Effect of Water Immersion on Raw and Expanded Ugandan Vermiculite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
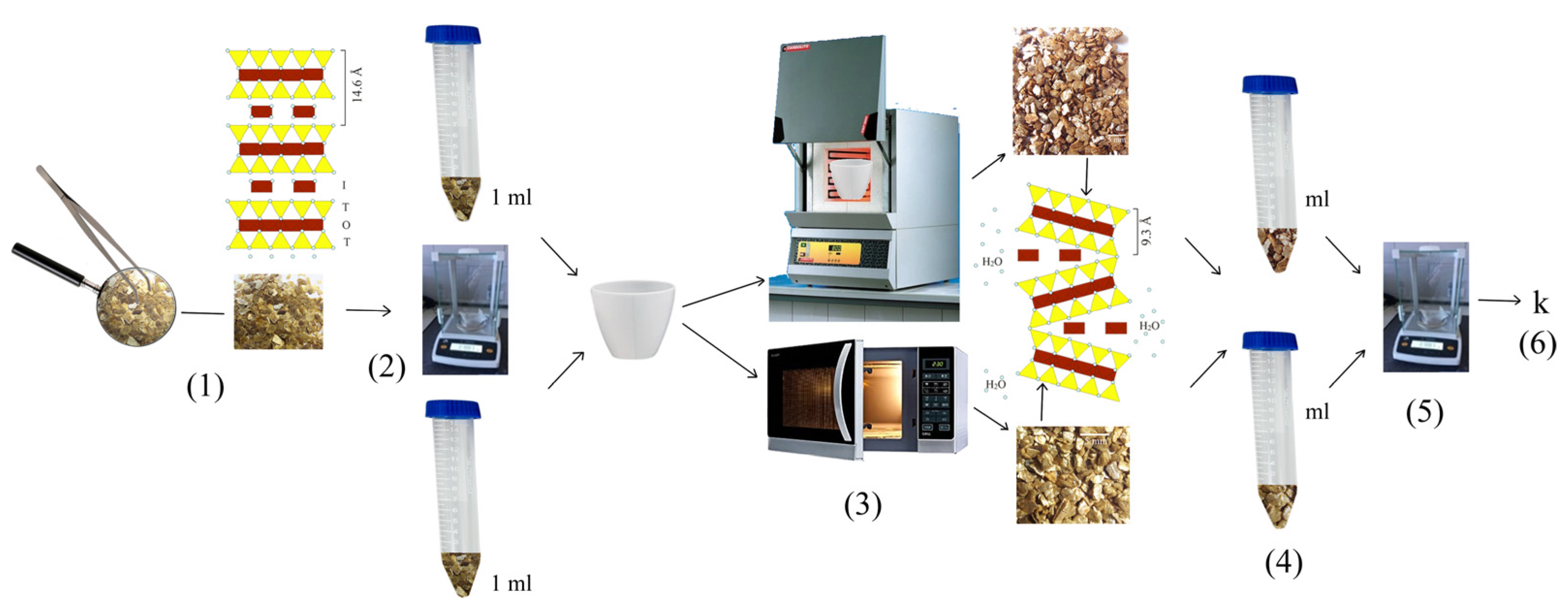
2.2.1. Vermiculite Expansion
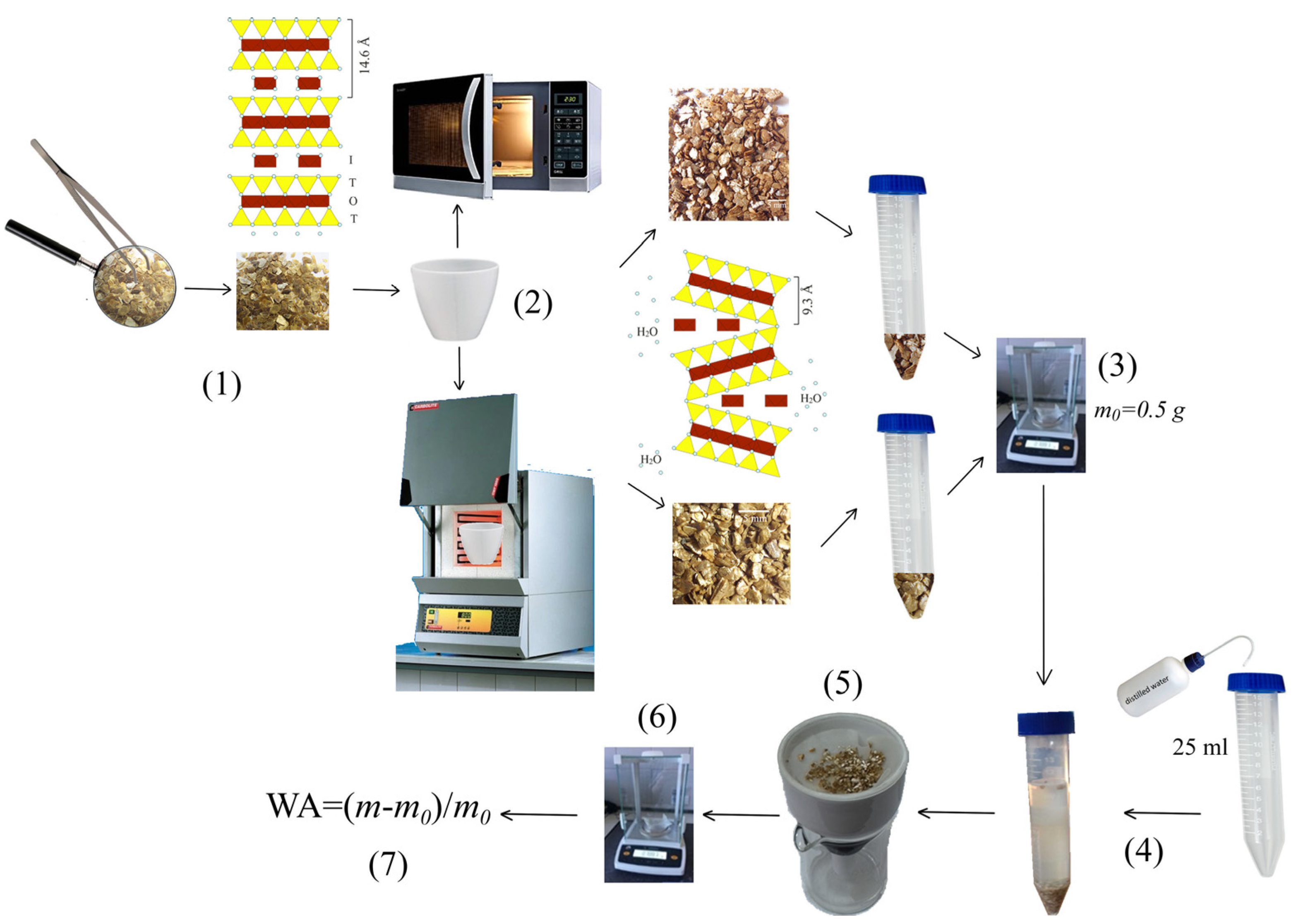
2.2.2. Water Immersion
2.3. Characterizations
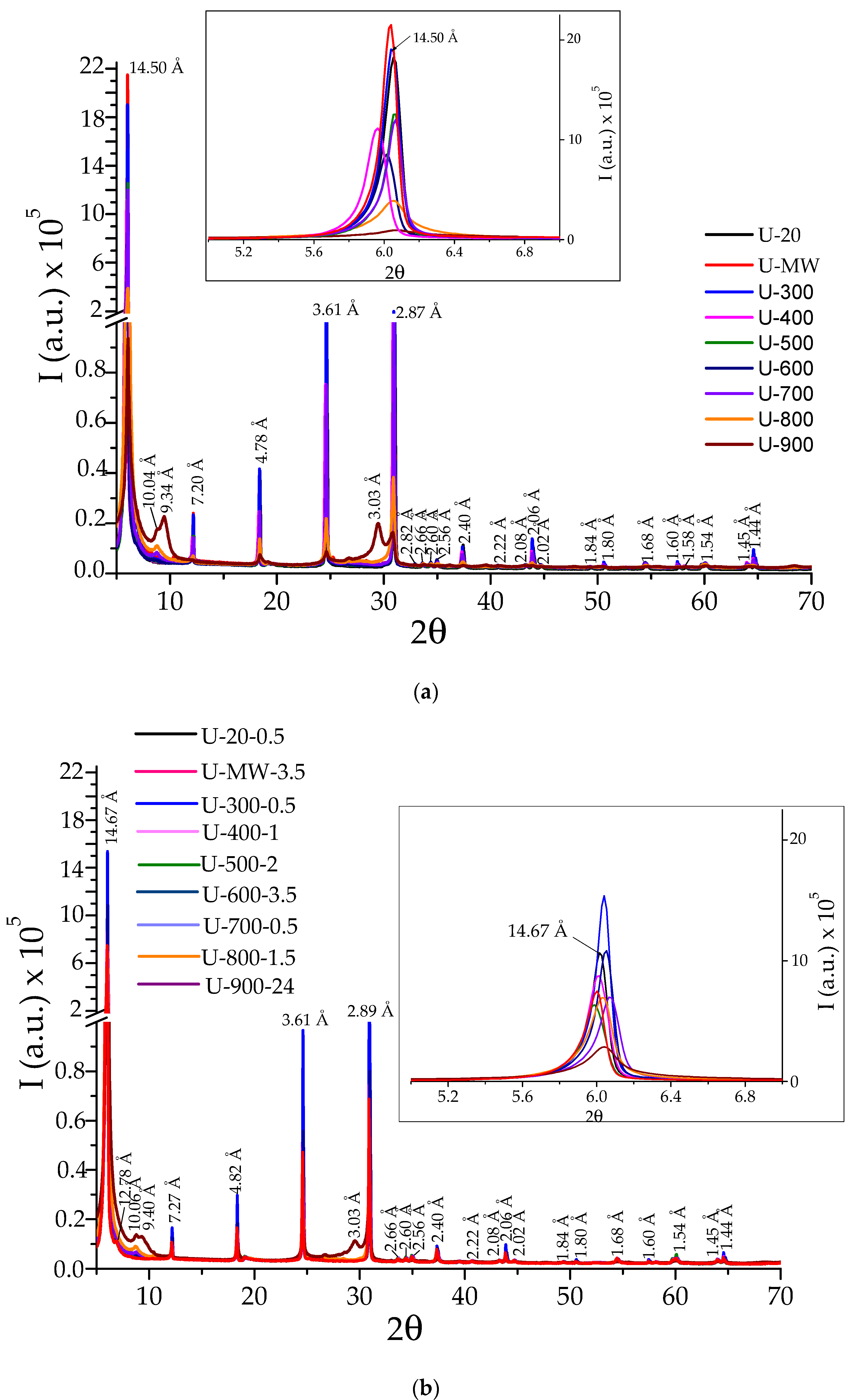
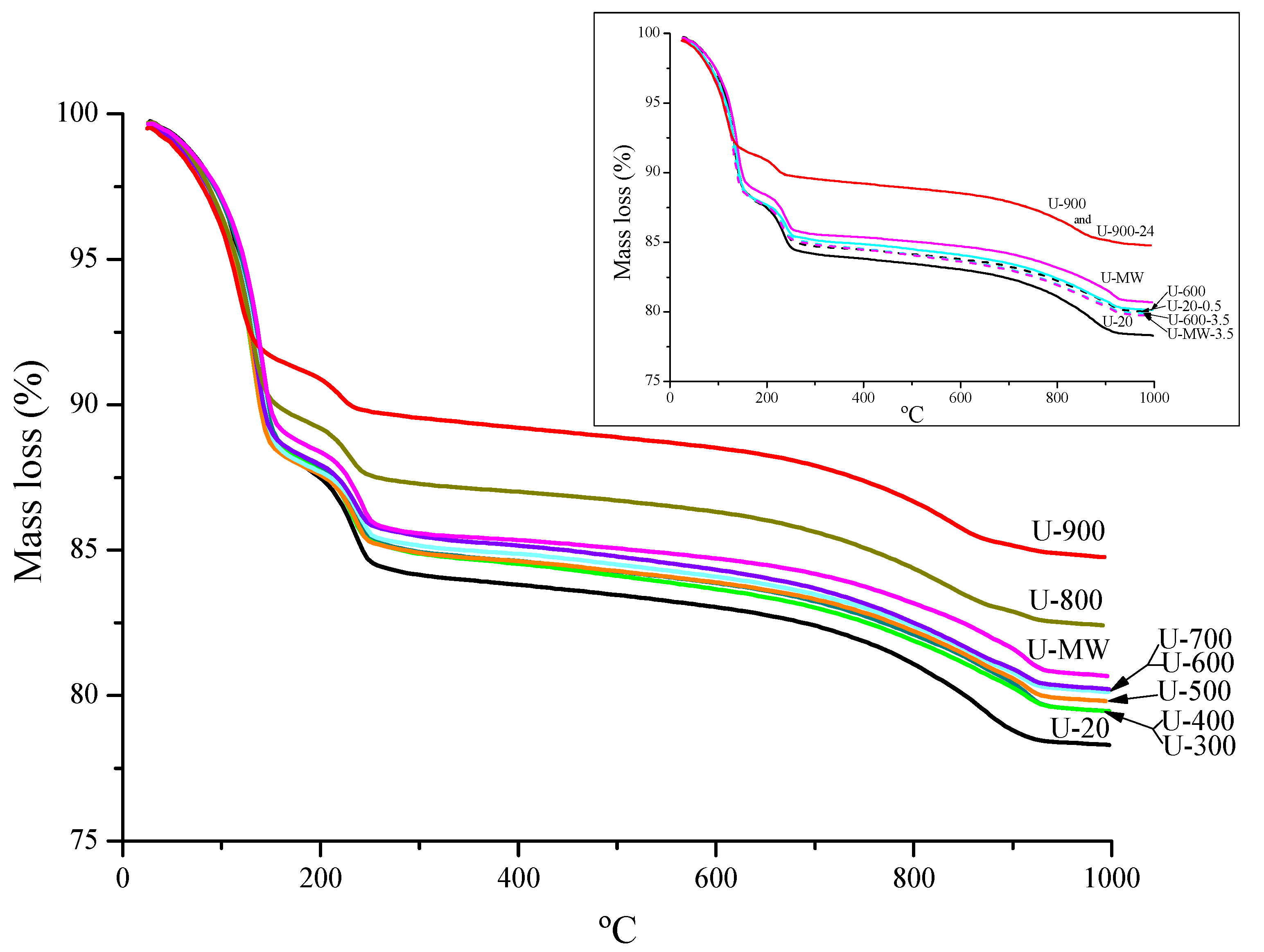
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Increase in the Number of Combined Tropical Nights (Minimum Temperature Exceeding 20 °C) and Hot Days (Maximum Temperature Exceeding 35 °C) under Present