Rapid Extraction Chemistry Using a Single Column for 230Th/U Dating of Quaternary Hydrothermal Sulfides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sample Digestion and U/Th Purification
2.3. Instrumental Analysis
3. Results and Discussion
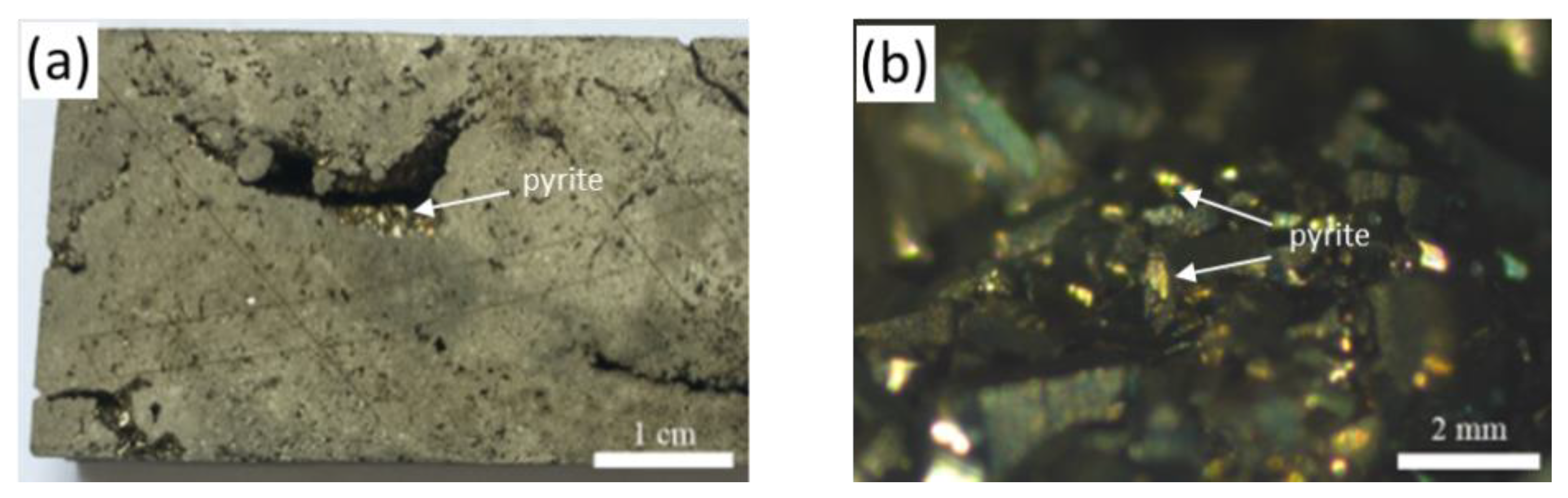
3.1. Impact of Dissolution Protocols on U and Isotopes
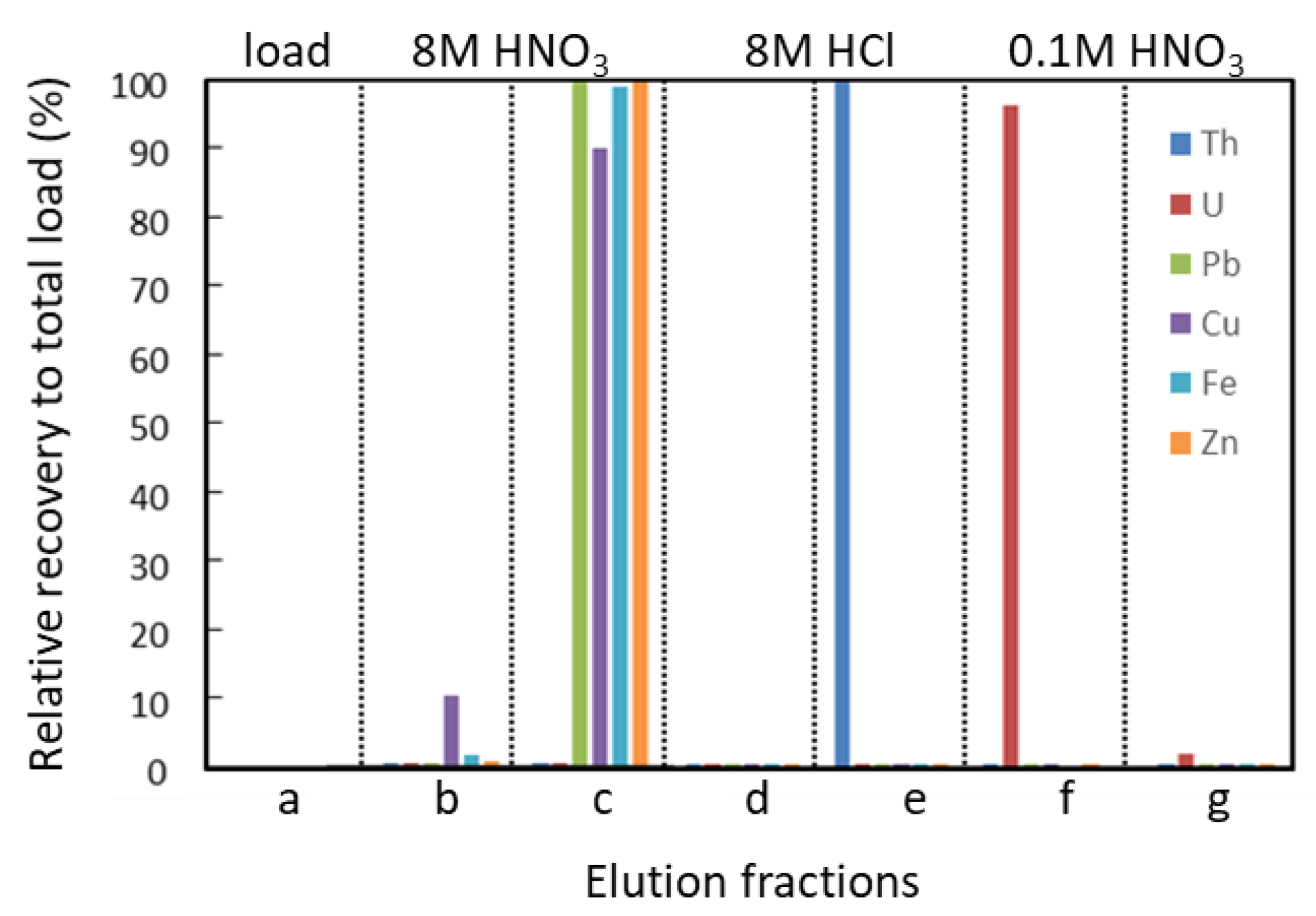
3.2. Yields of Th and U, and Leaching
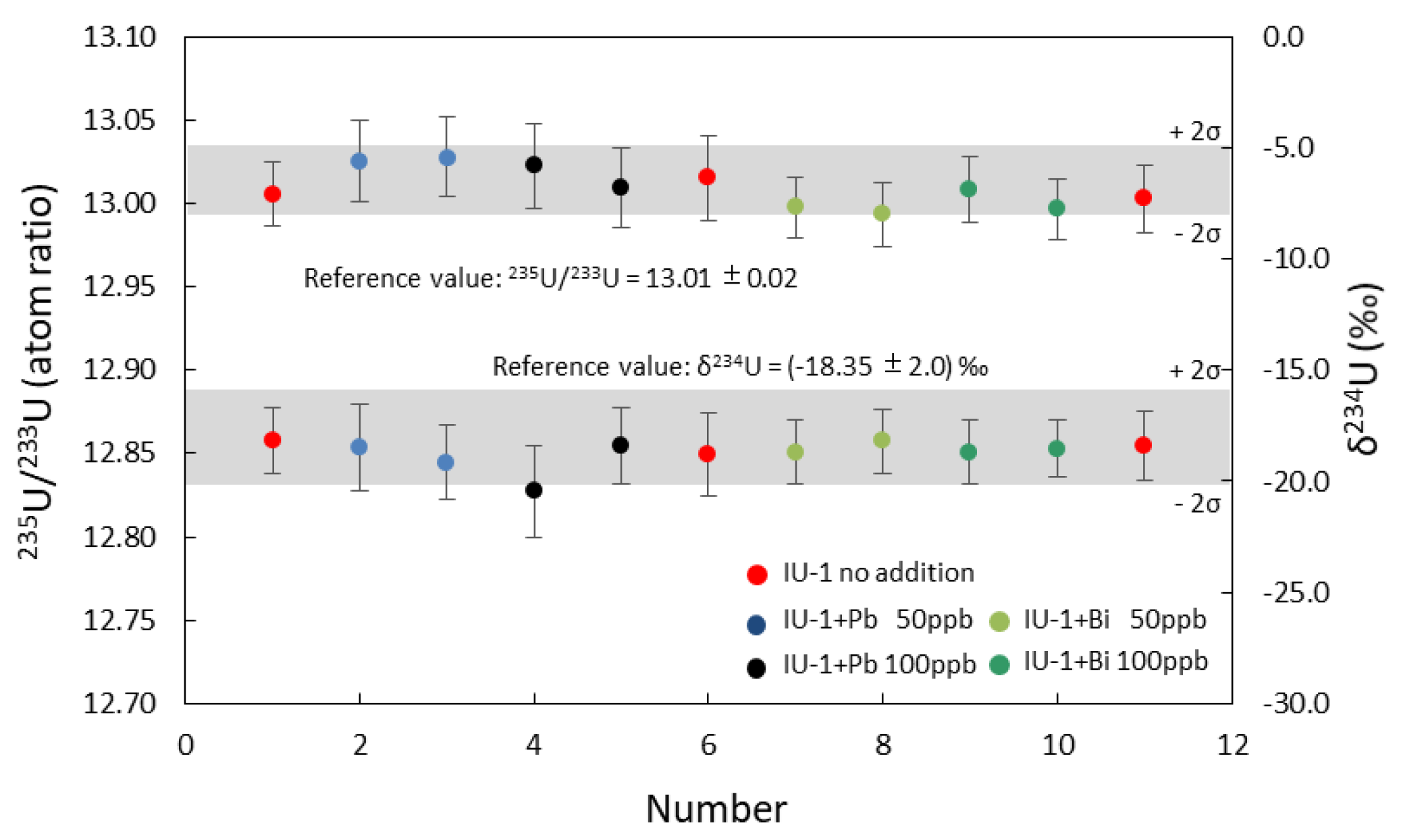
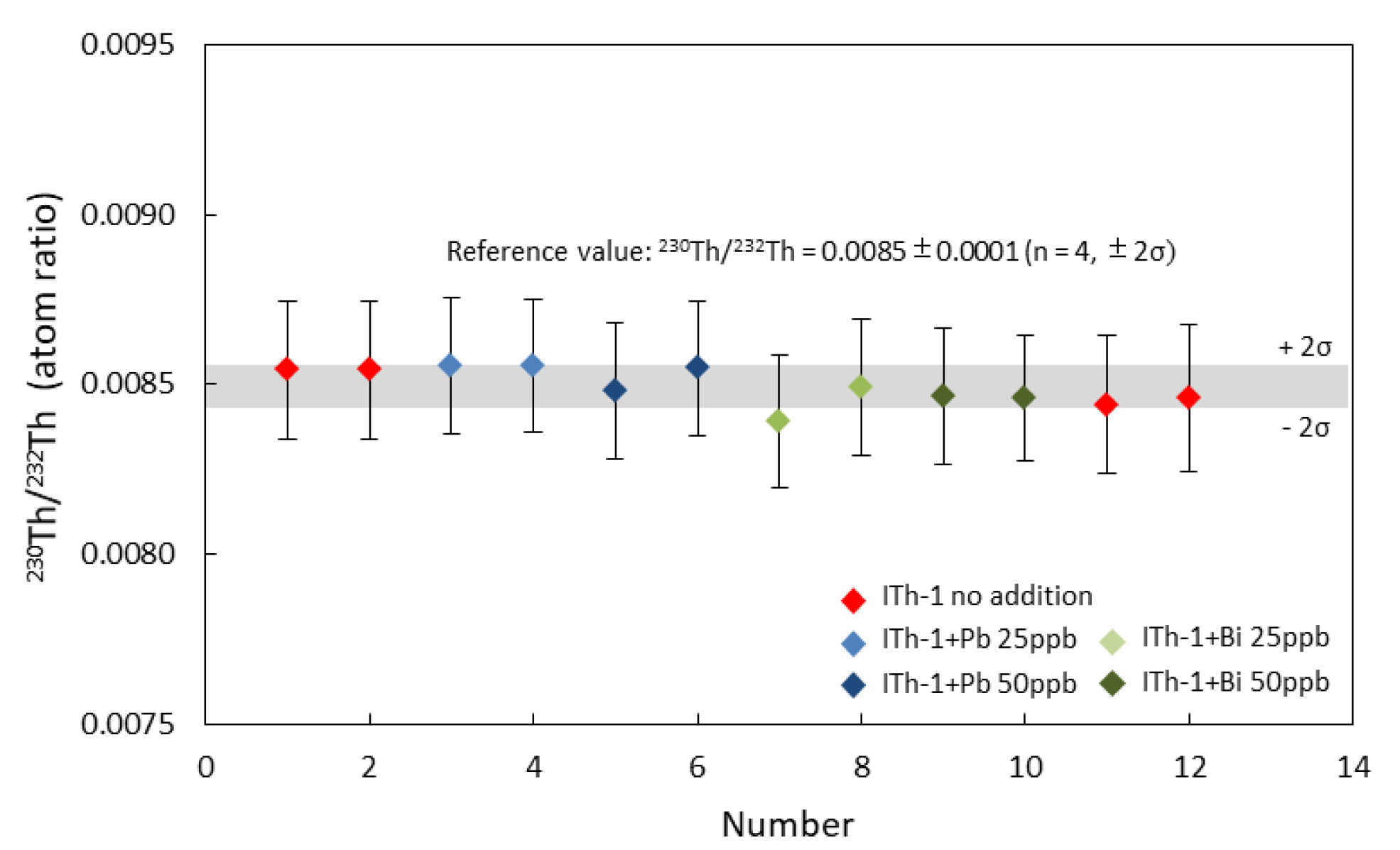
3.3. Impact of Pb and Bi on U-Th Isotopes
3.4. High-Precision 230Th/U Dating of Sulfide Standard and Geological Sample
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, H.; Edwards, R.L.; Shen, C.-C.; Polyak, V.J.; Asmerom, Y.; Woodhead, J.; Hellstrom, J.; Wang, Y.; Kong, X.; Spötl, C.; et al. Improvements in Th-230 dating, Th-230 and U-234 half-life values, and U-Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth Planet. Sci. Lett. 2013, 371, 82–91. [Google Scholar] [CrossRef]
- Edwards, R.L.; Chen, J.H.; Wasserburg, G.J. U-238 U-234-Th-230-Th-232 Systematics and the Precise Measurement of Time over the Past 500,000 Years. Earth Planet. Sci. Lett. 1987, 81, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-C.; Wu, C.-C.; Cheng, H.; Edwards, R.L.; Hsieh, Y.-T.; Gallet, S.; Chang, C.-C.; Li, T.-Y.; Doan Dinh, L.; Kano, A.; et al. High-precision and high-resolution carbonate Th-230 dating by MC-ICP-MS with SEM protocols. Geochim. Cosmochim. Acta 2012, 99, 71–86. [Google Scholar] [CrossRef]
- Lalou, C.; Brichet, E.; Hekinian, R. Age dating of sulfide deposits from axial and off-axial structures on the East Pacific Rise near 12°50′N. Earth Planet. Sci. Lett. 1985, 75, 59–71. [Google Scholar] [CrossRef]
