Induced Polarization as a Tool to Assess Alteration in Geothermal Systems: A Review
Abstract
:1. Introduction
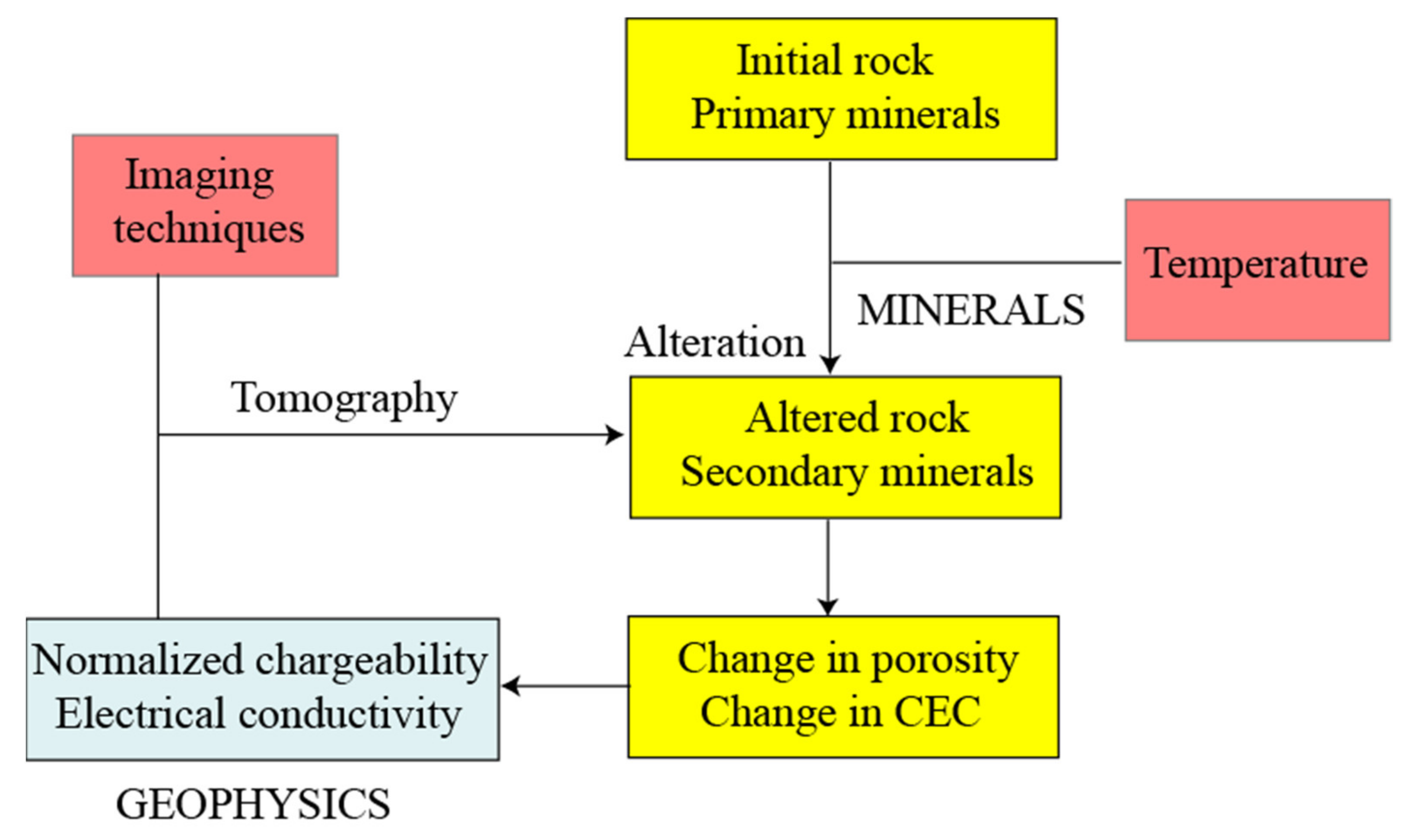
2. Alteration and Texture Changes

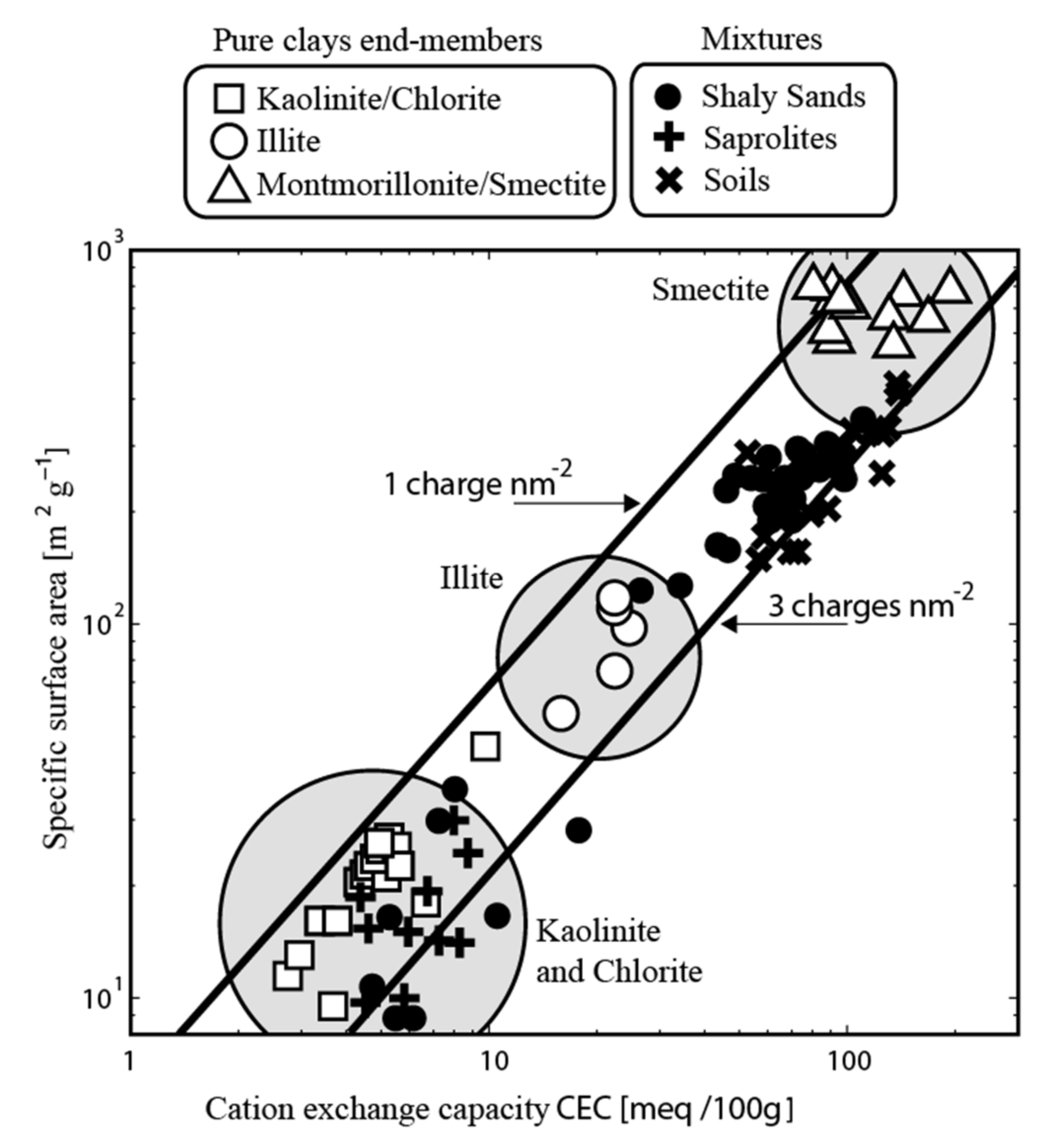
3. Induced Polarization
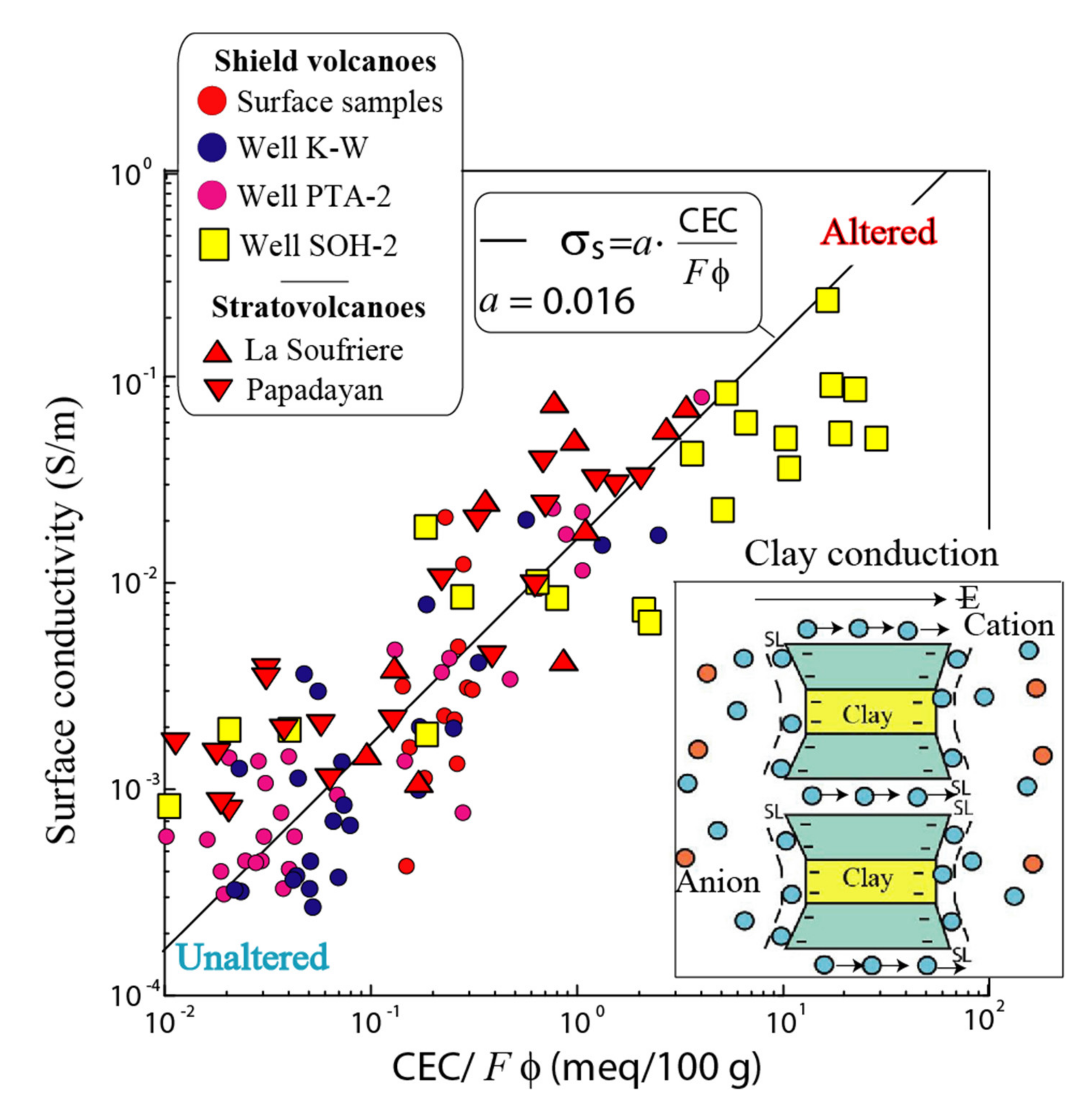
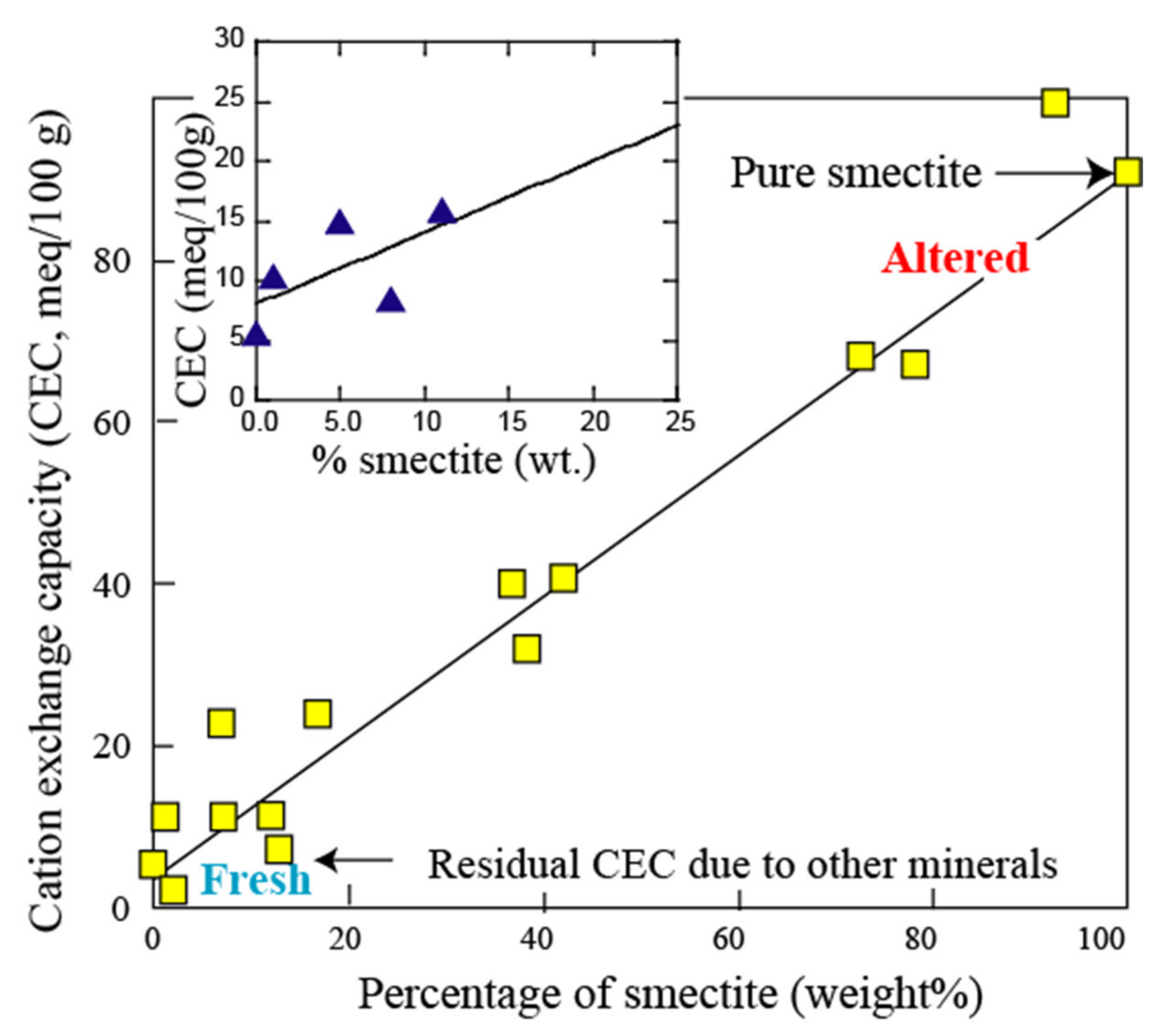
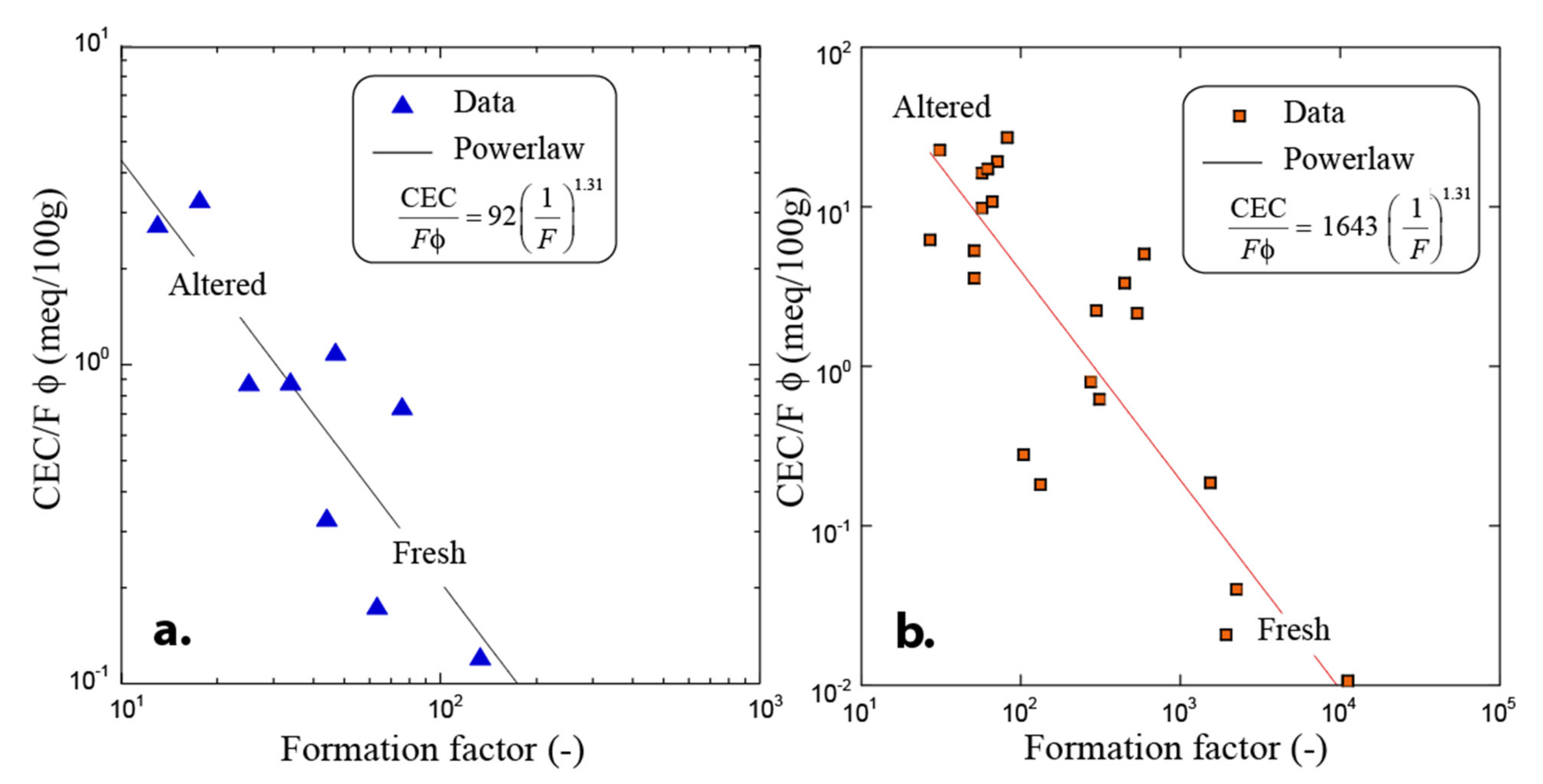
3.1. Electrical Conductivity
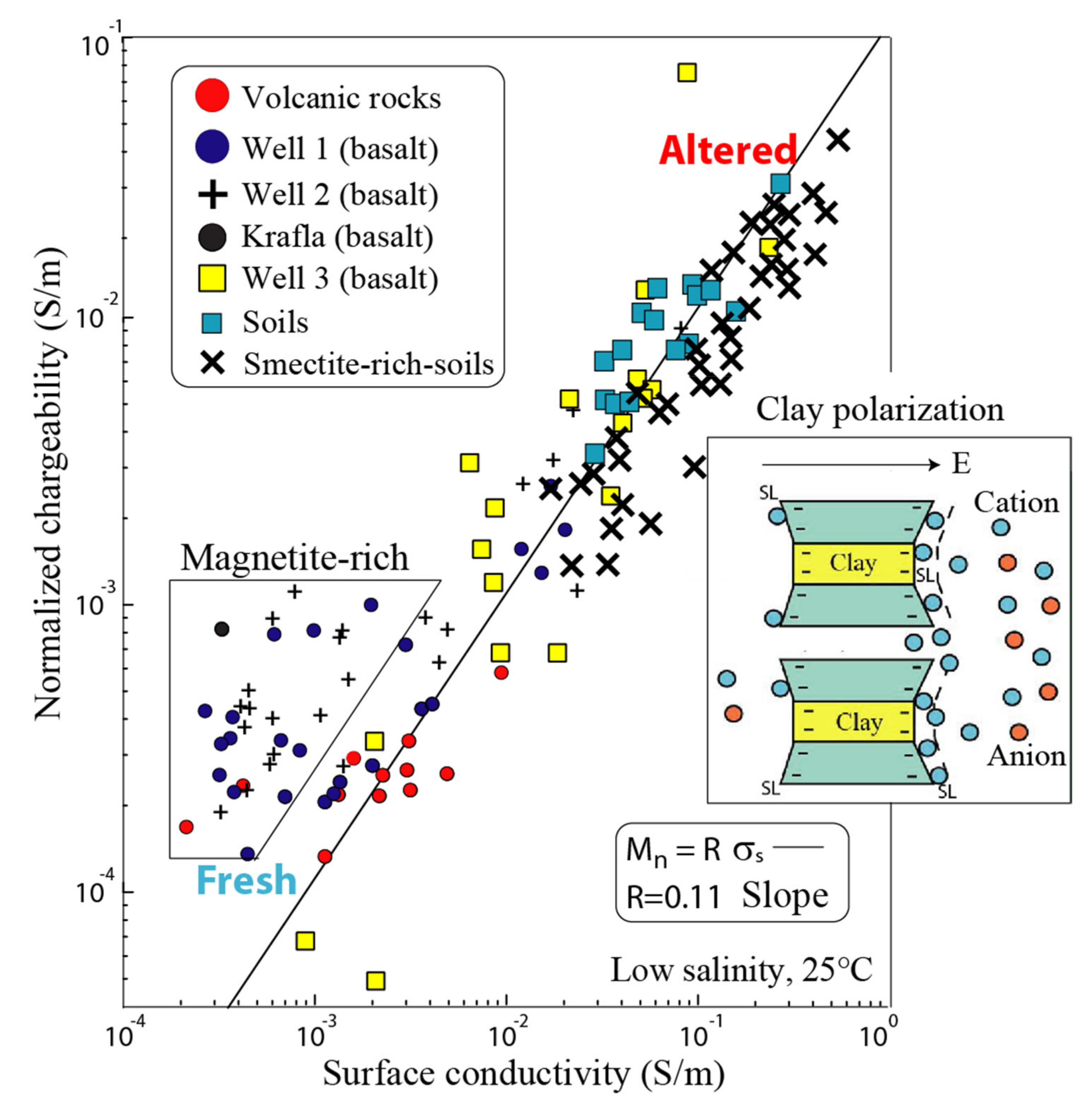
3.2. Normalized Chargeability
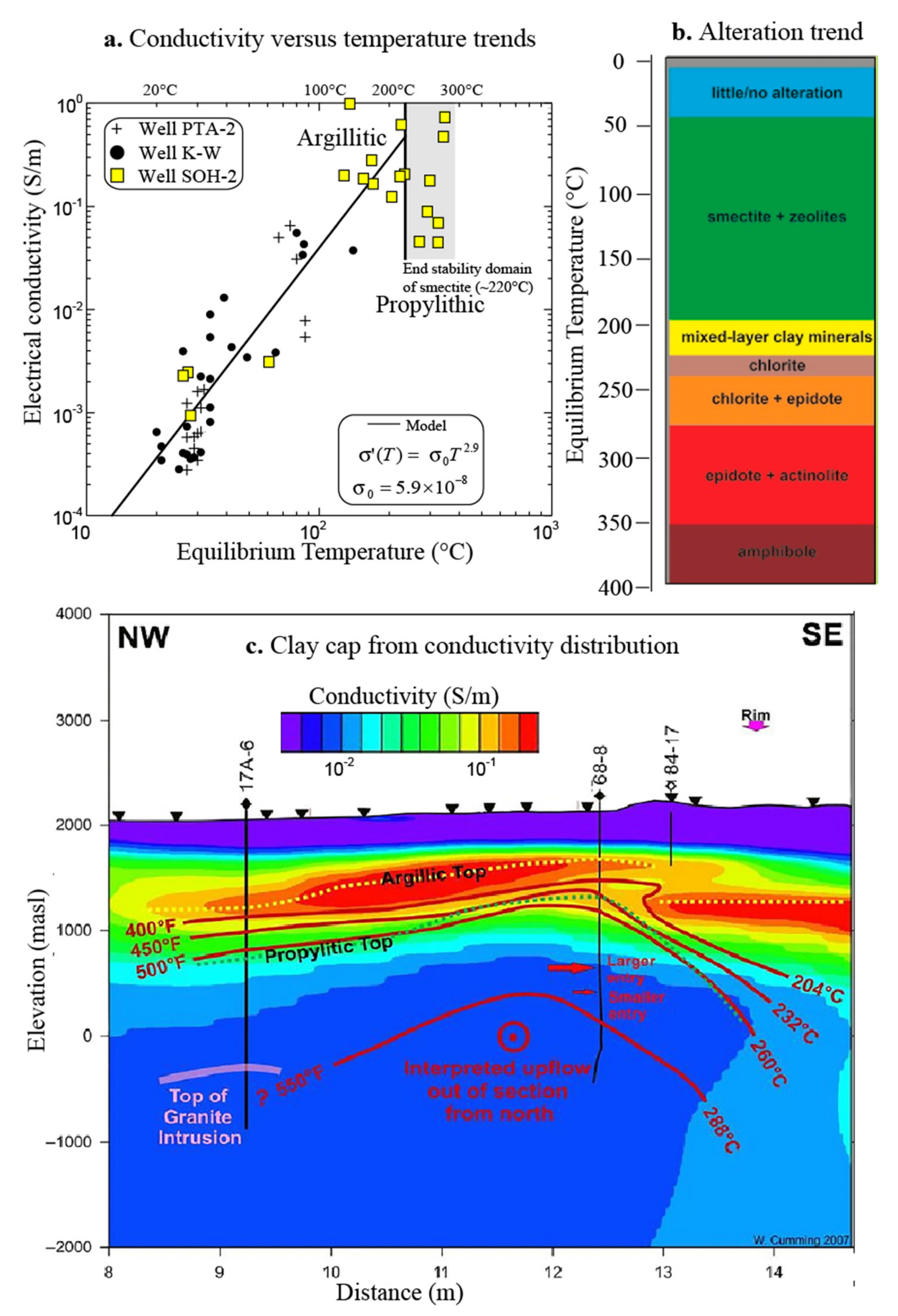
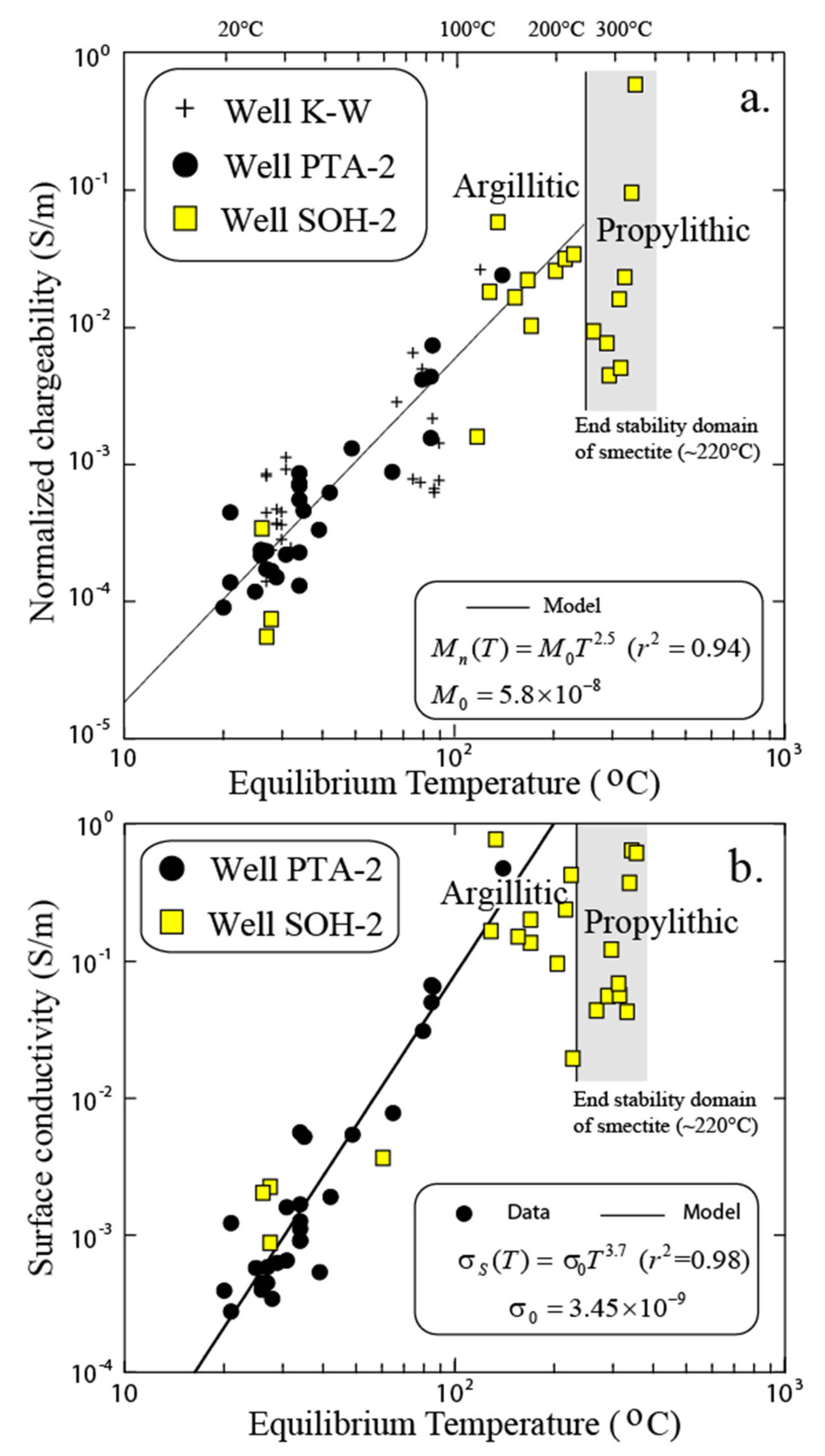
3.3. Influence of Temperature

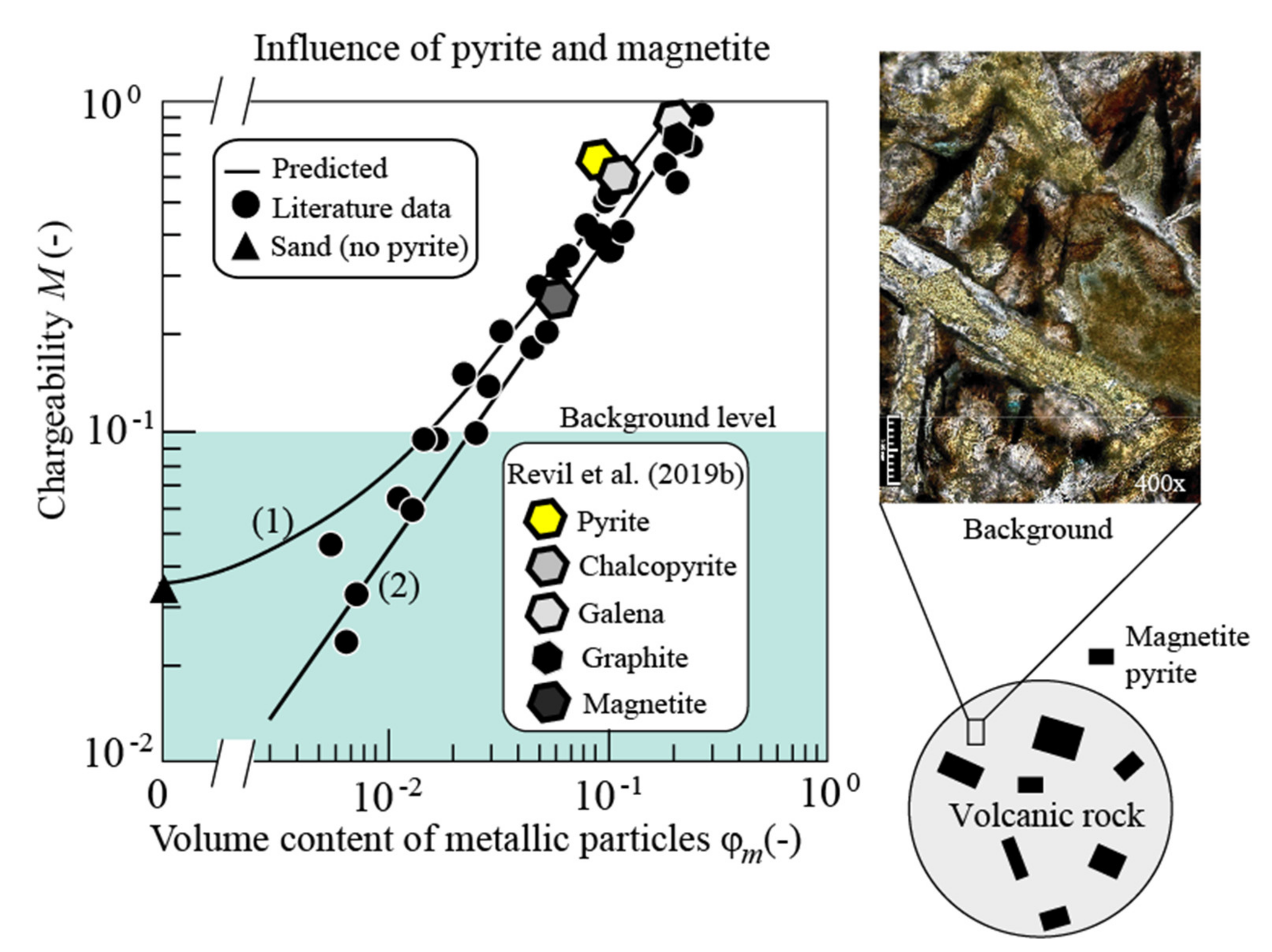
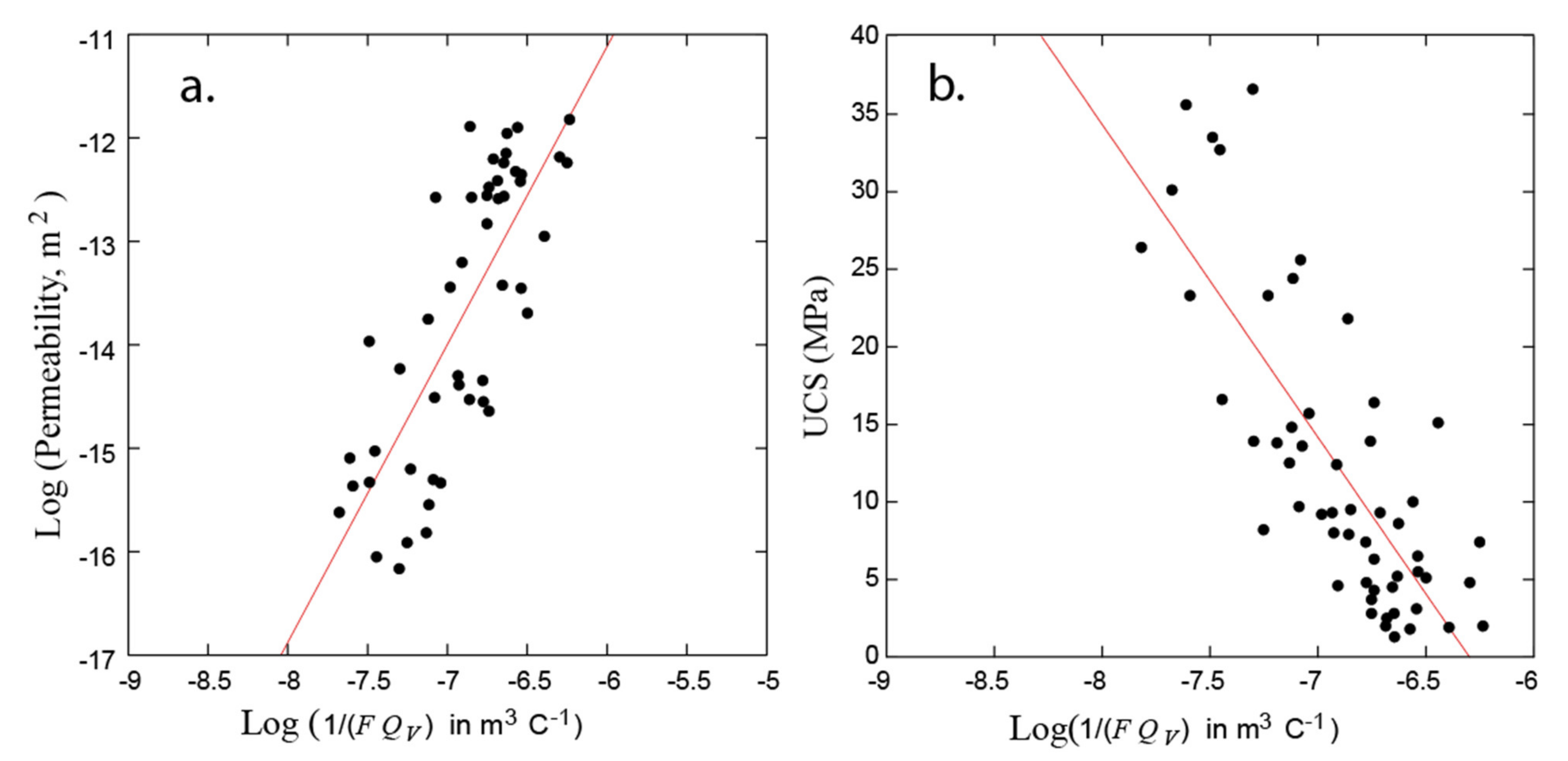
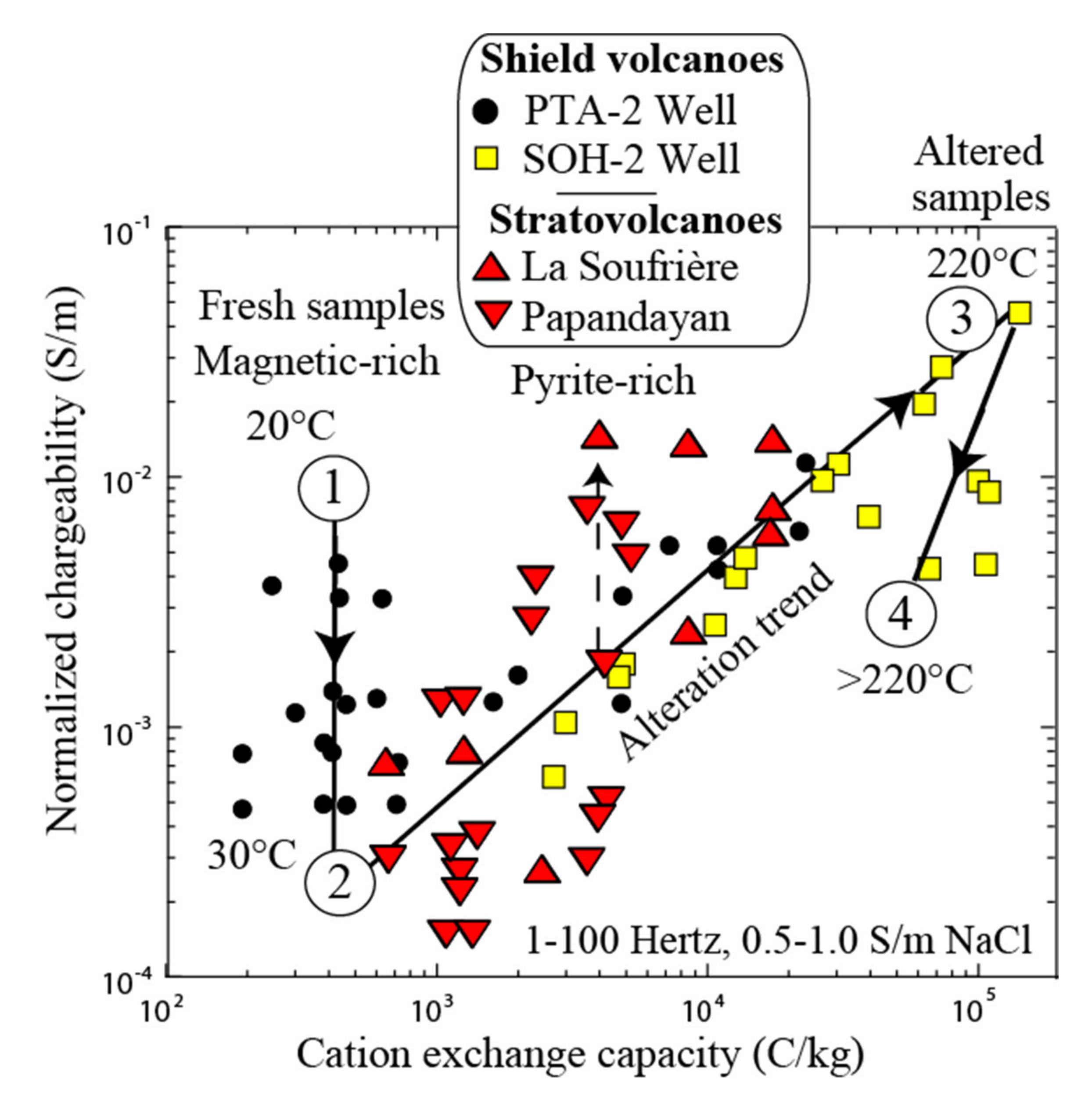
3.4. Influence of Magnetite and Pyrite
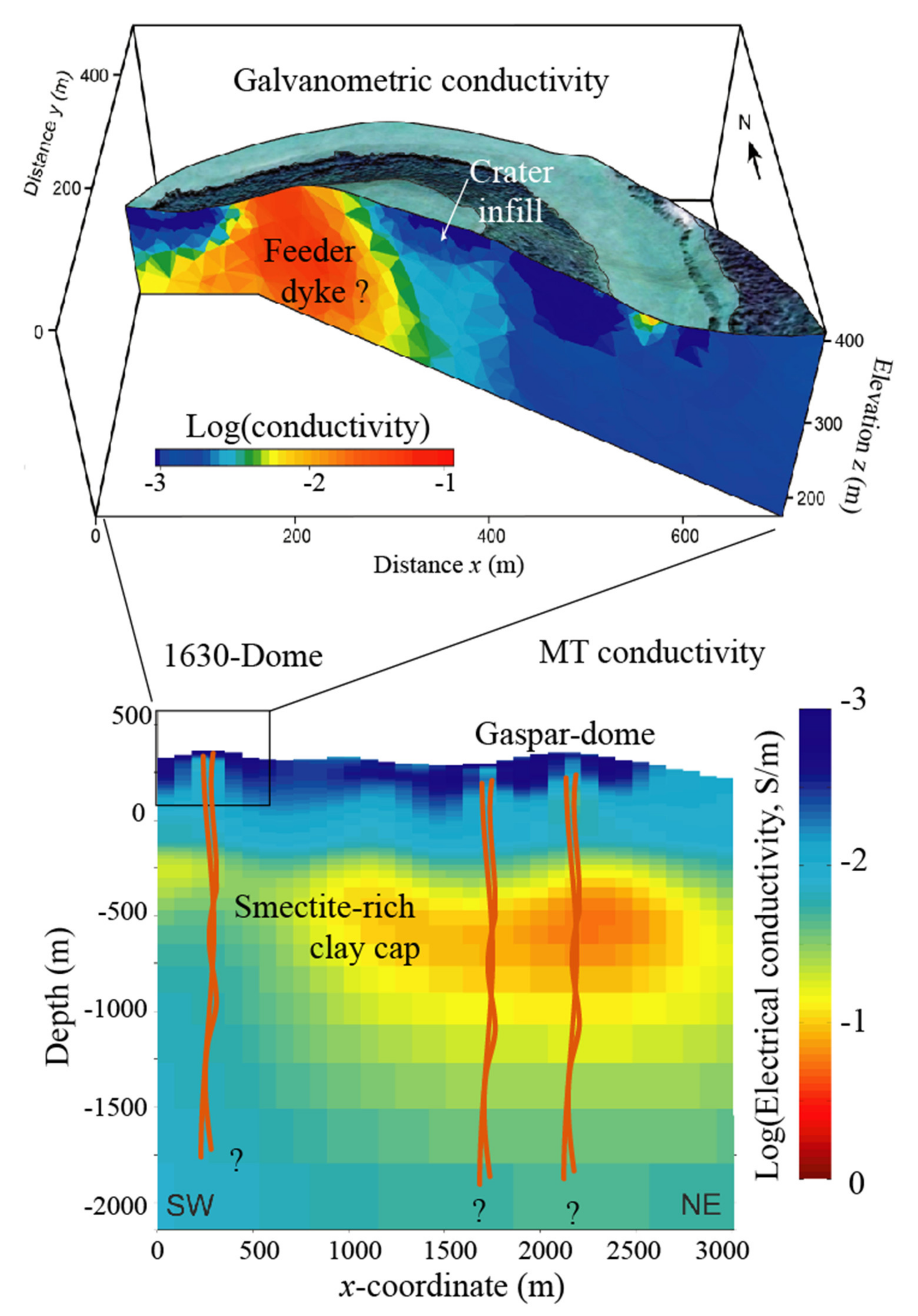
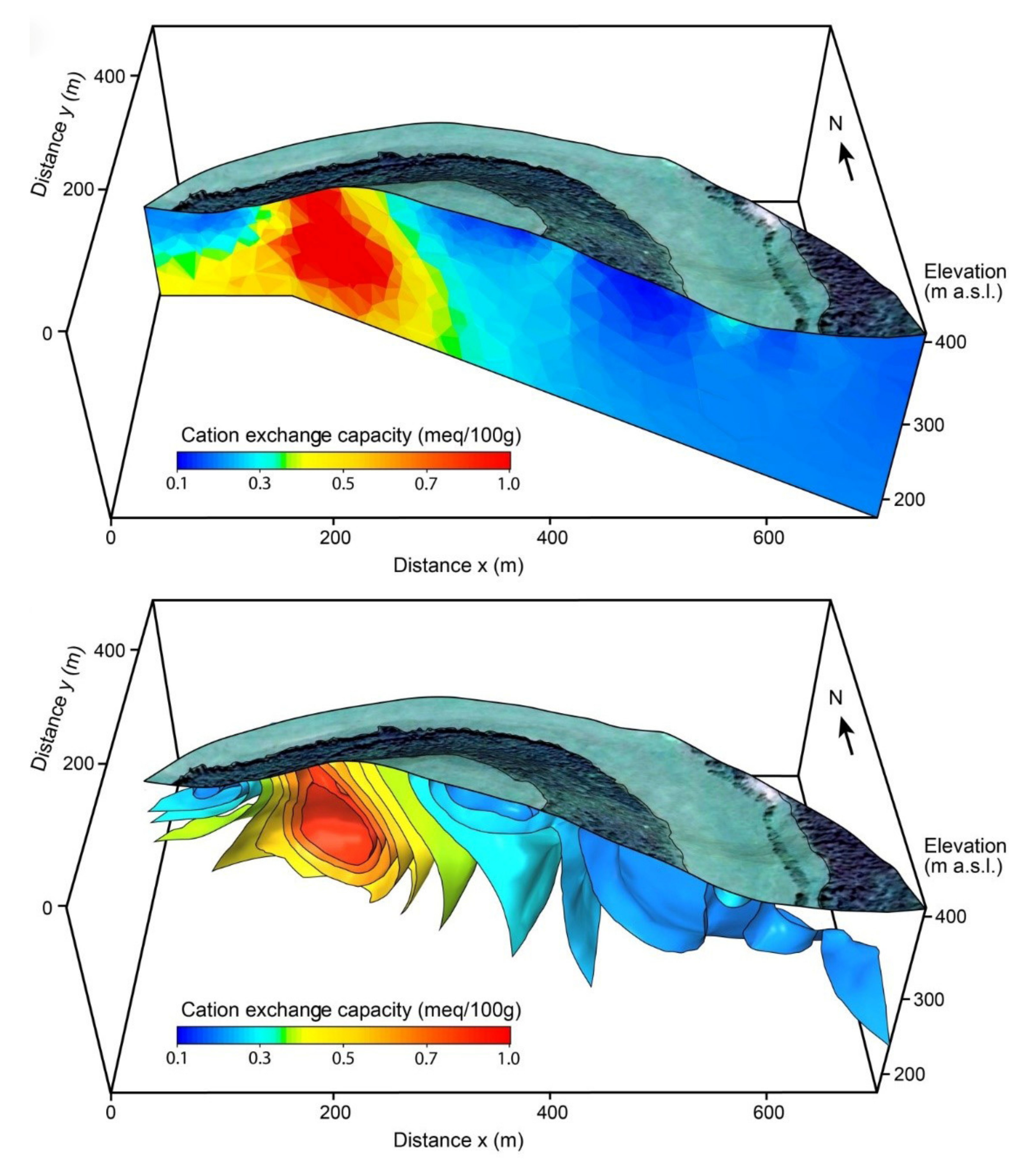
4. Applications
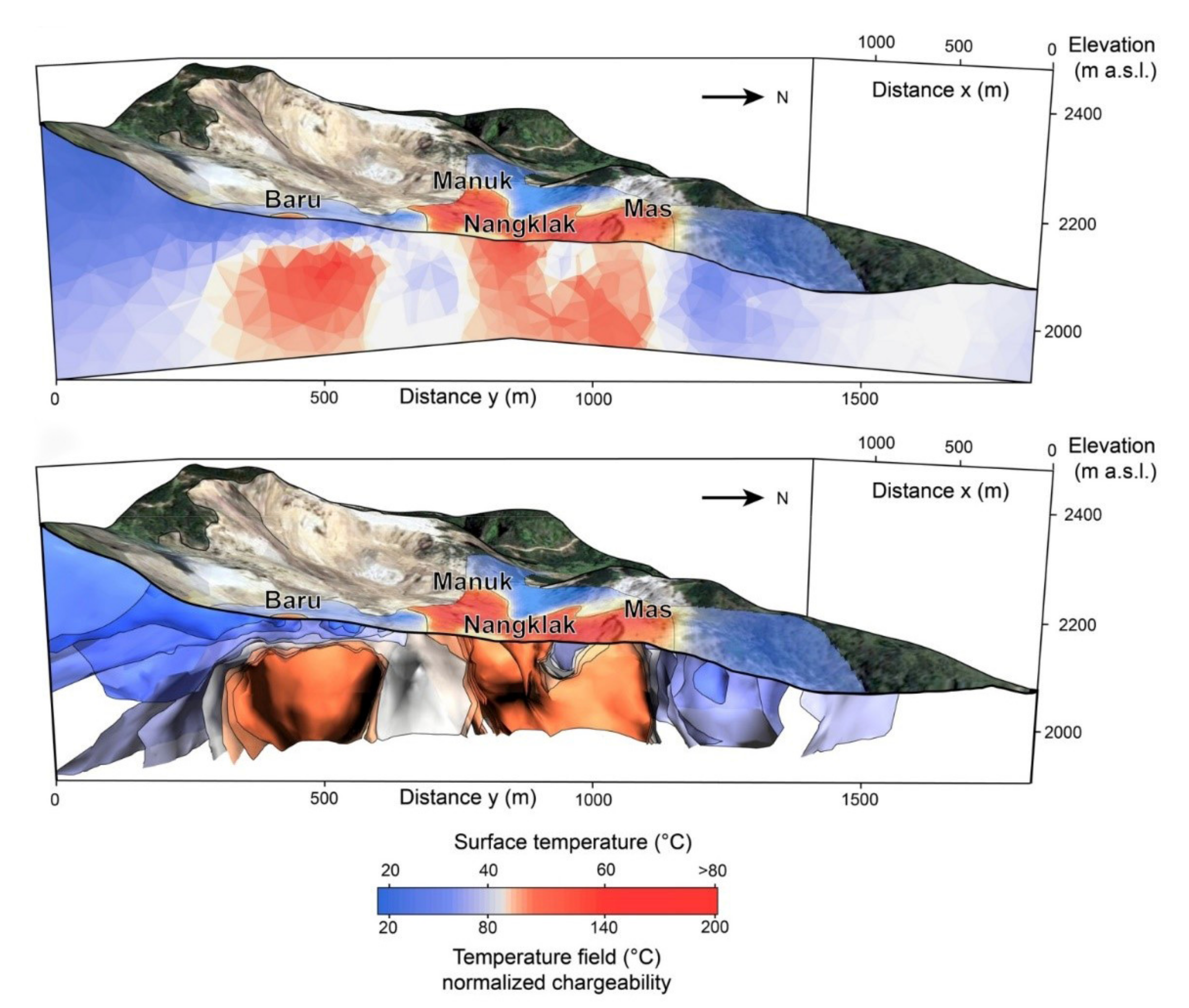


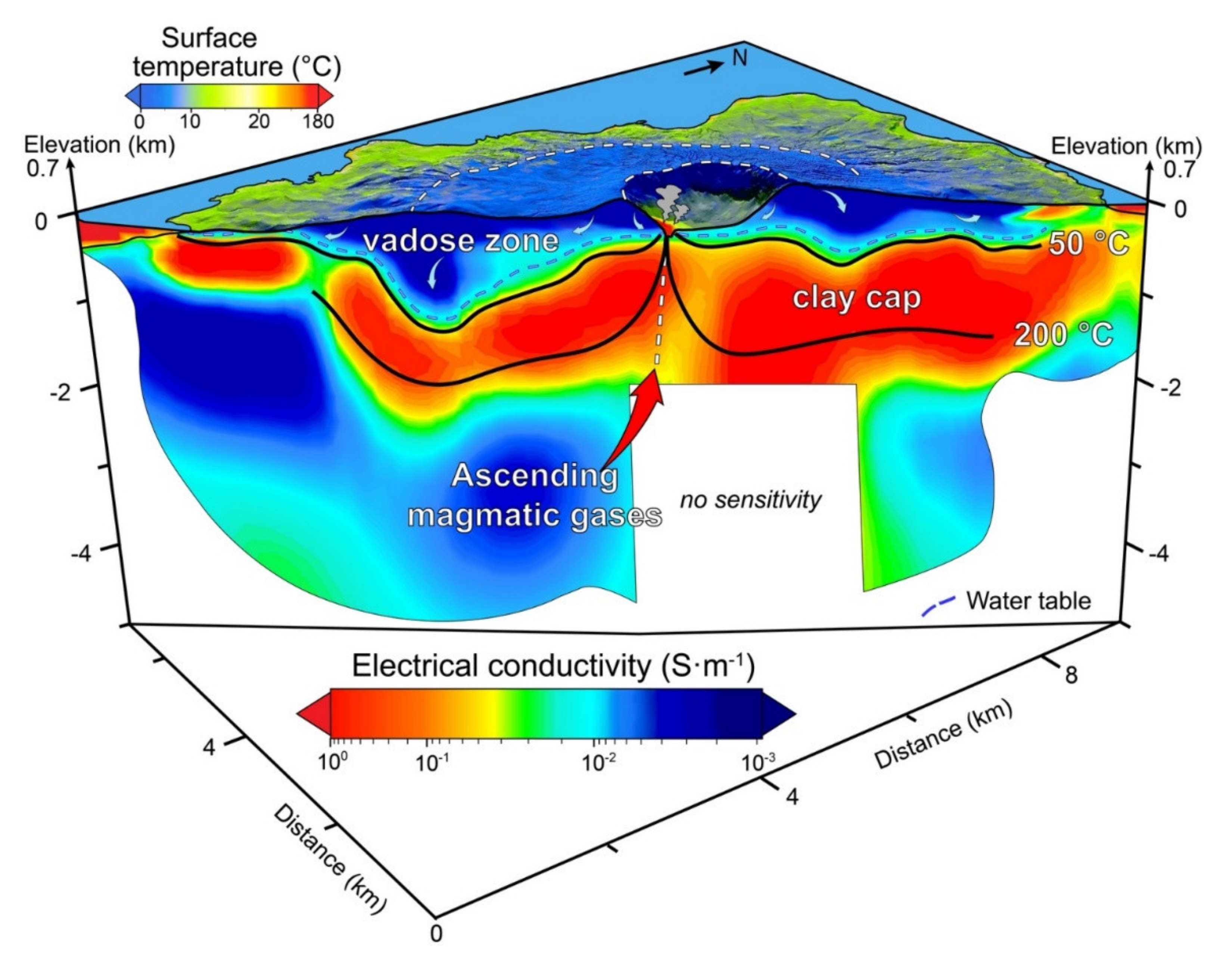
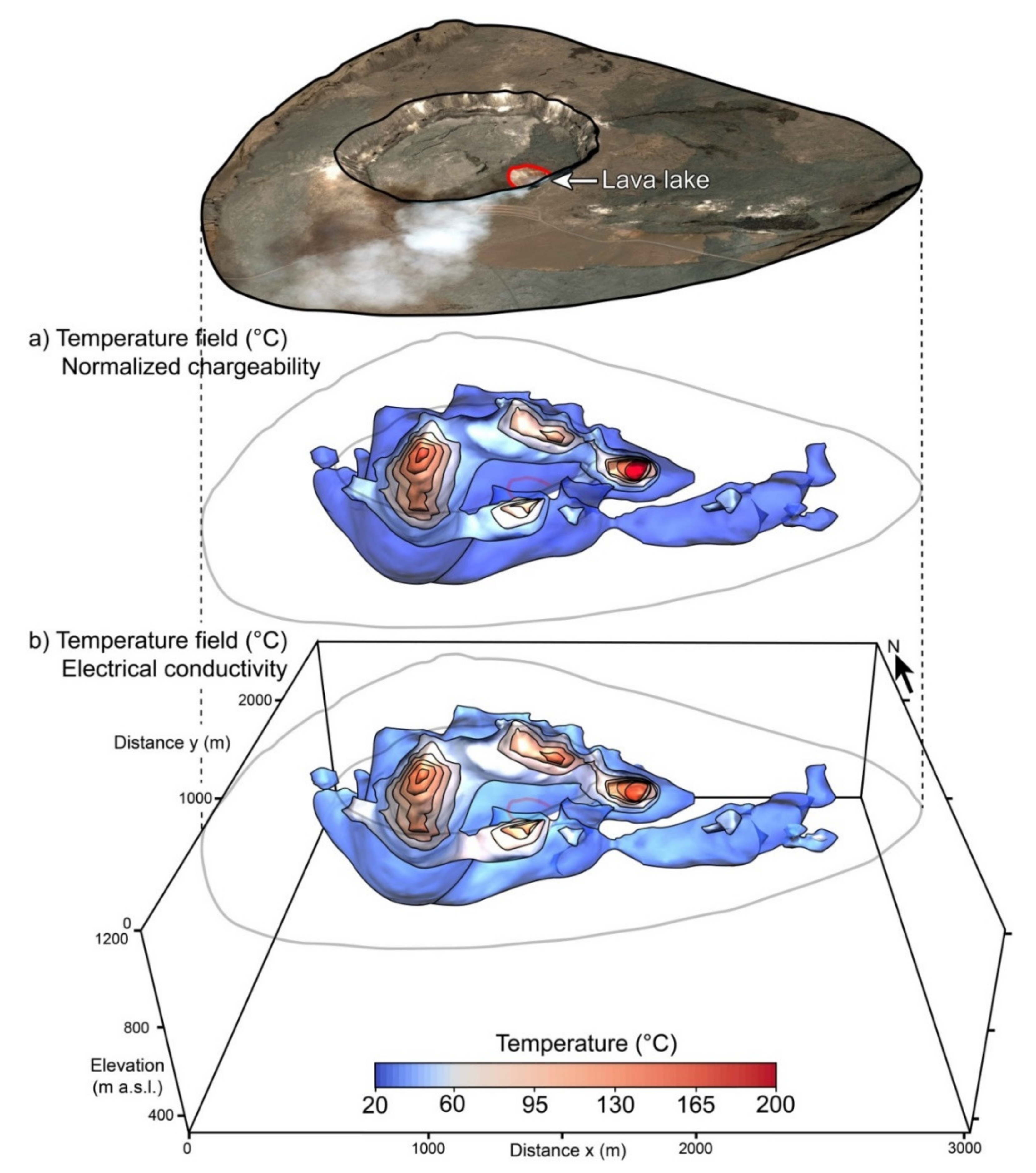
