Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance
Abstract
:1. Introduction
2. Thallium Ore Deposits and Tl Minerals
2.1. Thallium Ore Deposits
2.2. Thallium Minerals
2.2.1. Thallium Sulfide
2.2.2. Thallium Sulfosalt
2.2.3. Thallium Telluride and Selenides
2.2.4. Other Thallium Minerals
3. Secondary Sulfate Mineralogy in Tl Mineralization Areas
| Mineral (Ref.) | Chemical Formula | Mineral (Ref.) | Chemical Formula |
|---|---|---|---|
| Argentobaumhauerite [71] | (Ag,Tl)1.5Pb22As33.5S72 | Jentschite [72] | TlPbAs2SbS6 |
| Arsiccioite [73] | AgHg2TlAs2S6 | Kalithallite [74] | K3Tl3+Cl6·2H2O |
| Auerbakhite [75] | MnTl2As2S5 | Karpovite [76] | Tl2VO(SO4)2(H2O) |
| Avicennite [77] | Tl2O3 | Lafossaite [63] | Tl(Cl,Br) |
| Bernardite [78] | Tl(As,Sb)5S8 | Lanmuchangite [68] | TlAl(SO4)2·12H2O |
| Biagioniite [79] | Tl2SbS2 | Lorándite [80] | TlAsS2 |
| Boscardinite [81] | TlPb4(Sb7As2)S18 | Markhininite [82] | TlBi(SO4)2 |
| Bukovite [83] | Cu4Tl2Se4 | Nataliyamalikite [61] | TlI |
| Carlinite [49] | Tl2S | Parapierrotite [84] | Tl(Sb,As)5S8 |
| Chabournéite [85] | Tl4Pb2(Sb,As)20S34 | Perlialite [86] | K3TlAl3Si4O18·5H2O |
| Chalcothallite [83] | Tl2(Cu,Fe)6SbS4 | Philrothite [87] | TlAs3S5 |
| Christite [88] | TlHgAsS3 | Picotpaulite [89] | TlFe2S3 |
| Chrysothallite [74] | K6Cu6Tl3+Cl17(OH)4·H2O | Pierrotite [90] | Tl2(Sb,As)10S16 |
| Criddleite [91] | TlAg2Au3Sb10S10 | Pokhodyashinite [92] | Cu2Tl3Sb5As2S13 |
| Crookesite [93] | Cu7(Tl,Ag)Se4 | Protochabournéite [94] | Tl2Pb(Sb,As)10S17 |
| Cuprostibite [95] | Cu2(Sb,Tl) | Raberite [96] | Tl5Ag4As6SbS15 |
| Dalnegroite [97] | Tl4Pb2Sb8As12S34 | Raguinite [98] | TlFeS2 |
| Dekatriasartorite [99] | TlPb58As97S204 | Ralphcannonite [100] | AgZn2TlAs2S6 |
| Dorallcharite [101] | TlFe3(SO4)2(OH)6 | Rathite [102] | Ag2Pb12-xTlx/2As18+x/2S40 |
| Drechslerite [103] | Tl4(Sb4−xAsx)S8 | Rayite [104] | (Ag,Tl)2Pb8Sb8S21 |
| Écrinsite [105] | AgTl3Pb4As11Sb9S36 | Rebulite [106] | Tl5Sb5As8S22 |
| Edenharterite [107] | TlPbAs3S6 | Richardsollyite [108] | TlPbAsS3 |
| Ellisite [109] | Tl3AsS3 | Rohaite [83] | TlCu5SbS2 |
| Erniggliite [110] | Tl2SnAs2S6 | Routhierite [111] | TlCuHg2As2S6 |
| Enneasartorite [112] | Tl6Pb32As70S140 | Sabatierite [113] | Cu6TlSe4 |
| Evdokimovite [114] | Tl4(VO)3(SO4)5(H2O)5 | Saltonseaite [115] | (K,Tl)3NaMnCl6 |
| Fangite [116] | Tl3AsS4 | Sicherite [117] | TlAg2(As,Sb)3S6 |
| Ferrostalderite [118] | CuFe2TlAs2S6 | Simonite [119] | TlHgAs3S6 |
| Ferrovorontsovite [120] | (Fe5Cu)TlAs4S12 | Spaltiite [121] | Tl2Cu2As2S5 |
| Flinteite [122] | (K,Tl)2ZnCl4 | Stalderite [123] | TlCu(Zn,Fe,Hg)2As2S6 |
| Gabrielite [124] | Tl2AgCu2As3S7 | Steropesite [125] | Tl3BiCl6 |
| Gladkovskyite [126] | MnTlAs3S6 | Thalcusite [83] | Tl2Cu3FeS4 |
| Galkhaite [120] | Tl(Hg,Cu,Zn)6(As,Sb)4S12 | Thalfenisite [127] | Tl6(Fe,Ni,Cu)25S26Cl |
| Gillulyite [116] | Tl2(As,Sb)8S13 | Thalliomelane [103] | TlMn4+7.5Cu2+0.5O16 |
| Gungerite [128] | TlAs5Sb4S13 | Thalliumpharmacosiderite [121] | TlFe4[(AsO4)3(OH)4]·4H2O |
| Hatchite [129] | AgTlPbAs2S5 | Thunderbayite [130] | TlAg3Au3Sb7S6 |
| Hendekasartorite [112] | Tl2Pb48As82S172 | Tsygankoite [131] | Mn8Tl8Hg2(Sb21Pb2Tl)S48 |
| Hephaistosite [62] | TlPb2Cl5 | Twinnite [132] | Pb0.8Tl0.1Sb1.3As0.8S4 |
| Heptasartorite [112] | Tl7Pb22As55S108 | Vaughanite [133] | TlHgSb4S7 |
| Honeaite [60] | Au3TlTe2 | Voltaite [35] | (K,Tl)2Fe8Al(SO4)12·18H2O |
| Hutchinsonite [134] | (Pb,Tl)2As5S9 | Vorontsovite [120] | (Hg5Cu)TlAs4S12 |
| Imhofite [135] | Tl5.8As15.4S26 | Vrbaite [136] | Hg3Tl4As8Sb2S20 |
| Incomsartorite [137] | Tl6Pb144As246S516 | Wallisite [138] | CuPbTlAs2S5 |
| Jarosite [64] | (Tl,K)Fe3(SO4)2(OH)6 | Weissbergite [139] | TlSbS2 |
| Jankovicite [140] | Tl5Sb9(As,Sb)4S22 |
3.1. Secondary Thallium and Potassium Sulfate
3.2. Secondary Iron Sulfate
3.3. Secondary Calcium, Magnesium, Aluminum Sulfate
4. Environmental Significance of the Secondary Sulfate Minerals
4.1. Tl Sulfate
4.2. Calcium, Magnesium, Alumina, and Iron Sulfate
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Zhu, R.; Huang, L.; Pan, J.; Guo, C.; Cao, Y. Tracking observation for chronic thallium poisoning in Huilong Valley of Xingren County, China. Occup. Health Inj. 1986, 1, 52–54. (In Chinese) [Google Scholar]
- Viraraghavan, T.; Srinivasan, A. Thallium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier Inc.: Burlington, VT, USA, 2011; pp. 325–333. [Google Scholar]
- Liu, J.; Wang, J.; Tsang, D.C.W.; Xiao, T.; Chen, Y.; Hou, L. Emerging thallium pollution in China and source tracing by thallium isotopes. Environ. Sci. Technol. 2018, 52, 11977–11979. [Google Scholar] [CrossRef] [Green Version]
- Kazantzis, G. Thallium in the Environment and Health Effects. Environ. Geochem. Health 2000, 22, 275–280. [Google Scholar] [CrossRef]