- Lalou, C. Age of sub-bottom sulfide samples at the tag active mound. Proc. Ocean Drill. Program Sci. Results 1998, 158, 111–117. [Google Scholar]
- Wang, L.; Wang, X.; Ye, J.; Ma, Z.; Yang, W.; Xiao, J. Separation of Uranium and Thorium for Th-230-U Dating of Submarine Hydrothermal Sulfides. J. Vis. Exp. 2019, 147, 1833–1850. [Google Scholar] [CrossRef]
- Nakai, S.; Takamasa, A.; Fujiwara, T.; Toyoda, S.; Ishibashi, J.; Yoshizumi, R.; Urabe, T. Influence of Th-rich mineral phases on U–Th radioactive disequilibrium ages of sulfide deposits from the Okinawa Trough. Chem. Geol. 2018, 486, 61–72. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Tabuns, E.V.; Cherkashev, G.A.; Bel’tenev, V.E.; Maksimov, F.E.; Kuksa, K.A.; Lazareva, L.I.; Levchenkoa, S.B.; Zherebtsov, I.E. 230Th/U Chronology and Geochemistry of Irinovskoe Hydrothermal Field (Mid-Atlantic Ridge). Dokl. Earth Sci. 2018, 478, 26–30. [Google Scholar] [CrossRef]
- Takamasa, A.; Nakai, S.I.; Sato, F.; Toyoda, S.; Banerjee, D.; Ishibashi, J. U–Th radioactive disequilibrium and ESR dating of a barite-containing sulfide crust from South Mariana Trough. Quat. Geochronol. 2013, 15, 38–46. [Google Scholar] [CrossRef]
- Lalou, C.; Brichet, E. On the Isotopic Chronology of Submarine Hydrothermal Deposits. Chem. Geol. 1987, 65, 197–207. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Jin, X.; Qiu, Z.; Ma, Z.; Yang, H. Hydrothermal Activity Events at Kairei Field, Central Indian Ridge 25°S. Resour. Geol. 2012, 62, 208–214. [Google Scholar] [CrossRef]
- Ishibashi, J.-I.; Shimada, K.; Sato, F.; Uchida, A.; Toyoda, S.; Takamasa, A.; Nakai, S.I.; Hyodo, H.; Sato, K.; Kumagai, H.; et al. Dating of Hydrothermal Mineralization in Active Hydrothermal Fields in the Southern Mariana Trough. In Subseafloor Biosphere Linked to Hydrothermal Systems; Springer: Berlin, Germany, 2015; Volume 23, pp. 289–300. [Google Scholar]