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimbelman, D.R.; Rye, R.O.; Breit, G.N. Origin of secondary sulfate minerals on active andesitic stratovolcanoes. Chem. Geol. 2005, 215, 37–60. [Google Scholar] [CrossRef] [Green Version]
- Pola, A.; Crosta, G.; Fusi, N.; Barberini, V.; Norini, G. Influence of alteration on physical properties of volcanic rocks. Tectonophysics 2012, 566–567, 67–86. [Google Scholar] [CrossRef]
- Wyering, L.D.; Villeneuve, M.C.; Wallis, I.C.; Siratovich, P.A.; Kennedy, B.M.; Gravley, D.M.; Cant, J.L. Mechanical and physical properties of hydrothermally altered rocks, Taupo Volcanic Zone, New Zealand. J. Volcanol. Geotherm. Res. 2014, 288, 76–93. [Google Scholar] [CrossRef]
- Frolova, J.; Ladygin, V.; Rychagov, S.; Zukhubaya, D. Effects of hydrothermal alterations on physical and mechanical properties of rocks in the Kuril–Kamchatka island arc. Eng. Geol. 2014, 183, 80–95. [Google Scholar] [CrossRef]
- Revil, A.; Murugesu, M.; Prasad, M.; Le Breton, M. Alteration of volcanic rocks: A new non-intrusive indicator based on induced polarization measurements. J. Volcanol. Geotherm. Res. 2017, 341, 351–362. [Google Scholar] [CrossRef]
