Abstract
Eudialyte ores from Norra Kärr (Sweden) and Kringlerne (Greenland) are considered a potential source of rare-earth elements (REE) for the development of a sustainable REE industry outside China. Magnetic separation is successfully applicated to recover eudialyte as a magnetic fraction. In the case of the Norra Kärr deposit, up to 20% of the REE and up to 40% of the Zr are lost during mineral processing in the non-magnetic fraction. Zr and REE are associated with non-magnetic minerals such as catapleiite, low- or non-magnetic eudialyte species, and both their intergrowths. Besides zirconosilicates such as catapleiite and eudialyte, the non-magnetic fraction has valuable and already-liberated minerals such as alkali feldspars and nepheline, which should not be considered as tailings. In this investigation, a possible way to recover REE bearing zirconosilicates from the non-magnetic fraction using flotation is presented. First, a low-grade eudialyte concentrate (1.8% Zr, 0.94% REE) from ground ore was obtained using magnetic separation. The non-magnetic fraction was then treated using froth flotation, and a Zr-REE bearing product (9% Zr, 1.5% REE) was obtained as froth product. For this purpose, phosphoric acid esters were used as selective collectors for zirconosilicates at a pH between 3.5 and 4.5. The reagent regime could be proposed not only to recover Zr- and REE-bearing minerals, but also simultaneously to remove Fe, Ti, and other colored impurities from the nepheline-feldspar product and to minimize the tailings volume.
1. Introduction
The development of a modern green and low-carbon industry in developed countries requires a sustainable and fair supply chain of critical raw materials such as cobalt, lithium, vanadium, and rare earth elements (REE) [1,2,3]. To develop an independent REE industry, the Western world has invested in REE projects outside of China and has evaluated economically feasible REE projects [4]. Nowadays, alkaline igneous complexes are considered as potential sources for critical and other elements such as REE and high-field-strength elements (HFSE) such as Zr, Hf, Nb, and Ta. In this context, nepheline syenite rocks belong to alkaline igneous rocks, distinctly enriched in heavy REE (HREE) and HFSE [5].
Norra Kärr is a peralkaline nepheline-syenite intrusive complex located in south-central Sweden and is defined as comprising agpaitic rocks [6]. It is a unique deposit of REE and HFSE elements with a high ratio of HREE relative to total REE (TREE) represented by eudialyte group minerals (EGM). These are characterized by various chemical compositions and degrees of alteration [7]. The major rocks of the geological formation of the Norra Kärr deposit are lithologically subdivided into several main rock types, characterized by various texture types, alteration, crystal size, mineral composition, and amounts of zirconium and REE. They include pegmatitic, migmatitic, and catapleiite-containing grennaite (PGT), kaxtorpite, lakarpite, pulaskite, and mafic rocks [8]. EGM from Norra Kärr deposit are characterized by various chemical compositions and degrees of alteration. According to Sjöqvist et al. [7], in the Norra Kärr deposit, EGM is divided int othree main distinct groups: Fe-rich (REE-poor eudialyte from lakarpite), Fe-Mn-bisected (HREE-rich eudialyte from “pegmatitic” grennaite), and Mn-rich (LREE-rich eudialyte from “migmatitic” grennaite). Depending on the rock types, låvenite, rosenbuschite, zircon, catapleiite, mosandrite, lorenzenite, wöhlerite, britholite, cerite, and zircon, which are formed as complex pseudomorphic aggregates of secondary zirconosilicates, aluminosilicates, and various Nb and REE phases, were identified as minor accessory complex HFSE- and REE-bearing minerals [7].
According to the current state of the art, the main method for processing eudialyte ores is magnetic separation [9,10,11,12]. The main aims of Wet High-Intensity Magnetic Separation (WHIMS) are to concentrate REE-bearing minerals into the magnetic fraction and to remove acid soluble gangue minerals into non-magnetic products. A metallurgical treatment of eudialyte concentrates and leaching residues is based on hydrometallurgical methods and thermal decomposition followed by fuming, dry digestion, water leaching, and corresponding precipitation stages [13,14,15,16,17,18,19]. The non-magnetic fraction of the WHIMS stage is currently considered as tailings. About 24.8 Mt of tailings are disposed of in the projected tailings facility in Sweden, with an operational mine life of 20 years [20]. Due to the presence of non-magnetic zirconosilicates, most commonly catapleiite and other alteration products of EGM, an optimal recovery of Zr in the magnetic fraction cannot be achieved by magnetic separation alone.
Catapleiite is a rare, complex zirconosilicate (typomorphic postmagmatic mineral). It belongs to the catapleiite group, which consist of two International Mineralogical Association (IMA)-approved members: catapleiite (natrocatapleiite Na2ZrSi3O9·2H2O) and calciocatapleiite (CaZrSi3O9·2H2O). Structurally, the isolated [ZrO6] octahedra and three-membered [Si3O9] rings of tetrahedra form a zeolite-like heteropolyhedral framework. The framework cavities are filled with water molecules and sodium cations (frequently with substantial Ca) [21,22]. In the current work, the term “catapleiite“ from the Norra Kärr deposit shall be understood only as catapleiite–calciocatapleiite isomorphous series. It is commonly distributed in alkaline complexes as hydrothermal alteration products of EGM [8,23,24].
Currently, catapleiite is not processed on a commercial scale worldwide for the recovery of Zr and Hf. The recovery of catapleiite and other complex zirconosilicates such as wöhlerite, vlasovite, elpidite, and gittinsite from ores is investigated poorly so far. Froth flotation can be proposed for the recovery of zirconosilicates from gangue silicates such feldspars and nephelines. The floatability of already well-investigated zircon minerals such as zircon Zr(SiO4), baddeleyite ZrO2, eudialyte Na15Ca6Fe3Zr3Si(Si25O73)(O,OH,H2O)3(Cl,OH)2, and REE-containing silicates can also be helpful in the selection of a reagent system for the selective flotation of zirconsilicates. Potential collectors of these minerals could be fatty acids, sodium oleate, hydroxamic acids, amine, alkylsulfates, phosphonic acids, and monoalkyl phosphates [25,26,27,28].