- Xiao, T.; Boyle, D.; Guha, J.; Rouleau, A.; Hong, Y.; Zheng, B. Groundwater-related thallium transfer processes and their impacts on the ecosystem: Southwest Guizhou Province, China. Appl. Geochem. 2003, 18, 675–691. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Sun, Y.; Tsang, D.C.; Qi, J.; Zhang, W.; Li, N.; Yin, M.; Wang, J.; Lippold, H.; et al. Thallium pollution in China and removal technologies for waters: A review. Environ. Int. 2019, 126, 771–790. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhu, C.; Du, S.; Fan, Y.; Luo, C. Gallium (Ga), germanium (Ge), thallium (Tl) and cadmium (Cd) resources in China. Chin. Sci. Bull. 2020, 65, 3688–3699, (In Chinese with English abstract). [Google Scholar]
- Ikramuddin, M.; Besse, L.; Nordstrom, P.M. Thallium in the Carlin-type gold deposits. Appl. Geochem. 1986, 1, 493–502. [Google Scholar] [CrossRef]
- Janković, S. Sb-As-Tl mineral association in the Mediterranean region. Int. Geol. Rev. 1989, 31, 262–273. [Google Scholar] [CrossRef]
- Hofstra, A.H. Characteristics and models for Carlin-type gold deposits. Rev. Econ. Geol. 2000, 13, 163–220. [Google Scholar]
- Fan, Y.; Zhou, T.; Gabriel, V.; Hu, Q.; Yuan, F.; Zhang, X. Metallogenic regularities of thallium deposits. Geol. Sci. Technol. Inf. 2005, 24, 55–60. [Google Scholar]
- Xiao, T.; Yang, F.; Li, S.; Zheng, B.; Ning, Z. Thallium pollution in China: A geo-environmental perspective. Sci. Total Environ. 2012, 421, 51–58. [Google Scholar] [CrossRef]
- Jakubowska, M.; Pasieczna, A.; Zembrzuski, W.; Swit, Z.; Lukaszewski, Z. Thallium in fractions of soil formed on floodplain terraces. Chemosphere 2007, 66, 611–618. [Google Scholar] [CrossRef]
- Karbowska, B.; Zembrzuski, W.; Jakubowska, M.; Wojtkowiak, T.; Pasieczna, A.; Lukaszewski, Z. Translocation and mobility of thallium from zinc-lead ores. J. Geochem. Explor. 2014, 143, 127–135. [Google Scholar] [CrossRef]
- Xiong, Y.L. The aqueous geochemistry of thallium: Speciation and solubility of thallium in low temperature systems. Environ. Chem. 2009, 6, 441–451. [Google Scholar] [CrossRef]
- Vaněk, A.; Komárek, M.; Chrastný, V.; Galušková, I.; Mihaljevič, M.; Šebek, O.; Drahota, P.; Tejnecký, V.; Vokurková, P. Effect of low-molecular-weight organic acids on the leaching of thallium and accompanying cations from soil-a model rhizosphere solution approach. J. Geochem. Explor. 2012, 112, 212–217. [Google Scholar] [CrossRef]
- Zitko, V. Toxicity and pollution potential of thallium. Sci. Total Environ. 1975, 4, 185–192. [Google Scholar] [CrossRef]
- Jonsson, E.; Wagner, T. Ore Mineralogy of the Skrikerum Cu–Ag–Tl–(Au) Selenide Deposit, SE Sweden: Preliminary Results. In Proceedings of the 25th Nordic Geological Winter Meeting, Reykjavík, Iceland, 6–9 January 2002; 98. [Google Scholar]
- Zhang, Z.; Zhang, X.; Zhang, B. Elemental geochemistry and metallogenic model of Nanhua As-Tl deposit in Yunnan Province, China. Geochimica 1998, 27, 269–275, (In Chinese with English abstract). [Google Scholar]
- Yang, C.; Chen, Y.; Peng, P.; Li, C.; Chang, X.; Xie, C. Distribution of natural and anthropogenic thallium in the soils in an industrial pyrite slag disposing area. Sci. Total Environ. 2005, 341, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Li, G. A study of ore compositions and thallium occurrence in a mercury-thallium deposit at Lanmuchang in Xingren County in southwestern Guizhou. Guizhou Geol. 1996, 13, 24–37, (In Chinese with English abstract). [Google Scholar]
- Hu, R.; Peng, J.; Ma, D.; Su, W.; Shi, C.; Bi, X.; Tu, G. Epoch of large-scale low temperature mineralizations in southwestern Yangtze massif. Miner. Depos. 2007, 26, 583–596, (In Chinese with English abstract). [Google Scholar]
- Zhou, T.; Fan, Y.; Yuan, F.; Wu, M.; Hou, M.; Voicu, G.; Hu, Q.; Zhang, Q.; Yue, S. A preliminary geological and geochemical study of the Xiangquan thallium deposit, eastern China: The world’s first thallium-only mine. Miner. Petrol. 2005, 85, 243–251. [Google Scholar] [CrossRef]
- George, L.L.; Biagioni, C.; D’Orazio, M.; Cook, N.J. Textural and trace element evolution of pyrite during greenschist facies metamorphic recrystallization in the southern Apuan Alps (Tuscany, Italy): Influence on the formation of Tl-rich sulfosalt melt. Ore Geol. Rev. 2018, 102, 59–105. [Google Scholar] [CrossRef]
- Chang, X.; Chen, Y.; Liu, J.; Chen, N.W.Y.; Fu, S. Effect of thallium-bearing sulfide mineral utilization on environment in Yunfu of west Guangdong: Trace study of lead isotope. Acta Geosci. Sin. 2008, 29, 765–768, (In Chinese with English abstract). [Google Scholar]
- Jiang, K.; Yan, Y.; Zhu, C.; Zhang, L. The research on distribution of thallium and cadmium in the Jinding lead-zinc Deposit, Yunnan Province. Bull. Miner. Petrol. Geochem. 2014, 33, 753–758, (in Chinese with English abstract). [Google Scholar]
- Spinks, S.; Pearce, M.; Liu, W.; Kunzmann, M.; Ryan, C.; Moorhead, G.; Kirkham, R.; Blaikie, T.; Sheldon, H.; Schaubs, P.; et al. Carbonate replacement as the principal ore formation process in the Proterozoic McArthur River (HYC) sediment-hosted Zn-Pb Deposit, Australia. Econ. Geol. 2021, 116, 693–718. [Google Scholar] [CrossRef]