and Future Climate Conditions. Available online: https://www.eea.europa.eu/data-and-maps/figures/ (accessed on 24 April 2021).
- Feng, J.; Liu, M.; Mo, W.; Su, X. Heating temperature effect on the hygroscopicity of expanded vermiculite. Ceram. Int. 2021, 47, 25373–25380. [Google Scholar] [CrossRef]
- De Silva, G.H.M.J.S.; Surangi, M.L.C. Effect of waste rice husk ash on structural, thermal and run-off properties of clay roof tiles. Constr. Build. Mater. 2017, 154, 251–257. [Google Scholar] [CrossRef]
- Thomson, A.; Maskell, D.; Walker, P.; Lemke, M.; Shea, A.; Lawrence, R. Improving the hygrothermal properties of clay plasters. In Proceedings of the International Conference on 16th Non-Conventional Materials and Technologies, Winnipeg, MB, Canada, 10–13 August 2015. [Google Scholar]
- Nguyen, D.M.; Grillet, A.-C.; Diep, T.M.H.; Ha Thuc, C.N.; Woloszyn, M. Hygrothermal properties of bio-insulation building materials based on bamboo fibers and bio-glues. Constr. Build. Mater. 2017, 155, 852–866. [Google Scholar] [CrossRef]
- Padfield, T.; Jensen, L.A. Humidity buffering of building interiors by absorbent materials. In Proceedings of the 9th Nordic Symposium on Building Physics, Tampere, Finland, 29 May–2 June 2011. [Google Scholar]
- Rode, C. Moisture Buffering of Building Materials; Department of Civil Engineering Technical University of Denmark: Lyngby, Denmark, 2005. [Google Scholar]
- Padfield, T. The Role of Absorbent Building Materials in Moderating Changes of Relative Humidity. Ph.D. Thesis, The Technical University of Denmark, Lyngby, Denmark, 1998. Available online: https://www.conservationphysics.org/phd//phd-indx.html (accessed on 6 October 2021).