It is well known that crushing and grinding of raw materials consume up to 4% of the energy produced worldwide [29]. To enhance the efficiency and to reduce the energy consumption, ground raw materials as well as processed waste streams should be considered as a source for possible and recoverable products [30,31,32]. The main aim of this research work is to determine a reagent regime to recover Zr- and REE-bearing minerals from the Norra Kärr ore by flotation, which in this case are currently lost into the non-magnetic fraction. Additionally, with the use of froth flotation, it would be possible to simultaneously remove undesirable colored impurities and their intergrowths such as iron- and titanium-bearing minerals (titanite, micas (muscovite), pyroxenes, aegirine) from the non-magnetic fraction. In this case, the tailings product of the froth flotation must be considered as an additional marketable by-product such as nepheline syenite. It can be used in the glass, porcelain, and ceramics industries or as filling material [33]. Other researchers consider the nepheline syenite as source of potassium and aluminum using chloridizing–roasting technologies and leaching [34,35]. Thus, the second aim of this work is to investigate the possibility of producing nepheline syenite products from the non-magnetic fraction. This solution could lead to a minimization of the tailings volume to be disposed of when processing the Norra Kärr deposit according to the near-zero-waste principle [31].
The most critical requirements for nepheline syenite products are low contents of iron and titanium, which are the major undesirable impurities for the glass and ceramics industry [33]. A total Fe2O3 content of less than 0.1% is required for the production of clear glass, while a higher Fe2O3 content of up to 0.35% can be tolerated for colored glass [36]. For the removal of colored impurities and undesirable minerals from various nepheline syenites, flotation and magnetic separation are widely used, while leaching is less frequently used [37,38,39,40].
2. Materials and Methods
2.1. Materials
Representative samples were obtained during a sampling campaign at exploration works on the Norra Kärr deposit. The eudialyte ore sample was initially crushed and then dry ground with a vertical roller mill (VRM), (Loesche GmbH, Düsseldorf, Germany). The ground products were split among the subsamples, stored, and used for the various processing tests. According to wet-sieving analyses, the low-grade eudialyte ore sample was ground to 85% passing 90 µm (P50 = 45 µm and P25 = 25 µm). After dry grinding by VRM, the eudialyte ore sample was processed using WHIMS to obtain a low-grade eudialyte concentrate in the magnetic fraction. The non-magnetic fraction was then used as feed material for the flotation experiments to recover non-magnetic Zr- and REE-bearing minerals. The detailed characterization of the non-magnetic fraction is given in Section 3.1.
In order to identify the main minerals and components of the ground samples, mineralogical and chemical analyses were conducted. The results of the eudialyte ore sample are presented in Table 1.

Table 1.
Chemical components of interest of the Eudialyte ore sample (in wt.%).
With the molar proportion of (Na + K)/Al ratio at 1.12 (>1), the sample belongs to agpaitic rocks. The eudialyte ore sample contains 1.23% Zr and 0.403% REE, which are the major valuable components in this ore. Moreover, 50% of REEs belong to HREE. The distribution of individual REE in the eudialyte ore sample is shown in Figure 1.
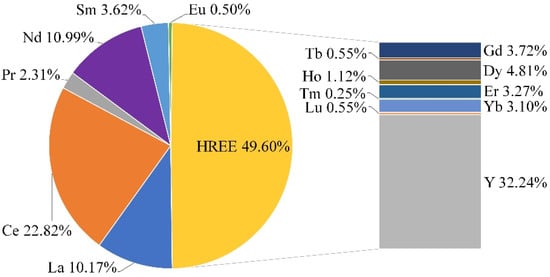
Figure 1.
Distribution of individual rare earth elements in eudialyte ore sample.
Eudialyte (7.2%) and catapleiite (2%) are identified as valuable Zr- and REE-bearing minerals (see Figure 2). The identified gangue minerals are feldspars (albite Na(AlSi3O8), microcline K(AlSi3O8), plagioclase (Na,Ca)[(Si,Al)AlSi2]O8, pyroxenes (aegirine-augite series (Na,Ca,Fe2+,Mg)(Fe3+,Al,Fe2+,Mg,)Si2O6) and nepheline Na3K(Al4Si4O16) with its alteration and replacement products (analcime Na(AlSi2O6)·H2O, natrolite Na2Al2Si3O10·2H2O, cancrinite Na6Ca2[(CO3)2|Al6Si6O24]·2H2O).
2.2. Sample Characterization
The chemical composition of the initial ore sample and products of the magnetic separation and some flotation products were externally determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Spectro Arcos 2013, SPECTRO Analytical Instruments GmbH, Kleven, Germany) after digestion of the samples. A portable/handheld X-ray fluorescence (XRF) analyzer (Niton XL3t, Thermo Fisher, Waltham, MA, USA) was used for routine measurements for elemental analyses of flotation products. The XRF system was calibrated using data validated by an external quality check by ICP analysis. The semi-quantitative X-ray Diffraction (XRD) analyses for the flotation concentrate were performed externally using a D2 PHASER X-ray diffractometer system (Siemens/Bruker D5000, MA, USA), equipped with DIFFRAC.EVA Software and DIFFRAC.TOPAS Software for mineral identification and quantification for Rietveld refinement, respectively. The quantitative evaluation of mineral compositions of the initial sample and products of the magnetic separation was performed by applying a Quanta 650-F QEM-SCAN© (FEI/Thermo Fisher, Hillsboro, OR, USA) scanning electron microscope (SEM). The particle size distribution in the feed sample and processed products were obtained by wet sieving analyses.
2.3. Magnetic Separation Stage
After sample preparation, 100 kg of dry ground eudialyte ore sample was treated using a wet, high-intensity magnetic separator JONES®WHIMS P40 (MBE-CMT GmbH, Cologne, Germany). The WHIMS separator matrix setting with a standard gap plate of 2.5 mm and an electric current of 7 A were chosen to achieve a magnetic-field strength of 1.2 T. The solid content of the pulp feed for WHIMS was set at 250 g/L. The generated magnetic and non-magnetic fractions were collected, then dried at 90 °C, weighted, and analyzed for the chemical composition. After drying, the non-magnetic fraction was homogenized and split into 1 kg of subsamples, which were stored for the flotation experiments.
2.4. Flotation Experiments
The small-scale flotation experiments with various pH levels and collectors were carried out in a laboratory-scale flotation machine (D12, Denver equipment, Denver, CO, USA) with a unit cell of 2.5 L. One kilogram of sample material (non-magnetic fraction of WHIMS stage) was mixed with Aachen tap water to obtain a flotation pulp with a solid content of 33%. The impeller speed was set to constant 1300 rpm during the flotation test. Prior to the collector dosage, pH regulators were added and agitated for 2 min. Then, the collector was agitated for 3 min followed by opening of the valve with an air flow of 5.5–6 L/min. The froth products were collected by an automatically driven froth-skimming device with a constant rate for all experiments. The collected products were dried overnight at 90 °C, weighted for mass pull calculation, and analyzed to calculate the recovery rates of Zr and Y. The results of the flotation tests were evaluated using the mass balance method and enrichment ratio, as described by Wills [41].