- Yang, W.; Tao, C.; Li, H.; Liang, J.; Liao, S.; Long, J.; Ma, Z.; Wang, L. Th-230/U-238 dating of hydrothermal sulfides from Duanqiao hydrothermal field, Southwest Indian Ridge. Mar. Geophys. Res. 2017, 38, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, K.; Ma, L.; Hishikawa, K.; Usui, A. Geochemistry and U-series dating of Holocene and fossil marine hydrothermal manganese deposits from the Izu-Ogasawara arc. Ore Geol. Rev. 2017, 87, 114–125. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Tabuns, E.V.; Kuksa, K.A.; Cherkashov, G.A.; Bel’tenev, V.E.; Arslanov, K.A.; Maksimov, F.E.; Lazareva, L.I.; Zhuravleva, A.I.; Petrov, A.Y.; et al. Chronology of Hydrothermal Activity Within the Yubileynoye Ore Field (Mid-Atlantic Ridge, 20A degrees 08′ N). Dokl. Earth Sci. 2018, 480, 700–704. [Google Scholar] [CrossRef]
- Chiang, H.-W.; Lu, Y.; Wang, X.; Lin, K.; Liu, X. Optimizing MC-ICP-MS with SEM protocols for determination of U and Th isotope ratios and 230Th ages in carbonates. Quat. Geochronol. 2019, 50, 75–90. [Google Scholar] [CrossRef]
- Andersen, M.B.; Stirling, C.H.; Potter, E.K.; Halliday, A.N. Toward epsilon levels of measurement precision on U-234/U-238 by using MC-ICPMS. Int. J. Mass Spectrom. 2004, 237, 107–118. [Google Scholar] [CrossRef]
- Shen, C.-C.; Lin, K.; Duan, W.; Jiang, X.; Partin, J.W.; Edwards, R.L.; Cheng, H.; Tan, M. Testing the annual nature of speleothem banding. Sci. Rep. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Edwards, R.L.; Sinha, A.; Spoetl, C.; Yi, L.; Chen, S.; Kelly, M.; Kathayat, G.; Wang, X.; Li, X.; et al. The Asian monsoon over the past 640,000 years and ice age terminations. Nature 2016, 534, 640–646. [Google Scholar] [CrossRef]