- Kim, H.J.; Kim, S.S.; Lee, Y.G.; Song, K.D. The Hygric Performances of Moisture Adsorbing/Desorbing Building Materials. Aerosol Air Qual. Res. 2010, 2010, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Fořt, J.; Doleželová, M.; Černý, R. Moisture Buffering Potential of Plasters for Energy Efficiency in Modern Buildings. In Proceedings of the International Environmental Engineering Conference, Vilnius, Lithuania, 27–28 April 2017. [Google Scholar]
- Okada, K.; Matsui, S.; Isobe, T.; Kameshima, Y.; Nakajima, A. Water-retention properties of porous ceramics prepared from mixtures of allophane and vermiculite for materials to counteract heat island effects. Ceram. Int. 2008, 34, 345–350. [Google Scholar] [CrossRef]
- Valášková, M.; Martynková, G.S.; Smetana, B.; Študentová, S. Influence of vermiculite on the formation of porous cordierites. Appl. Clay Sci. 2009, 46, 196–201. [Google Scholar] [CrossRef]
- Tarnai, M.; Mizuguchi, H. Design, construction and recent applications of porous concrete in Japan. In Proceedings of the JCI Symposium on Design, Construction and Recent Applications of Porous Concrete; Concrete Institute: Tokyo, Japan, 2004; pp. 1–10. [Google Scholar]
- Bhutta, M.A.R.; Hasanah, N. Properties of porous concrete from waste crushed concrete (recycled aggregate). Constr. Build. Mater. 2013, 47, 1243–1248. [Google Scholar] [CrossRef]
- Mohan Ram, P.; Shanmugam, V.; Senthilkumar, P. Impact of particle size of solid desiccant mould on moisture absorption and regeneration time. Renew. Sust. Energy Rev. 2014, 6, 013124. [Google Scholar] [CrossRef]
- Midgley, H.G.; Midgley, C.M. The mineralogy of some commercial vermiculites. Clay Miner. Bull. 1960, 23, 142–150. [Google Scholar] [CrossRef]
- Couderc, P.; Douillet, P. Les vermiculites industrielles: Exfoliation, caracteristiques mineralogiques et chimiques. Bull. Soc. Fr. Céram. 1973, 99, 51–59. [Google Scholar]
- Justo, A.; Perez-Rodriguez, J.L.; Sanchez-Soto, P.J. Thermal studies of vermiculites and mica-vermiculite interstratifications. J. Therm. Anal. 1993, 40, 59–65. [Google Scholar] [CrossRef]
- Marcos, C.; Arango, Y.C.; Rodriguez, I. X-ray diffraction studies of the termal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Marcos, C.; Rodriguez, I. Expansion behaviour of commercial vermiculites at 1000 °C. Appl. Clay Sci. 2010, 48, 492–498. [Google Scholar] [CrossRef]