- Mordensky, S.P.; Heap, M.J.; Kennedy, B.M.; Gilg, H.A.; Villeneuve, M.C.; Farquharson, J.I.; Gravley, D.M. Influence of alteration on the mechanical behaviour and failure mode of andesite: Implications for shallow seismicity and volcano monitoring. Bull. Volcanol. 2019, 81, 44. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer: Amsterdam, The Netherlands, 2008; p. 1250. [Google Scholar]
- Bach, W.; Paulick, H.; Garrido, C.J.; Ildefonse, B.; Meurer, W.P.; Humphris, S.E. Unraveling the sequence of serpentinization reactions: Petrography, mineral chemistry, and petrophysics of serpentinites from MAR 15°N (ODP Leg 209, Site 1274). Geophys. Res. Lett. 2006, 33, 13. [Google Scholar] [CrossRef] [Green Version]
- Bonnemains, D.; Carlut, J.; Escartín, J.; Mével, C.; Andreani, M.; Debret, B. Magnetic signatures of serpentinization at ophiolite complexes. Geochem. Geophys. Geosystems 2016, 17, 2969–2986. [Google Scholar] [CrossRef] [Green Version]
- Eichelberger, J.C.; Carrigan, C.R.; Westrich, H.R.; Price, R.H. Non-explosive silicic volcanism. Nature 1986, 323, 598–602. [Google Scholar] [CrossRef]
