Bentonite Alteration in Batch Reactor Experiments with and without Organic Supplements: Implications for the Disposal of Radioactive Waste
Abstract
:1. Introduction
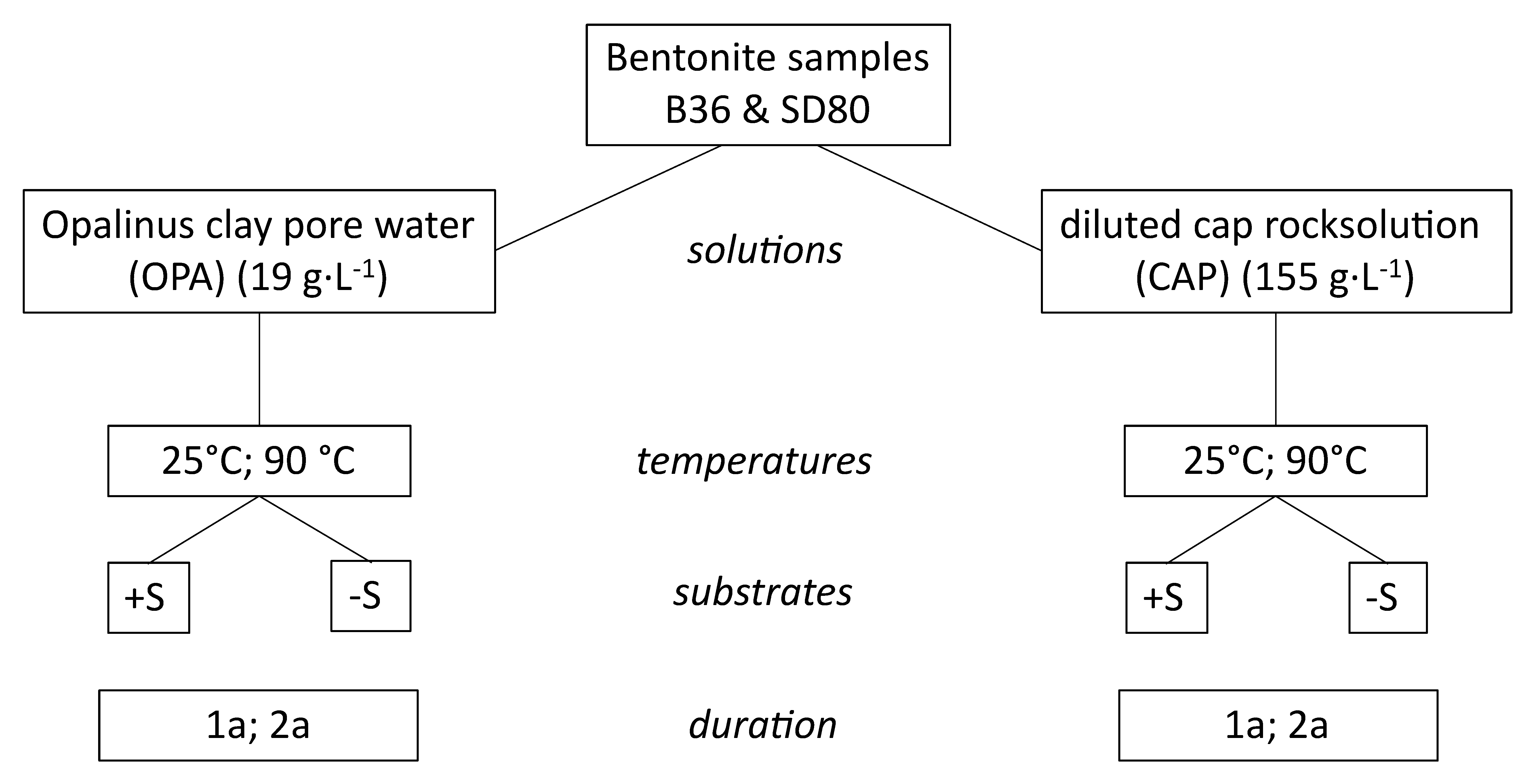
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Bentonite Characterization (XRF, CEC, XRD)
2.3. Smectite Characterization (SEM-EDX)
2.4. Solution Chemistry (pH, ICP-OES)
2.5. DNA Extraction, Amplification of 16S rRNA Gene and Sequencing
3. Results
3.1. Characterization of Starting Material
3.2. Characterization of the Reacted Bentonites
3.2.1. Visual Changes of the Bentonite Batches
3.2.2. Microbial Diversity Analysis
3.2.3. X-ray Diffraction
3.2.4. Smectite Layer Charge Distribution
3.2.5. Solution Chemistry of the Supernatant
4. Discussion
4.1. Smectite Alteration Mechanisms
4.1.1. Interlayer Cation Exchange
4.1.2. Tetrahedral and Octahedral Charge Distribution
4.2. Microbial Diversity and Its Potential Influence on the Mineralogy
4.3. Implications for a Real Repository Scenario
5. Conclusions
- After experimentation, no neoformation of minerals was observed. Mineralogical and chemical changes can be attributed to interlayer cation exchange reactions, particle delamination and tetrahedral as well as octahedral metal ion substitutions. These changes are more pronounced at higher salinity and elevated temperatures.
