Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Elaboration of Samples
2.3. Characterization Techniques
2.4. Photocatalytic Experiments
2.5. Photoluminescence Experiments
3. Results and Discussion
3.1. X-ray Diffraction Analyses
3.2. Scanning Electron Microscopy: Morphology of the Powders
3.3. Raman Spectroscopy Study
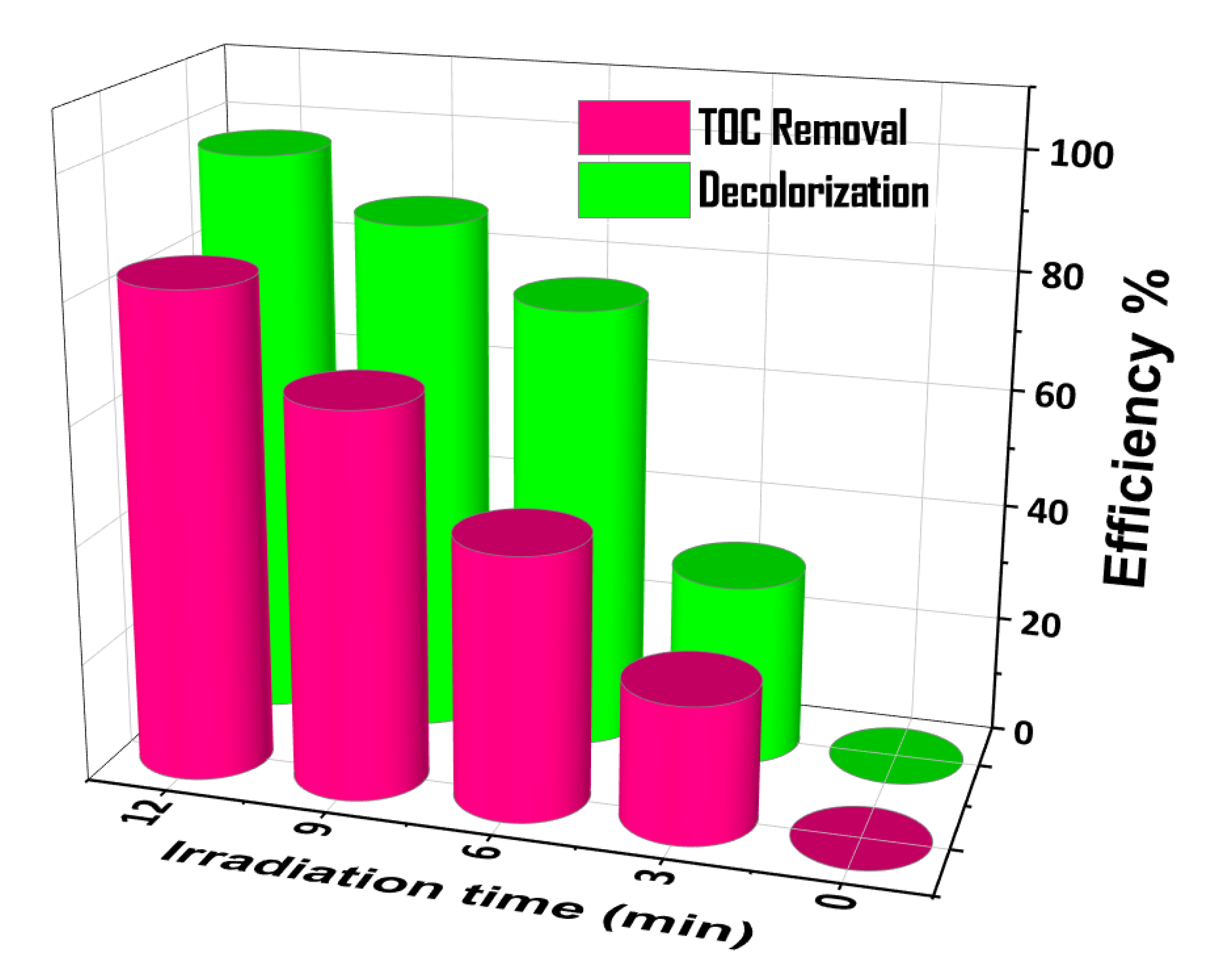
4. Photocatalytic Properties
| BiPO4 | → | BiPO4 (h+, e−) |
| BiPO4 (e−) + O2 | → | O2•− |
| BiPO4 (h+) + H2O | → | OH− + H+ |
| BiPO4 (h+) + OH− | → | OH• |
| BiPO4 (h+) + RhB | → | RhB+ |
| RhB + O2− + h+ + OH• | → | CO2 + H2O + simple molecules |
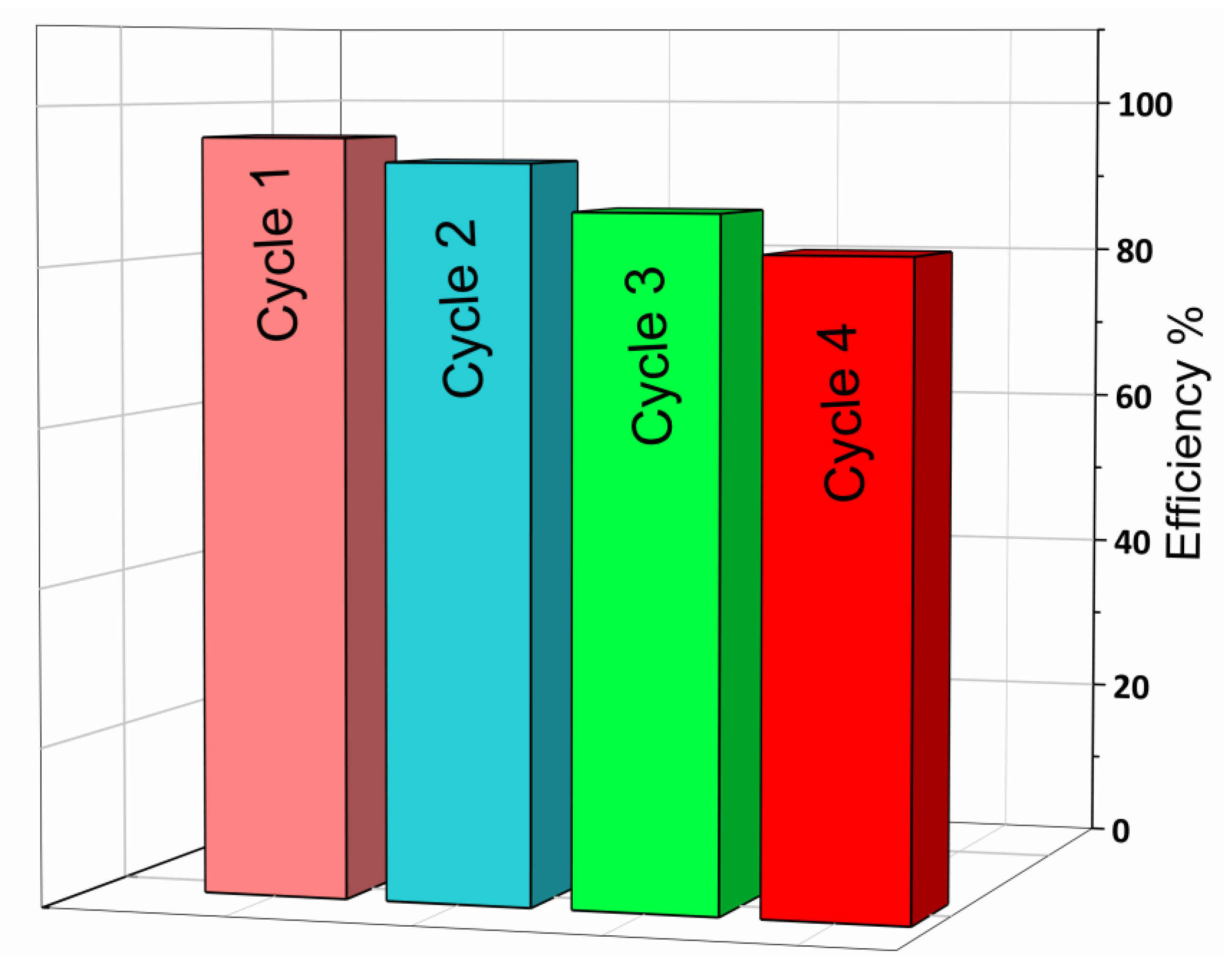
Stability of the BiP-500 °C Catalyst
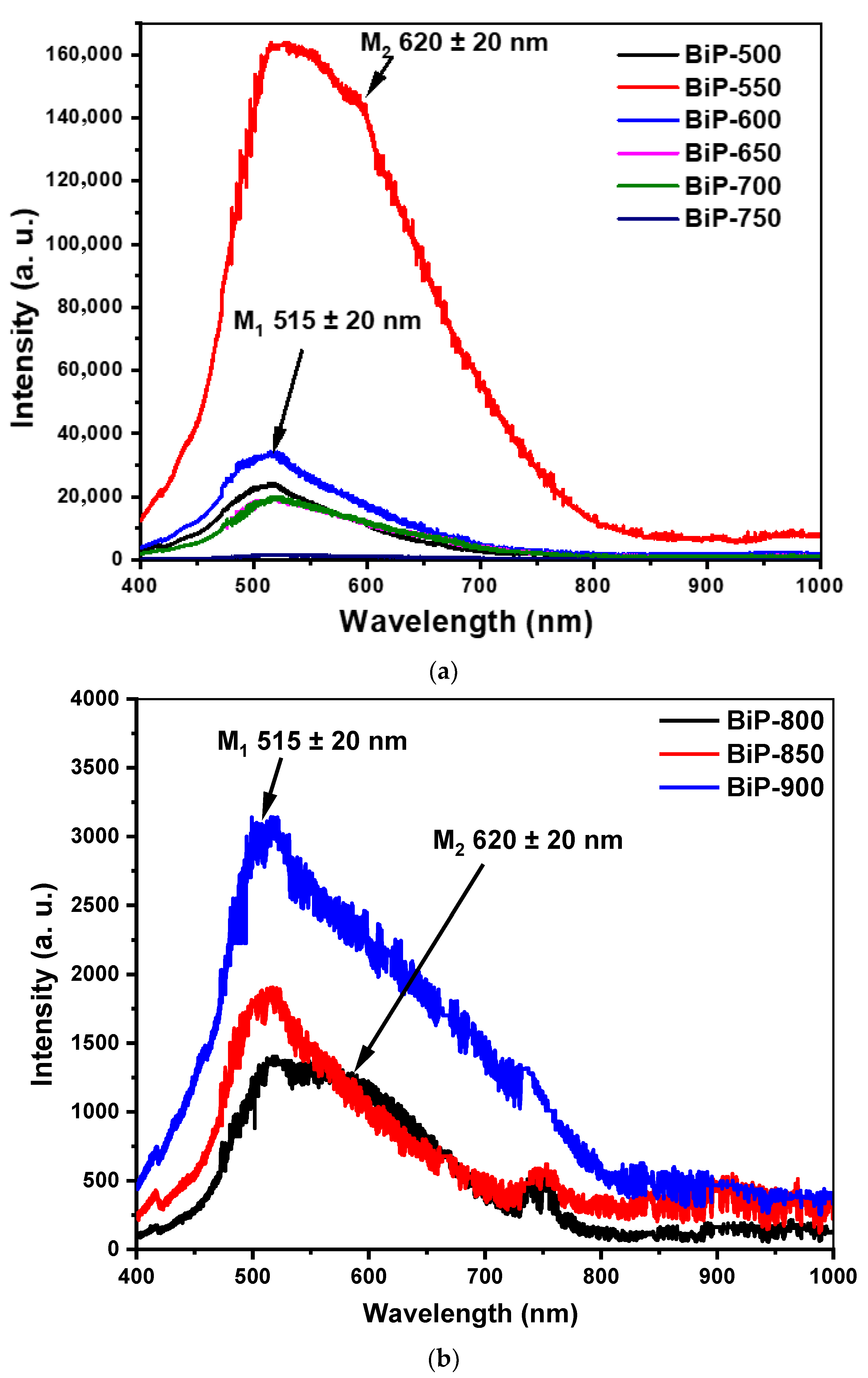
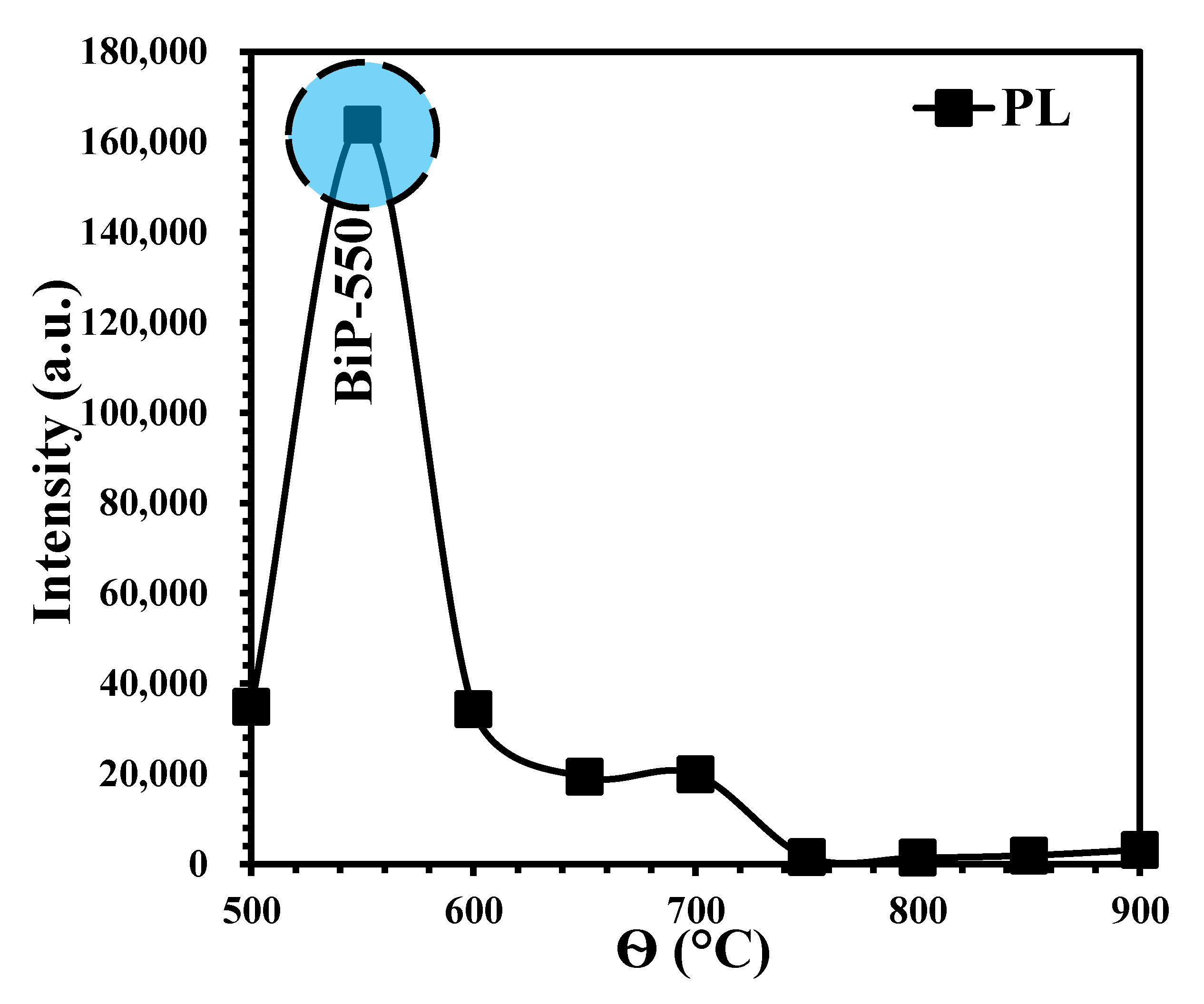
5. Photoluminescent Properties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Mamun, M.; Kader, S.; Islam, M.; Khan, M. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Tounsadi, H.; Metarfi, Y.; Taleb, M.; El Rhazi, K.; Rais, Z. Impact of chemical substances used in textile industry on the employee’s health: Epidemiological study. Ecotoxicol. Environ. Saf. 2020, 197, 110594. [Google Scholar] [CrossRef]
- Haounati, R.; Ouachtak, H.; El Haouti, R.; Akhouairi, S.; Largo, F.; Akbal, F.; Benlhachemi, A.; Jada, A.; Addi, A.A. Elaboration and properties of a new SDS/CTAB@Montmorillonite organoclay composite as a superb adsorbent for the removal of malachite green from aqueous solutions. Sep. Purif. Technol. 2020, 255, 117335. [Google Scholar] [CrossRef]
- Chennah, A.; Naciri, Y.; Taoufyq, A.; Bakiz, B.; Bazzi, L.; Guinneton, F.; Villain, S.; Gavarri, J.R.; Benlhachemi, A. Electrodeposited zinc phosphate hydrate electrodes for electrocatalytic applications. J. Appl. Electrochem. 2018, 49, 163–177. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Ajmal, Z.; Bouddouch, A.; Bakiz, B.; Navío, J.; Albourine, A.; Valmalette, J.-C.; Ezahri, M.; Benlhachemi, A. Influence of Sr-doping on structural, optical and photocatalytic properties of synthesized Ca3(PO4)2. J. Colloid Interface Sci. 2020, 572, 269–280. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Taoufyq, A.; Bakiz, B.; Guinneton, F.; Villain, S.; Valmalette, J.; Gavarri, J.; Benlhachemi, A. Photocatalytic and photoluminescent properties of a system based on SmPO4 nanostructure phase. Mater. Today Proc. 2020, 27, 3139–3144. [Google Scholar] [CrossRef]
- Souza, R.P.; Ambrosio, E.; Souza, M.T.F.; Freitas, T.K.F.S.; Ferrari-Lima, A.M.; Garcia, J.C. Solar photocatalytic degradation of textile effluent with TiO2, ZnO, and Nb2O5 catalysts: Assessment of photocatalytic activity and mineralization. Environ. Sci. Pollut. Res. 2017, 24, 12691–12699. [Google Scholar] [CrossRef]