2.5. Reagents
The flotation reagents used in this study are shown in Table 2. Besides commercial phosphoric acid collectors from Clariant International Ltd. (Muttenz, Switzerland), additional phosphoric acid esters for the flotation experiments were synthesized and provided by ILCO Chemikalien GmbH (Erkelenz, Germany). The sulfuric acid and sodium hydroxide, supplied from Carl Roth GmbH, were used to maintain the required pH level. All reagent solutions were freshly prepared with distilled water.

Table 2.
Reagents used in the flotation experiments.
3. Results and Discussion
3.1. Characterization of the Non-Magnetic Fraction (Flotation Feed)
Magnetic separation is a well-known separation process to recover pyroxenes (aegirine), eudialyte, and other iron-bearing minerals. In the case of the Norra Kärr deposit, the efficiency of magnetic separation depends not only on the liberation degree of eudialyte, but also on the liberation degree of iron-bearing minerals from gangue minerals. Initially for this study, the main aim of the magnetic separation was the preparation of feed materials (non-magnetic fraction), which contain lost non-recoverable Zr- and REE-bearing minerals. The result of the WHIMS experiment is shown in Table 3.

Table 3.
The results of WHIMS for Eudialyte ore sample from Norra Kärr.
The magnetic fraction with a mass pull of 32.2% is a low-grade eudialyte concentrate (0.936% REE and 1.83% Zr), which is suitable for a further metallurgical treatment to extract REE [16,17]. Thus, WHIMS is a suitable method to recover iron-bearing minerals such as eudialyte and aegirine from the eudialyte ore sample as well as to remove acid-consuming minerals into the non-magnetic fraction. The mineral composition of this eudialyte ore sample and the corresponding non-magnetic fraction is shown in Figure 2.
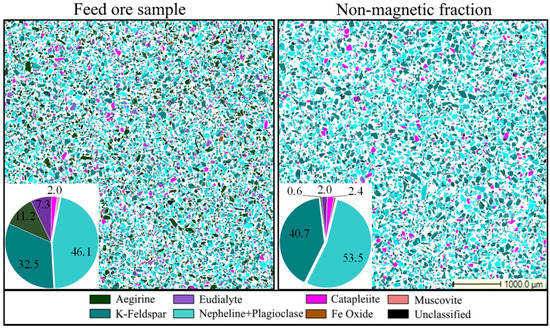
Figure 2.
Detailed section views and modal composition of the eudialyte ore sample and non-magnetic fraction.
The losses of Zr (52%) and REE (20%) in the non-magnetic fraction belong to valuable zirconosilicates such as catapleiite (2.4%) and eudialyte (2%). The result thus indicates that the recovery of Zr-bearing minerals by magnetic separation alone is not efficient. Main gangue minerals of the non-magnetic fraction (over 94%) are feldspars and nepheline and its alteration products. Other iron-bearing impurities in the non-magnetic fraction are aegirine, muscovite, and clay minerals such as illite in negligible amounts. The zirconosilicate minerals lost in non-magnetic tailings are mostly released. Seventy and fifty percent, respectively, of the catapleiite and eudialyte particles in the non-magnetic fraction occur as liberated grains, while 20% of the catapleiite particles are associated with eudialyte. Thus, the zirconosilicates can be recovered by further treatment using froth flotation. The components of the non-magnetic fraction are listed in Table 4.

Table 4.
Chemical components of interest of the non-magnetic fraction (in wt.%).
The Zr and REE contents in the non-magnetic fraction reach 0.95% and 0.118%, respectively. According to the granulometric distribution, obtained by wet sieving analyses, the d80 value of particles of the non-magnetic fraction was 100 µm (P35 = 45 µm and P20 = 25 µm). Over 45% of Zr and 60% of Y are distributed in the size fraction of 0–45 µm. The content of radioactive impurities (U, Th) is negligible at less than 0.001%. The distribution of individual REE in the non-magnetic fraction is shown in Figure 3. About 50% of REEs are represented by HREEs. The distributions of Nd and Pr as more valuable elements from LREE are 10.65% and 1.27%, respectively.
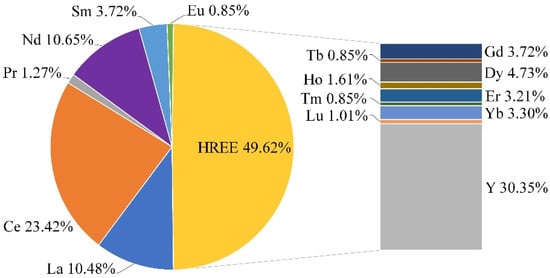
Figure 3.
Distribution of individual rare earth elements in non-magnetic fraction.
3.2. Flotation of Zirconosilicate Minerals from Non-Magnetic Fraction
3.2.1. Selection of Collectors for the Flotation of Zirconosilicate Minerals
The first set of flotation tests with different collectors was conducted at a pH of 4, 7, and 10. The dosage of collectors, listed in Table 2, was set at 500 g/t. The flotation time was 3 min. The performance of different commercial reagents for the recovery of Zr and Y at the pH of 4 is demonstrated in Figure 4.
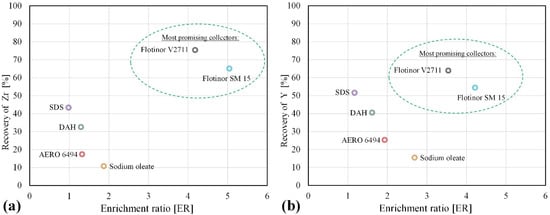
Figure 4.
Selection of collectors for the selective flotation of zirconosilicates at the pH of 4: Zr (a) and Y (b).
The results in Figure 4 are represented by the recovery of Zr (Figure 4a) and Y (Figure 4b) as a function of the enrichment ratio. The most promising results, indicated by the green oval in the graph, were achieved at a pH of 4 with phosphoric acid esters as collectors, supplied by Clariant. With Flotinor SM 15, the recovery of Zr was between 60 and 70% with an enrichment ratio of over 5. With Flotinor V2711, the recovery of Zr was between 70% and 80% with an enrichment ratio of over 5. Compared to Zr, the recovery of Y was 10% lower. Other collectors did not show satisfactory results at pH 4.