- The initial charge distribution determines the reactivity of the smectite, with octahedral charge dominated smectites (e.g., SD80) being less susceptible to these alterations. However, the influence of accessory minerals (e.g., feldspar, calcite, pyrite) on the environment and smectite alteration should not be neglected with regard to the long-term stability of the bentonite barrier.
- Considering the microbial influence on a potential HLW repository, the detected genera in SD80 appear to be more important than the specialized microorganisms detected in bentonite B36 due to their potential to reduce sulfate in order to form H2S, and thus, promoting the corrosion of metal canisters. Further, it should be noted that the microbial diversity changed with respect to the bentonite and to the applied conditions used in this study. As a result, bentonite-inherent microorganisms may have a potential negative long-term effect on the barrier system. This should be considered when selecting bentonites as buffer material.
- The reaction kinetics of smectite alteration as well as the precise role of microbes could not be determined due to the complexity of bentonite mineral assemblages and the large number of influencing factors. Further experimentation using simpler mineral mixtures and the addition of single substrates (hydrogen gas, lactate or acetate) at lower concentrations are required. The measurement and quantification of metabolites, e.g., the formation and consumption of organic acids and gases, is necessary to understand further the microbial metabolic potential within the bentonites and its impact on the barrier system.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilg, H.A.; Kaufhold, S.; Ufer, K. Smectite and bentonite terminology, classification, and genesis. In Bentonites: Characterization, Geology, Mineralogy, Analysis, Mining, Processing and Uses; Kaufhold, S., Ed.; Schweizbart Science Publishers: Stuttgart, Germany, 2021; ISBN 978-3-510-96859-6. in press. [Google Scholar]
- Christidis, G.E.; Huff, W.D. Geological aspects and genesis of bentonites. Elements 2009, 5, 93–98. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management—A review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. Distinguishing between more and less suitable bentonites for storage of high-level radioactive waste. Clay Miner. 2016, 51, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Savage, D. An Assessment of the Impact of the Degradation of Engineered Structures on the Safety-Relevant Functions of the Bentonite Buffer in a HLW Repository. NTB-13-02, Wettingen, Switzerland. 2014. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:48088313 (accessed on 21 June 2021).