- Maleki, A.; Safari, M.; Shahmoradi, B.; Zandsalimi, Y.; Daraei, H.; Gharibi, F. Photocatalytic degradation of humic substances in aqueous solution using Cu-doped ZnO nanoparticles under natural sunlight irradiation. Environ. Sci. Pollut. Res. 2015, 22, 16875–16880. [Google Scholar] [CrossRef]
- Frederichi, D.; Scaliante, M.H.N.O.; Bergamasco, R. Structured photocatalytic systems: Photocatalytic coatings on low-cost structures for treatment of water contaminated with micropollutants—A short review. Environ. Sci. Pollut. Res. 2021, 28, 23610–23633. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: State of the art and present applications In honor of Pr. R.L. Burwell Jr. (1912–2003), Former Head of Ipatieff Laboratories, Northwestern University, Evanston (Ill). Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Im, J.-K.; Son, H.-S.; Kang, Y.-M.; Zoh, K.-D. Carbamazepine Degradation by Photolysis and Titanium Dioxide Photocatalysis. Water Environ. Res. 2012, 84, 554–561. [Google Scholar] [CrossRef]
- Chi, C.; Pan, J.; You, M.; Dong, Z.; Zhao, W.; Song, C.; Zheng, Y.; Li, C. The porous TiO2 nanotubes/Ag3PO4 heterojunction for enhancing sunlight photocatalytic activity. J. Phys. Chem. Solids 2018, 114, 173–178. [Google Scholar] [CrossRef]
- Farbod, M.; Khademalrasool, M. Synthesis of TiO2 nanoparticles by a combined sol–gel ball milling method and investigation of nanoparticle size effect on their photocatalytic activities. Powder Technol. 2011, 214, 344–348. [Google Scholar] [CrossRef]
- Radwan, E.K.; Yu, L.; Achari, G.; Langford, C.H. Photocatalytic ozonation of pesticides in a fixed bed flow through UVA-LED photoreactor. Environ. Sci. Pollut. Res. 2016, 23, 21313–21318. [Google Scholar] [CrossRef] [PubMed]
- Amaterz, E.; Tara, A.; Bouddouch, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Jbara, O. Photo-electrochemical degradation of wastewaters containing organics catalysed by phosphate-based materials: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 843–872. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, A.W.; Zhu, Y. Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Haounati, R.; El Guerdaoui, A.; Ouachtak, H.; El Haouti, R.; Bouddouch, A.; Hafid, N.; Bakiz, B.; Santos, D.; Taha, M.L.; Jada, A.; et al. Design of direct Z-scheme superb magnetic nanocomposite photocatalyst Fe3O4/Ag3PO4@Sep for hazardous dye degradation. Sep. Purif. Technol. 2021, 277, 119399. [Google Scholar] [CrossRef]
- Ellouzi, I.; Bouddouch, A.; Bakiz, B.; Benlhachemi, A.; Oualid, H.A. Glucose-assisted ball milling preparation of silver-doped biphasic TiO2 for efficient photodegradation of Rhodamine B: Effect of silver-dopant loading. Chem. Phys. Lett. 2021, 770, 138456. [Google Scholar] [CrossRef]