- Douville, E.; Salle, E.; Frank, N.; Eisele, M.; Pons-Branchu, E.; Ayrault, S. Rapid and accurate U-Th dating of ancient carbonates using inductively coupled plasma-quadrupole mass spectrometry. Chem. Geol. 2010, 272, 1–11. [Google Scholar] [CrossRef]
- Stirling, C.H.; Andersen, M.B. Uranium-series dating of fossil coral reefs: Extending the sea-level record beyond the last glacial cycle. Earth Planet. Sci. Lett. 2009, 284, 269–283. [Google Scholar] [CrossRef]
- Hoffmann, D.L. Th-230 isotope measurements of femtogram quantities for U-series dating using multi ion counting (MIC) MC-ICPMS. Int. J. Mass Spectrom. 2008, 275, 75–79. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Maksimov, F.; Zheleznov, A.; Cherkashov, G.; Bel’Tenev, V.; Lazareva, L. 230Th/U chronology of ore formation within the semyenov hydrothermal district (13°31′ N) at the Mid-Atlantic ridge. Geochronometria 2011, 38, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Cherkashov, G.; Kuznetsov, V.; Kuksa, K.; Tabuns, E.; Maksimov, F.; Bel’tenev, V. Sulfide geochronology along the Northern Equatorial Mid-Atlantic Ridge. Ore Geol. Rev. 2017, 87, 147–154. [Google Scholar] [CrossRef]
- Ivanovich, M.; Harmon, R.S. Uranium Series Disequilibrium: Applications to Environmental Problems; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Kuznetsov, V.; Cherkashev, G.; Lein, A.; Shilov, V.; Maksimov, F.; Arslanov, K.; Stepanova, T.; Baranova, N.; Chernov, S.; Tarasenko, D. Th-230/U dating of massive sulfides from the Logatchev and Rainbow hydrothermal fields (Mid-Atlantic Ridge). Geochronometria 2006, 25, 51–55. [Google Scholar]