- Kaufhold, S.; Klimke, S.; Schloemer, S.; Alpermann, T.; Renz, F.; Dohrmann, R. About the corrosion mechanism of metal iron in contact with bentonite. ACS Earth Space Chem. 2020, 4, 711–721. [Google Scholar] [CrossRef]
- Gaucher, E.C.; Blanc, P. Cement/clay interactions—A review: Experiments, natural analogues, and modeling. Waste Manag. 2006, 26, 776–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, S.; Shimbashi, M.; Minato, D.; Watanabe, Y.; Jenni, A.; Mäder, U. Alteration of bentonite reacted with cementitious materials for 5 and 10 years in the Mont Terri Rock Laboratory (CI Experiment). Minerals 2021, 11, 251. [Google Scholar] [CrossRef]
- Pusch, R. Transport of radionuclides in smectite clay. In Environmental Interactions of Clays: Clays and the Environment; Parker, A., Rae, J.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 7–35. ISBN 978-3-662-03651-8. [Google Scholar]
- Herbert, H.-J.; Kasbohm, J.; Sprenger, H.; Fernández, A.M.; Reichelt, C. Swelling pressures of MX-80 bentonite in solutions of different ionic strength. Phys. Chem. Earth Parts A/B/C 2008, 33, S327–S342. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. Stability of bentonites in salt solutions | sodium chloride. Appl. Clay Sci. 2009, 45, 171–177. [Google Scholar] [CrossRef]
- Hofmann, H.; Bauer, A.; Warr, L.N. Behavior of smectite in strong salt brines under conditions relevant to the disposal of low- to medium-grade nuclear waste. Clays Clay Miner. 2004, 52, 14–24. [Google Scholar] [CrossRef]
- Stroes-Gascoyne, S.; Hamon, C.J.; Maak, P. Limits to the use of highly compacted bentonite as a deterrent for microbiologically influenced corrosion in a nuclear fuel waste repository. Phys. Chem. Earth Parts A/B/C 2011, 36, 1630–1638. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; Fernández, A.M.; García-Gutiérrez, M. Colloidal properties of different smectite clays: Significance for the bentonite barrier erosion and radionuclide transport in radioactive waste repositories. Appl. Geochem. 2018, 97, 157–166. [Google Scholar] [CrossRef]
- Meleshyn, A. Microbial Processes Relevant for Long-Term Performance of Radioactive Waste Repositories in Clays; GRS-291; GRS: Köln, Germany, 2011. [Google Scholar]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- El Mendili, Y.; Abdelouas, A.; Bardeau, J.-F. Insight into the mechanism of carbon steel corrosion under aerobic and anaerobic conditions. Phys. Chem. Chem. Phys. 2013, 15, 9197–9204. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Bengtsson, A.; Blom, A.; Johansson, L.; Taborowski, T. Mobility and reactivity of sulphide in bentonite clays–Implications for engineered bentonite barriers in geological repositories for radioactive wastes. Appl. Clay Sci. 2017, 146, 495–502. [Google Scholar] [CrossRef]
- Lantenois, S.; Lanson, B.; Muller, F.; Bauer, A.; Jullien, M.; Plançon, A. Experimental study of smectite interaction with metal Fe at low temperature: 1. Smectite destabilization. Clays Clay Miner. 2005, 53, 597–612. [Google Scholar] [CrossRef]
- Soltermann, D. Ferrous Iron Uptake Mechanisms at the Montmorillonite-Water Interface under Anoxic and Electrochemically Reduced Conditions. Ph.D. Thesis, ETH, Zurich, Switzerland, 2014. [Google Scholar]
- Ufer, K.; Stanjek, H.; Roth, G.; Dohrmann, R.; Kleeberg, R.; Kaufhold, S. Quantitative phase analysis of bentonites by the Rietveld method. Clays Clay Miner. 2008, 56, 272–282. [Google Scholar] [CrossRef]
- Kaufhold, S.; Stucki, J.W.; Finck, N.; Steininger, R.; Zimina, A.; Dohrmann, R.; Ufer, K.; Pentrák, M.; Pentráková, L. Tetrahedral charge and Fe content in dioctahedral smectites. Clay Miner. 2017, 52, 51–65. [Google Scholar] [CrossRef]
- Wersin, P.; Leupin, O.X.; Mettler, S.; Gaucher, E.C.; Mäder, U.; de Cannière, P.; Vinsot, A.; Gäbler, H.E.; Kunimaro, T.; Kiho, K.; et al. Biogeochemical processes in a clay formation in situ experiment: Part A–Overview, experimental design and water data of an experiment in the Opalinus Clay at the Mont Terri Underground Research Laboratory, Switzerland. Appl. Geochem. 2011, 26, 931–953. [Google Scholar] [CrossRef]
- Meleshyn, A. Mechanisms of Transformation of Bentonite Barriers (UMB); GRS-930; GRS: Köln, Germany, 2019. [Google Scholar]
- Matschiavelli, N.; Drozdowski, J.; Kluge, S.; Arnold, T.; Cherkouk, A. Joint project: Umwandlungsmechanismen in Bentonitbarrieren—Subproject B: Einfluss von mikrobiellen Prozessen auf die Bentonitumwandlung, HZDR-103, Dresden, Germany. 2019. Available online: https://www.hzdr.de/db/!Publications?pNid=head&pSelMenu=0&pSelTitle=29398 (accessed on 21 June 2021).