- Mueller, S.; Scheu, B.; Spieler, O.; Dingwell, D.B. Permeability control on magma fragmentation. Geology 2008, 36, 399–402. [Google Scholar] [CrossRef]
- Collinson, A.S.D.; Neuberg, J.W. Gas storage, transport and pressure changes in an evolving permeable volcanic edifice. J. Volcanol. Geotherm. Res. 2012, 243–244, 1–13. [Google Scholar] [CrossRef]
- Cassidy, M.; Manga, M.; Cashman, K.; Bachmann, O. Controls on explosive-effusive volcanic eruption styles. Nat. Commun. 2018, 9, 2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, M.E. Massive collapse of volcano edifices triggered by hydrothermal pressurization. Geology 2004, 32, 373–376. [Google Scholar] [CrossRef]
- Ball, J.L.; Taron, J.; Reid, M.E.; Hurwitz, S.; Finn, C.; Bedrosian, P. Combining Multiphase Groundwater Flow and Slope Stability Models to Assess Stratovolcano Flank Collapse in the Cascade Range. J. Geophys. Res. Solid Earth 2018, 123, 2787–2805. [Google Scholar] [CrossRef]
- Revil, A.; Coperey, A.; Heap, M.J.; Carbillet, L. A geophysical index to map alteration, permeability, and mechanical properties within volcanoes. Application to the soft volcanic rocks from Whakaari/White Island (New Zealand). J. Volcanol. Geotherm. Res. 2020, 401, 106945. [Google Scholar] [CrossRef]
- Romero, J.E.; Polacci, M.; Watt, S.; Kitamura, S.; Tormey, D.; Sielfeld, G.; Arzilli, F.; La Spina, G.; Franco, L.; Burton, M.; et al. Volcanic Lateral Collapse Processes in Mafic Arc Edifices: A Review of Their Driving Processes, Types and Consequences. Front. Earth Sci. 2021, 9, 325. [Google Scholar] [CrossRef]
- Stix, J. Understanding Fast and Slow Unrest at Volcanoes and Implications for Eruption Forecasting. Front. Earth Sci. 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Mannen, K.; Tanada, T.; Jomori, A.; Akatsuka, T.; Kikugawa, G.; Fukazawa, Y.; Yamashita, H.; Fujimoto, K. Source constraints for the 2015 phreatic eruption of Hakone volcano, Japan, based on geological analysis and resistivity structure. Earth Planets Space 2019, 71, 135. [Google Scholar] [CrossRef] [Green Version]