- Dehnavi, A.; Mcfarlane, C.; Lentz, D.; Walker, J. Assessment of pyrite composition by LA-ICP-MS techniques from massive sulfide deposits of the Bathurst Mining Camp, Canada: From textural and chemical evolution to its application as a vectoring tool for the exploration of VMS deposits. Ore Geol. Rev. 2018, 92, 656–671. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Muntean, J.L. The Carlin gold system: Applications to exploration in Nevada and beyond. Rev. Econ. Geol. 2018, 20, 39–88. [Google Scholar]
- Chen, D. Discovery of rich-thallium ore body in paragenesis ore deposit of mercury and thallium and research of its mineralization mechanism. J. Guizhou Inst. Technol. 1989, 18, 1–19, (In Chinese with English abstract). [Google Scholar]
- Hofstra, A.H.; Christensen, O.D. Comparison of Carlin-type Au deposits in the United States, China, and Indonesia—Implications for genetic models and exploration. US Geol. Surv. Open-File Report. 2002, 02-131, 64–94. [Google Scholar]
- Nevolko, P.A.; Hnylko, O.M.; Mokrushnikov, V.P.; Gibsher, A.S.; Redin, Y.O.; Zhimulev, F.I.; Karavashkin, M.I. Geology and geochemistry of the Kadamzhai and Chauvai gold-antimony- mercury deposits: Implications for new province of Carlin-type gold deposits at the Southern Tien Shan (Kyrgyzstan). Ore Geol. Rev. 2019, 105, 551–571. [Google Scholar] [CrossRef]
- Vikentyev, I.V.; Tyukova, E.E.; Vikent’Eva, O.V.; Chugaev, A.V.; Dubinina, E.O.; Prokofiev, V.Y.; Murzin, V.V. Vorontsovka Carlin-style gold deposit in the North Urals: Mineralogy, fluid inclusion and isotope data for genetic model. Chem. Geol. 2019, 508, 144–166. [Google Scholar] [CrossRef]
- Biagioni, C.; D’Orazio, M.; Fulignati, P.; George, L.L.; Mauro, D.; Zaccarini, F. Sulfide melts in ore deposits from low-grade metamorphic settings: Insights from fluid and Tl-rich sulfosalt microinclusions from the Monte Arsiccio mine (Apuan Alps, Tuscany, Italy). Ore Geol. Rev. 2020, 123, 103589. [Google Scholar] [CrossRef]
- Zen, X.; Wu, Y. Polygenetic mechanism for compound enriched Yunfu pyrite, Guangdong Province. Geotect. Metallogen. 1998, 22, 242–251. [Google Scholar]
- Biagioni, C.; D’Orazio, M.; Vezzoni, S.; Dini, A.; Orlandi, P. Mobilization of Tl-Hg-As-Sb-(Ag,Cu)-Pb sulfosalt melts during low-grade metamorphism in the Alpi Apuane (Tuscany, Italy). Geology 2013, 41, 747–751. [Google Scholar] [CrossRef]
- Hettmann, K.; Kreissig, K.; Rehkämper, M.; Wenzel, T.; Mertz-Kraus, R.; Markl, G. Thallium geochemistry in the metamorphic Lengenbach sulfide deposit, Switzerland: Thallium-isotope fractionation in a sulfide melt. Am. Mineral. 2014, 99, 793–803. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Bagas, L.; Evans, N.J.; Chen, J.; Du, B. Mineralization processes at the giant Jinding Zn–Pb deposit, Lanping Basin, Sanjiang Tethys Orogen: Evidence from in situ trace element analysis of pyrite and marcasite. Geol. J. 2018, 53, 1279–1294. [Google Scholar] [CrossRef]
- Wu, M. Study of geologic characteristics of the Xiangquan thallium deposit, Anhui Province. J. Hefei Univ. Technol. 2006, 29, 1571–1576. [Google Scholar]
- Muntean, J.; Cline, J.; Simon, A.; Longo, A. Magmatic-hydrothermal origin of Nevada’s Carlin-type gold deposits. Nat. Geosci. 2011, 4, 122–127. [Google Scholar] [CrossRef]
- Su, W.; Dong, W.; Zhang, X.; Shen, N.; Hu, R.; Hofstra, A.; Cheng, L.; Xia, Y.; Yang, K. Carlin-type gold deposits in the Dian-Qian-Gui “Golden Triangle” of Southwest China. Econ. Geol. 2018, 20, 157–185. [Google Scholar]
- Xiong, Y.L. Hydrothermal thallium mineralization up to 300 °C: A thermodynamic approach. Ore Geol. Rev. 2007, 32, 291–313. [Google Scholar] [CrossRef]
- Sobott, R.; Klaes, R.; Moh, G. Thallium-containing mineral systems. Part I: Natural assemblages of Tl-sulfosalts and related laboratory experiments. Chen. Erde. 1987, 47, 195–218. [Google Scholar]
- Chen, D.; Ren, D.; Hua, W.; Zou, Z.; Yun, Q. Where is the second thallium ore deposit of Lanmuchang type?-the discovery of Yangjiawan thallium prospect and its prospecting potentiality. J. Guizhou Inst. Technol. 1989, 27, 18–26, (In Chinese with English abstract). [Google Scholar]
- Palinkaš, S.; Hofstra, A.; Percival, T.; Šoštaric, S.; Palinkaš, L.; Bermanec, V.; Pecskay, Z.; Boev, B. Comparison of the Allchar Au-As-Sb-Tl Deposit, Republic of Macedonia, with Carlin-type gold deposits. Rev. Econ. Geol. 2018, 20, 335–363. [Google Scholar]
- Rader, S.T.; Mazdab, F.K.; Barton, M.D. Mineralogical thallium geochemistry and isotope variations from igneous, metamorphic, and metasomatic systems. Geochim. Cosmochim. Acta 2018, 243, 42–65. [Google Scholar] [CrossRef]
- Heinrichs, H.; Schulz-Dobrick, B.; Wedepohl, K. Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochim. Cosmochim. Acta 1980, 44, 1519–1533. [Google Scholar] [CrossRef]
- Radtke, A.S.; Dicksonc, F.W. Carlinite, Tl2S, a new mineral from Nevada. Am. Mineral. 1975, 60, 559–565. [Google Scholar]
- Herrmann, J.; Voegelin, A.; Palatinus, L.; Mangold, S.; Majzlan, J. Secondary Fe-As-Tl mineralization in soils near Buus in the Swiss Jura Mountains. Eur. J. Mineral. 2018, 30, 887–898. [Google Scholar] [CrossRef]