- Meier, L.P.; Kahr, G. Determination of the Cation Exchange Capacity (CEC) of clay minerals using the complexes of copper (II) Ion with Triethylenetetramine and Tetraethylenepentamine. Clays Clay Miner. 1999, 47, 386–388. [Google Scholar] [CrossRef]
- Stanjek, H.; Künkel, D. CEC determination with Cu-triethylenetetramine: Recommendations for improving reproducibility and accuracy. Clay Miner. 2016, 51, 1–17. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Christidis, G.E. Validity of the structural formula method for layer charge determination of smectites: A re-evaluation of published data. Appl. Clay Sci. 2008, 42, 1–7. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science, 2nd ed.; Springer: New York, NY, USA; London, UK, 2009; ISBN 978-0-387-76501-3. [Google Scholar]
- Velde, B. Electron microprobe analysis of clay minerals. Clay Miner. 1984, 19, 243–247. [Google Scholar] [CrossRef]
- Van der Pluijm, B.A. Analytical electron microscopy and the problem of potassium diffusion1. Clays Clay Miner. 1988, 36, 498–504. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Electron-excited X-ray microanalysis by energy dispersive spectrometry at 50: Analytical accuracy, precision, trace sensitivity, and quantitative compositional mapping. Microsc. Microanal. 2019, 25, 1075–1105. [Google Scholar] [CrossRef]
- Stevens, R.E. A system for calculating analyses of micas and related minerals to end members. US Geol. Surv. Bull. 1946, 950, 101–119. [Google Scholar]
- Wolters, F. Classification of Montmorillonites. Ph.D. Thesis, Universität Karlsruhe, Karlsruhe, Germany, 2005. [Google Scholar]
- Matschiavelli, N.; Kluge, S.; Podlech, C.; Standhaft, D.; Grathoff, G.; Ikeda-Ohno, A.; Warr, L.N.; Chukharkina, A.; Arnold, T.; Cherkouk, A. The year-long development of microorganisms in uncompacted bavarian bentonite slurries at 30 and 60 °C. Environ. Sci. Technol. 2019, 53, 10514–10524. [Google Scholar] [CrossRef] [Green Version]
- Warr, L.N. IMA-CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997; ISBN 978-0-19-508713-0. [Google Scholar]
- Emmerich, K.; Wolters, F.; Kahr, G.; Lagaly, G. Clay Profiling: The classification of montmorillonites. Clays Clay Miner. 2009, 57, 104–114. [Google Scholar] [CrossRef]
- Stucki, J.W. A review of the effects of iron redox cycles on smectite properties. Comptes Rendus Geosci. 2011, 343, 199–209. [Google Scholar] [CrossRef]
- Klemps, R.; Cypionka, H.; Widdel, F.; Pfennig, N. Growth with hydrogen, and further physiological characteristics of Desulfotomaculum species. Arch. Microbiol. 1985, 143, 203–208. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, M.J.; Lafay, B.; Christen, R.; Fernandez-Linares, L.C.; Acquaviva, M.; Bonin, P.; Bertrand, J.-C. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int. J. Syst. Bacteriol. 1992, 42, 568–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, K. Microbial Processes in Radioactive Waste Disposal, SKB TR-00-04, Stockholm, Sweden. 2000. Available online: https://www.osti.gov/etdeweb/servlets/purl/21330059 (accessed on 14 June 2021).