- Wang, L.; Ma, Z.; Cheng, H.; Duan, W.; Xiao, J. Determination of 230Th Dating Age of Uranium-Series Standard Samples by Multiple Collector Inductively Coupled Plasma Mass Spectrometry. J. Chin. Mass Spectrom. Soc. 2016, 37, 262–272. [Google Scholar]
- Wang, L.; Ma, Z.; Sun, Z.; Wang, Y.; Wang, X.; Cheng, H.; Xiao, J. U concentration and 234U/238 U of seawater from the Okinawa Trough and Indian Ocean using MC-ICPMS with SEM protocols. Mar. Chem. 2017, 196, 71–80. [Google Scholar] [CrossRef]
- Terashima, S.; Sato, K.; Taniguchi, M.; Okai, T.; Imai, N. The preparation and characterisation of two new Geological Survey of Japan geochemical reference materials: Copper ore JCu-1 and zinc ore JZn-1. Geostand. Newsl. J. Geostand. Geoanal. 2003, 27, 259–271. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Petersen, S.; Frische, M.; Qiu, Z.; Li, H.; Li, H.; Wu, Z.; Cui, R. Mineralogy and trace element geochemistry of sulfide minerals from the Wocan Hydrothermal Field on the slow-spreading Carlsberg Ridge, Indian Ocean. Ore Geol. Rev. 2017, 84, 1–19. [Google Scholar] [CrossRef]
- Shen, C.C.; Edwards, R.L.; Cheng, H.; Dorale, J.A.; Thomas, R.B.; Moran, S.B.; Weinstein, S.E.; Edmonds, H.N. Uranium and thorium isotopic and concentration measurements by magnetic sector inductively coupled plasma mass spectrometry. Chem. Geol. 2002, 185, 165–178. [Google Scholar] [CrossRef]
- Hiess, J.; Condon, D.J.; McLean, N.; Noble, S.R. U-238/U-235 Systematics in Terrestrial Uranium-Bearing Minerals. Science 2012, 335, 1610–1614. [Google Scholar] [CrossRef] [Green Version]
- Jaffey, A.H.; Flynn, K.F.; Glendenin, L.E.; Bentley, W.C.; Essling, A.M. Precision measurement of half-lives and specific activities of U-235 and U-238. Phys. Rev. C 1971, 4, 1889. [Google Scholar] [CrossRef]