- Nowacki, W.; Edenharter, A.; Engel, P.; Gostojić, M.; Nagl, A. On the Crystal Chemistry of Some Thallium Sulphides and Sulfosalts. In Ore Genesis-The State of Art; Amstutz, G.C., El Goresdy, A., Frenzel, G., Kluth, C., Moh, G., Wauschkuhn, A., Zimmermann, R.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 689–704. [Google Scholar]
- Zemann, J. Thallium in Mineralogie und Geochemie. Mitteilungen der Österreich. Miner. Gesselschaft. 1993, 138, 75–91. [Google Scholar]
- Gržetić, I.; Balić-Žunić, T. The photoelectron spectra of some Tl-Sb sulphosalts. Phys. Chem. Miner. 1993, 20, 285–296. [Google Scholar] [CrossRef]
- Makovicky, E. Modular crystal chemistry of thallium sulfosalts. Minerals 2018, 8, 478. [Google Scholar] [CrossRef] [Green Version]
- Raber, T.; Roth, P. The Lengenbach quarry in Switzerland: Classic locality for rare thallium sulfosalts. Minerals 2018, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, B.A.; Knill, M.D. Geochemistry and genesis of the Lengenbach Pb-Zn-As-Tl-Ba-mineralisation, Binn Valley, Switzerland. Miner. Deposita 1996, 31, 319–339. [Google Scholar] [CrossRef]
- Jovanovski, G.; Boev, B.; Makreski, P.; Stafilov, T.; Boev, I. Intriguing minerals: Lorandite, TlAsS2, a geochemical detector of solar neutrinos. ChemTexts 2019, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Mao, J.; Han, Y.; Jian, W.; Han, J. Discovery of lorandite TIAsS2 at the distal Au-Tl deposit in a skarn system, Fengshan Area, Middle Lower Yangtze River, Eastern China. Acta Geol. Sinica 2017, 91, 1493–1494. (In English) [Google Scholar] [CrossRef]
- Welch, M.D.; Still, J.W.; Rice, C.M.; Stanley, C.J. A new telluride topology: The crystal structure of honeaite Au3TlTe2. Mineral. Mag. 2017, 81, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Rice, C.M.; Welch, M.D.; Still, J.W.; Criddle, A.J.; Stanley, C.J. Honeaite, a new gold-thallium-telluride from the Eastern Goldfields, Yilgarn Craton, Western Australia. Eur. J. Mineral. 2016, 28, 979–990. [Google Scholar] [CrossRef]
- Okrugin, V.; Favero, M.; Liu, A.; Etschmann, B.; Plutachina, E.; Mills, S.; Brugger, J. Smoking gun for thallium geochemistry in volcanic arcs: Nataliyamalikite, TlI, a new thallium mineral from an active fumarole at Avacha Volcano, Kamchatka Peninsula, Russia. Am. Mineral. 2017, 102, 1736–1746. [Google Scholar] [CrossRef]
- Campostrini, I.; Demartin, F.; Gramaccioli, C.M.; Orlandi, P. Hephaistosite. TlPb2Cl5, a new thallium mineral species from La Fossa crater, Vulcano, Aeolian Islands, Italy. Can. Mineral. 2008, 46, 701–708. [Google Scholar] [CrossRef]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.N.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik Volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Voegelin, A.; Pfenninger, N.; Petrikis, J.; Majzlan, J.; Plötze, M.; Senn, A.C.; Göttlicher, J. Thallium speciation and extractability in a thallium-and arsenic-rich soil developed from mineralized carbonate rock. Environ. Sci. Technol. 2015, 49, 5390–5398. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernández, Y.; Villalobos, M.; Marcus, M.A.; Pi-Puig, T.; Zanella, R.; Martínez-Villegas, N. Tl(I) sorption behavior on birnessite and its implications for mineral structural changes. Geochim. Cosmochim. Acta 2019, 248, 356–369. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Shvedov, G.I.; Polonyankin, A.A.; Martin, R.F.; O’Driscoll, B. New and unusual Pd-Tl-bearing mineralization in the Anomal’nyi deposit, Kondyor concentrically zoned complex, northern Khabarovskiy kray, Russia. Mineral. Mag. 2017, 81, 679–688. [Google Scholar] [CrossRef]
- Hammarstrom, J.M.; Seal II, R.R.; Meier, A.L.; Kornfeld, J.M. Secondary sulfate minerals associated with acid drainage in the eastern US: Recycling of metals and acidity in surficial environments. Chem. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wang, G.; Zou, Z.; Chen, Y.M. A new mineral—Lanmuchangite. Acta Mineral. Sinica. 2001, 21, 271–277, (In Chinese with English abstract). [Google Scholar]
- D’Orazio, M.; Mauro, D.; Valerio, M.; Biagioni, C. Secondary sulfates from the Monte Arsiccio Mine (Apuan Alps, Tuscany, Italy): Trace-element budget and role in the formation of acid mine drainage. Minerals 2021, 11, 206. [Google Scholar] [CrossRef]
- Bermanec, V.; Palinkaš, L.A.; Fiket, Ž.; Hrenović, J.; Plenković-Moraj, A.; Kniewald, G.; Boev, B. Interaction of acid mine drainage with biota in the Allchar Carlin-type As-Tl-Sb-Au deposit, Macedonia. J. Geochem. Explor. 2018, 194, 104–119. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E. Argentobaumhauerite: Name, chemistry, crystal structure, comparison with baumhauerite, and position in the Lengenbach mineralization sequence. Mineral. Mag. 2016, 80, 819–840. [Google Scholar] [CrossRef]
- Graeser, S.; Edenharter, A. Jentschite (TlPbAs2SbS6)-a new sulphosalt mineral from Lengenbach, Binntal (Switzerland). Mineral. Mag. 1992, 72, 293–305. [Google Scholar]