- Jan, T.; Azmat, S.; Wahid, B.; Adil, M.; Alawadhi, H.; Mansoor, Q.; Farooq, Z.; Ilyas, S.; Ahmad, I.; Ismail, M. Chemically synthesized ZnO-Bi2O3 (BZO) nanocomposites with tunable optical, photoluminescence and antibacterial characteristics. Mater. Sci. Semicond. Process. 2018, 84, 71–75. [Google Scholar] [CrossRef]
- Bao, N.; Liu, Y.; Li, Z.-W.; Yu, H.; Bai, H.-T.; Xia, L.; Feng, D.-W.; Zhang, H.-B.; Dong, X.-T.; Wang, T.-Y.; et al. Construction of order mesoporous (Eu–La)/ZnO composite material and its luminescent characters. J. Lumin. 2016, 177, 409–415. [Google Scholar] [CrossRef]
- Ajmal, M.; Ali, T.; Mian, S.A.; Khan, M.A.; Ahmad, S.; Khan, A.A. Effects of Ce3+-doping concentration on the luminescent properties of La2O3: Ce3+ phosphors. Mater. Today Proc. 2017, 4, 4924–4929. [Google Scholar] [CrossRef]
- Amaterz, E.; Bouddouch, A.; Tara, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Jbara, O. Correlation between photoluminescence and photoelectrochemical properties of SrHPO4/BaHPO4/FTO anode material. Opt. Mater. 2020, 109, 110268. [Google Scholar] [CrossRef]
- Ansari, A.A.; Aldalbahi, A.; Labis, J.P.; El-Toni, A.M.; Ahamed, M.; Manthrammel, M. Highly biocompatible, monodispersed and mesoporous La(OH)3:Eu@mSiO2 core-shell nanospheres: Synthesis and luminescent properties. Colloids Surf. B Biointerfaces 2018, 163, 133–139. [Google Scholar] [CrossRef]
- García-Murillo, A.; Carrillo-Romo, F.D.J.; Oliva-Uc, J.; Esquivel-Castro, T.A.; de la Torre, S.D. Effects of Eu content on the luminescent properties of Y2O3:Eu3+ aerogels and Y(OH)3/Y2O3:Eu3+@SiO2 glassy aerogels. Ceram. Int. 2017, 43, 12196–12204. [Google Scholar] [CrossRef]
- Taoufyq, A.; Mauroy, V.; Guinneton, F.; Bakiz, B.; Villain, S.; Hallaoui, A.; Benlhachemi, A.; Nolibe, G.; Lyoussi, A.; Gavarri, J.-R. Role of the chemical substitution on the luminescence properties of solid solutions Ca(1 − x)Cd(x)WO4 (0 ≤ x ≤ 1). Mater. Res. Bull. 2015, 70, 40–46. [Google Scholar] [CrossRef]
- Bakiz, B.; Hallaoui, A.; Taoufyq, A.; Benlhachemi, A.; Guinneton, F.; Villain, S.; Ezahri, M.; Valmalette, J.-C.; Arab, M.; Gavarri, J.-R. Luminescent properties under X-ray excitation of Ba(1 − x)PbxWO4 disordered solid solution. J. Solid State Chem. 2018, 258, 146–155. [Google Scholar] [CrossRef]
- Hallaoui, A.; Taoufyq, A.; Arab, M.; Bakiz, B.; Benlhachemi, A.; Bazzi, L.; Villain, S.; Valmalette, J.-C.; Guinneton, F.; Gavarri, J.-R. Influence of chemical substitution on the photoluminescence of Sr(1−x)PbWO4 solid solution. J. Solid State Chem. 2015, 227, 186–195. [Google Scholar] [CrossRef]
- Hallaoui, A.; Taoufyq, A.; Arab, M.; Bakiz, B.; Benlhachemi, A.; Bazzi, L.; Valmalette, J.-C.; Villain, S.; Guinneton, F.; Gavarri, J.-R. Structural, vibrational and photoluminescence properties of Sr(1−x)PbxMoO4 solid solution synthesized by solid state reaction. Mater. Res. Bull. 2016, 79, 121–132. [Google Scholar] [CrossRef]