- Savage, D. The Effects of High Salinity Groundwater on the Performance of Clay Barriers; SKI-R-05-54; Swedish Nuclear Power Inspectorate: Stockholm, Sweden, 2005. [Google Scholar]
- Tertre, E.; Prêt, D.; Ferrage, E. Influence of the ionic strength and solid/solution ratio on Ca (II)-for-Na+ exchange on montmorillonite. Part 1: Chemical measurements, thermodynamic modeling and potential implications for trace elements geochemistry. J. Colloid Interface Sci. 2011, 353, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Warr, L.; Berger, J. Hydration of bentonite in natural waters: Application of “confined volume” wet-cell X-ray diffractometry. Phys. Chem. Earth Parts A/B/C 2007, 32, 247–258. [Google Scholar] [CrossRef]
- Christidis, G.E.; Blum, A.E.; Eberl, D.D. Influence of layer charge and charge distribution of smectites on the flow behaviour and swelling of bentonites. Appl. Clay Sci. 2006, 34, 125–138. [Google Scholar] [CrossRef]
- Laird, D.A. Influence of layer charge on swelling of smectites. Appl. Clay Sci. 2006, 34, 74–87. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Koch, D.; Houben, G. The pH of aqueous bentonite suspensions. Clays Clay Miner. 2008, 56, 338–343. [Google Scholar] [CrossRef]
- Beaufort, D.; Berger, G.; Lacharpagne, J.C.; Meunier, A. An experimental alteration of montmorillonite to a di + trioctahedral smectite assemblage at 100 °C and 200 °C. Clay Miner. 2001, 36, 211–225. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Protonation–deprotonation of dioctahedral smectites. Appl. Clay Sci. 2001, 20, 27–38. [Google Scholar] [CrossRef]
- Drits, V.A. A Model for the mechanism of Fe3+ to Fe2+ reduction in dioctahedral smectites. Clays Clay Miner. 2000, 48, 185–195. [Google Scholar] [CrossRef]
- Rozalen, M.; Huertas, F.J.; Brady, P.V. Experimental study of the effect of pH and temperature on the kinetics of montmorillonite dissolution. Geochim. Cosmochim. Acta 2009, 73, 3752–3766. [Google Scholar] [CrossRef]
- Birgersson, M.; Karnland, O.; Korkeakoski, P.; Sellin, P.; Mäder, U.; Wersin, P. Montmorillonite Stability Under Near-Field Conditions; NTB-14-12; National Cooperative for the Disposal of Radioactive Waste (NAGRA): Wettingen, Switzerland, 2014; pp. 1015–2636. [Google Scholar]
- Ye, W.-M.; He, Y.; Chen, Y.G.; Chen, B.; Cui, Y.-J. Thermochemical effects on the smectite alteration of GMZ bentonite for deep geological repository. Environ. Earth Sci. 2016, 75, 906. [Google Scholar] [CrossRef]
- Nguyen-Thanh, L.; Herbert, H.-J.; Kasbohm, J.; Hoang-Minh, T.; Mählmann, R.F. Effects of chemical structure on the stability of smectites in short-term alteration experiments. Clays Clay Miner. 2014, 62, 425–446. [Google Scholar] [CrossRef] [Green Version]
- Stroes-Gascoyne, S.; Sergeant, C.; Schippers, A.; Hamon, C.J.; Nèble, S.; Vesvres, M.-H.; Barsotti, V.; Poulain, S.; Le Marrec, C. Biogeochemical processes in a clay formation in situ experiment: Part D–Microbial analyses–Synthesis of results. Appl. Geochem. 2011, 26, 980–989. [Google Scholar] [CrossRef]