Increasing the pH level to 7 (Figure 5a) and 10 (Figure 5b) did not improve the flotation results for any collectors. SDS, DAH, and Flotinor SM 15 demonstrate a high mass pull in the froth product at pH of 7 without an enrichment of Zr and Y. At a pH of 10, the recovery of Zr and Y with Flotinor SM 15 decreased to 30% with an enrichment ratio of 1.7–2. Other collectors such as sodium oleate or hydroxamate (AERO 6494), which were especially recommended for the recovery of Zr- and REE-bearing minerals such as eudialyte [42], showed a low selectivity and recovery of 30–40% at a pH of 7 and 10. The poor results for this experiment conditions can be related to the surface properties of silicate minerals after dry and simultaneously iron-free grinding, harmful effects of slimes, and the ionic composition of the flotation pulp. In order to achieve more satisfactory results with these collectors, the influence of desliming, collector consumption, pH level, and suitable activators and depressants must further be investigated.
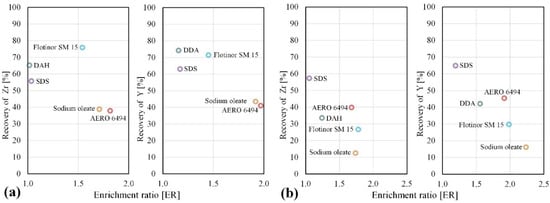
Figure 5.
Selection of collectors for the selective flotation of zirconosilicates at the pH of 7 (a) and 10 (b).
The difference between the recovery of Zr and Y can be explained by the presence of two forms of zirconosilcates: eudialyte as a Y (REE)-bearing mineral with 9% Zr and catapleiite as a more enriched Zr mineral with 22.7% Zr. It can also be observed that for all collectors, excluding phosphoric acid esters, the recovery and enrichment ratio of Y are higher than for Zr.
Due to the unknown composition and actual structure of commercial phosphoric acid esters from Clariant, the second set of flotation experiments were conducted with collectors with a known composition, supplied by ILCO Chemikalien GmbH. The experiments with phosphoric acid esters (500 g/t) were conducted at a pH of 4 with a flotation time of 3 min. Sulfuric acid, aimed to maintain the pH level, serves also as depressor of silicate minerals. The collectors differ in length of aliphatic, linear, and branched hydrocarbon chains and in the degree of ethoxylation (EO). The ratio of mono- and diesters was unknown. The results are plotted in Figure 6.
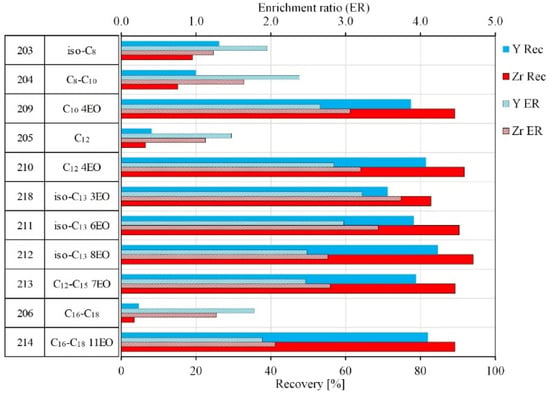
Figure 6.
Results of collectors based on phosphoric acid esters.
As can be seen in Figure 6, the structure of phosphoric acid esters and their degrees of ethoxylation affects flotation recoveries of zirconosilicate minerals. Non-ethoxylated collectors (Ilcophos 203–206) did not have collective properties at these experiment conditions (500 g/t), while ethoxylated collectors showed a significant recovery with a selectivity towards zirconium minerals. The recovery of Zr and Y with ethoxylated phosphoric acid esters (Ilcophos 209–212, 218) reached 75–90% with an enrichment ratio of 2.5–3.5.
The ethoxylation degree within the same hydrocarbon chain length also affects the flotation results. With an increase of EO moles from 3 to 8 for the isotridecyl chain (Ilcophos 218, 211, 212), the recovery of Zr and Y increased by 12–14%, while the enrichment ratio decreased. As result, a phosphoric acid ester with a high ethoxylation degree should be used for a selective flotation of zirconosilicate minerals.
Preliminary experiments with conventional selective collectors showed a low selectivity. In comparison to conventional supported collectors for the recovery of REE- and Zr-bearing minerals such as sodium oleate and hydroxamate acids, the most suitable performance was demonstrated by phosphoric acid ester collectors. Phosphoric acid esters were proposed for the flotation of dark colored heavy minerals from sands (micas, zircon, rutile, titanite, etc.), calcareous minerals (fluorite, calcite, apatite), and lithium-containing phase [25,43,44].
Phosphoric acid esters can differ in ratio of mono- and diesters and in the structure of non-polar hydrocarbon chains (aromatic, aliphatic, linear, branched, ethoxylated/propoxylated) [25,45]. The presence or absence of ethoxylated chains near the phosphorus group as well as the structure of carbon chains or its steric effects of the chain structure can affect the interaction of phosphoric acid esters with the mineral surface. The general structure of non-ethoxylated and ethoxylated phosphoric acid esters is presented in Figure 7.
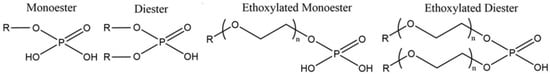
Figure 7.
The general formulas for non-ethoxylated and ethoxylated phosphoric acid esters.
Some researchers have reported the following interaction mechanism of phosphoric acid esters with cations of the mineral surface. In the case of zircon flotation, Bjelopavlic [26] identified the interaction of monoalkyl phosphates (C8) with zirconium cation as a bidentate chelate complex. Espiritu et al. [46] proposed the possible bonding of the phosphoric group with metals on the mineral surface. Besides the approaching of the metal cation at the mineral surface, the forming of a covalent bond, bidentate complex, and binuclear complex with the metal cation is possible. Further investigations of this collector type can help to better understand the interaction mechanism of ethoxylated and non-ethoxylated forms of phosphoric acid esters as well as the optimal ratio of mono- and diesters on the mineral surface. Since Flotinor SM 15 provides comparable results to Ilcophos collectors and is available as a commercially phosphoric acid ester collector, which has an approved and reliable formulation, Flotinor SM 15 was selected for further flotation experiments.
3.2.2. Effect of Collector Dosage and pH Value
It was decided to investigate in detail the influence of pH and collector consumption with the extended flotation time, considering the presence of acid soluble minerals, the consumption of the pH regulator, and the additional objective of purifying the nepheline syenite product from unwanted colored impurities. The single-collector dose-flotation experiments were conducted in a single stage with a flotation time of 7 min. The effect of a single collector dosage (Flotinor SM 15) on the flotation of zirconosilicate minerals was evaluated by adding 250, 350, 500, 750, and 1000 g/t of collector. The pH in all tests was 3.5–4. The results are shown in Figure 8a.