- Biagioni, C.; Bonaccorsi, E.; Moëlo, Y.; Orlandi, P.; Bindi, L.; D’Drazio, M.; Vezzoni, S. Mercury-arsenic sulfosalts from the Apuan Alps (Tuscany, Italy). II. Arsiccioite, AgHg2TlAs2S6, a new mineral from the Monte Arsiccio mine: Occurrence, crystal structure and crystal chemistry of the routhierite isotypic series. Mineral. Mag. 2014, 78, 101–111. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Belakovskiy, D.I.; Yapaskurt, V.O.; Vigasina, M.F.; Lykova, I.S.; Sidorov, E.G.; Pushcharovsky, D.Y. Chrysothallite K6Cu6Tl3+Cl17(OH)4∙H2O, a new mineral species from the Tolbachik volcano, Kamchatka, Russia. Mineral. Mag. 2015, 79, 365–376. [Google Scholar] [CrossRef]
- Miyawaki, R.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) Newsletter 57. Eur. J. Mineral. 2020, 32, 498. [Google Scholar] [CrossRef]
- Siidra, O.I.; Vergasova, L.P.; Kretser, Y.L.; Polekhovsky, Y.S.; Filatov, S.K.; Krivovichev, S.V. Unique thallium mineralization in the fumaroles of Tolbachik volcano, Kamchatka Peninsula, Russia. II. Karpovite, Tl2VO(SO4)2(H2O). Mineral. Mag. 2014, 78, 1699–1709. [Google Scholar] [CrossRef]
- Radtke, A.S.; Dickson, F.W.; Slack, J.F. Occurrence and formation of avicennite, Tl2O3, as a secondary mineral at the Carlin gold deposit, Nevada. Res. U.S. Geol. Surv. 1978, 6, 241–246. [Google Scholar]
- Pašava, J.; Pertlik, F.; Stumpfl, E.F.; Zemann, J. Bernardite, a new thallium arsenic sulphosalt from Allchar, Macedonia, with a determination of the crystal structure. Mineral. Mag. 1989, 53, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Bindi, L.; Moëlo, Y. Biagioniite, Tl2SbS2, from the Hemlo gold deposit, Marathon, Ontario, Canada: Occurrence and crystal structure. Mineral. Mag. 2015, 79, 1089–1098. [Google Scholar] [CrossRef]
- Radtke, A.S.; Taylor, C.M.; Erd, R.C.; Dickson, F. Occurrence of lorandite, TlAsS2, at the Carlin gold deposit, Nevada. Econ. Geol. 1974, 69, 121–123. [Google Scholar] [CrossRef]
- Orlandi, P.; Biagioni, C.; Bonaccorsi, E.; Moëlo, Y.; Paar, W.H. Lead-antomony sulfosalts from Tuscany (Italy). XII. Boscardinite, TlPb4(Sb7As2)9S18, a new species from Monte Arsiccio mine: Occurrence and crystal structure. Can. Mineral. 2012, 50, 235–251. [Google Scholar] [CrossRef]
- Siidra, O.I.; Vergasova, L.P.; Krivovichev, S.V.; Kretser, Y.L.; Zaitsev, A.N.; Filatov, S.K. Unique thallium mineralization in the fumaroles of Tolbachik volcano, Kamchatka Peninsula, Russia. I. Markhininite, TlBi(SO4)2. Mineral. Mag. 2014, 78, 1687–1698. [Google Scholar] [CrossRef]
- Makovicky, E.; Johan, Z.; Karup-Møeller, S. New data on bukovite, thalcusite, chalcothallite and rohaite. Neues Jahrb. Mineral. Abh. 1980, 138, 122–146. [Google Scholar]
- Biagioni, C.; Moëlo, Y.; Perchiazzi, N.; Demitri, N.; Zaccarini, F. Parapierrotite from the Monte Arsiccio mine (Apuan Alps, Tuscany, Italy): Occurrence and new data on its crystal-chemistry. Eur. J. Mineral. 2019, 31, 1055–1065. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Biagioni, C.; Moëlo, Y.; Orlandi, P. Chabournéite from Monte Arsiccio Mine (Apuan Alps, Tuscany, Italy): Occurrence and Crystal Structure. In Proceedings of the 20th General Meeting of the IMA (IMA2010), Budapest, Hungary, 21–27 August 2010. [Google Scholar]
- Artioli, G.; Kvick, A. Synchrotron X-ray Rietveld study of perlialite, the natural counterpart of synthetic zeolite-L, Sample: Data collected with synchrotron radiation. Eur. J. Mineral. 1990, 2, 749–759. [Google Scholar] [CrossRef]
- Bindi, L.; Nestola, F.; Makovicky, E.; Guastoni, A.; De Battisti, L. Tl-bearing sulfosalt from the Lengenbach quarry, Binn Valley, Switzerland: Philrothite, TlAs3S5. Mineral. Mag. 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Radtke, A.S.; Dickson, F.W.; Slack, J.F.; Brown, K.L. Christite, a new thallium mineral from the Carlin gold deposit, Nevada. Am. Mineral. 1977, 62, 421–425. [Google Scholar]
- Balić-Žunić, T.; Karanovic, L.; Poleti, D. Crystal structure of picotpaulite, TlFe2S3 from Allchar, FYR Macedonia. Acta Chim. Slov. 2008, 55, 801–809. [Google Scholar]
- Engel, P.; Gostojić, M.; Nowacki, W. The crystal structure of pierrotite, Tl2(Sb,As)10S16. Z. Krist.-Cryst. Mater. 1983, 165, 209–215. [Google Scholar]
- Harris, D.C.; Roberts, A.C.; Laflamme, J.H.G.; Stanley, C.J. Criddleite, TlAg2Au3Sb10S10, a new gold-bearing mineral from Hemlo, Ontario, Canada. Mineral. Mag. 1988, 52, 691–697. [Google Scholar] [CrossRef]
- Miyawaki, R.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) Newsletter 55. Mineral. Mag. 2020, 84, 486. [Google Scholar] [CrossRef]
- Palache, C.; Berman, H.; Frondel, C. The System of Mineralogy, Volume I: Elements, Sulfides, Sulfosalts, Oxides, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1944; Volume 183, pp. 484–485. [Google Scholar]
- Orlandi, P.; Biagioni, C.; Moëlo, Y.; Bonaccorsi, E.; Paar, W.H. Lead-antimony sulfosalts from Tuscany (Italy). XIII. Protochabournéite, ~Tl2Pb(Sb9–8As1–2)∑10S17, from the Monte Arsiccio mine: Occurrence, crystal structure and relationship with chabournéite. Can. Mineral. 2013, 51, 475–494. [Google Scholar] [CrossRef]
