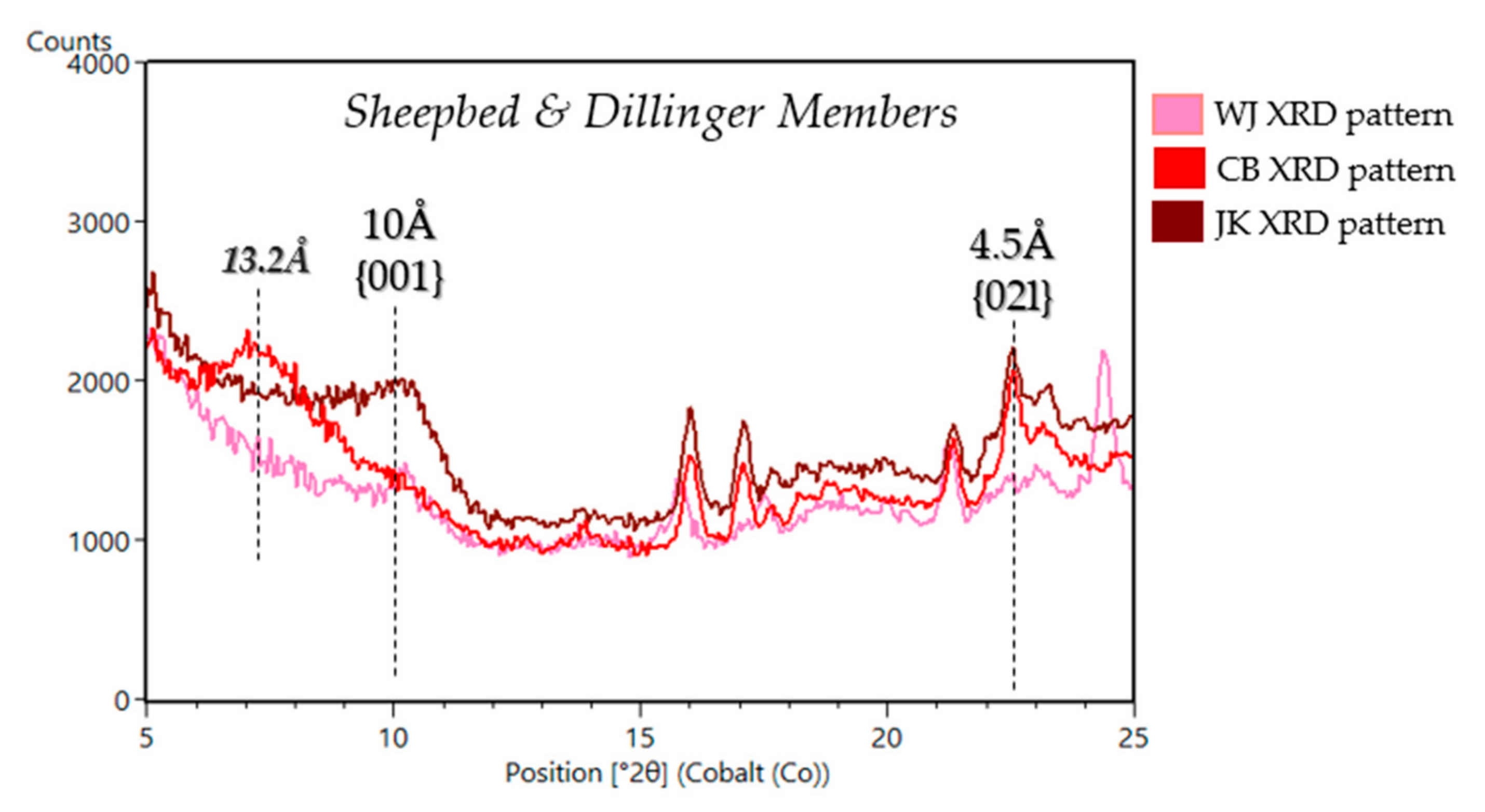
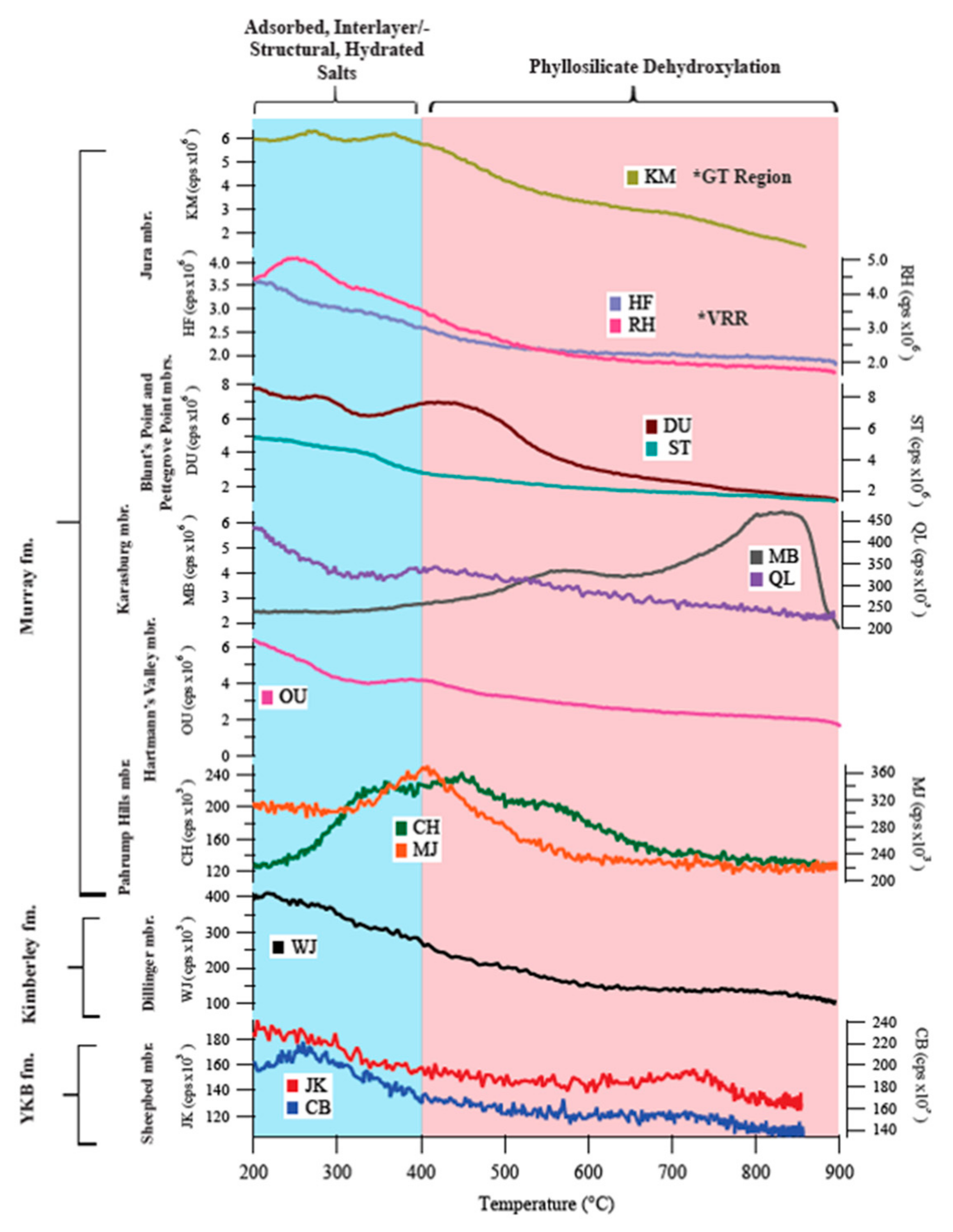
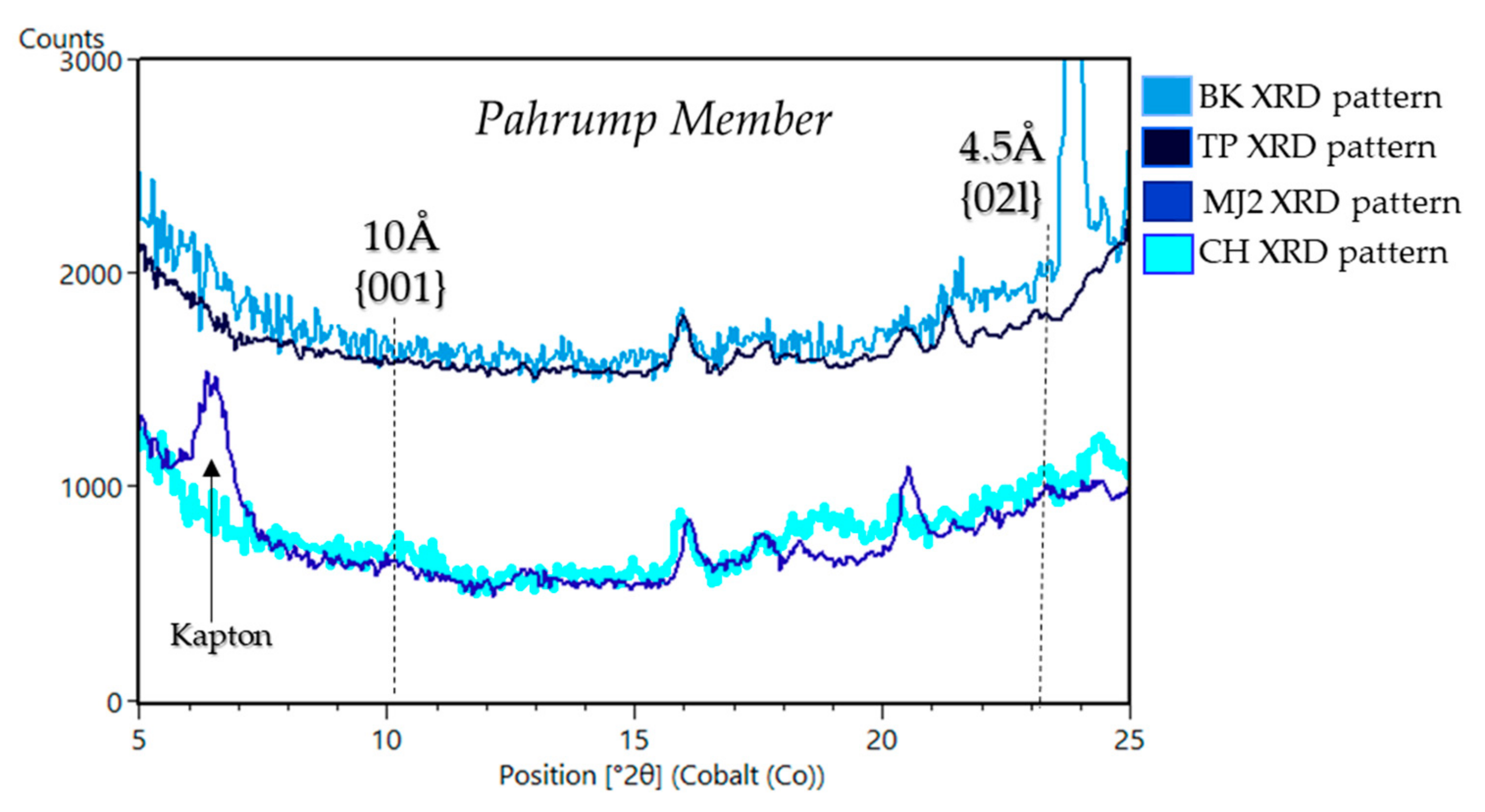
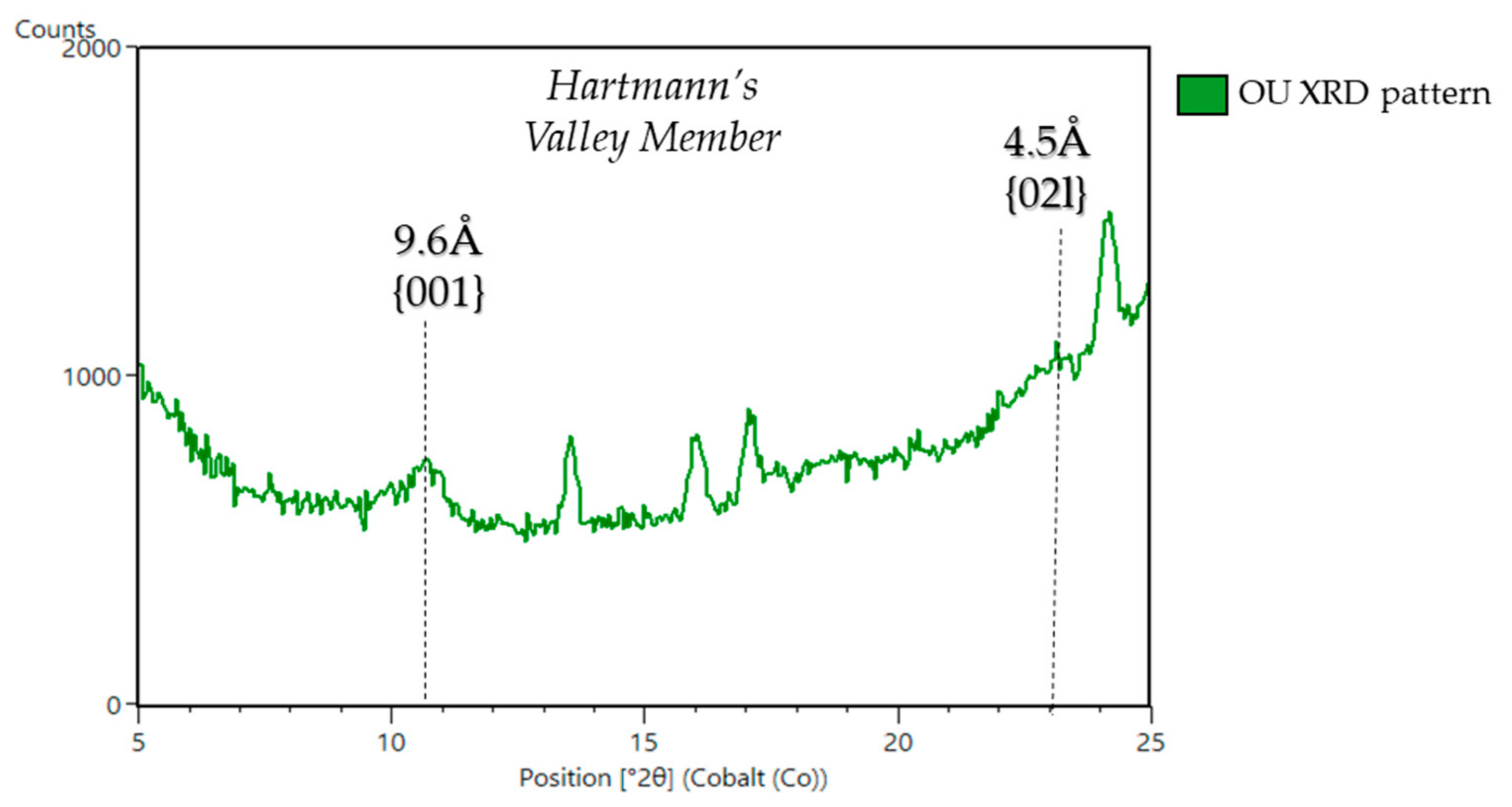
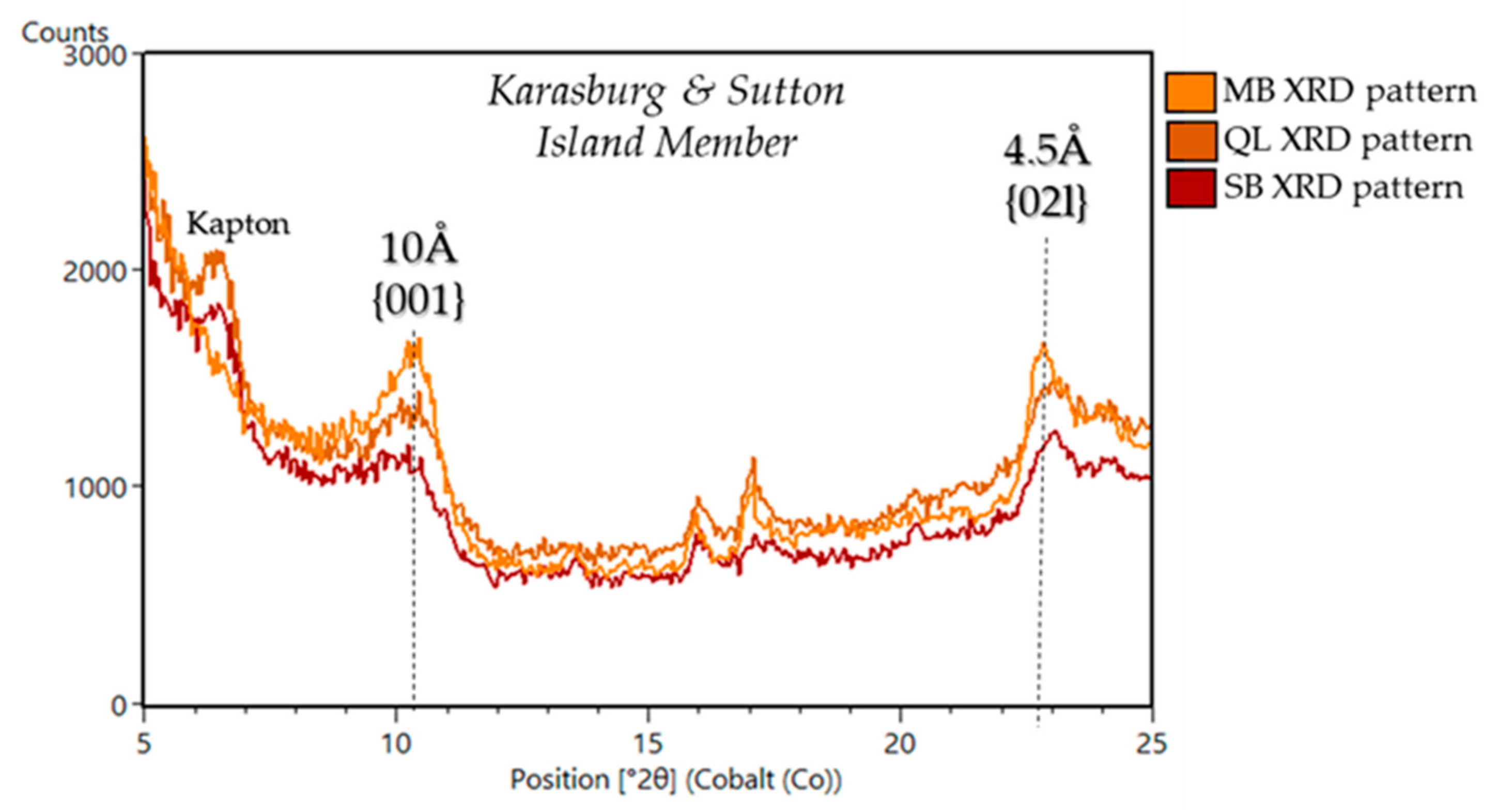
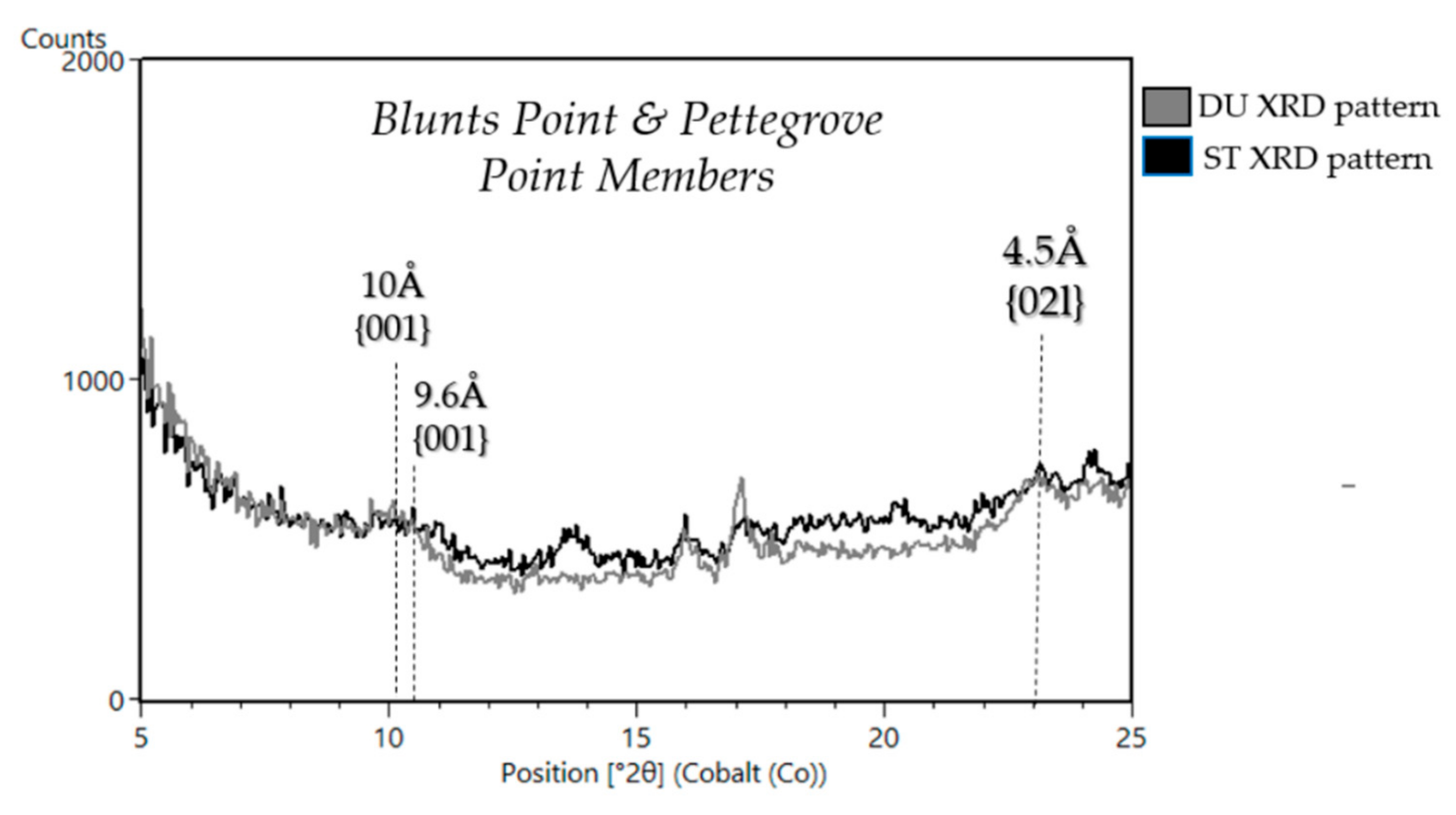
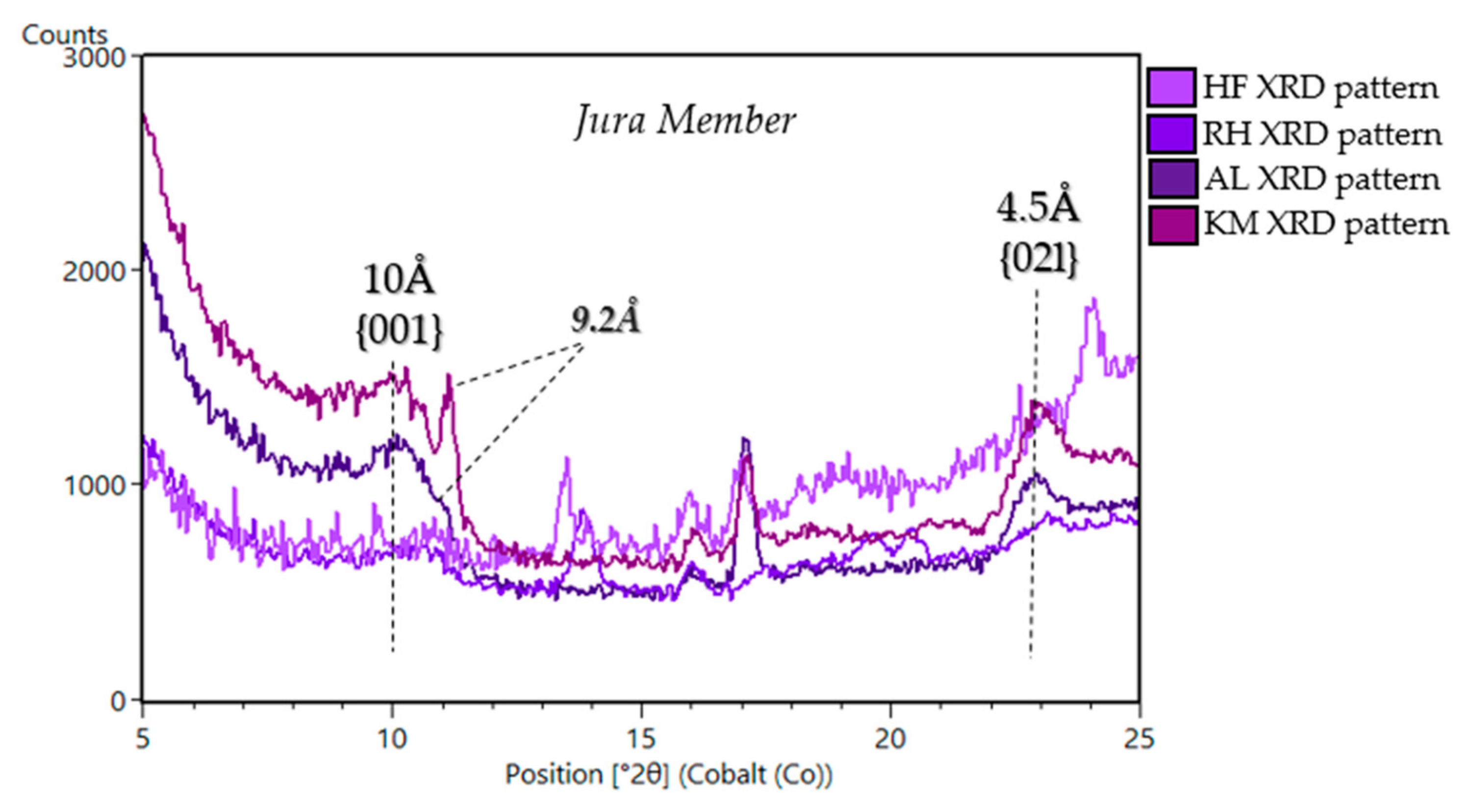
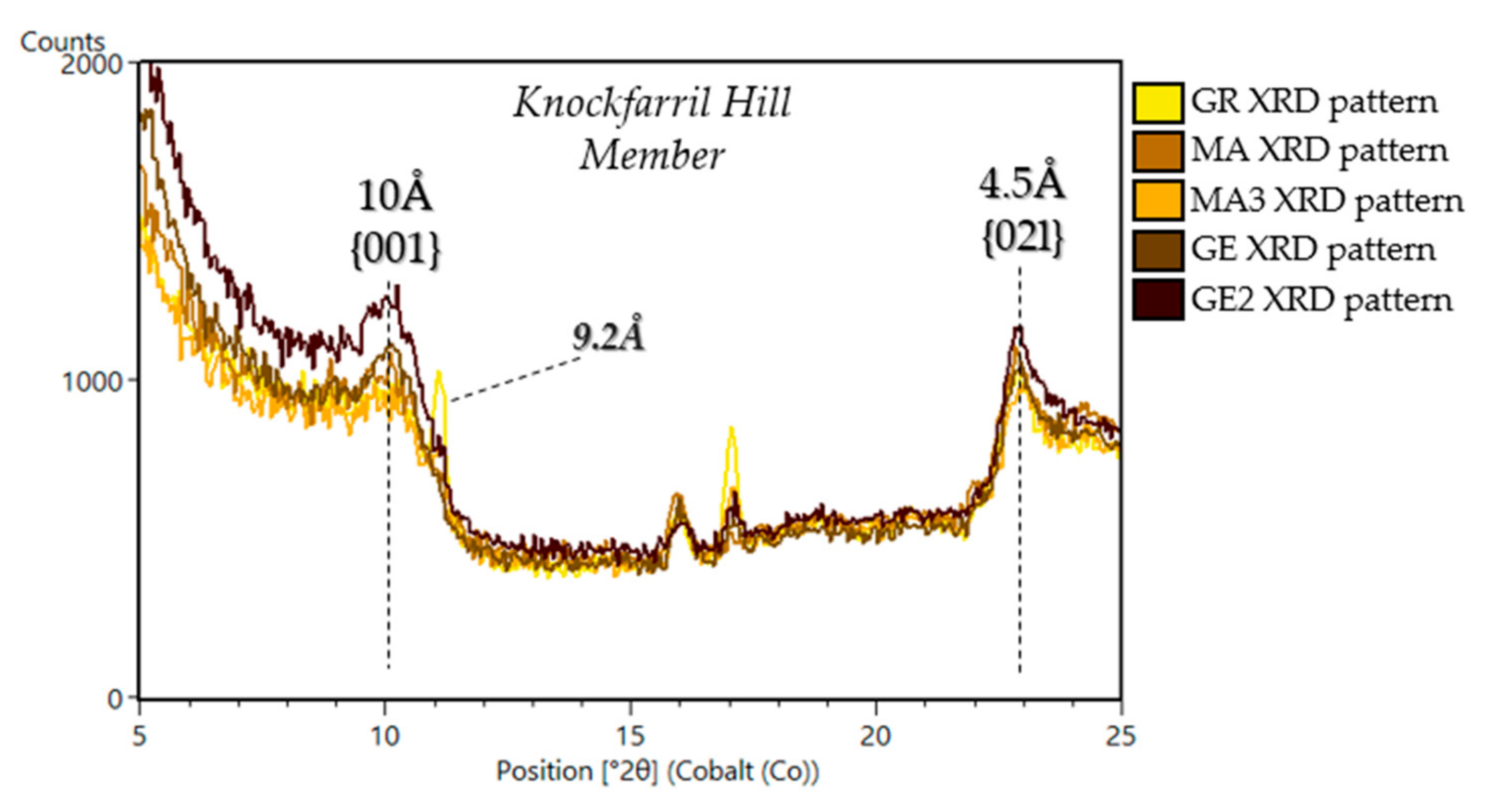
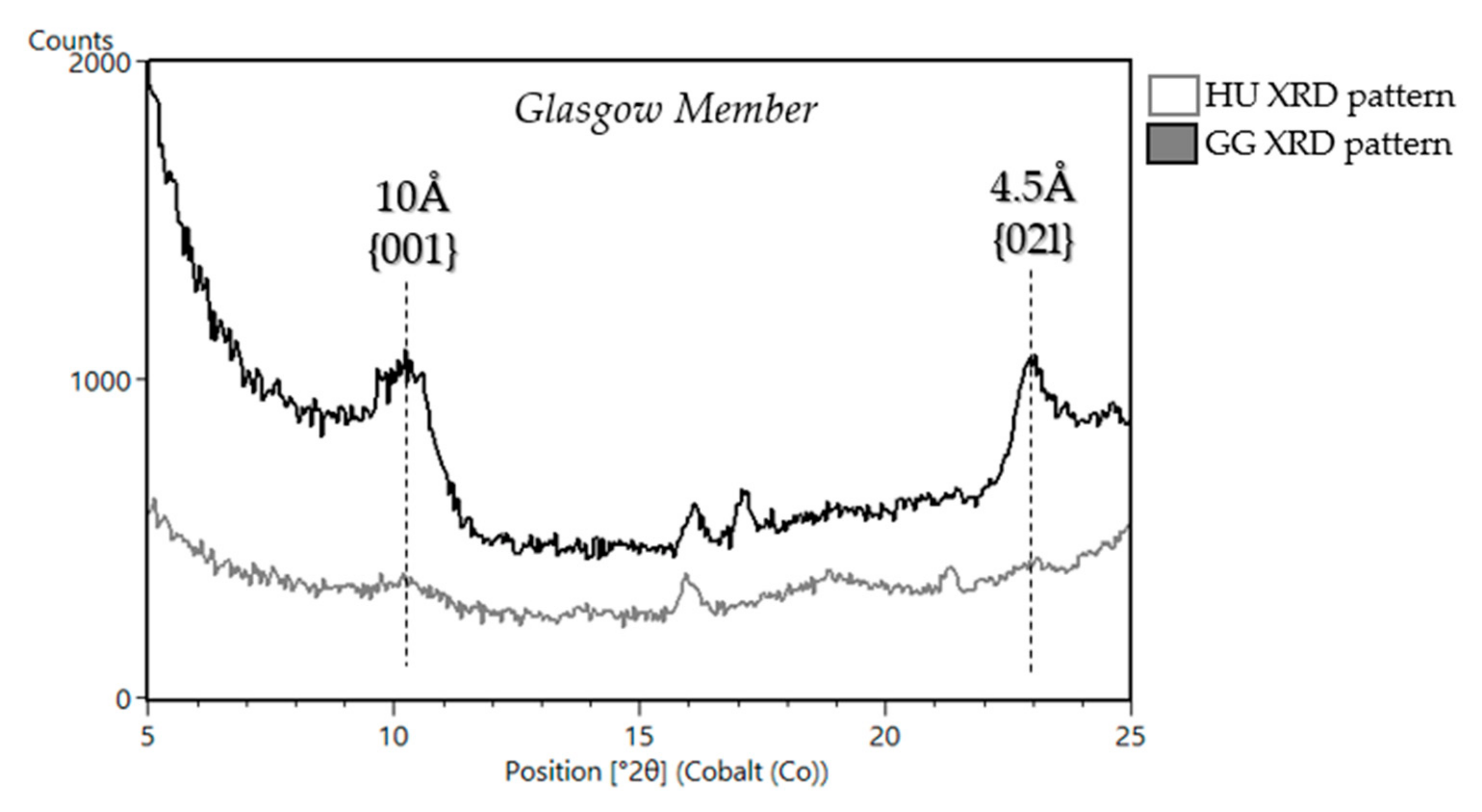
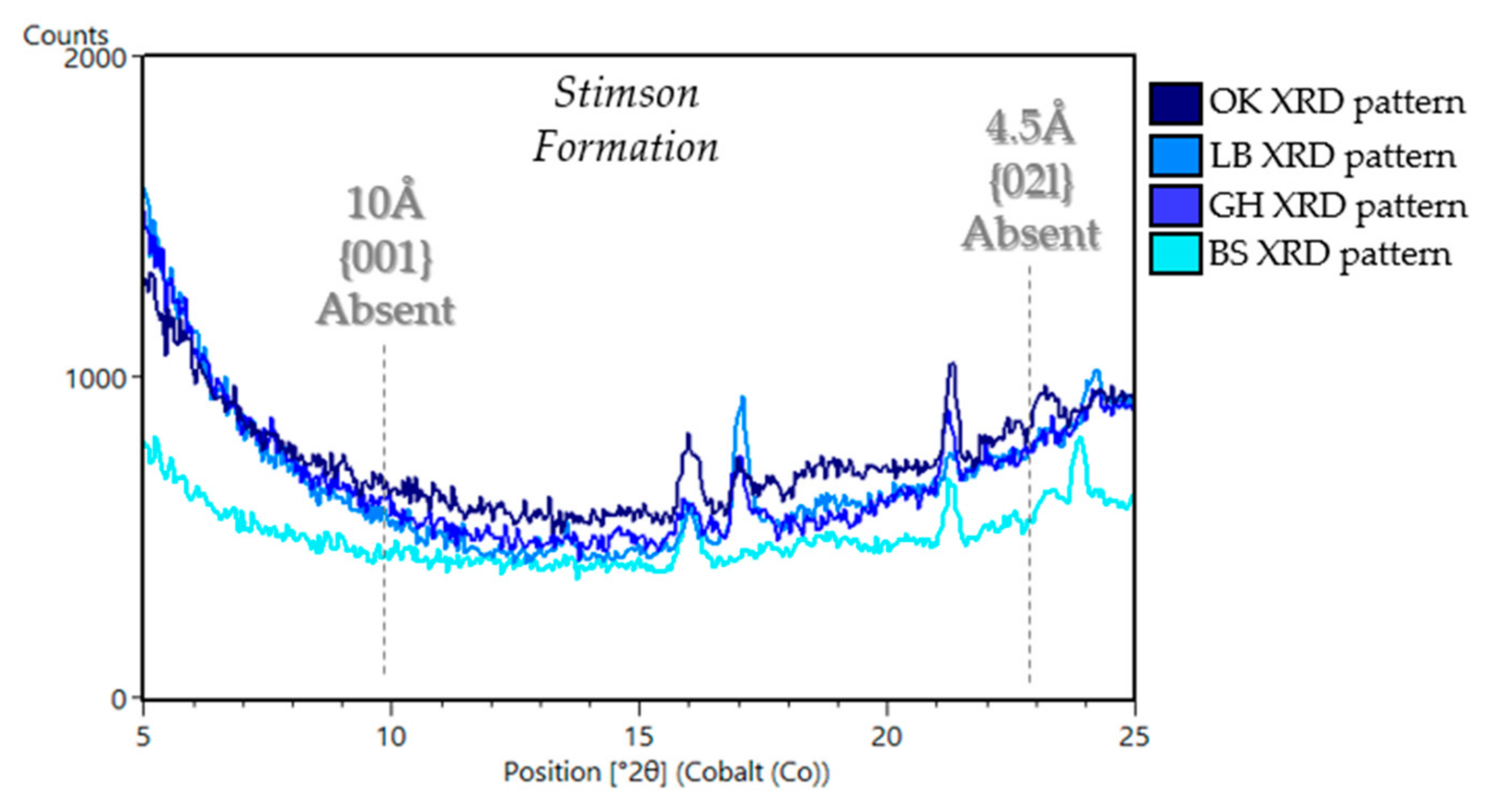
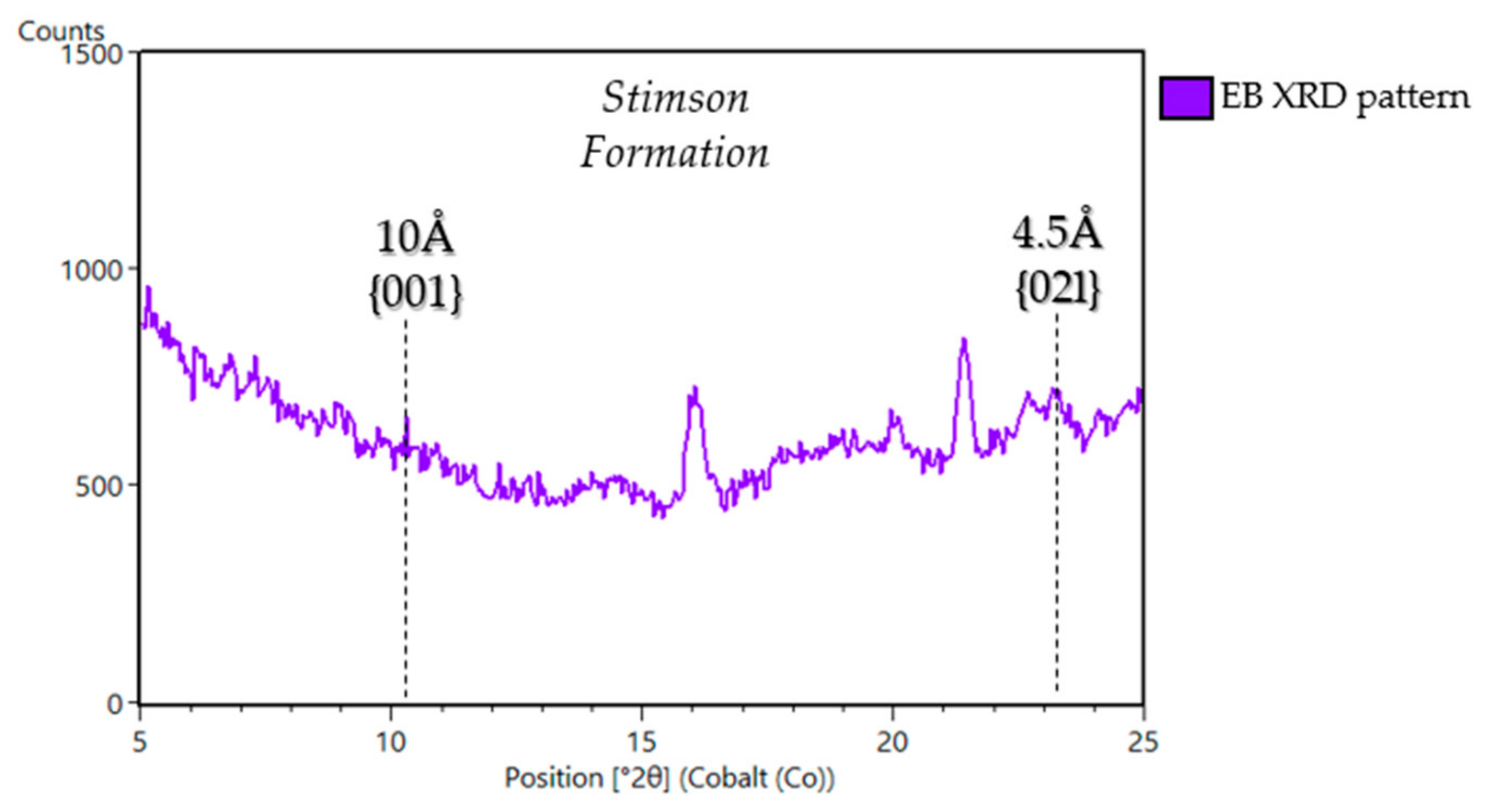
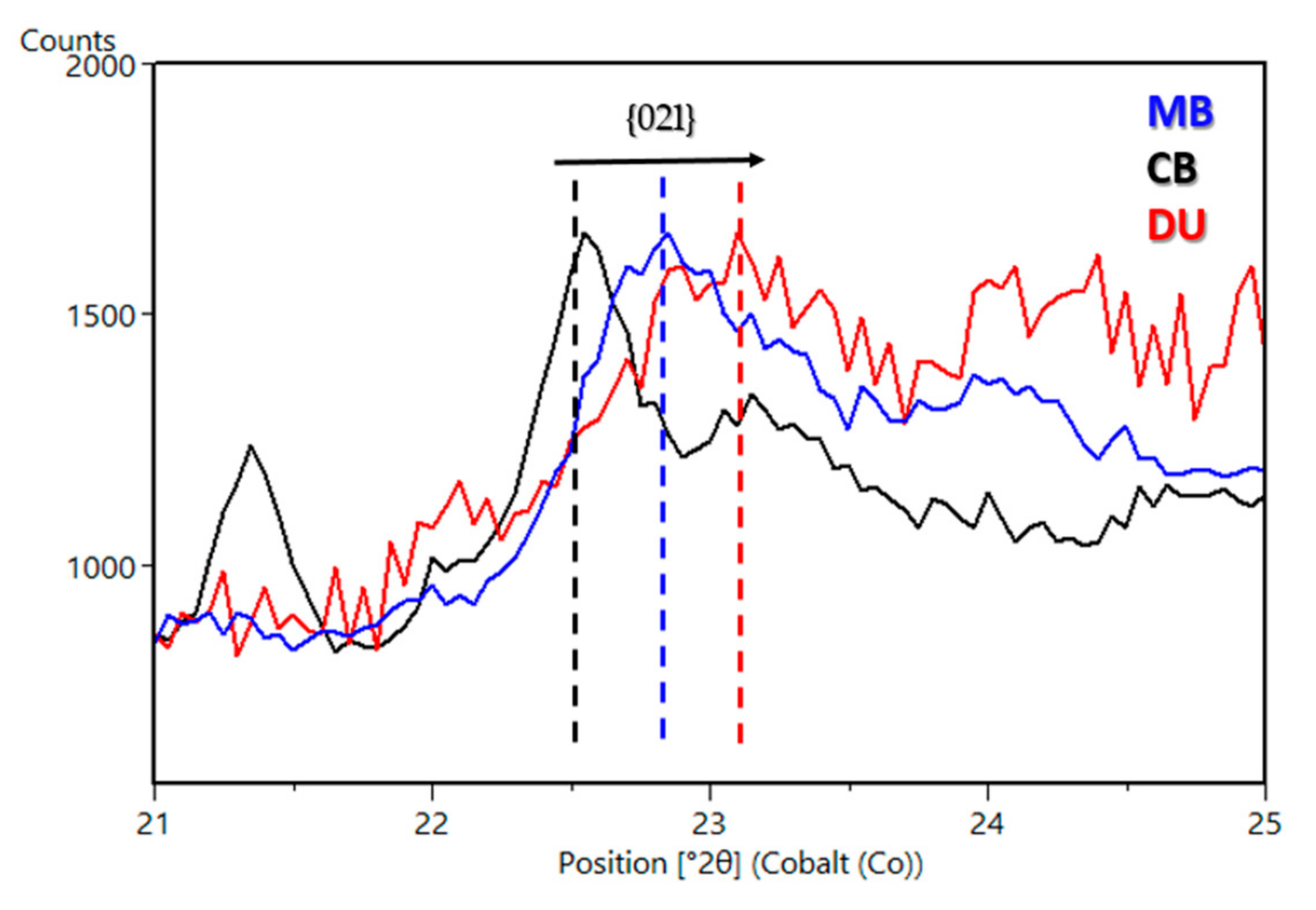
| Sample Name | Group | Formation | Member | Depositional Environment | Elevation (m) | Drill Sol | Clay (wt. %) | Clay Type | d(001)/ d(02l) |
|---|---|---|---|---|---|---|---|---|---|
| Rocknest *1 | n/a | n/a | n/a | Modern aeolian/Recently inactive | −4516.9 | 61, 66, 69, 74 | 0 | n/a | n/a |