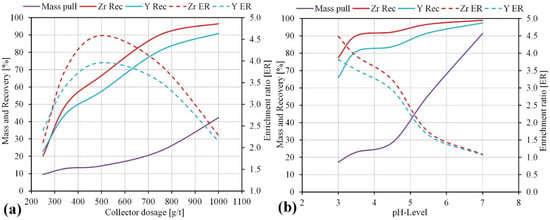
Figure 8.
Flotation recovery and enrichment ratio as function of collector dosage (a) and pH level (b).
At lower dosages (250–350 g/t), the amount of collector is likely insufficient to recover Zr-bearing minerals. The maximal enrichment ratios of Zr and Y (4.6 and 4) were achieved at the collector dosage of 500 g/t, while the recoveries reached 67% and 57%, respectively. The dosage of 750 g/t was the most efficient to recover Zr-bearing minerals. The recovery of Zr and Y reached 90% and 80% at a mass pull in concentrate of 23%. Further increasing of the collector dosage to over 750 g/t resulted in a significant increase of mass pull into concentrate of up to 43% and a recovery of Zr and Y in excess of 90%, although at lower enrichment. Thus, when the dosage of Flotinor SM15 exceeded 750 g/t, the effect of selective flotation of zirconosilicate minerals significantly decreased.
In order to determine the appropriate pH value for the flotation, the tests at various pH levels (3, 3.5, 4.5, 5.5, and 7) were provided with Flotinor SM 15 (750 g/t). They were conducted in a single stage with a flotation time of 7 min. The recovery and enrichment ratio of Zr and Y as a function of different pH values from 3.0 to 7.0, adjusted with sulfuric acid, are illustrated in Figure 8b.
At the pH level of 3.5, the mass pull reached 22% with recoveries of 90% and 80% for Zr and Y, respectively, which that this is an optimal flotation condition. Lowering the pH below 3.5 results in a 10% reduction in recovery with a corresponding improvement in concentrate quality. The influence of the pH level on the flotation performance is consistent with the maximum floatability of pure catapleiite and eudialyte in the narrow pH range of 3.5–4.5 using isooctylphosphate, as first reported by Ney [47]. Naifonov et al. [48] also reported a maximum floatability of eudialyte at pH levels of 3.5–4.5 using sodium oleate and phosphoric acid esters.
As the pH level increases from 4.5 to 7, the mass pull into the froth product rapidly increases from 28% to 90%. When the pH value exceeds 7, a selective flotation of zirconosilicate minerals from gangue minerals is not efficient. Thus, the pH level maintained by sulfuric acid has a strong influence on the selective flotation of zirconsilicate minerals from feldspars and nepheline. Moreover, pH levels of 4–5.5 may be recommended for rougher and scavenger flotation stages, while the cleaner flotation of rougher concentrates is possible after a slightly acidification to pH levels of 3–3.5. Furthermore, it is worth considering that the dissolution of acid soluble minerals such as nepheline and its alteration species causes a high consumption of an acidifying agent (sulfuric acid), instable flotation process, losses of rare earth metals, and an enrichment of process water with dissolved cations (Al3+, Ca2+).
3.2.3. Characterization of Flotation Products
To obtain a rougher concentrate for cleaner flotation tests, the procedure of the rougher flotation described in Section 2.4 was repeated three times at a pH of 4.5, collector dosage of 750 g/t (Flotinor SM 15), and flotation time of 7 min. The four-stage cleaner flotation of the rougher concentrate were provided in 2.5 L and 1.25 L unit cells and twice in a 0.75 L unit cell at a pH of 3.5, respectively. The flotation time was 5 min in each cleaner stage. Figure 9 demonstrates the effect of the cleaner flotation on recovery and enrichment ratio of Zr and Y in the cleaner concentrates.
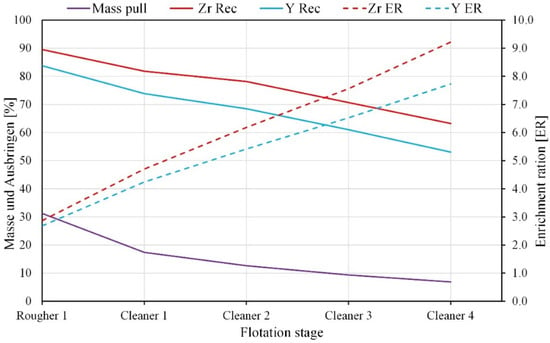
Figure 9.
Effect of cleaning stage number on flotation results.
With increasing cleaning stages, the enrichment ratios of the Zr and Y content in the froth product increased up to 9 and 7.5, while recoveries decreased by 25%. The mass pull into the froth product from the non-magnetic fraction also decreased by 25% to 7.5%. The concentrate of the fourth cleaner flotation and the tailings of the rougher flotation are named Zr-REE bearing concentrate and nepheline syenite product, respectively. The flotation products obtained from the experiments were used for quantitative mineralogical and chemical analyses.
According to the chemical composition in Table 5, common value components of the Zr-REE bearing concentrate are Zr (9.12%), Hf (0.2%), Nb (0.0746%), and REE (1.52%). Looking closer to the distribution of individual REE in Figure 10 reveals that 51.5% of the REEs belong to HREEs as an elements fraction with greater value.

Table 5.
Common value components of the Zr-REE bearing concentrate (in wt.%).
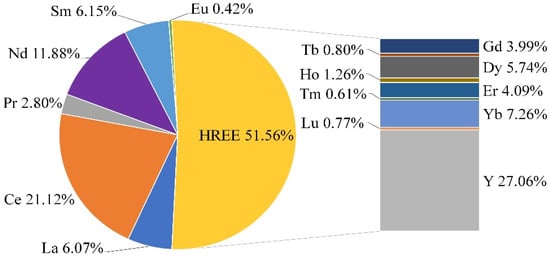
Figure 10.
Distribution of individual rare earth elements in Zr-REE-bearing concentrate.
The quantitative XRD analysis was performed to determine the main mineral phases of the cleaner concentrates, as illustrated in Figure 11.
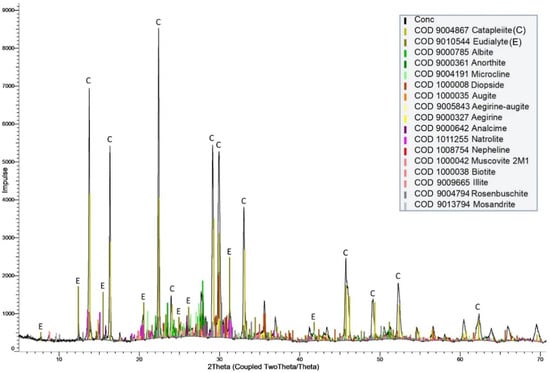
Figure 11.
XRD pattern of Zr-REE-bearing concentrate.