- Fru, E.C.; Athar, R. In situ bacterial colonization of compacted bentonite under deep geological high-level radioactive waste repository conditions. Appl. Microbiol. Biotechnol. 2008, 79, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Huang, Q.; Zhang, X.; Chen, H. Adsorption of DNA on clay minerals and various colloidal particles from an Alfisol. Soil Biol. Biochem. 2006, 38, 471–476. [Google Scholar] [CrossRef]
- Greaves, M.P.; Wilson, M.J. The adsorption of nucleic acids by montmorillonite. Soil Biol. Biochem. 1969, 1, 317–323. [Google Scholar] [CrossRef]
- Hobbie, S.N.; Li, X.; Basen, M.; Stingl, U.; Brune, A. Humic substance-mediated Fe (III) reduction by a fermenting Bacillus strain from the alkaline gut of a humus-feeding scarab beetle larva. Syst. Appl. Microbiol. 2012, 35, 226–232. [Google Scholar] [CrossRef]
- Bai, Y.; Mellage, A.; Cirpka, O.A.; Sun, T.; Angenent, L.T.; Haderlein, S.B.; Kappler, A. AQDS and Redox-Active NOM enables microbial Fe (III)-mineral reduction at cm-scales. Environ. Sci. Technol. 2020, 54, 4131–4139. [Google Scholar] [CrossRef] [PubMed]
- Jaisi, D.P.; Kukkadapu, R.K.; Eberl, D.D.; Dong, H. Control of Fe (III) site occupancy on the rate and extent of microbial reduction of Fe (III) in nontronite. Geochim. Cosmochim. Acta 2005, 69, 5429–5440. [Google Scholar] [CrossRef] [Green Version]
- Pankhania, I.P.; Spormann, A.M.; Hamilton, W.A.; Thauer, R.K. Lactate conversion to acetate, CO2 and H2 in cell suspensions of Desulfovibrio vulgaris (Marburg): Indications for the involvement of an energy driven reaction. Arch. Microbiol. 1988, 150, 26–31. [Google Scholar] [CrossRef]
- Thauer, R.K.; Möller-Zinkhan, D.; Spormann, A.M. Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu. Rev. Microbiol. 1989, 43, 43–67. [Google Scholar] [CrossRef]
- Daniels, L.; Sparling, R.; Sprott, G. The bioenergetics of methanogenesis. Biochim. Biophys. Acta (BBA)—Rev. Bioenerg. 1984, 768, 113–163. [Google Scholar] [CrossRef]
- Courdouan, A.; Christl, I.; Meylan, S.; Wersin, P.; Kretzschmar, R. Isolation and characterization of dissolved organic matter from the Callovo–Oxfordian formation. Appl. Geochem. 2007, 22, 1537–1548. [Google Scholar] [CrossRef]
- Ramos, M.E.; Huertas, F.J. Adsorption of lactate and citrate on montmorillonite in aqueous solutions. Appl. Clay Sci. 2014, 90, 27–34. [Google Scholar] [CrossRef]
- Grigoryan, A.A.; Jalique, D.R.; Medihala, P.; Stroes-Gascoyne, S.; Wolfaardt, G.M.; McKelvie, J.; Korber, D.R. Bacterial diversity and production of sulfide in microcosms containing uncompacted bentonites. Heliyon 2018, 4, e00722. [Google Scholar] [CrossRef] [Green Version]










| Sample | LOI | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | TiO2 | Na2O | MnO | P2O5 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD80 | 16.5 | 51.7 | 17.9 | 4.8 | 2.9 | 2.5 | 0.9 | 0.7 | 0.7 | 0.1 | 0.2 | 99.4 |
| B36 | 13.9 | 58.6 | 16.3 | 7.4 | 1.5 | 1.2 | 1.5 | 0.8 | 0.4 | 0.1 | 0.1 | 101.8 |
| Sample | Sme | Fsp | Qz | Kln | Crs | Ant | Cal | Py | Brt |
|---|---|---|---|---|---|---|---|---|---|