- Makishima, A.; Nakamura, E. High-resolution MC-ICPMS employing amplifiers with a 10 ohm resistor for bulk sulfur determination in biological and geological samples. J. Anal. At. Spectrom. 2012, 27, 891–895. [Google Scholar] [CrossRef]
- Andersen, M.B.; Stirling, C.H.; Zimmermann, B.; Halliday, A.N. Precise determination of the open ocean 234U/238U composition. Geochem. Geophys. Geosyst. 2010, 11, 1–8. [Google Scholar] [CrossRef]

| Step | Acid Condition | Volume (mL) | Elements Eluted | Fractions |
|---|---|---|---|---|
| Clean | 8 M HNO3 | 1.0 | ||
| 0.5 | ||||
| 8 M HCl | 1.0 | |||
| 0.5 | ||||
| H2O (18.2 MΩ·cm) | 1.0 | |||
| 1.0 | ||||
| Condition | 8 M HNO3 | 1.0 | ||
| 1.0 | ||||
| Load Sample | 8 M HNO3 | 0.2 | a | |
| Wash | 8 M HNO3 | 1.0 | matrix elements | b |
| 0.5 | matrix elements | c | ||
| Elute Th | 8 M HCl | 1.0 | Th | d |
| 0.5 | Th | e | ||
| Elute U | 0.1 M HNO3 | 1.0 | U | f |
| 1.0 | U | g |
| Instrument | Parameter | Setting |
|---|---|---|
| MC-ICPMS | RF power | 1250 W |
| Cooling gas | 16 L/min | |
| Auxiliary gas | 1.8 L/min | |
| Sample gas | 1 L/min | |
| Extraction voltage | −2000 V | |
| Low resolution | 300 | |
| CETAC Aridus II | Sample injection rate | 50 μL/min |
| Ar sweep gas | 2 L/min | |
| Nitrogen gas | 3 mL/min | |
| Spray chamber temperature | 110 °C | |
| Membrane oven temperature | 160 °C |
| Element | L1 | C(SEM) | H1 | H2 | H3 |
|---|---|---|---|---|---|
| U | 233U | ||||
| 234U | |||||
| 235U | 238U | ||||
| 236U | 238U | ||||
| Th | 229Th | ||||
| 230Th | 232Th |
| No. | Mass (g) | Dissolution Acid | Resin | Dissolution Time (Day) | Instrument | Reference |