According to the XRD analysis results, the Zr-REE bearing concentrate consisted of catapleiite, eudialyte, feldspars, pyroxenes, and nepheline, including its alteration products. The predominance of catapleiite peaks indicates that catapleiite was more abundant in the Zr-REE bearing concentrate, which was obtained by froth flotation. According to results of Rietveld refinement, catapleiite (35%) and eudialyte (15%) are Zr-, Hf-, and REE-bearing minerals in the Zr-REE-bearing concentrate. Due to the solubility of eudialyte and catapleiite in inorganic acids, the Zr-REE-bearing concentrate could be processed by hydrometallurgical methods to recover REE, Zr, and Hf from the eudialyte concentrate and its leach residues [14,16]. The chemical composition of the flotation tailings (nepheline syenite products) is given in Table 6.

Table 6.
Chemical composition of flotation tailings (nepheline syenite products) (in wt.%).
According to mineralogical and chemical analyses, the flotation tailings, obtained after the recovery of Zr-REE bearing minerals, should not be considered as waste materials for a disposal in a tailings dam. The mass pull of the flotation tailings from the non-magnetic fraction is 68%. The flotation tailings can be characterized as nepheline syenite product, consisting of feldspars (47%), nepheline (20%), and natrolite (25%). Analcime, sodalite, cancrinite, muscovite, and illite were also identified. The d80-value of particles in the flotation tailings is 100 µm, while the content of fine fractions below 25 µm is 10%. The low content of impurities such as Fe2O3 (0.17%) and TiO2 (17 ppm) as well as the high alkali content (Na2O + K2O > 15%) are an advantage for the industrial application of nepheline syenite products, especially in refractories, glass, ceramic filler, and pigment industries. Moreover, WHIMS could be suggested as a solution to reduce the iron content.
3.3. Development of an Alternative Beneficiation Flowsheet for Norra Kärr Deposit
In this study, the case of catapleiite-bearing rocks of the Norra Kärr deposit was considered. The processing of a low-grade eudialyte ore from the Norra Kärr deposit was investigated by a consecutive pre-concentration of paramagnetic REE-bearing phases using magnetic separation and a scavenger treatment of non-magnetic fraction to recover Zr- and REE-bearing minerals using froth flotation. The newly developed flowsheet to process eudialyte ore from Norra Kärr aims to minimize the tailing volumes and is introduced in Figure 12.
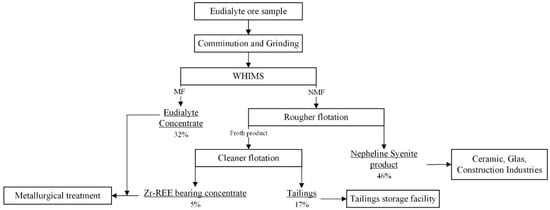
Figure 12.
Proposed flowsheet for the processing of a eudialyte ore sample from Norra Kärr deposit.
After comminution and dry grinding of the eudialyte ore by a vertical roller mill, an eudialyte concentrate was recovered using WHIMS with a magnetic field intensity of 1–1.2 T. The non-magnetic fraction is fed to the selective flotation (rougher and cleaner stages) of zirconosilicate minerals, as well as iron-bearing minerals and colored impurities. The dry and simultaneously iron-free ore grinding by VRM or high-pressure grinding roll (HPGR) could be considered as an advantage for the further selective flotation [49]. Phosphoric acid esters such as the commercial collector (Flotinor SM 15) are used as selective collectors for zirconosilicate minerals such as catapleiite and eudialyte in a weak acid circuit, maintained by sulfuric acid. The obtained Zr-REE-bearing concentrate is the source of Zr, Hf, and REE for a further metallurgical treatment. The flotation tailings of the rougher flotation should be considered as nepheline syenite product. If required, grinding and/or WHIMS could be considered as further treatment methods for the reduction of iron in nepheline syenite product to meet the corresponding industry requirements. Thus, the proposed flowsheet for beneficiation of the investigated low-grade eudialyte ore aims to improve the recoveries of Zr and REE, to meet the quality requirements for nepheline syenite products from the non-magnetic fraction, and simultaneously to minimize the storage tailings volume by 50%, as calculated from the running of the mine. Furthermore, due to the complexity of Norra Kärr deposits and the lithological division of the rock types and subtypes, the additional approbation of the proposed flowsheet for the recovery of Zr- and REE-bearing minerals could be required or considered.
4. Conclusions
The proposed flowsheet for processing of the eudialyte ore from the Norra Kärr deposit, given in Figure 12, consists of comminution, grinding, and magnetic separation stages. As result, a low-grade eudialyte concentrate from the Norra Kärr deposit is recoverable and usable for further hydrometallurgical treatments. In this study, it was aimed to recover complex rare zirconium minerals such as non-magnetic eudialyte species and catapleiite using froth flotation. These minerals normally get lost during wet, high-magnetic separation stages into the non-magnetic fraction. The non-magnetic fraction commonly bears non-magnetic valuable gangue minerals such as alkali feldspars and nepheline.
At the beginning of the flotation tests for Zr- and REE-bearing minerals, conventional and thus well-known collectors such as sodium oleate and alkyl hydroxamate were tested. It was concluded that selective collectors for Zr-bearing minerals are phosphoric acid esters in an acidic pH value of below 4. Moreover, the ethoxylated phosphoric acid esters are recommended for the selective flotation of Zr-bearing minerals. The optimal pH value was between 3.5 and 4.5 with a collector consumption (Flotinor SM 15) of 750 g/t. As pH-regulator, sulfuric acid is recommended. The optimal flotation time for the rougher flotation was ascertained to be 7 min. Recoveries of Zr and Y reached 90% and 80%, respectively. After the fourth cleaner flotation stage, the Zr-REE bearing concentrate containing 9.2% of Zr and 1.5% of REE was obtained. This concentrate can be potentially used to recover Zr, Hf, and REE in hydrometallurgical stages with a low-grade eudialyte concentrate. Simultaneously, as a result of the froth flotation of the Zr minerals, the non-magnetic fraction is purified from colored impurities such as Fe- and Ti-bearing minerals. The flotation tailings should be considered as nepheline-feldspars products with low amounts of Fe and Ti for the production in the ceramic and glass industries or as filling materials. By a combination of selective flotation and magnetic separation, a nepheline syenite product with 0.17% Fe2O3 and 23% Al2O3 was obtained from the eudialyte ore sample of the Norra Kärr deposit. Thus, a near zero-waste concept for the Norra Kärr deposit and an improvement of the process efficiency could be suggested if, besides the eudialyte concentrate (REE-Zr-Nb-product), the Zr-REE bearing concentrate and the nepheline syenite products are considered as recovered marketable byproducts.