| John Klein 2 | Bradbury | YKB | Sheepbed | Fluvio-Lacustrine | −4519.5 | 182 | 22 | trioctahedral | ~10Å/4.5Å |
| Cumberland 2 | Bradbury | YKB | Sheepbed | Fluvio-Lacustrine | −4519.5 | 279 | 18 | trioctahedral | ~13.2Å/4.5Å |
| Windjana 3 | Bradbury | Kimberley | Dillinger | Reworked aeolian/ fluvial | −4481.5 | 621 | 10 | trioctahedral | ~10Å/4.5Å |
| Confidence Hills 4 | Mount Sharp | Murray | Pahrump Hills | Fluvio-Lacustrine | −4460.3 | 759 | 8 | trioctahedral | ~10Å/4.5Å |
| Mojave 2 4 | Mount Sharp | Murray | Pahrump Hills | Fluvio-Lacustrine | −4459.4 | 882 | 5 | dioctahedral | ~10Å/4.5Å |
| Telegraph Peak 4 | Mount Sharp | Murray | Pahrump Hills | Fluvio-Lacustrine | −4453.2 | 908 | 0 | n/a | n/a |
| Buckskin 5 | Mount Sharp | Murray | Pahrump Hills | Fluvio-Lacustrine | −4446.8 | 1060 | 0 | n/a | n/a |