| SD80 | 89 | 7 | <1 | n.d. | n.d. | <1 | <1 | <1 | 1 |
| B36 | 65 | 15 | 12 | 4 | 4 | <1 | n.d. | n.d. | n.d. |
| Sample | d001 [nm] | d060 [nm] | d001 [nm] | d001 FWHM [Δ° 2θ] | ||
|---|---|---|---|---|---|---|
| RP | RP | AD | EG | AD | EG | |
| SD80 | 1.52 | 0.150 | 1.45 | 1.68 | 1.8 | 0.6 |
| B36 | 1.50 | 0.150 | 1.48 | 1.68 | 1.4 | 0.8 |
| Sample | Tetrahedral Cations | ξtet | Octahedral Cations | ξoct | Interlayer Cations | ξ | ξtet | ||||||
| Si4+ | Al3+ | Al3+ | Fe3+ | Mg2+ | Ca2+ | Na+ | K+ | Mg2+ | % | ||||
| SD80 | 3.94 | 0.06 | −0.06 | 1.44 | 0.26 | 0.30 | −0.30 | 0.09 | 0.01 | 0.03 | 0.06 | −0.36 | 17 |
| B36 | 3.79 | 0.21 | −0.21 | 1.37 | 0.46 | 0.16 | −0.16 | 0.11 | 0.01 | 0.03 | 0.06 | −0.38 | 56 |
| Sample | CEC | Na+ | K+ | Ca2+ | Mg2+ | Σcations |
|---|---|---|---|---|---|---|
| cmol∙kg−1 | cmol∙kg−1 | cmol∙kg−1 | cmol∙kg−1 | cmol∙kg−1 | cmol∙kg−1 | |
| SD80 | 87 ± 2.2 | 17 ± 0.4 | 2 ± 0.06 | 43 ± 2.4 | 23 ± 2.3 | 86 ± 2.6 |
| B36 | 54 ± 2.0 | 1 ± 0.03 | 1 ± 0.04 | 30 ± 1.4 | 14 ± 0.4 | 46 ± 1.8 |
| Sample | Solution | 0 d | 1 d | 8 d | 30 d |
|---|---|---|---|---|---|
| - | OPA | 7.8 | - | - | - |
| SD80 | OPA | 7.4 | 7.2 | 7.3 | - |
| B36 | OPA | 6.3 | 5.4 | 5.6 | 5.7 |
| - | CAP | 7.3 | - | - | - |
| SD80 | CAP | 7.1 | 6.9 | 6.9 | 6.9 |
| B36 | CAP | 5.7 | 5.0 | 5.0 | 5.0 |
| Sample | DUR | T | SOL | Su | pH | Si | Mg | Ca | Na | K | S | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | (°C) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | ||||
| 0 | 25 | OPA | – | 7.8 | 0.1 ± 0.1 | 14.5 ± 0.6 | 25.9 ± 1.4 | 226 ± 11 | 1.7 ± 0.1 | 14.7 ± 1.1 | 308 ± 13 | |
| SD80 | 1 | 25 | OPA | – | n.d. | n.d. | 35.0 | 46.4 | 274 | n.d. | 22.1 | 372 |
| SD80 | 1 | 25 | OPA | + | n.d. | 1.8 | 41.7 | 39.7 | 379 | 3.1 | 0.3 | n.d. |
| SD80 | 1 | 90 | OPA | – | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SD80 | 1 | 90 | OPA | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SD80 | 2 | 25 | OPA | – | n.d. | 0.9 | 36.3 | 42.5 | 254 | 3.4 | 21.0 | n.d. |
| SD80 | 2 | 25 | OPA | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| SD80 | 2 | 90 | OPA | – | n.d. | 2.5 | 31.4 | 56.7 | 313 | 6.9 | 26.2 | 447 |
| SD80 | 2 | 90 | OPA | + | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 0 | 25 | CAP | – | 7.3 | 0.2 ± 0.2 | n.d. | 11.6 ± 3.0 | 2389 ± 70 | 6.0 ± 0.6 | 16.4 ± 0.4 | 2562 ± 5 | |
| SD80 | 1 | 25 | CAP | – | 7.4 | 0.3 | 63.2 | 93.9 | 2352 | 7.3 | 22.1 | 2642 |
| SD80 | 1 | 25 | CAP | + | 7.4 | 0.6 | 82.0 | 97.7 | 2773 | 7.4 | 23.8 | 2652 |
| SD80 | 1 | 90 | CAP | – | 7.4 | 1.3 | 64.6 | 113.5 | 2729 | 9.5 | 18.9 | 2877 |
| SD80 | 1 | 90 | CAP | + | 7.2 | 1.7 | 78.0 | 117.2 | 2642 | 10.1 | 23.6 | 2750 |
| SD80 | 2 | 25 | CAP | – | 7.4 | 0.5 | 69.1 | 99.3 | 2481 | 7.3 | 23.5 | 2636 |
| SD80 | 2 | 25 | CAP | + | 7.6 | 0.7 | 79.8 | 96.3 | 2503 | 7.7 | 22.5 | 2667 |
| SD80 | 2 | 90 | CAP | – | 7.5 | 1.5 | 64.9 | 98.1 | 2446 | 10.0 | 14.6 | 2907 |
| SD80 | 2 | 90 | CAP | + | n.d. | 1.0 | 79.9 | 136.0 | 2698 | 9.8 | 20.5 | n.d. |