- Franzson, H.; Kristjánsson, B.R.; Gunnarsson, G.; Björnsson, G.; Hjartarson, A.; Steingrímsson, B.; Gunnlaugsson, E.; Gísla-son, G. The Hengill–Hellisheiði geothermal field. Development of a conceptual geothermal model. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Revil, A.; Qi, Y.; Ghorbani, A.; Coperey, A.; Ahmed, A.S.; Finizola, A.; Ricci, T. Induced polarization of volcanic rocks. 3. Imaging clay cap properties in geothermal fields. Geophys. J. Int. 2019, 218, 1398–1427. [Google Scholar] [CrossRef]
- Weisenberger, T.B.; Ingimarsson, H.; Hersir, G.P.; Flóvenz, Ó.G. Cation-Exchange Capacity Distribution within Hydrothermal Systems and Its Relation to the Alteration Mineralogy and Electrical Resistivity. Energies 2020, 13, 5730. [Google Scholar] [CrossRef]
- Blundy, J.; Afanasyev, A.; Tattitch, B.; Sparks, S.; Melnik, O.; Utkin, I.; Rust, A. The economic potential of metalliferous sub-volcanic brines. R. Soc. Open Sci. 2021, 8, 202192. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, C. Study of Underground Electrical Prospecting. Available online: https://archive.org/details/studyofundergrou00schlrich/page/n21/mode/2up (accessed on 12 June 2021).