- Hålenius, U.; Ålinder, C. Occurrence and formation of cuprostibite in a Zn–Pb–Ag mineralized siliceous dolomite at Långsjön, central Sweden. Neues Jahrb. Mineral. Monatsh. 1982, 5, 201–215. [Google Scholar]
- Bindi, L.; Nestola, F.; Guastoni, A.; Peruzzo, L.; Ecker, M.; Carampin, R. Raberite, Tl5Ag4As6SbS15, a new Tl-bearing sulfosalt from Lengenbach quarry, Binn valley, Switzerland: Description and crystal structure. Mineral. Mag. 2012, 76, 1153–1163. [Google Scholar] [CrossRef]
- Nestola, F.; Guastoni, L.; Bindi, L.; Secco, L. Dalnegroite, Tl5-xPb2x(As,Sb)21-xS34, a new thallium sulphosalt from Lengenbach quarry, Binntal, Switzerland. Mineral. Mag. 2009, 73, 1027–1032. [Google Scholar] [CrossRef]
- Laurent, Y.; Picot, P.; Pierrot, R.; Ivanov, T. La Raguinite, TlFeS2, une nouvelle espèce minérale et Ie problème de l’allcharite. Bull. Minéralogie. 1969, 92, 38–48, (In French with English abstract). [Google Scholar]
- Hålenius, U.; Hatert, F. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) Newsletter 40. Eur. J. Mineral. 2017, 29, 1084. [Google Scholar]
- Bindi, L.; Biagioni, C.; Raber, T.; Roth, P.; Nestola, F. Ralphcannonite, AgZn2TlAs2S6, a new mineral of the routhierite isotypic series from Lengenbach, Binn Valley, Switzerland. Mineral. Mag. 2015, 79, 1089–1098. [Google Scholar] [CrossRef]
- Balic-Žunic, T.; Moëlo, Y.; Loncar, Z.; Micheelsen, H. Dorallcharite, Tl0.8K0.2Fe3(SO4)2(OH)6, a new member of the jarosite-alunite family. Eur. J. Mineral. 1994, 6, 255–263. [Google Scholar]
- Berlepsch, P.; Armbruster, T.; Topa, D. Structural and chemical variations in rathite, Pb8Pb4−x(Tl2As2)x(Ag2As2)As16S40: Modulations of a parent structure. Z. Krist.-Cryst. Mater. 2002, 217, 580–590. [Google Scholar] [CrossRef]
- Miyawak, R.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) Newsletter 52. Eur. J. Mineral. 2020, 32, 3–4. [Google Scholar]
- Basu, K.; Bortinikov, N.S.; Mookherjee, A.; Mozgova, N.N.; Tsepin, A.I.; Vyal’sov, L.N. Rare minerals from Rajpura-Dariba, Rajasthan, India. IV: A new Pb–Ag–Tl–Sb sulfosalt, rayite. Neues Jahrb. Mineral. Monatsh. 1983, 7, 296–304. [Google Scholar]
- Topa, D.; Kolitsch, U.; Makovicky, E.; Stanley, C. Écrinsite, AgTl3Pb4As11Sb9S36, a new thallium-rich homeotype of baumhauerite from the Jas Roux sulphosalt deposit, Parc National des Écrins, Hautes-Alpes, France. Eur. J. Mineral. 2017, 29, 689–700. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Šcavničar, S.; Engel, P. The crystal structure of rebulite, Tl5Sb5As8S22. Z. Krist.-Cryst. Mater. 1982, 160, 109–126. [Google Scholar]
- Graeser, S.; Schwander, H. Edenharterite (TlPbAs3S6): A new mineral from Lengenbach, Binntal (Switzerland). Eur. J. Mineral. 1992, 4, 1265–1270. [Google Scholar] [CrossRef]
- Meisser, N.; Roth, P.; Nestola, F.; Biagioni, C.; Bindi, L.; Robyr, M. Richardsollyite, TlPbAsS3, a new sulfosalt from the Lengenbach quarry, Binn Valley, Switzerland. Eur. J. Mineral. 2017, 29, 679–688. [Google Scholar] [CrossRef]
- Dickson, F.W.; Radtke, A.S.; Peterson, J.A. Ellisite, Tl3AsS3, a new mineral from the Carlin gold deposit, Nevada, and associated sulfide and sulfosalt minerals. Am. Mineral. 1979, 64, 701–770. [Google Scholar]
- Graeser, S.; Schwaner, H.; Wulf, R.; Edenharter, A. Erniggliite (Tl2SnAs2S6), a new mineral from Lengenbach, Binntal (Switzerland): Description and crystal stucture determination based on data from synchrotron radiation. Schweiz. Mineral. Petrogr. Mitteilungen. 1992, 5, 259–260. [Google Scholar]
- Biagioni, C.; Bonaccorsi, E.; Moëlo, Y.; Orlandi, P. Mercury-arsenic sulfosalts from Apuan Alps (Tuscany, Italy). I. Routhierite, (Cu0.8Ag0.2)Hg2Tl(As1.4Sb0.6)∑=2S6, from Monte Arsiccio mine: Occurrenceand crystal structure. Eur. J. Mineral. 2014, 26, 163–170. [Google Scholar] [CrossRef]
- Topa, D.; Makovicky, E.; Stöger, B.; Stanley, C. Heptasartorite, Tl7Pb22As55S108, enneasartorite, Tl6Pb32As70S140 and hendekasartorite, Tl2Pb48As82S172, three members of the anion-omission series of ‘sartorites’ from the Lengenbach quarry at Binntal, Wallis, Switzerland. Eur. J. Mineral. 2017, 29, 701–712. [Google Scholar] [CrossRef]
- Johan, Z.; Kvacek, M.; Picot, P. La sabatierite, un nouveau seléniure de cuivre et de thalliumJ. Bull. Mineral. 1978, 101, 557–560. [Google Scholar]
- Siidra, O.I.; Vergasova, L.P.; Kretser, Y.L.; Polekhovsky, Y.S.; Filatov, S.K.; Krivovichev, S.V. Unique thallium mineralization in the fumaroles of Tolbachik volcano, Kamchatka Peninsula, Russia. III. Evdokimovite, Tl4(VO)3(SO4)5(H2O)5. Mineral. Mag. 2014, 78, 1711–1724. [Google Scholar] [CrossRef]
- Turchkova, A.G.; Pekov, I.V.; Yapaskurt, V.O.; Sidorov, E.G.; Britvin, S.N. Manganese mineralization in fumarole deposits at the Tolbachik volcano (Kamchatka, Russia). IX Intern. Sympos. Miner. Divers. Res. Preserv. Sofia 2017, 9. [Google Scholar]