|---|---|---|---|---|---|---|
| 1 | 2.0–4.0 | aqua regia | AG 1 × 8 or AV-17 | >2 | α-couting and MC-ICP-MS | Lalou et al., 1985, 1986 [4,5]; Kuznetsov et al., 2006 [26]; Wang et al., 2019 [6] |
| 2 | 1.0–2.0 | HNO3 + H2O | AG1-X8 and U/TEVA | 2–7 | ICPMS and MC-ICP-MS | Takamasa et al., 2013 [9]; Ishibashi et al., 2015 [12]; Nakai et al., 2018 [7] |
| 3 | <0.1 | HCl + HF followed by HNO3 | AG 1-X8 | <1 | MC-ICP-MS | This study |
| Standard | Mass | 238U | 232Th | 230Th/232Th | δ234U * | 230Th/238U | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg) | (ppb) | (ppb) | (AT × 10−6) | (Measured) | (Activity Ratio) | ||||||
| JZn-1 | 53 | 2033 | ±2 | 9218 | ±47 | 3.64 | ±0.03 | 19 | ±1 | 1.00 | ±0.01 |
| 50 | 2038 | ±2 | 9178 | ±47 | 3.65 | ±0.03 | 20 | ±1 | 1.00 | ±0.01 | |
| 52 | 2037 | ±2 | 9185 | ±47 | 3.64 | ±0.03 | 17 | ±1 | 1.00 | ±0.01 | |
| 53 | 2042 | ±2 | 9189 | ±47 | 3.64 | ±0.03 | 20 | ±1 | 1.00 | ±0.01 | |
| 57 | 2034 | ±2 | 9207 | ±47 | 3.64 | ±0.03 | 19 | ±1 | 1.00 | ±0.01 | |
| Mean | 2037 | ±7 | 9195 | ±33 | 3.64 | ±0.01 | 19 | ±2 | 1.00 | ±0.01 | |
| Element | Wt. % | 100 Elt./Fe |
|---|---|---|
| Fe | 33.0 | 100.0 |
| Na | 1.8 | 5.5 |
| Mg | 0.4 | 1.2 |
| Si | 0.4 | 1.2 |
| Al | 0.2 | 0.6 |
| Ca | 0.2 | 0.6 |
| Cu | 0.1 | 0.3 |
| Zn | 0.1 | 0.3 |
| Sample | 238U | 232Th | 230Th / 232Th | δ234U | 230Th/238U | 230Th Age (yr, UC) c | 230Th Age (yr, C) d | δ234UInitial c (C) | 230Th Age (yr BP, C) e | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | (ppb) | (ppt) | (AT × 10−6) a | (Measured) b | (Activity) | |||||||||||||
| 1 | 4872 | ±14 | 2780 | ±56 | 223.4 | ±4.7 | 146 | ±2 | 0.0077 | ±0.0001 | 738 | ±5 | 723 | ±11 | 147 | ±2 | 703 | ±11 |