- Titov, K.; Komarov, V.; Tarasov, V.; Levitski, A. Theoretical and experimental study of time domain-induced polarization in water-saturated sands. J. Appl. Geophys. 2002, 50, 417–433. [Google Scholar] [CrossRef]
- Kemna, A.; Binley, A.; Cassiani, G.; Niederleithinger, E.; Revil, A.; Slater, L.; Williams, K.H.; Orozco, A.F.; Haegel, F.-H.; Hördt, A.; et al. An overview of the spectral induced polarization method for near-surface applications. Near Surf. Geophys. 2012, 10, 453–468. [Google Scholar] [CrossRef] [Green Version]
- Gross, L.; Soueid Ahmed, A.; Revil, A. Induced polarization of volcanic rocks. 4. Large-scale induced polarization imaging. Geophys. J. Int. 2021, 225, 950–967. [Google Scholar] [CrossRef]
- Revil, A.; Finizola, A.; Piscitelli, S.; Rizzo, E.; Ricci, T.; Crespy, A.; Angeletti, B.; Balasco, M.; Barde Cabusson, S.; Bennati, L.; et al. Inner structure of La Fossa di Vulcano (Vulcano Island, southern Tyrrhenian Sea, Italy) revealed by high-resolution electric resistivity tomography coupled with self-potential, temperature, and CO2 diffuse degassing measurements. J. Geophys. Res. Solid Earth 2008, 113, B7. [Google Scholar] [CrossRef] [Green Version]
- Revil, A.; Johnson, T.C.; Finizola, A. Three-dimensional resistivity tomography of Vulcan’s forge, Vulcano Island, southern Italy. Geophys. Res. Lett. 2010, 37, 15. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Carbajal, M.; Komorowski, J.-C.; Nicollin, F.; Gibert, D. Volcano electrical tomography unveils edifice collapse hazard linked to hydrothermal system structure and dynamics. Sci. Rep. 2016, 6, 29899. [Google Scholar] [CrossRef] [Green Version]
- Revil, A.; Finizola, A.; Ricci, T.; Delcher, E.; Peltier, A.; Barde-Cabusson, S.; Avard, G.; Bailly, T.; Bennati, L.; Byrdina, S.; et al. Hydrogeology of Stromboli volcano, Aeolian Islands (Italy) from the interpretation of resistivity tomograms, self-potential, soil temperature and soil CO2 concentration measurements. Geophys. J. Int. 2011, 186, 1078–1094. [Google Scholar] [CrossRef] [Green Version]
- Gresse, M.; Vandemeulebrouck, J.; Byrdina, S.; Chiodini, G.; Revil, A.; Johnson, T.C.; Ricci, T.; Vilardo, G.; Mangiacapra, A.; Lebourg, T.; et al. Three-Dimensional Electrical Resistivity Tomography of the Solfatara Crater (Italy): Implication for the Multiphase Flow Structure of the Shallow Hydrothermal System. J. Geophys. Res. Solid Earth 2017, 122, 8749–8768. [Google Scholar] [CrossRef]
- Lyklema, J.; Dukhin, S.S.; Shilov, V.N. The relaxation of the double layer around colloidal particles and the low-frequency dielectric dispersion: Part I. Theoretical considerations. J. Electroanal. Chem. Interfacial Electrochem. 1983, 143, 1–21. [Google Scholar] [CrossRef]
- Vinegar, H.J.; Waxman, M.H. Induced polarization of shaly sands. Geophysics 1984, 49, 1267–1287. [Google Scholar] [CrossRef]
- Leroy, P.; Revil, A.; Kemna, A.; Cosenza, P.; Ghorbani, A. Complex conductivity of water-saturated packs of glass beads. J. Colloid Interface Sci. 2008, 321, 103–117. [Google Scholar] [CrossRef]
- Archie, G.E. The electrical resistivity log as an aid in determining some reservoir characteristics. Pet. Trans. AIME 1942, 146, 54–62. [Google Scholar] [CrossRef]
- Revil, A.; Hermitte, D.; Spangenberg, E.; Cochemé, J.J. Electrical properties of zeolitized volcaniclastic materials. J. Geophys. Res. Solid Earth 2002, 107, ECV 3-1–ECV 3-17. [Google Scholar] [CrossRef]
- Ghorbani, A.; Revil, A.; Coperey, A.; Soueid Ahmed, A.; Roque, S.; Heap, M.J.; Grandis, H.; Viveiros, F. Complex conductivity of volcanic rocks and the geophysical mapping of alteration in volcanoes. J. Volcanol. Geotherm. Res. 2018, 357, 106–127. [Google Scholar] [CrossRef]
- Revil, A.; Breton, M.L.; Niu, Q.; Wallin, E.; Haskins, E.; Thomas, D.M. Induced polarization of volcanic rocks. 2. Influence of pore size and permeability. Geophys. J. Int. 2017, 208, 814–825. [Google Scholar] [CrossRef]
- Revil, A.; Le Breton, M.; Niu, Q.; Wallin, E.; Haskins, E.; Thomas, D.M. Induced polarization of volcanic rocks—1. Surface versus quadrature conductivity. Geophys. J. Int. 2017, 208, 826–844. [Google Scholar] [CrossRef]
- Árnason, K.; Eysteinsson, H.; Hersir, G.P. Joint 1D inversion of TEM and MT data and 3D inversion of MT data in the Hengill area, SW Iceland. Geothermics 2010, 39, 13–34. [Google Scholar] [CrossRef]
- Flóvenz, Ó.G.; Hersir, G.P.; Sæmundsson, K.; Ármannsson, H.; Friðriksson, Þ. 7.03-Geothermal Energy Exploration Techniques. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012; pp. 51–95. [Google Scholar]
- Nieto, F.; Velilla, N.; Peacor, D.R.; Huertas, M.O. Regional retrograde alteration of sub-greenschist facies chlorite to smectite. Contrib. Mineral. Petrol. 1994, 115, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Salaün, A.; Villemant, B.; Gérard, M.; Komorowski, J.-C.; Michel, A. Hydrothermal alteration in andesitic volcanoes: Trace element redistribution in active and ancient hydrothermal systems of Guadeloupe (Lesser Antilles). J. Geochem. Explor. 2011, 111, 59–83. [Google Scholar] [CrossRef]
- Finizola, A.; Ricci, T.; Deiana, R.; Cabusson, S.B.; Rossi, M.; Praticelli, N.; Giocoli, A.; Romano, G.; Delcher, E.; Suski, B.; et al. Adventive hydrothermal circulation on Stromboli volcano (Aeolian Islands, Italy) revealed by geophysical and geochemical approaches: Implications for general fluid flow models on volcanoes. J. Volcanol. Geotherm. Res. 2010, 196, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Aran, D.; Maul, A.; Masfaraud, J.-F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Ciesielski, H.; Sterckeman, T.; Santerne, M.; Willery, J.P. Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agronomie 1997, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Stimac, J.; Goff, F.; Goff, C.J. Chapter 46-Intrusion-Related Geothermal Systems A2-Sigurdsson, Haraldur. In The Encyclopedia of Volcanoes, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 799–822. [Google Scholar]
- Richards, J.P. Magmatic to hydrothermal metal fluxes in convergent and collided margins. Ore Geol. Rev. 2011, 40, 1–26. [Google Scholar] [CrossRef]
- Henley, R.W.; Ellis, A.J. Geothermal systems ancient and modern: A geochemical review. Earth-Sci. Rev. 1983, 19, 1–50. [Google Scholar] [CrossRef]
- Mas, A.; Guisseau, D.; Patrier Mas, P.; Beaufort, D.; Genter, A.; Sanjuan, B.; Girard, J.P. Clay minerals related to the hydrothermal activity of the Bouillante geothermal field (Guadeloupe). J. Volcanol. Geotherm. Res. 2006, 158, 380–400. [Google Scholar] [CrossRef]
- Revil, A.; Qi, Y.; Ghorbani, A.; Gresse, M.; Thomas, D.M. Induced polarization of volcanic rocks. 5. Imaging the temperature field of shield volcanoes. Geophys. J. Int. 2021. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sawaguchi, T.; Iwaya, S.; Horiuchi, M. Delineation of Prospecting Targets for Kuroko Deposits Based on Modes of Volcanism of Underlying Dacite and Alteration Haloes. Min. Geol. 1976, 26, 105–117. [Google Scholar] [CrossRef]
- Galley, A.; Hannington, M.D.; Jonasson, I. Volcanogenic massive sulphide deposits, in mineral deposits of Canada: A synthesis of major deposit types. Geol. Assoc. Can. Miner. Depos. Div. Spec. Publ. 2007, 5, 141–162. [Google Scholar]
- Gibson, H.L.; Galley, A.; Goodfellow, W.D. Volcanogenic massive sulphide deposits of the Archean, Noranda district, Québec. Geol. Assoc. Can. Miner. Depos. Div. Spec. Publ. 2007, 5, 533–552. [Google Scholar]
- Goodfellow, W. Metallogeny of the Bathurst Mining Camp, northern New Brunswick. Geol. Assoc. Can. Miner. Depos. Div. Spec. Publ. 2007, 5, 449–469. [Google Scholar]
- Chittoori, B.; Puppala, J. Quantitative Estimation of Clay Mineralogy in Fine-Grained Soils. J. Geotech. Geoenvironmental Eng. 2011, 137, 997–1008. [Google Scholar] [CrossRef]
- Patchett, J.G. An investigation of shale conductivity. In Society of Professional Well Logging Analysis 16th Logging Symposium; Society of Professional Well Log Analysts: Houston, TX, USA, 1975. [Google Scholar]
- Lipsicas, M. Molecular and surface interactions in clay intercalates. In Physics and Chemistry of Porous Media; Johnson, D.L., Sen, P.N., Eds.; American Institute of Physics: College Park, MD, USA, 1984; pp. 191–202. [Google Scholar]
- Zundel, J.P.; Siffert, B. Mécanisme de rétention de l’octylbenzene sulfonate de sodium sur les minéraux argileux. In Solid-Liquid Interactions in Porous Media; Technip: Paris, France, 1985; pp. 447–462. [Google Scholar]