- Wilson, J.R.; Sen Gupta, P.K.; Robinson, P.D.; Criddle, A.J. Fangite, Tl3AsS4, a new thallium arsenic sulfosalt from the Mercur Au deposit, Utah, and revised optical data for gillulyite. Am. Mineral. 1993, 78, 1096–1103. [Google Scholar]
- Graeser, S.; Berlepsch, P.; Makovvicky, E.; Balić-Žunić, T. Sicherite, TlAg2(As,Sb)3S6, a new sulfosalt mineral from Lengenbach (Binntal, Switzerland): Description and structure determination. Mineral. Mag. 2001, 86, 1087–1093. [Google Scholar] [CrossRef]
- Biagioni, C.; Bindi, L.; Nestola, F.; Cannon, R.; Roth, P.; Raber, T. Ferrostalderite, CuFe2TlAs2S6, a new mineral from Lengenbach, Switzerland: Occurrence, crystal structure, and emphasis on the role of iron in sulfosalts. Mineral. Mag. 2014, 80, 175–186. [Google Scholar] [CrossRef]
- Engel, P.; Nowacki, W. The crystal structure of simonite, TIHgAs3S6. Z. Krist.-Cryst. Mater. 1982, 161, 159–166. [Google Scholar]
- Kasatkin, A.; Nestola, F.; Agakhanov, A.A.; Škoda, R.; Karpenko, V.Y.; Tsyganko, M.; Plášil, J. Vorontsovite, (Hg5Cu)S6TlAs4S12, and Ferrovorontsovite, (Fe5Cu)S6TlAs4S12: The Tl- and Tl-Fe-analogues of galkhaite from the Vorontsovskoe Gold Deposit, Northern Urals, Russia. Minerals 2018, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Willams, P.A.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) – Newsletter 20. Mineral. Mag. 2014, 78, 553–557. [Google Scholar]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Britvin, S.N.; Vigasina, M.F.; Sidorov, E.G.; Pushcharovsky, D.Y. New zinc and potassium chlorides from fumaroles of the Tolbachik volcano, Kamchatka, Russia: Mineral data and crystal chemistry. II. Flinteite, K2ZnCl4. Eur. J. Mineral. 2015, 27, 581–588. [Google Scholar] [CrossRef]
- Graeser, S.; Schwander, H.; Wulf, R. Stalderite TlCu (Zn,Fe,Hg)2As2S6—A new mineral related to routhierite: Description and crystal structure determination. Schweiz. Mineral. Petrogr. Mitt. 1995, 75, 337–345. [Google Scholar]
- Graeser, S.; Topa, D.; Balić-Žunić, T.; Makovicky, E. Gabrielite, Tl2AgCu2As3S7, a new species of thallium sulfosalt from Lengenbach, Binntal, Switzerland. Can. Mineral. 2006, 44, 135–140. [Google Scholar] [CrossRef]
- Demartin, F.; Gramaccioli, C.M.; Campostrini, I. Steropesite, Tl3BiCl6, a new thallium bismuth chloride from La Fossa crater, Vulcano, Aeolian islands, Italy. Can. Mineral. 2009, 47, 373–380. [Google Scholar] [CrossRef]
- Kasatkin, A.V.; Makovicky, E.; Plášil, J.; Škoda, R.; Chukanov, N.V.; Stepanov, S.Y.; Agakhanov, A.A.; Nestola, F. Gladkovskyite, MnTlAs3S6, a new thallium sulfosalt from the Vorontsovskoe gold deposit, Northern Urals, Russia. J. Geosci. 2019, 64, 207–218. [Google Scholar] [CrossRef]
- Rudashevskiy, N.S. Thalfenisite, the thallium analog of djerfisherite. Int. Geol. Rev. 1982, 24, 116–122. [Google Scholar] [CrossRef]
- Miyawaki, R.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) Newsletter 56. Mineral. Mag. 2020, 84, 623. [Google Scholar] [CrossRef]
- Marumo, F.; Nowacki, W. The crystal structure of hatchite, PbTlAgAs2S5. Z. Krist.-Cryst. Mater. 1967, 125, 249–265. [Google Scholar]
- Bindi, L.; Roberts, A.C. Thunderbayite, TlAg3Au3Sb7S6, a new gold-bearing mineral from the Hemlo gold deposit, Marathon, Ontario, Canada. Mineral. Mag. 2020, 84, 805–812. [Google Scholar] [CrossRef]
- Kasatkin, A.V.; Makovicky, E.; Plášil, J.; Škoda, R.; Agakhanov, A.A.; Karpenko, V.Y.; Nestola, F. Tsygankoite, Mn8Tl8Hg2(Sb21Pb2Tl)Σ24S48, a New Sulfosalt from the Vorontsovskoe Gold Deposit, Northern Urals, Russia. Minerals 2018, 8, 218. [Google Scholar] [CrossRef] [Green Version]
- Makovicky, E.; Topa, D. Twinnite, Pb0.8Tl0.1Sb1.3As0.8S4, the OD character and the question of its polytypism. Z. Krist.-Cryst. Mater. 2012, 227, 468–475. [Google Scholar]
- Harris, D.C.; Roberts, A.C.; Criddle, A.J. Vaughanite (TlHgSb4S7), a new mineral from the Hemlo gold deposit, Hemlo, Ontario, Canada. Mineral. Mag. 1989, 53, 79–83. [Google Scholar] [CrossRef]
- Takéuchi, Y.; Ghose, S.; Nowacki, W. The crystal structure of hutchinsonite, (Tl,Pb)2As5S9. Z. Krist.-Cryst. Mater. 1965, 121, 321–348. [Google Scholar]
- Balić-Žunić, T.; Makovicky, E. Contributions to the crystal chemistry of thallium sulphosalts I: The O–D nature of imhofite. Neues Jahrb. Mineral. Abh. 1993, 165, 317–330. [Google Scholar]
- Ohmasa, M.; Nowacki, W. The crystal structure of vrbaite, Hg3Tl4As8Sb2S20. Z. Krist.-Cryst. Mater. 1971, 134, 360–380. [Google Scholar]
- Hålenius, U.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) Newsletter 33. Mineral. Mag. 2016, 80, 1136. [Google Scholar]
- Takéuchi, Y.; Ohmasa, M. The crystal structure of wallisite, PbTlCuAs2S5, the Cu analogue of hatchite PbTlAgAs2S5. Z. Krist.-Cryst. Mater. 1968, 127, 349–365. [Google Scholar] [CrossRef]
- Dickson, F.W.; Radtke, A.S. Weissbergite, TlSbS2, a new mineral from the Carlin gold deposit, Nevada. Am. Mineral. 1978, 63, 720–724. [Google Scholar]