- Hillier, S.; Marwa, E.M.M.; Rice, C.M. On the mechanism of exfoliation of ‘Vermiculite’. Clay Miner. 2013, 48, 563–582. [Google Scholar] [CrossRef] [Green Version]
- Justo, A.; Maqueda, C.; Pérez Rodriguez, J.L.; Morillo, E. Expansibility of some vermiculites. Appl. Clay Sci. 1989, 4, 509–519. [Google Scholar] [CrossRef]
- de la Calle, C.; Suquet, H. Vermiculite. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1988. [Google Scholar]
- Argüelles, A.; Leoni, M.; Blanco, J.A.; Marcos, C. Structure and microstructure of Mg-vermiculite. Z. Kristallogr. Suppl. 2009, 30, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Argüelles, A.; Leoni, M.; Blanco, J.; Marcos, C. Semi-ordered crystalline structure of the Sta. Olalla vermiculite inferred from X-ray powder diffraction. Am. Mineral. 2010, 95, 126–134. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Justo, A.; Maqueda, C.; Pérez Rodriguez, J.L. Estudio Químico de Vermiculitas de Andalucia y Badajoz. Bol. Soc. Esp. Mineral. 1986, 9, 123–129. [Google Scholar]
- Velde, B. High temperature or metamorphic vermiculites. Contrib. Mineral. Petrol. 1978, 66, 319–323. [Google Scholar] [CrossRef]
- Marcos, C. Structural Changes in Vermiculites Induced by Temperature, Pressure, Irradiation, and Chemical Treatments. In Clay Science and Technology; Morari Do Nascimento, G., Ed.; IntechOpen Book Series; IntechOpen: London, UK, 2021. [Google Scholar]
- Marcos, C.; Rodríguez, I. Expansibility of vermiculites irradiated with microwaves. Appl. Clay Sci. 2011, 51, 33–37. [Google Scholar] [CrossRef]
- Melero Tur, S.; García Morales, S.; Neila Gonzalez, F.J. Design and evaluation of a dehumidifying plaster panel for passive architecture integration. Rev. Constr. 2015, 14, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fazio, P.; Rao, J. An investigation of moisture buffering performance of wood paneling at room level and its buffering effect on a test room. Build. Environ. 2012, 47, 205–216. [Google Scholar] [CrossRef]




| Element Oxides | Mass% |
|---|---|
| SiO2 | 34.96 |
| Al2O3 | 12.05 |
| Fe2O3 | 8.38 |
| MnO | 0.11 |
| MgO | 21.52 |
| CaO | 0.36 |
| Na2O | 0.14 |
| K2O | 0.36 |
| TiO2 | 1.42 |
| P2O5 | 0.11 |
| L.O.I. | 20.52 |
| TOTAL | 99.92 |
| T °C | K | 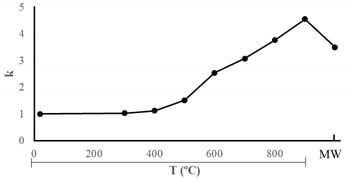 |
| U-20 | 1.00 | |
| U-300 | 1.03 | |