| 2 | 4997 | ±14 | 2621 | ±50 | 246.0 | ±4.9 | 144 | ±2 | 0.0078 | ±0.0001 | 749 | ±5 | 735 | ±11 | 144 | ±2 | 715 | ±11 |
| 3 | 4853 | ±13 | 2770 | ±56 | 225.5 | ±4.7 | 147 | ±2 | 0.0078 | ±0.0001 | 745 | ±5 | 730 | ±11 | 147 | ±2 | 710 | ±11 |
| 4 | 5010 | ±14 | 2739 | ±55 | 231.6 | ±4.8 | 144 | ±2 | 0.0077 | ±0.0001 | 735 | ±5 | 721 | ±11 | 144 | ±2 | 701 | ±11 |
| 5 | 4921 | ±14 | 3090 | ±62 | 203.7 | ±4.3 | 145 | ±2 | 0.0078 | ±0.0001 | 741 | ±5 | 725 | ±12 | 145 | ±2 | 705 | ±12 |
| 6 | 5083 | ±10 | 2895 | ±79 | 225.2 | ±6.4 | 147 | ±2 | 0.0078 | ±0.0001 | 742 | ±6 | 728 | ±12 | 147 | ±2 | 702 | ±15 |
| 7 | 4980 | ±12 | 3039 | ±61 | 208.6 | ±4.4 | 146 | ±2 | 0.0077 | ±0.0001 | 737 | ±5 | 721 | ±12 | 147 | ±2 | 701 | ±12 |
| 8 | 4933 | ±11 | 2634 | ±73 | 238.8 | ±6.9 | 147 | ±2 | 0.0077 | ±0.0001 | 737 | ±6 | 724 | ±11 | 148 | ±2 | 704 | ±11 |
| Mean | 4956 | ±153 | 2821 | ±347 | 225.4 | ±28.3 | 146 | ±3 | 0.0078 | ±0.0001 | 740 | ±9 | 726 | ±10 | 146 | ±3 | 705 | ±10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-S.; Wang, Y.-J.; Ye, J.; Wang, X.-F.; Xiao, J.-L.; Ma, Z.-B. Rapid Extraction Chemistry Using a Single Column for 230Th/U Dating of Quaternary Hydrothermal Sulfides. Minerals 2021, 11, 983. https://doi.org/10.3390/min11090983
Wang L-S, Wang Y-J, Ye J, Wang X-F, Xiao J-L, Ma Z-B. Rapid Extraction Chemistry Using a Single Column for 230Th/U Dating of Quaternary Hydrothermal Sulfides. Minerals. 2021; 11(9):983. https://doi.org/10.3390/min11090983
Chicago/Turabian StyleWang, Li-Sheng, Ye-Jian Wang, Jun Ye, Xue-Feng Wang, Ju-Le Xiao, and Zhi-Bang Ma. 2021. "Rapid Extraction Chemistry Using a Single Column for 230Th/U Dating of Quaternary Hydrothermal Sulfides" Minerals 11, no. 9: 983. https://doi.org/10.3390/min11090983