- Cvetković, L.; Boronikhin, V.A.; Pavićević, M.K.; Krajnović, D.; Gržetić, I.; Libowitzky, E.; Tillmanns, E. Jankovićite, Tl5Sb9(As,Sb)4S22, a new Tl-sulfosalt from Allchar, Macedonia. Miner. Petrol. 1995, 53, 125–131. [Google Scholar] [CrossRef]
- Johan, Z.; Udubasa, G.; Zemann, J. “Monsmedite”, a discredited potassium thallium sulphate mineral from Baia Sprie and its identity with voltaite: The state of the art. Neues Jahrb. Mineral. Abh. 2009, 186, 63–66. [Google Scholar] [CrossRef]
- Bourgoin, V.; Favreau, G.; Boulliard, J.C. Jas Roux: Un gisement exceptionnel à minéraux de thallium. Cah. Micromon. 2011, 3, 2–91. [Google Scholar]
- Makreski, P.; Stefov, S.; Pejov, L.; Jovanovski, G. Minerals from Macedonia. XXIX. Experimental and theoretical study of the vibrational spectra of extremely rare Tl-sulfate mineral from Allchar–Dorallcharite. Vib. Spectrosc. 2017, 89, 85–91. [Google Scholar] [CrossRef]
- Kovács-Pálffy, P.; Muske, J.; Földváric, M.; Kónyac, M.; Homonnayc, Z.; Ntaflosc, T.; Papp, G.; Király, E.; Sajó, I.; Szilágyi, V.; et al. Detailed study of “monsmedite” specimens from the original (1963) find, Baia Sprie, Baia Mare ore district (Romania). Carpathian J. Earth Environ. Sci. 2011, 6, 321–330. [Google Scholar]
- Lin, J.; Yin, M.; Wang, J.; Liu, J.; Tsang, D.C.; Wang, Y.; Chen, Y. Geochemical fractionation of thallium in contaminated soils near a large-scale Hg-Tl mineralised area. Chemosphere 2020, 239, 124775. [Google Scholar] [CrossRef]
- Long, D.; Fegan, N.E.; McKee, J.D.; Lyons, W.B.; Hines, M.E.; Macumber, P.G. Formation of alunite, jarosite and hydrous iron oxides in a hypersaline system: Lake Tyrrell, Victoria, Australia. Chem. Geol. 1992, 96, 183–202. [Google Scholar] [CrossRef]
- Smeaton, C.M.; Fryer, B.J.; Weisener, C.G. Intracellular precipitation of Pb by Shewanella putrefaciens CN32 during the reductive dissolution of Pb-jarosite. Environ. Sci. Technol. 2009, 43, 8091–8096. [Google Scholar] [CrossRef]
- Smith, A.M.L.; Dubbin, W.E.; Wright, K.; Hudson-Edwards, K.A. Dissolution of lead- and lead-arsenic-jarosites at pH 2 and 8 and 20 degrees C: Insights from batch experiments. Chem. Geol. 2006, 229, 344–361. [Google Scholar] [CrossRef]
- Weisener, C.G.; Babechuk, M.G.; Fryer, B.J.; Maunder, C. Microbial dissolution of silver jarosite: Examining its trace metal behaviour in reduced environments. Geomicrobiol. J. 2008, 25, 415–424. [Google Scholar] [CrossRef]
- Bingjie, O.; Xiancai, L.; Huan, L.; Juan, L.; Tingting, Z.; Xiangyu, Z.; Jianjun, L.; Rucheng, W. Reduction of jarosite by Shewanella oneidensis MR-1 and secondary mineralization. Geochim. Cosmochim. Acta 2014, 124, 54–71. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, C.; Lu, G.; Yi, X.; Wang, H.; Dang, Z. Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan mine. Sci. Total Environ. 2018, 616, 647–657. [Google Scholar] [CrossRef]
- Gao, K.; Jiang, M.; Guo, C.; Zeng, Y.; Fan, C.; Zhang, J.; Reinfelder, J.R.; Huang, W.; Lu, G.; Dang, Z. Reductive dissolution of jarosite by a sulfate reducing bacterial community: Secondary mineralization and microflora development. Sci. Total Environ. 2019, 690, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Yang, D.; Zhang, W.; Wang, H.; Zeng, R. Anaerobic reductive bio-dissolution of jarosites by Acidithiobacillus ferrooxidans using hydrogen as electron donor. Sci. Total Environ. 2019, 686, 869–877. [Google Scholar] [CrossRef]
- Casiot, C.; Egal, M.; Bruneel, O.; Verma, N.; Parmentier, M.; Elbaz-Poulichet, F. Predominance of aqueous Tl (I) species in the river system downstream from the abandoned Carnoulès mine (Southern France). Environ. Sci. Technol. 2011, 45, 2056–2064. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate- determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Gu, X.; Heaney, P.J.; Reis, F.D.A.; Brantley, S.L. Deep abiotic weathering of pyrite. Science 2020, 370, 1–8. [Google Scholar] [CrossRef] [PubMed]
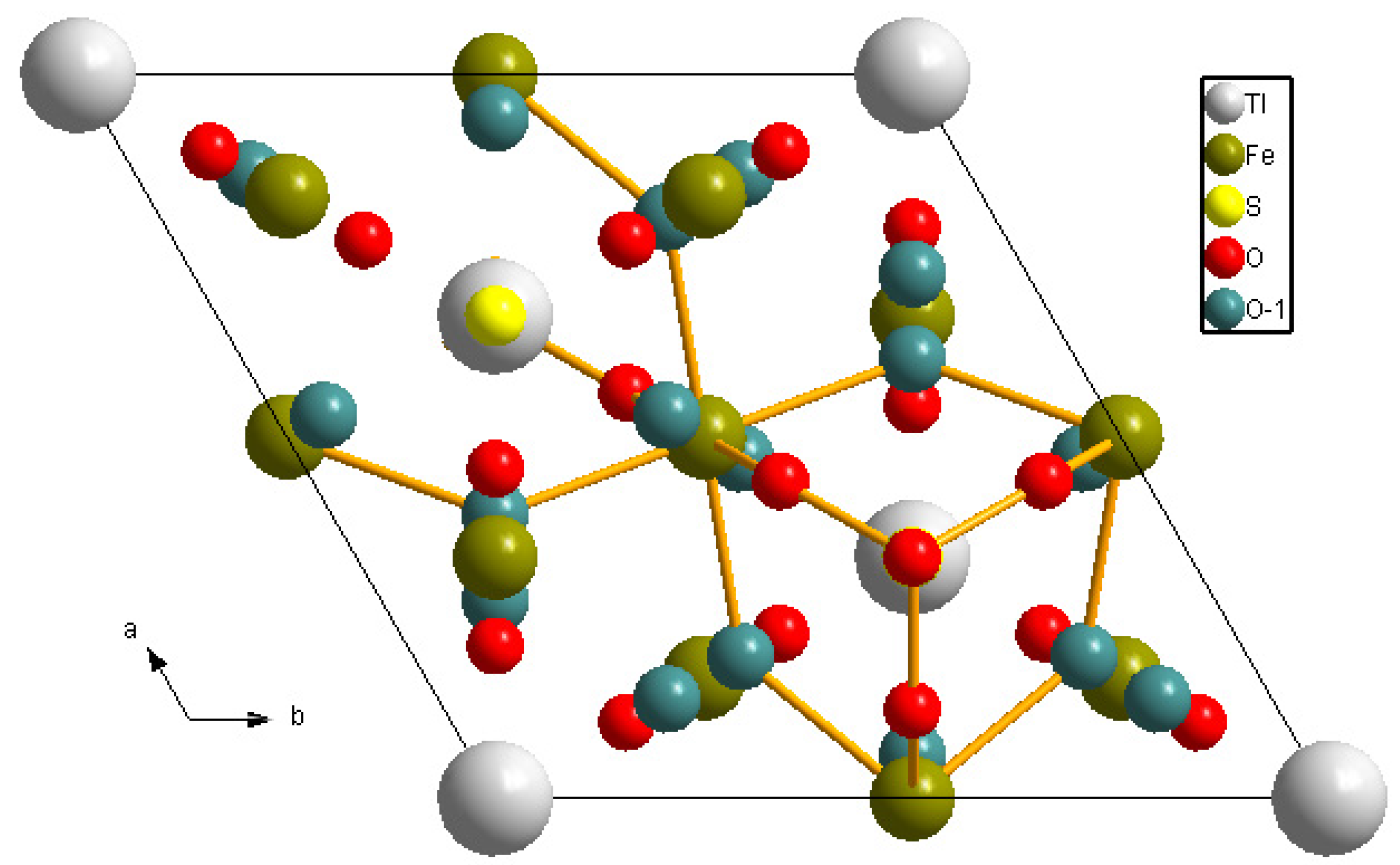

| Mineral | Chemical Formula | Crystal System | Unit-Cell Parameters |
|---|---|---|---|
| Lanmuchangite | TlAl(SO4)2·12H2O | Isometric | a = 12.212 Å, V = 1871 Å3 |
| Dorallcharite | TlFe3(SO4)2(OH)6 | Trigonal | a = 7.3301, c = 17.6631 Å, V = 821.73 Å3, |
| Evdokimovite | Tl4(VO)3(SO4)5·(H2O)5 | Monoclinic | a = 6.2958, b = 10.110, c = 39.426 Å, V = 2509.4 Å3, β = 90.347° |
| Karpovite | Tl2VO(SO4)2(H2O) | Monoclinic | a = 4.6524, b = 11.0757, c = 9.3876 Å, V = 478.60 Å3, β = 98.353° |
| Markhininite | TlBi(SO4)2 | Triclinic | a = 7.378, b = 10.657, c = 10.657 Å, V = 680.2 Å3, α = 61.31, β = 70.964, γ = 70.964° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Gu, S. Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance. Minerals 2021, 11, 855. https://doi.org/10.3390/min11080855
Zhao F, Gu S. Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance. Minerals. 2021; 11(8):855. https://doi.org/10.3390/min11080855
Chicago/Turabian StyleZhao, Fengqi, and Shangyi Gu. 2021. "Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance" Minerals 11, no. 8: 855. https://doi.org/10.3390/min11080855