| Big Sky 6,7,9 | Siccar Point | Stimson | n/a | Ancient aeolian | −4434.7 | 1119 | 0 | n/a | n/a |
| Greenhorn 9 | Siccar Point | Stimson | n/a | Ancient aeolian | −4434.5 | 1137 | 0 | n/a | n/a |
| Gobabeb ** | n/a | n/a | n/a | Modern/ Active aeolian | −4423.8 | 1224 | 0 | n/a | n/a |
| Lubango 9 | Siccar Point | Stimson | n/a | Ancient aeolian | −4429 | 1320 | 0 | n/a | n/a |
| Okoruso 9 | Siccar Point | Stimson | n/a | Ancient aeolian | −4429.3 | 1332 | 0 | n/a | n/a |
| Oudam 6,7 | Mount Sharp | Murray | Hartmann's Valley | Reworked aeolian/ fluvial | −4435.5 | 1361 | 3 | Ferri-pyrophyllite | ~9.6Å/4.5Å |
| Marimba 6,7 | Mount Sharp | Murray | Karasburg | Fluvio-Lacustrine | −4410.4 | 1422 | 28 | trioctahedral/ dioctahedral | ~10Å/4.5Å |
| Quela 6,7 | Mount Sharp | Murray | Karasburg | Fluvio-Lacustrine | −4379.7 | 1464 | 16 | trioctahedral/ dioctahedral | ~10Å/4.5Å |
| Sebina 6,7 | Mount Sharp | Murray | Sutton Island | Fluvio-Lacustrine | −4360.8 | 1495 | 19 | trioctahedral/ dioctahedral | ~10Å/4.5Å |
| Ogunquit Beach *** 10 | n/a | n/a | n/a | Modern aeolian | −4300 | 1651 | 0 | n/a | n/a |
| Duluth 8 | Mount Sharp | Murray | Blunts Point | Fluvio-Lacustrine | −4192.5 | 2057 | 15 | dioctahedral | ~10Å/4.5Å |
| Stoer 8 (VRR) | Mount Sharp | Murray | Pettegrove Point | Fluvio-Lacustrine | −4169.9 | 2136 | 10 | dioctahedral | ~10Å/4.5Å |
| Highfield 8 (VRR) | Mount Sharp | Murray | Jura | Fluvio-Lacustrine | −4147 | 2224 | 5 | dioctahedral | ~9.6Å/4.5Å |