| Sample | DUR | T | SOL | Su | pH | Si | Mg | Ca | Na | K | S | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | (°C) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | (mmol·L−1) | ||||
| 0 | 25 | OPA | – | 7.8 | 0.1 ± 0.1 | 14.5 ± 0.6 | 25.9 ± 1.4 | 226 ± 11 | 1.7 ± 0.1 | 14.7 ± 1.1 | 308 ± 13 | |
| B36 | 1 | 25 | OPA | – | 7.3 | 1.1 | 28.7 | 50.9 | 232 | 2.5 | 15.6 | 353 |
| B36 | 1 | 25 | OPA | + | 6.9 | 1.4 | 30.4 | 60.7 | 283 | 2.4 | 15.9 | 345 |
| B36 | 1 | 90 | OPA | – | 5.1 | 2.7 | 22.5 | 58.6 | 231 | 3.5 | 14.7 | 358 |
| B36 | 1 | 90 | OPA | + | 5.2 | 3.1 | 25.9 | 59.3 | 300 | 3.1 | 14.5 | 347 |
| B36 | 2 | 25 | OPA | – | 7.1 | 1.1 | 28.4 | 58.3 | 232 | 2.8 | 15.1 | 355 |
| B36 | 2 | 25 | OPA | + | 7.5 | 1.1 | 27.7 | 60.2 | 282 | 2.1 | 11.8 | 333 |
| B36 | 2 | 90 | OPA | – | 5.0 | 3.0 | 27.3 | 62.0 | 250 | 4.5 | 16.5 | 385 |
| B36 | 2 | 90 | OPA | + | 5.2 | 3.2 | 24.1 | 55.5 | 288 | 3.8 | 14.6 | 342 |
| 0 | 25 | CAP | – | 7.3 | 0.2 ± 0.2 | n.d. | 11.6 ± 3.0 | 2389 ± 70 | 6.0 ± 0.6 | 16.4 ± 0.4 | 2562 ± 5 | |
| B36 | 1 | 25 | CAP | – | 6.7 | 0.5 | 35.8 | 96.1 | 2351 | 7.5 | 16.4 | 2590 |
| B36 | 1 | 25 | CAP | + | 7.5 | 0.7 | 40.9 | 107.8 | 2617 | 7.1 | 17.6 | 2621 |
| B36 | 1 | 90 | CAP | – | 4.5 | 2.2 | 38.6 | 107.3 | 2672 | 8.4 | 18.1 | 2787 |
| B36 | 1 | 90 | CAP | + | 4.7 | 2.0 | 36.7 | 107.6 | 2637 | 7.5 | 17.6 | 2659 |
| B36 | 2 | 25 | CAP | – | 7.0 | 0.6 | 34.5 | 92.7 | 2514 | 6.8 | 17.5 | 2576 |
| B36 | 2 | 25 | CAP | + | 7.1 | 0.7 | 38.1 | 93.1 | 2336 | 6.9 | 15.6 | 2597 |
| B36 | 2 | 90 | CAP | – | 4.6 | 2.4 | 37.8 | 101.1 | 2575 | 8.8 | 15.2 | 2777 |
| B36 | 2 | 90 | CAP | + | 4.8 | 2.5 | 36.2 | 97.7 | 2429 | 7.6 | 16.3 | 2615 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlech, C.; Matschiavelli, N.; Peltz, M.; Kluge, S.; Arnold, T.; Cherkouk, A.; Meleshyn, A.; Grathoff, G.; Warr, L.N. Bentonite Alteration in Batch Reactor Experiments with and without Organic Supplements: Implications for the Disposal of Radioactive Waste. Minerals 2021, 11, 932. https://doi.org/10.3390/min11090932
Podlech C, Matschiavelli N, Peltz M, Kluge S, Arnold T, Cherkouk A, Meleshyn A, Grathoff G, Warr LN. Bentonite Alteration in Batch Reactor Experiments with and without Organic Supplements: Implications for the Disposal of Radioactive Waste. Minerals. 2021; 11(9):932. https://doi.org/10.3390/min11090932
Chicago/Turabian StylePodlech, Carolin, Nicole Matschiavelli, Markus Peltz, Sindy Kluge, Thuro Arnold, Andrea Cherkouk, Artur Meleshyn, Georg Grathoff, and Laurence N. Warr. 2021. "Bentonite Alteration in Batch Reactor Experiments with and without Organic Supplements: Implications for the Disposal of Radioactive Waste" Minerals 11, no. 9: 932. https://doi.org/10.3390/min11090932
APA StylePodlech, C., Matschiavelli, N., Peltz, M., Kluge, S., Arnold, T., Cherkouk, A., Meleshyn, A., Grathoff, G., & Warr, L. N. (2021). Bentonite Alteration in Batch Reactor Experiments with and without Organic Supplements: Implications for the Disposal of Radioactive Waste. Minerals, 11(9), 932. https://doi.org/10.3390/min11090932