- Xue, F.; Li, H.; Zhu, Y.; Xiong, S.; Zhang, X.; Wang, T.; Liang, X.; Qian, Y. Solvothermal synthesis and photoluminescence properties of BiPO4 nano-cocoons and nanorods with different phases. J. Solid State Chem. 2009, 182, 1396–1400. [Google Scholar] [CrossRef]
- Du, G.; Kan, X.; Han, Y.; Sun, Z.; Guo, W. Structure and luminescence of YPO4:Dy3+ microflowers. Mater. Lett. 2012, 74, 229–231. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, H.; Dong, J.; Yang, C.; Gan, S.; Zou, L. Facile hydrothermal synthesis and luminescent properties of Sm3+/Eu3+ codoped GdPO4 phosphors. J. Phys. Chem. Solids 2017, 111, 355–363. [Google Scholar] [CrossRef]
- Kirubanithy, M.; Irudayaraj, A.A.; Raj, A.D.; Manikandan, S. Synthesis, Characterization and Photoluminescence Behaviours of CePO4 and Tb-doped CePO4 Nanostructures. Mater. Today Proc. 2015, 2, 4344–4347. [Google Scholar] [CrossRef]
- Zhang, W.; NI, Y.; Huang, W.; Lu, C.; Xu, Z. Hydrothermal synthesis, structure study and luminescent properties of YbPO4:Tb3+ nanoparticles. J. Rare Earths 2010, 28, 299–302. [Google Scholar] [CrossRef]
- Bühler, G.; Feldmann, C. Microwave-Assisted Synthesis of Luminescent LaPO4:Ce, Tb Nanocrystals in Ionic Liquids. Angew. Chem. Int. Ed. 2006, 45, 4864–4867. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.-R.; Valmalette, J.-C.; Benlhachemi, A. Customized synthesis of functional bismuth phosphate using different methods: Photocatalytic and photoluminescence properties enhancement. Nanotechnol. Environ. Eng. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Valmalette, J.; Gavarri, J.; Benlhachemi, A. Photocatalytic and photoluminescence properties of CePO4 nanostructures prepared by coprecipitation method and thermal treatment. Optik 2021, 238, 166683. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Bouziani, A.; Djellabi, R.; Ajmal, Z.; Laabd, M.; Navío, J.A.; Mills, A.; Bianchi, C.L.; Li, H.; et al. Photocatalytic oxidation of pollutants in gas-phase via Ag3PO4-based semiconductor photocatalysts: Recent progress, new trends, and future perspectives. Crit. Rev. Environ. Sci. Technol. 2021, 1–44. [Google Scholar] [CrossRef]
- Binas, V.; Sambani, K.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Synthesis and photocatalytic activity of Mn-doped TiO2 nanostructured powders under UV and visible light. Appl. Catal. B Environ. 2012, 113–114, 79–86. [Google Scholar] [CrossRef]