Author Contributions
Conceptualization, I.S. and C.B.; experimental work I.S. and C.B.; methodology, I.S. and D.G.; validation, L.W.; formal analysis, I.S., D.G. and L.W.; investigation, I.S. and C.B.; resources, I.S. and H.W.; writing—original draft preparation, I.S.; writing—review and editing, D.G. and L.W.; visualization, D.G. and L.W.; supervision, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are partially available on request from the corresponding author.
Acknowledgments
We thank the ILCO Chemikalien GmbH (Corvin Volkholz) for the supported chemicals reagents.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sovacool, B.K.; Ali, S.H.; Bazilian, M.; Radley, B.; Nemery, B.; Okatz, J.; Mulvaney, D. Sustainable minerals and metals for a low-carbon future. Science 2020, 367, 30–33. [Google Scholar] [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability evaluation of essential critical raw materials: Cobalt, niobium, tungsten and rare earth elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef] [Green Version]
- Silin, I.; Hahn, K.; Gürsel, D.; Kremer, D.; Gronen, L.; Stopić, S.; Friedrich, B.; Wotruba, H. Mineral processing and metallurgical treatment of lead vanadate ores. Minerals 2020, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Jaroni, M.S.; Friedrich, B.; Letmathe, P. Economical feasibility of rare earth mining outside China. Minerals 2019, 9, 576. [Google Scholar] [CrossRef] [Green Version]
- Dostal, J. Rare Earth Element Deposits of Alkaline Igneous Rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Marks, M.A.; Markl, G. A global review on agpaitic rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Sjöqvist, A.; Cornell, D.; Andersen, T.; Erambert, M.; Ek, M.; Leijd, M. Three Compositional Varieties of Rare-Earth Element Ore: Eudialyte-Group Minerals from the Norra Kärr Alkaline Complex, Southern Sweden. Minerals 2013, 3, 94–120. [Google Scholar] [CrossRef]
- Atanasova, P.; Marks, M.A.W.; Heinig, T.; Krause, J.; Gutzmer, J.; Markl, G. Distinguishing Magmatic and Metamorphic Processes in Peralkaline Rocks of the Norra Kärr Complex (Southern Sweden) Using Textural and Compositional Variations of Clinopyroxene and Eudialyte-group Minerals. J. Petrol. 2017, 58, 361–384. [Google Scholar] [CrossRef] [Green Version]
- Vaccarezza, V.; Anderson, C. An Overview of Beneficiation and Hydrometallurgical Techniques on Eudialyte Group Minerals. Min. Metall. Explor. 2020, 37, 39–50. [Google Scholar] [CrossRef]
- Stark, T.; Silin, I.; Wotruba, H. Mineral Processing of Eudialyte Ore from Norra Kärr. J. Sustain. Metall. 2017, 3, 32–38. [Google Scholar] [CrossRef]
- Forrester, K.; Leijd, M.; Oczlon, M.; Holmström, H.; Saxon, M. Beneficiation of rare earth element enriched eudialyte from the Norra Kärr peralkaline intrusion with wet high intensity magnetic separation. In Proceedings of the Conference of Metallurgists, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Silin, I.; Stark, T.; Wolfrum, S.; Wotruba, H. Method and Device for Producing an Eudialyte Concentrate by Direct Flotation. European Patent Number 3150283A1, 30 September 2015. [Google Scholar]
- Voßenkaul, D.; Birich, A.; Müller, N.; Stoltz, N.; Friedrich, B. Hydrometallurgical Processing of Eudialyte Bearing Concentrates to Recover Rare Earth Elements Via Low-Temperature Dry Digestion to Prevent the Silica Gel Formation. J. Sustain. Metall. 2017, 3, 79–89. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Friedrich, B. Hydrometallurgical Treatment of an Eudialyte Concentrate for Preparation of Rare Earth Carbonate. Johns. Matthey Technol. Rev. 2019, 63, 2–13. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Friedrich, B. Recovery of Zr, Hf, Nb from eudialyte residue by sulfuric acid dry digestion and water leaching with H2O2 as a promoter. Hydrometallurgy 2018, 181, 206–214. [Google Scholar] [CrossRef]
- Balinski, A.; Wiche, O.; Kelly, N.; Reuter, M.A.; Scharf, C. Separation of rare earth elements from contaminants and valuable components by in-situ precipitation during the hydrometallurgical processing of eudialyte concentrate. Hydrometallurgy 2020, 194, 105345. [Google Scholar] [CrossRef]
- Balinski, A.; Atanasova, P.; Wiche, O.; Kelly, N.; Reuter, M.A.; Scharf, C. Selective Leaching of Rare Earth Elements (REEs) from Eudialyte Concentrate after Sulfation and Thermal Decomposition of Non-REE Sulfates. Minerals 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Cheremisina, O.V.; Volkova, O.; Litvinova, T.E. Influence of anion nature on acid leaching of silicate minerals and solvent extraction of rare and rare-earth elements. Geochemistry 2020, 80, 125507. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Bunin, I.Z.; Ryazantseva, M.V.; Chanturiya, E.L.; Samusev, A.L.; Anashkina, N.E. Mechanism of the Change in the Structural, Chemical, and Technological Properties of Eudialyte upon Combined Energy Effects. Bull. Russ. Acad. Sci. Phys. 2019, 83, 716–720. [Google Scholar] [CrossRef]
- Davidson, T. Amended and Restated Prefeasibility Study. NI 43-101. Technical report for the Norra Kärr Rare Earth Element Deposit.; GBM: Gränna, Sweden, 2015. [Google Scholar]
- Zubkova, N.V.; Ksenofontov, D.A.; Chukanov, N.V.; Pekov, I.V.; Artamonova, A.A.; Koshlyakova, N.N.; Bychkov, A.Y.; Pushcharovsky, D.Y. Crystal Chemistry of the Microporous Zirconosilicate Na6Zr3[Si9O27], a Product of High-Temperature Transformation of Catapleiite, and Its Ag-Exchanged Form. Minerals 2020, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Ksenofontov, D.A.; Grebenev, V.V.; Zubkova, N.V.; Pekov, I.V.; Kabalov, Y.K.; Chukanov, N.V.; Pushcharovsky, D.Y.; Artamonova, A.A. Behavior of Catapleiite under Heating and Crystal Structure of its High-Temperature Transformation Product, a New Phase Na6Zr3[Si9O27] with Nine-membered Rings of SiO4 Tetrahedra. Geol. Ore Depos. 2019, 61, 696–705. [Google Scholar] [CrossRef]