- Lockhart, N.C. Electrical properties and the surface characteristics and structure of clays. II. Kaolinite—a nonswelling clay. J. Colloid Interface Sci. 1980, 74, 520–529. [Google Scholar] [CrossRef]
- Sinitsyn, V.A.; Aja, S.U.; Kulik, D.A.; Wood, S.A. Acid–base surface chemistry and sorption of some lanthanides on K+-saturated Marblehead illite: I. results of an experimental investigation. Geochim. Cosmochim. Acta 2000, 64, 185–194. [Google Scholar] [CrossRef]
- Avena, M.J.; De Pauli, C.P. Proton Adsorption and Electrokinetics of an Argentinean Montmorillonite. J. Colloid Interface Sci. 1998, 202, 195–204. [Google Scholar] [CrossRef]
- Shainberg, I.; Alperovitch, N.; Keren, R. Effect of Magnesium on the Hydraulic Conductivity of Na-Smectite-Sand Mixtures. Clays Clay Miner. 1988, 36, 432–438. [Google Scholar] [CrossRef]
- Su, Q.; Feng, Q.; Shang, Z. Electrical impedance variation with water saturation in rock. Geophysics 2000, 65, 68–75. [Google Scholar] [CrossRef]
- Ma, C.; Eggleton, R.A. Cation Exchange Capacity of Kaolinite. Clays Clay Miner. 1999, 47, 174–180. [Google Scholar] [CrossRef]
- Revil, A. Effective conductivity and permittivity of unsaturated porous materials in the frequency range 1 mHz–1GHz. Water Resour. Res. 2013, 49, 306–327. [Google Scholar] [CrossRef] [Green Version]
- Weller, A.; Nordsiek, S.; Debschütz, W. Estimating permeability of sandstone samples by nuclear magnetic resonance and spectral-induced polarization. Geophysics 2010, 75, E215–E226. [Google Scholar] [CrossRef]
- Weller, A.; Zhang, Z.; Slater, L.; Kruschwitz, S.; Halisch, M. Induced polarization and pore radius-A discussion. Geophysics 2016, 81, D519–D526. [Google Scholar] [CrossRef]
- Johnson, T.C.; Versteeg, R.J.; Ward, A.; Day-Lewis, F.D.; Revil, A. Improved hydrogeophysical characterization and monitoring through parallel modeling and inversion of time-domain resistivity and induced-polarization. Geophysics 2010, 75, 4. [Google Scholar] [CrossRef]
- Soueid Ahmed, A.; Revil, A.; Byrdina, S.; Coperey, A.; Gailler, L.; Grobbe, N.; Viveiros, F.; Silva, C.; Jougnot, D.; Ghorbani, A.; et al. 3D electrical conductivity tomography of volcanoes. J. Volcanol. Geotherm. Res. 2018, 356, 243–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Kruschwitz, S.; Weller, A.; Halisch, M.; Prinz, C. Enhanced pore space analysis by use of μ-CT, MIP, NMR, and SIP. In Proceedings of the Annual Symposium of the Society of Core Analysts, Vienna, Austria, 27 August–1 September 2017. [Google Scholar]
- Wang, H.; Revil, A. Surface Conduction Model for Fractal Porous Media. Geophys. Res. Lett. 2020, 47, e2020GL087553. [Google Scholar] [CrossRef]
- Revil, A.; Skold, M.; Hubbard, S.S.; Wu, Y.; Watson, D.B.; Karaoulis, M. Petrophysical properties of saprolites from the Oak Ridge Integrated Field Research Challenge site, Tennessee. Geophysics 2013, 78, D21–D40. [Google Scholar] [CrossRef]
- Revil, A.; Razdan, M.; Julien, S.; Coperey, A.; Abdulsamad, F.; Ghorbani, A.; Gasquet, D.; Sharma, R.; Rossi, M. Induced polarization response of porous media with metallic particles — Part 9: Influence of permafrost. Geophysics 2019, 84, E337–E355. [Google Scholar] [CrossRef]
- Harvey, C.C.; Browne, P.R.L. Mixed-Layer Clay Geothermometry in the Wairakei Geothermal Field, New Zealand. Clays Clay Miner. 1991, 39, 614–621. [Google Scholar] [CrossRef]
- Morata, D.; Vázquez, M.; Nieto, F. HR-TEM studies in geothermal alteration mineralogy: From the nano scale to conceptual models. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 17–19 April 2015. [Google Scholar]
- Bauer, K.; Muñoz, G. Joint Seismic and Magnetotelluric Exploration around the Geothermal Research Well Gross Schönebeck (NE German Basin). Eur. Assoc. Geosci. Eng. 2011. [Google Scholar] [CrossRef]
- Kousehlar, M.; Weisenberger, T.; Tutti, F.; Mirnejad, H. Fluid control on low-temperature mineral formation in volcanic rocks of Kahrizak, Iran. Geofluids 2012, 12, 295–311. [Google Scholar] [CrossRef]
- Cumming, W.; Mackie, R. 3D MT resistivity imaging for geothermal resource assessment and environmental mitigation at the glass mountain KGRA, California. Geothe Res. Counc. 2007, 31, 331–334. [Google Scholar]
- Ushijima, K.; Jotaki, H.; Mizunaga, H. Magnetotelluric Soundings in the Takigami Geothermal Area, Japan. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Hersir, G.P.; Árnason, K.; Vilhjálmsson, A.M.; Saemundsson, K.; Ágústsdóttir, Þ.; Friðleifsson, G.Ó. Krýsuvík high temperature geothermal area in SW Iceland: Geological setting and 3D inversion of magnetotelluric (MT) resistivity data. J. Volcanol. Geotherm. Res. 2020, 391, 106500. [Google Scholar] [CrossRef]
- Tugawin, R.J.; Rigor, D.M.; Banos, C.; Layugan, D. Resistivity Model Based on 2D Inversion of Magnetotelluric Sounding Data in Bacon- Manito, Southern Luzon, Philippines. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 19–25 April 2015. [Google Scholar]
- Gasperikova, E.; Rosenkjaer, G.K.; Arnason, K.; Newman, G.A.; Lindsey, N.J. Resistivity characterization of the Krafla and Hengill geothermal fields through 3D MT inverse modeling. Geothermics 2015, 57, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Revil, A.; Florsch, N.; Mao, D. Induced polarization response of porous media with metallic particles-Part 1: A theory for disseminated semiconductors. Geophysics 2015, 80, D525–D538. [Google Scholar] [CrossRef]
- Revil, A.; Abdel Aal, G.Z.; Atekwana, E.A.; Mao, D.; Florsch, N. Induced polarization response of porous media with metallic particles — Part 2: Comparison with a broad database of experimental data. Geophysics 2015, 80, D539–D552. [Google Scholar] [CrossRef]
- Pelton, W.H.; Ward, S.H.; Hallof, P.G.; Sill, W.R.; Nelson, P.H. Mineral discrimination and removal of inductive coupling with multifrequency IP. Geophysics 1978, 43, 588–609. [Google Scholar] [CrossRef]
- Mahan, M.K.; Redman, J.D.; Strangway, D.W. Complex resistivity of synthetic sulfide bearing rocks. Geophys. Prospect. 1986, 34, 743–768. [Google Scholar] [CrossRef]
- Phillips, C.R. Experimental Study of the Induced Polarization Effect Using Cole-Cole and GEMTIP Models. Master’s Thesis, University of Utah, Salt Lake City, UT, USA, 2010. [Google Scholar]
- Gurin, G.; Titov, K.; Ilyin, Y.; Tarasov, A. Induced polarization of disseminated electronically conductive minerals: A semi-empirical model. Geophys. J. Int. 2015, 200, 1555–1565. [Google Scholar] [CrossRef] [Green Version]
- Hogg, C.; Kiyan, D.; Rath, V.; Byrdina, S.; Vandemeulebrouck, J.; Revil, A.; Viveiros, F.; Carmo, R.; Silva, C.; Ferreira, T. 3-D interpretation of short-period magnetotelluric data at Furnas Volcano, Azores Islands. Geophys. J. Int. 2017, 213, 371–386. [Google Scholar] [CrossRef]
- Revil, A.; Qi, Y.; Ghorbani, A.; Soueid Ahmed, A.; Ricci, T.; Labazuy, P. Electrical conductivity and induced polarization investigations at Krafla volcano, Iceland. J. Volcanol. Geotherm. Res. 2018, 368, 73–90. [Google Scholar] [CrossRef]
- Raguenel, M.; Driesner, T.; Bonneau, F. Numerical modeling of the geothermal hydrology of the Volcanic Island of Basse-Terre, Guadeloupe. Geotherm. Energy 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Gresse, M.; Uyeshima, M.; Koyama, T.; Hase, H.; Aizawa, K.; Yamaya, Y.; Morita, Y.; Weller, D.; Rung-Arunwan, T.; Kaneko, T.; et al. Hydrothermal and Magmatic System of a Volcanic Island Inferred From Magnetotellurics, Seismicity, Self-potential, and Thermal Image: An Example of Miyakejima (Japan). J. Geophys. Res. Solid Earth 2021, 126, e2021JB022034. [Google Scholar] [CrossRef]
- Lambert, J.M.; Simkovich, G.; Walker, P.L. Production of pyrrhotites by pyrite reduction. Fuel 1980, 59, 687–690. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revil, A.; Gresse, M. Induced Polarization as a Tool to Assess Alteration in Geothermal Systems: A Review. Minerals 2021, 11, 962. https://doi.org/10.3390/min11090962
Revil A, Gresse M. Induced Polarization as a Tool to Assess Alteration in Geothermal Systems: A Review. Minerals. 2021; 11(9):962. https://doi.org/10.3390/min11090962
Chicago/Turabian StyleRevil, Andre, and Marceau Gresse. 2021. "Induced Polarization as a Tool to Assess Alteration in Geothermal Systems: A Review" Minerals 11, no. 9: 962. https://doi.org/10.3390/min11090962
APA StyleRevil, A., & Gresse, M. (2021). Induced Polarization as a Tool to Assess Alteration in Geothermal Systems: A Review. Minerals, 11(9), 962. https://doi.org/10.3390/min11090962