- Zhu, Y.; Ling, Q.; Liu, Y.; Wang, H.; Zhu, Y. Photocatalytic performance of BiPO4 nanorods adjusted via defects. Appl. Catal. B Environ. 2016, 187, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Li, D.; Ma, X.; Chen, Y.; Zhu, Y. Effects of distortion of PO4 tetrahedron on the photocatalytic performances of BiPO4. Catal. Sci. Technol. 2011, 1, 1399–1405. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, Y. A review of BiPO4, a highly efficient oxyacid-type photocatalyst, used for environmental applications. Catal. Sci. Technol. 2015, 5, 3071–3083. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Lv, Y.; Ling, Q.; Liu, D.; Zhu, Y. Enhancement of photocatalytic activity for BiPO4via phase junction. J. Mater. Chem. A 2014, 2, 13041–13048. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Ling, Q.; Zhu, Y. Enhancement of full-spectrum photocatalytic activity over BiPO4/Bi2WO6 composites. Appl. Catal. B Environ. 2017, 200, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Selvaraj, R.; Sillanpää, M.; Kim, Y.; Tai, C.-W. The influence of operating parameters on heterogeneous photocatalytic mineralization of phenol over BiPO4. Chem. Eng. J. 2014, 245, 117–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Sillanpää, M.; Obregón, S.; Colón, G. A novel two-steps solvothermal synthesis of nanosized BiPO4 with enhanced photocatalytic activity. J. Mol. Catal. A Chem. 2015, 402, 92–99. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Xu, J.; Bai, X.; Zong, R.; Zhu, Y. Degradation and mineralization mechanism of phenol by BiPO4 photocatalysis assisted with H2O2. Appl. Catal. B Environ. 2013, 142–143, 561–567. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Lu, Q.; Sun, H.; Xiu, Z. Synthesis of Mesoporous BiPO4 Nanofibers by Electrospinning with Enhanced Photocatalytic Performances. Ind. Eng. Chem. Res. 2014, 53, 13023–13029. [Google Scholar] [CrossRef]
- Lv, Y.; Zhu, Y.; Zhu, Y. Enhanced Photocatalytic Performance for the BiPO4–x Nanorod Induced by Surface Oxygen Vacancy. J. Phys. Chem. C 2013, 117, 18520–18528. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Chang, H.-W.; Lu, S.-Y.; Liu, C.W. Preparation, Characterization, and Electrophysical Properties of Nanostructured BiPO4 and Bi2Se3 Derived from a Structurally Characterized, Single-Source Precursor Bi[Se2P(OiPr)2]3. J. Phys. Chem. C 2007, 111, 18538–18544. [Google Scholar] [CrossRef]
- Li, G.; Ding, Y.; Zhang, Y.; Lu, Z.; Sun, H.; Chen, R. Microwave synthesis of BiPO4 nanostructures and their morphology-dependent photocatalytic performances. J. Colloid Interface Sci. 2011, 363, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Hou, W.-H.; Lv, Y.-N.; Zhu, J.-J.; Chen, H.-Y. One-Dimensional BiPO4Nanorods and Two-Dimensional BiOCl Lamellae: Fast Low-Temperature Sonochemical Synthesis, Characterization, and Growth Mechanism. Inorg. Chem. 2005, 44, 8503–8509. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Xu, J.; Chen, Y.; Zhu, Y. Influence of OH-related defects on the performances of BiPO4 photocatalyst for the degradation of rhodamine B. Appl. Catal. B Environ. 2012, 115–116, 314–319. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, Y. New Type of BiPO4 Oxy-Acid Salt Photocatalyst with High Photocatalytic Activity on Degradation of Dye. Environ. Sci. Technol. 2010, 44, 5570–5574. [Google Scholar] [CrossRef]