- Borst, A.M.; Friis, H.; Andersen, T.; Nielsen, T.F.D.; Waight, T.E.; Smit, M.A. Zirconosilicates in the kakortokites of the Ilímaussaq complex, South Greenland: Implications for fluid evolution and high-field-strength and rare-earth element mineralization in agpaitic systems. Mineral. Mag. 2016, 80, 5–30. [Google Scholar] [CrossRef] [Green Version]
- van de Ven, M.; Borst, A.; Davies, G.; Hunt, E.; Finch, A. Hydrothermal Alteration of Eudialyte-Hosted Critical Metal Deposits: Fluid Source and Implications for Deposit Grade. Minerals 2019, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice; Flotation of Industrial Minerals; Elsevier: Kidlington, UK, 2015; Volume 3, ISBN 9780444530837. [Google Scholar]
- Bjelopavlic, M.; Ralston, J.; Reynolds, G. Adsorption of Monoalkyl Phosphates at the Zircon-Aqueous Solution Interface. J. Colloid Interface Sci. 1998, 208, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Marion, C.; Li, R.; Williams, H.; Hundt, D.; Waters, K.E. A physico-chemical investigation into the separation of zircon and quartz by flotation. In Proceedings of the 29th International Mineral Processing Congress (IMPC 2018), Moscow, Russia, 17–21 September 2018; pp. 4062–4072. [Google Scholar]
- Sobieraj, S.; Ralston, J.; Smart, R.S.C. Flotation of Zircon from Mineral Sands. Flotacja cyrkonu z piasków mineralnych (in Polish). Physicochem. Probl. Miner. Process. 1991, 24, 233–243. [Google Scholar]
- Jeswiet, J.; Szekeres, A. Energy Consumption in Mining Comminution. Procedia CIRP 2016, 48, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Silin, I.; Rubinstein, Y.; Volobayev, I.; Liono, A.; Gürsel, D.; Wotruba, H. Valorisation of Abandoned Low Grade Tailings in Albania for Recovery of Metal Concentrates and Mineral Products. Russ. J. Non Ferr. Met. 2021, 62, 483–494. [Google Scholar] [CrossRef]
- Spooren, J.; Binnemans, K.; Björkmalm, J.; Breemersch, K.; Dams, Y.; Folens, K.; González-Moya, M.; Horckmans, L.; Komnitsas, K.; Kurylak, W.; et al. Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: Technology development trends. Resour. Conserv. Recycl. 2020, 160, 104919. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Garzón, E.; Pérez-Villarejo, L.; Angelopoulos, G.N.; Eliche-Quesada, D. Mining Wastes of an Albite Deposit as Raw Materials for Vitrified Mullite Ceramics. Minerals 2021, 11, 232. [Google Scholar] [CrossRef]
- Lorenz, W.; Gwosdz, W. Quarzrohstoffe: Mit 69 Tabellen; Schweizerbart: Stuttgart, Germany, 1999; ISBN 978-3-510-95839-9. [Google Scholar]
- Bagani, M.; Balomenos, E.; Panias, D. Nepheline syenite as an alternative source for aluminum production. Minerals 2021, 11, 734. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Nepheline Syenite—An Alternative Source for Potassium and Aluminium. In Rare Metal Technology 2019; Azimi, G., Kim, H., Alam, S., Ouchi, T., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–159. ISBN 978-3-030-05739-8. [Google Scholar]
- Everard, L.J. An Assessment of the Resource Potential for Nepheline Syenite at Cygnet and Elsewhere in Tasmania: Record 1996/13; Tasmanian Geological Survey: Tasmania, Australia, 1998.
- Burat, F.; Kangal, O.; Onal, G. An alternative mineral in the glass and ceramic industry: Nepheline syenite. Miner. Eng. 2006, 19, 370–371. [Google Scholar] [CrossRef]
- Çinar, M.; Durgut, E. Mineral beneficiation of Kırşehir nepheline syenite with combination of dry magnetic separation and flotation methods. Physicochem. Probl. Miner. Process. 2019, 55, 1227–1238. [Google Scholar] [CrossRef]
- Kangal, M.O.; Bulut, G.; Yeşilyurt, Z.; Basturkcu, H.; Burat, F. Characterization and production of Turkish nepheline syenites for industrial applications. Physicochem. Probl. Miner. Process. 2019, 55, 605–616. [Google Scholar] [CrossRef]
- Ahmed, H.A.M. Dry versus wet upgrading of nepheline syenite ores. Physicochem. Probl. Miner. Process. 2011, 46, 107–118. [Google Scholar]
- Wills, B.A. Wills’ Mineral. Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral. Recovery, 8th ed.; Elsevier Science: Amsterdam, The Netherlands, 2015; ISBN 9780080970530. [Google Scholar]
- Ivanova, V.; Mitrofanova, G. Flotation of eudialyte: Correlation of experimental data with the results of quantum-chemical calculations. In Proceedings of the XVI Balkan Mineral Processing Congress, Belgrade, Serbia, 15–17 June 2015; pp. 347–351. [Google Scholar]
- Qiu, H.; Kersebaum, J.; Wollmann, A.; Feuge, N.; Haas, A.; Goldmann, D.; Wilhelm, R. Improvement of the froth flotation of LiAlO2 and melilite solid solution via pre-functionalization. Sci. Rep. 2021, 11, 20443. [Google Scholar] [CrossRef] [PubMed]
- Baudet, G.; Morteani, G.; Strub, M.P. Application of Phosphoric Esters to Flotation of Finely Divided Oxidized Ores: Final Report, C.E.C. Research Contract MA1M-0059-C; Department Mineralurgie: Orleans, France, 1992. [Google Scholar]
- O’Lenick, A.J. Surfactants: Strategic Personal Care Ingredients; Allured Publ. Corp.: Carol Stream, IL, USA, 2005; ISBN 978-1932633085. [Google Scholar]
- Espiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Ney, P. Zeta-Potentiale und Flotierbarkeit von Mineralen; Springer: Vienna, Austria; New York, NY, USA, 1973; ISBN 978-3-211-81104-7. [Google Scholar]
- Nayfonov, T.; Belborodov, V.; Zakharova, I. Flotation technology for beneficiation of eudialyte ore. In Proceedings of the XVII International Mineral Processing Congress, Dresden, Germany, 23–28 September 1991; Volume 4, pp. 131–138. [Google Scholar]
- Silin, I.; Huben, J.; Wotruba, H.; Ognyanova, A. Study on the Characterisation and Processing of Iron Ore after Grinding by HPGR. In Proceedings of the 29th International Mineral Processing Congress (IMPC 2018), Moscow, Russia, 17–21 September 2018; pp. 2388–2397. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).