| U-400 | 1.12 | |
| U-500 | 1.51 | |
| U-600 | 2.54 | |
| U-700 | 3.07 | |
| U-800 | 3.76 | |
| U-900 | 4.55 | |
| U-MW | 3.50 |
| Time (h) | U-700 | U-800 | U-900 |
|---|---|---|---|
| 0.5 | 4.7 | 71.1 | 106.3 |
| 1 | 0.8 | 79.3 | 105.6 |
| 1.5 | 4.1 | 121.5 | 68.5 |
| 2 | 3.0 | 66.2 | 112.5 |
| 2.5 | 2.4 | 67.0 | 88.8 |
| 3.5 | 2.6 | 69.2 | 29.3 |
| 5.5 | 4.6 | 68.8 | 38.3 |
| 7.5 | 2.2 | 65.5 | 127.1 |
| 9.5 | 2.0 | 67.5 | 101.9 |
| 11.5 | 3.5 | 69.7 | 84.6 |
| 23 | 2.0 | 70.6 | 67.0 |
| 24 | 4.1 | 72.0 | 129.6 |
| Mass Loss (%) | ||
|---|---|---|
| Sample | 1st Step | 2nd Step |
| U-20 | 15.6 | 4.5 |
| U-0.5 | 15.0 | 3.6 |
| U-300 | 14.9 | 4.3 |
| U-400 | 14.7 | 4.0 |
| U-500 | 14.8 | 4.0 |
| U-600 | 14.5 | 3.9 |
| U-600-3.5 | 14.9 | 3.6 |
| U-700 | 14.1 | 4.0 |
| U-800 | 12.5 | 4.1 |
| U-900 | 6.3 | 3.6 |
| U-900-24 | 9.9 | 3.7 |
| U-MW | 14.2 | 4.0 |
| U-MW-3.5 | 14.8 | 4.0 |
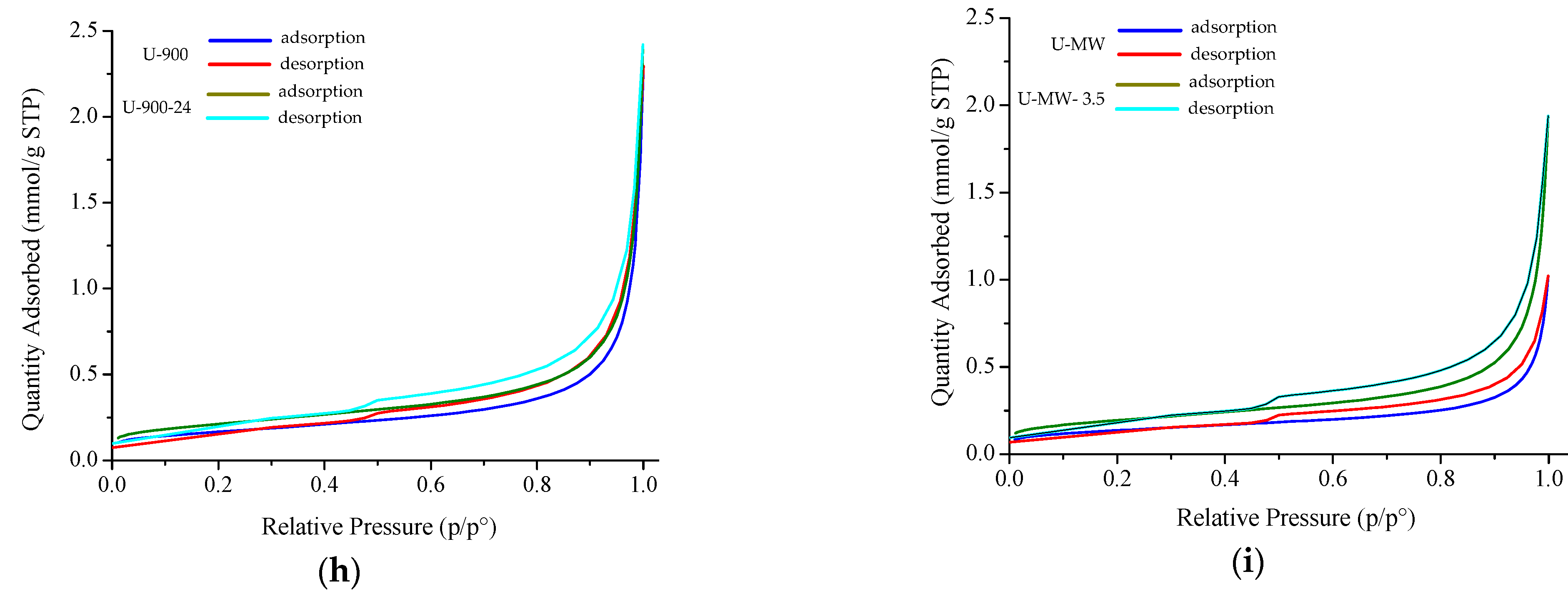
| Sample | SBET (m2/g) | Qm (mmol/g STP) | Vp (mmol/g STP) | Pore Size (nm) | C | R2 |
|---|---|---|---|---|---|---|
| U-20 | 11.7 ± 0.1 | 0.12 | 0.005 | 2.93 | 55.1 | 0.9999 |
| U-20-0.5 | 15.7 ± 0.1 | 0.16 | 0.04 | 2.93 | 109.3 | 0.9998 |
| U-300 | 8.0 ± 0.0 | 0.08 | 0.02 | 2.93 | 303.6 | 0.9999 |
| U-400 | 6.6 ± 0.1 | 0.07 | 0.03 | 2.93 | 60.6 | 0.9996 |
| U-500 | 14.6 ± 0.0 | 0.15 | 0.04 | 2.79 | 164.6 | 0.9999 |
| U-600 | 18.1 ± 0.0 | 0.19 | 0.05 | 2.52 | 180.6 | 0.9999 |
| U-600-3.5 | 17.9 ± 0.1 | 0.18 | 0.05 | 2.52 | 156.6 | 0.9999 |
| U-700 | 12.2 ± 0.1 | 0.13 | 0.04 | 2.52 | 135.3 | 0.9999 |
| U-800 | 18.3 ± 0.0 | 0.18 | 0.06 | 2.93 | 282.6 | 0.9999 |
| U-900 | 13.2 ± 0.0 | 0.14 | 0.06 | 2.52 | 184.7 | 0.9999 |
| U-900-24 | 16.9 ± 0.0 | 0.17 | 0.06 | 2.52 | 163.2 | 0.9999 |
| U-MW | 11.0 ± 0.0 | 0.11 | 0.02 | 3.24 | 168.4 | 0.9999 |
| U-MW-3.5 | 15.6 ± 0.0 | 0.16 | 0.04 | 2.79 | 144.4 | 0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos, C. Effect of Water Immersion on Raw and Expanded Ugandan Vermiculite. Minerals 2022, 12, 23. https://doi.org/10.3390/min12010023
Marcos C. Effect of Water Immersion on Raw and Expanded Ugandan Vermiculite. Minerals. 2022; 12(1):23. https://doi.org/10.3390/min12010023
Chicago/Turabian StyleMarcos, Celia. 2022. "Effect of Water Immersion on Raw and Expanded Ugandan Vermiculite" Minerals 12, no. 1: 23. https://doi.org/10.3390/min12010023