- Becerro, A.I.; Criado, J.; Gontard, L.C.; Obregón, S.; Fernández, A.; Colón, G.; Ocaña, M. Bifunctional, Monodisperse BiPO4-Based Nanostars: Photocatalytic Activity and Luminescent Applications. Cryst. Growth Des. 2014, 14, 3319–3326. [Google Scholar] [CrossRef] [Green Version]
- Yan-Yan, Z.; Yan-Fang, L.; Yan-Hui, L.; Hua, W.; Qiang, L.; Yong-Fa, Z. Reflux Preparation and Photocatalytic Performance of Bismuth Phosphate Nanorods. Acta Phys.-Chim. Sin. 2013, 29, 576–584. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, H.; Li, N.; Chen, M.; Teng, F. Controllable growth of novel BiPO4 dendrites by an innovative approach and high energy facets-dependent photocatalytic activity. CrystEngComm 2014, 16, 8334–8339. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Guo, C.; Zhang, Y. Removal of rhodamine B from aqueous solution by BiPO4 hierarchical architecture. Front. Environ. Sci. Eng. 2013, 7, 382–387. [Google Scholar] [CrossRef]
- Ye, H.; Lin, H.; Cao, J.; Chen, S.; Chen, Y. Enhanced visible light photocatalytic activity and mechanism of BiPO4 nanorods modified with AgI nanoparticles. J. Mol. Catal. A Chem. 2014, 397, 85–92. [Google Scholar] [CrossRef]
- Cheng, L.-W.; Tsai, J.-C.; Huang, T.-Y.; Huang, C.-W.; Unnikrishnan, B.; Lin, Y.-W. Controlled synthesis, characterization and photocatalytic activity of BiPO4 nanostructures with different morphologies. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.; Ezahri, M.; Valmalette, J.; Benlhachemi, A. Role of thermal decomposition process in the photocatalytic or photoluminescence properties of BiPO4 polymorphs. Water Environ. Res. 2020, 92, 1874–1887. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, L.; Li, G.; Li, L.; Zheng, J. Exploring the unique electrical properties of metastable BiPO4 through switchable phase transitions. CrystEngComm 2013, 15, 609–615. [Google Scholar] [CrossRef]
- Poloznikova, M.E.; Fomichev, V.V. The vibrational spectra and characteristic features of the orthophosphates of Group I–III elements. Russ. Chem. Rev. 1994, 63, 399–409. [Google Scholar] [CrossRef]
- Silva, E.; Ayala, A.P.; Guedes, I.; Paschoal, C.; Moreira, R.; Loong, C.-K.; Boatner, L. Vibrational spectra of monazite-type rare-earth orthophosphates. Opt. Mater. 2006, 29, 224–230. [Google Scholar] [CrossRef]