| Rockhall 8 (VRR) | Mount Sharp | Murray | Jura | Fluvio-Lacustrine | −4143.8 | 2261 | 13 | dioctahedral | ~9.6Å/4.5Å |
| Aberlady 11 (GT) | Mount Sharp | Murray | Jura | Fluvio-Lacustrine | −4157.94 | 2370 | 28 | dioctahedral | ~9.22Å/ ~10Å /4.5Å |
| Kilmarie 11 (GT) | Mount Sharp | Murray | Jura | Fluvio-Lacustrine | −4157.95 | 2384 | 28 | dioctahedral | ~9.22Å/ ~10Å /4.5Å |
| Glen Etive 11 (GT) | Mount Sharp | Murray | Knockfarril Hill | Fluvio-Lacustrine | −4133.36 | 2486 | 34 | dioctahedral | ~10Å/4.5Å |
| Glen Etive 2 11 (GT) | Mount Sharp | Murray | Knockfarril Hill | Fluvio-Lacustrine | −4129.62 | 2527 | 26 | dioctahedral | ~10Å/4.5Å |
| Hutton 12 | Mount Sharp | Murray | Glasgow | Fluvio-Lacustrine | −4095.37 | 2668 | 6.3 | dioctahedral | ~10Å /4.5Å |
| Edinburgh A | Siccar Point | Stimson | n/a | Ancient aeolian | −4088.69 | 2711 | 7 | dioctahedral | ~10Å/4.5Å/Future publication |
| Glasgow A | Mount Sharp | Murray | Glasgow | Fluvio-Lacustrine | −4107.93 | 2754 | 24 | dioctahedral | ~10Å/4.5Å/Future publication |
| Mary Anning 12 | Mount Sharp | Murray | Knockfarril Hill | Fluvio-Lacustrine | −4128.06 | 2839 | 28 | dioctahedral | ~10Å/4.5Å |
| Mary Anning 3 12 | Mount Sharp | Murray | Knockfarril Hill | Fluvio-Lacustrine | −4128.06 | 2872 | 30 | dioctahedral | ~10Å/4.5Å |
| Groken12 | Mount Sharp | Murray | Knockfarril Hill | Fluvio-Lacustrine | −4127.91 | 2914 | Future publication | dioctahedral | ~9.22Å/~10Å /4.5Å |
| Nontron | Mount Sharp | Murray | Glasgow | Fluvio-Lacustrine | −4066.51 | 3064 | Future publication | Future publication | Future publication |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, V.M.; Rampe, E.B.; Bristow, T.F.; Thorpe, M.T.; Clark, J.V.; Castle, N.; Fraeman, A.A.; Edgar, L.A.; McAdam, A.; Bedford, C.; et al. A Review of the Phyllosilicates in Gale Crater as Detected by the CheMin Instrument on the Mars Science Laboratory, Curiosity Rover. Minerals 2021, 11, 847. https://doi.org/10.3390/min11080847
Tu VM, Rampe EB, Bristow TF, Thorpe MT, Clark JV, Castle N, Fraeman AA, Edgar LA, McAdam A, Bedford C, et al. A Review of the Phyllosilicates in Gale Crater as Detected by the CheMin Instrument on the Mars Science Laboratory, Curiosity Rover. Minerals. 2021; 11(8):847. https://doi.org/10.3390/min11080847
Chicago/Turabian StyleTu, Valerie M., Elizabeth B. Rampe, Thomas F. Bristow, Michael T. Thorpe, Joanna V. Clark, Nicholas Castle, Abigail A. Fraeman, Lauren A. Edgar, Amy McAdam, Candice Bedford, and et al. 2021. "A Review of the Phyllosilicates in Gale Crater as Detected by the CheMin Instrument on the Mars Science Laboratory, Curiosity Rover" Minerals 11, no. 8: 847. https://doi.org/10.3390/min11080847
APA StyleTu, V. M., Rampe, E. B., Bristow, T. F., Thorpe, M. T., Clark, J. V., Castle, N., Fraeman, A. A., Edgar, L. A., McAdam, A., Bedford, C., Achilles, C. N., Blake, D., Chipera, S. J., Craig, P. I., Des Marais, D. J., Downs, G. W., Downs, R. T., Fox, V., Grotzinger, J. P., ... Bridges, J. C. (2021). A Review of the Phyllosilicates in Gale Crater as Detected by the CheMin Instrument on the Mars Science Laboratory, Curiosity Rover. Minerals, 11(8), 847. https://doi.org/10.3390/min11080847