- Heuser, J.; Bukaemskiy, A.; Neumeier, S.; Neumann, A.; Bosbach, D. Raman and infrared spectroscopy of monazite-type ceramics used for nuclear waste conditioning. Prog. Nucl. Energy 2014, 72, 149–155. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Dong, S.-Y.; Sun, J.-H.; Feng, J.-L.; Wu, Q.-H.; Sun, S.-P. Microwave-assisted preparation, characterization and photocatalytic properties of a dumbbell-shaped ZnO photocatalyst. J. Hazard. Mater. 2010, 179, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effects of ZnO Nanocrystals on Photocatalytic Activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wang, N.; Quan, X.; Chen, Y. Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities. Sep. Purif. Technol. 2008, 62, 727–732. [Google Scholar] [CrossRef]

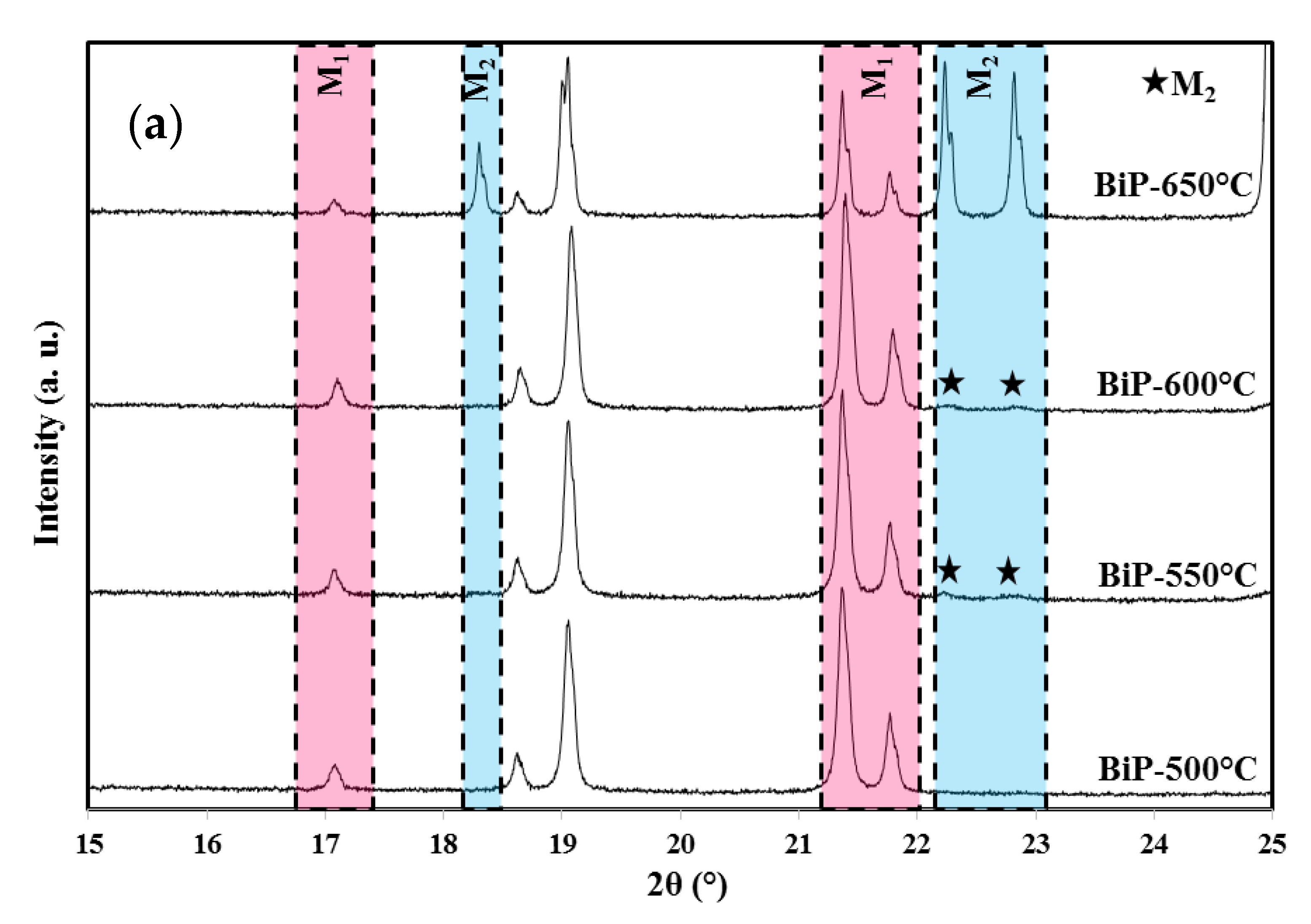
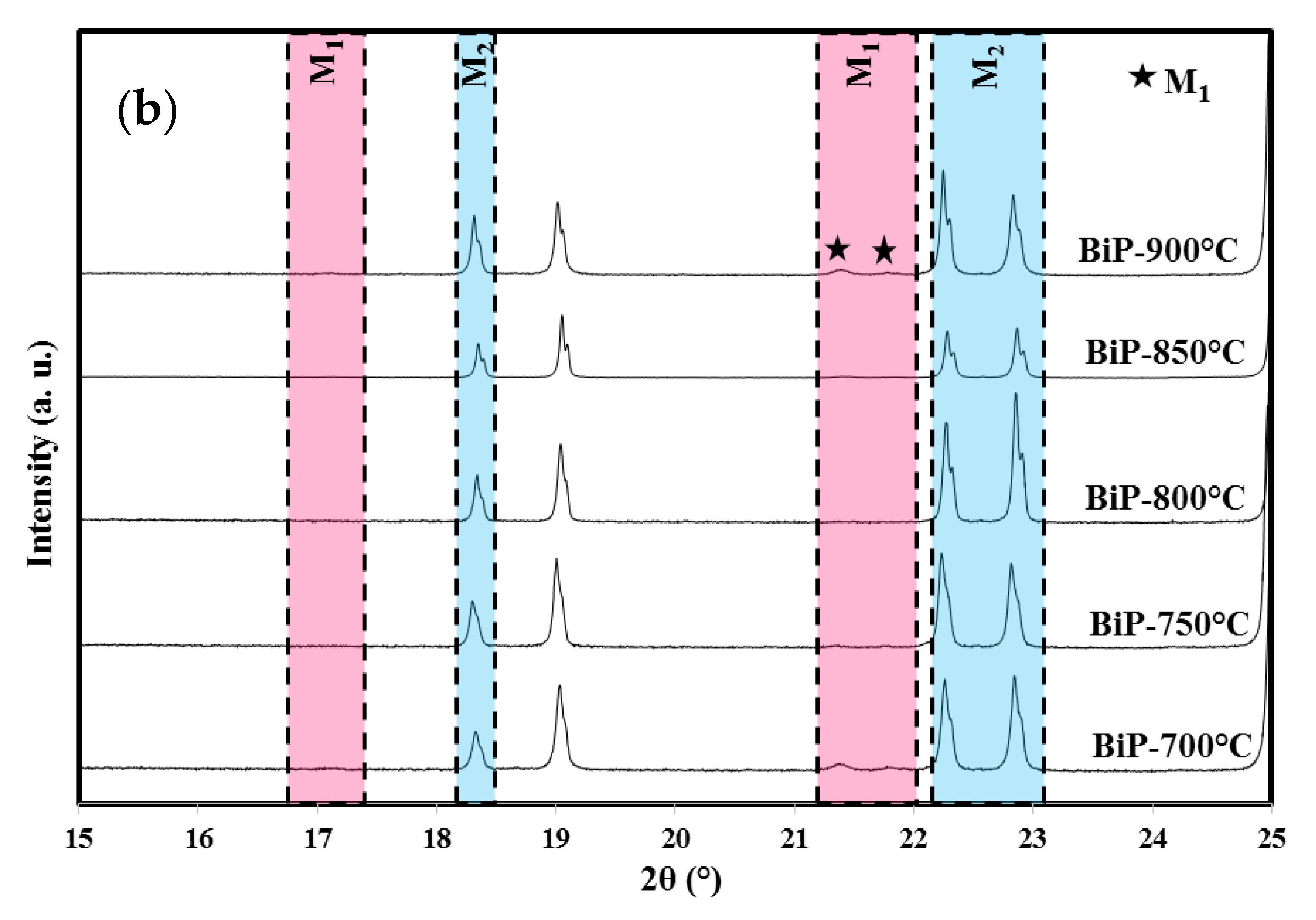

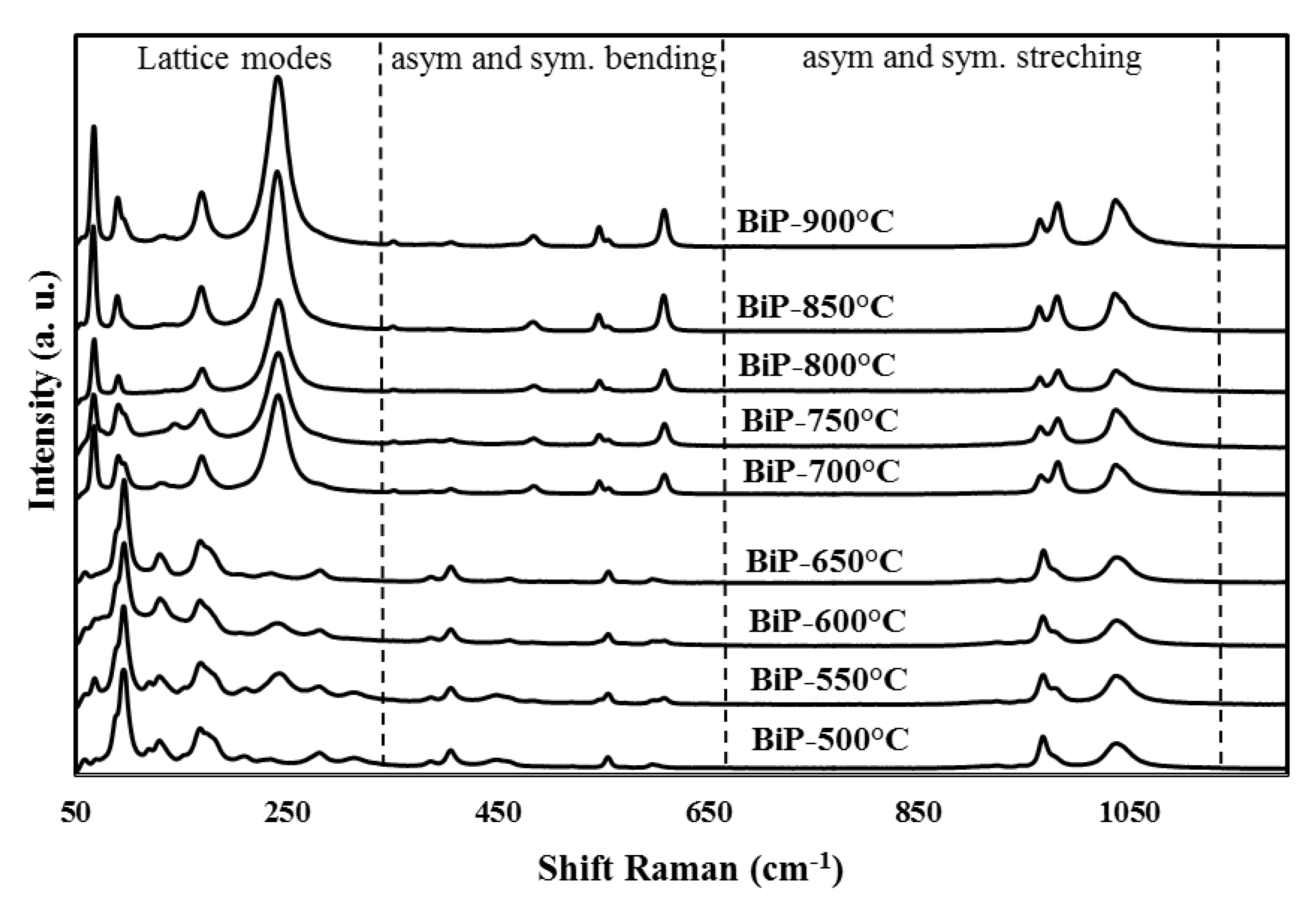

| Methods (Preparation Method/Raw Materials/Optimized Conditions) | Photocatalytic Activity (in Comparison to the Apparent Kinetics Constant kapp) | Reference |
|---|---|---|
| Hydrothermal Reaction/Na3PO4 + Bi(NO3)3/pH = 1, 180 °C, 96 h | kapp = 0.1225 min−1 for [RhB] = 10−5 M, (UV-254 11 W, cat. 0.5 g/L); as twice as that of P25 | [53] |
| Hydrothermal Reaction/Na3PO4 + Bi(NO3)3/pH = 1, 180 °C, 72 h | kapp = 0.1839 min−1 for [RhB] = 10−5 M, (UV-254 11 W, cat. 0.5 g/L); as twice as that of P25 | [53,54] |
| Hydrothermal Reaction/Na3PO4 + Bi(NO3)3/pH = 1, 180 °C, 48 h | kapp = 0.0858 min−1 for [RhB] = 10−5 M, (UV-254 11 W, cat. 0.5 g/L); as twice as that of P25 | [53] |
| Hydrothermal Reaction/Na3PO4 + Bi(NO3)3/pH = 1, 180 °C, 12 h | kapp = 0.0594 min−1 for [RhB] = 10−5 M, (UV-254 11 W, cat. 0.5 g/L); as twice as that of P25 | [53] |
| Solvothermal reaction/H3PO4 + Bi(NO3)3 + CA-Na/EG-glycerol solution, 120 °C, 20 h | kapp = 0.53 h−1 for [RhB] = 10−5 M and 0.12 h−1 for 10 ppm MB (UV-254 4 W × 6, cat. 1 g/L) | [55] |
| Flux/Bi(NO3)3 + NaH2PO4/pH = 2.2, Bi3+/PO43− = 1:5, 48 h | kapp = 0.193 min−1 (UV-254 11 W, 0.5 g/L, [MB] = 10−5 M; | [56] |
| Hydrothermal Reaction/NaH2PO4 + Bi(NO3)3/160 °C, 24 h | kapp = 0.016 min−1 for 50 ppm phenol (UV-254 11 W, cat. 0.5 g/L); Or kapp = 0.036 min−1 by adding 60 ppm H2O2 | [47] |
| Microwave synthesis/NaH2PO4 + Bi(NO3)3/glycerol, EG or DEG aq, 800 W, 15 min | kapp = 0.035 min−1 for 10 mg/L MO (500 W Xe lamp, cat. 1 g/L) | [51] |
| Hydrothermal reaction/Na3PO4 + Bi(NO3)3 + EDTA/HNO3, 180 °C, 24 h | kapp = 0.0294 min−1 for [MB] = 10−5 M (Xe 500 W, 0.5 g/L, cat. 0.8 g/L) | [57] |
| Electrospinning method/Bi(NO3)3 + (NH4)3PO4·+ Citric acid/PVP 0.1 g/mL, pH = 1, Electrospinning voltage 25 kV, speed 1.5 µm/s | kapp = 0.01638 min−1 for 10−5 M APMP pulping effluent (Xe 500 W λ ≤ 400 nm, 0.5 g/L, cat. 1.3 g/L) as 3.7 times as that of P25 | [48] |
| Coprecipitation method/Bi(NO3)3∙5H2O + NaH2PO4∙2H2O + glycerol − H2O dried at 120 °C for 12 h, calcination in 500 °C for 6 h | kapp = 0.1089 min−1 (UV-254 15 W, 0.5 g/L, [MB] = 3.10−5 M; | [43] |
| Hydrothermal Reaction/Na3PO4∙12 H2O+ Bi(NO3)3∙5H2O/, 180 °C, 72 h | kapp = 0.071 min−1 for [RhB] = 10−1 M (mercury lamp 100 W, cat. 1 g/L) | [58] |
| Hydrothermal Reaction/Na2HPO4∙12 H2O + Bi(NO3)3∙5H2O/pH = 3, 180 °C, 24 h | kapp = 0.001 min−1 for 10 mg/L RhB (500 W Xe lamp, cat. 1.6 g/L) | [59] |
| Hydrothermal reaction + ultrasonic irradiation (20 kHz)/Na3PO4 + Bi(NO3)3 + HNO3/strong sonicated for 30 min and followed by 180 °C, 24 h | kapp = 0.1589 min−1 for 5 mg/L MB (UV-253.9 10 W Xe lamp, cat. 4 g/L) | [60] |
| Process Parameters | Weight Fraction | ||
|---|---|---|---|
| Calcined Powder | Sintering Temperature (°C) | M1 | M2 |
| M1 | 500 | 1 | 0 |
| 550 | 0.91 | 0.09 | |
| 600 | 0.86 | 0.14 | |
| 650 | 0.44 | 0.56 | |
| 700 | 0.34 | 0.66 | |
| 750 | 0.19 | 0.81 | |
| M2 | 800 | 0.02 | 0.98 |
| 850 | 0.07 | 0.93 | |
| 900 | 0.26 | 0.74 | |
| Photocatalyst | Atomic Percentage of Bi | Atomic Percentage of P | Experimental Ratio |
|---|---|---|---|
| BiP-500 | 50.13 | 49.87 | 1.005 |
| BiP-550 | 49.96 | 50.04 | 1.001 |
| BiP-600 | 50.46 | 49.54 | 1.010 |
| BiP-650 | 50.91 | 49.09 | 1.037 |
| BiP-700 | 50.40 | 49.60 | 1.016 |
| BiP-750 | 50.43 | 49.57 | 1.017 |
| BiP-800 | 51.72 | 48.28 | 1.071 |
| BiP-850 | 50.01 | 49.99 | 1.000 |
| BiP-900 | 51.73 | 48.27 | 1.071 |
| Raman Data. Wavenumbers in cm−1. | ||
|---|---|---|
| M1 | M2 | Assignments |
| 1039 | 1037 | PO4 Asym. Stretching. mode ν3 |
| 983 | ||
| 969 | 965 | PO4 Sym. Stretching. mode ν1 |
| 929 | ||
| 597 | 609 | ν4 Bending modes of PO4 |
| 556 | 547 | |
| 461 | 485 | ν2 Bending modes of PO4 |
| 388 | 352 | |
| 281 | 242 | Bending modes of O–Bi–O |
| 169 | 169 | Stretching vibrations of Bi–O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouddouch, A.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Guinneton, F.; Villain, S.; Gavarri, J.-R.; Valmalette, J.-C.; Benlhachemi, A. Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B. Minerals 2021, 11, 1007. https://doi.org/10.3390/min11091007
Bouddouch A, Amaterz E, Bakiz B, Taoufyq A, Guinneton F, Villain S, Gavarri J-R, Valmalette J-C, Benlhachemi A. Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B. Minerals. 2021; 11(9):1007. https://doi.org/10.3390/min11091007
Chicago/Turabian StyleBouddouch, Abdessalam, Elhassan Amaterz, Bahcine Bakiz, Aziz Taoufyq, Frédéric Guinneton, Sylvie Villain, Jean-Raymond Gavarri, Jean-Christophe Valmalette, and Abdeljalil Benlhachemi. 2021. "Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B" Minerals 11, no. 9: 1007. https://doi.org/10.3390/min11091007
APA StyleBouddouch, A., Amaterz, E., Bakiz, B., Taoufyq, A., Guinneton, F., Villain, S., Gavarri, J.-R., Valmalette, J.-C., & Benlhachemi, A. (2021). Phase Transformation, Photocatalytic and Photoluminescent Properties of BiPO4 Catalysts Prepared by Solid-State Reaction: Degradation of Rhodamine B. Minerals, 11(9), 1007. https://doi.org/10.3390/min11091007







