Study on Double-Layer Ignition Sintering Process Based on Autocatalytic Denitrification of Sintering Layer
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials
2.1.1. Raw Materials for Catalytic Reduction
2.1.2. Raw Materials for Double-Layer Ignition Sintering
2.2. Experimental Methods and Devices
2.2.1. Catalytic Reduction
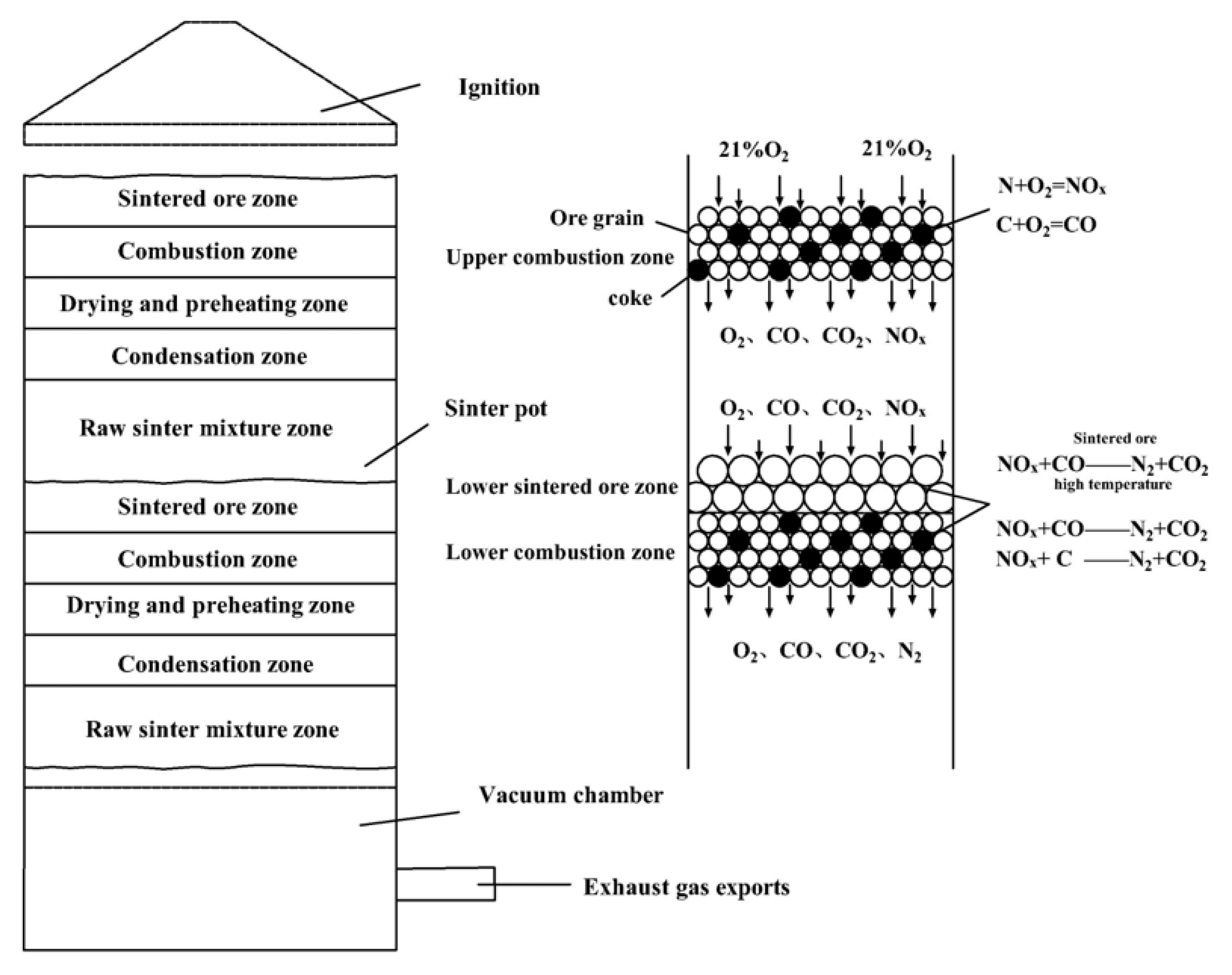
2.2.2. Double-Layer Ignition Sintering
2.3. Calculation of NOx
2.4. Sintering Indices
3. Results and Discussion
3.1. Catalytic Reduction
3.1.1. Effect of Temperature on Catalytic Reduction of NO in Sintered Ore
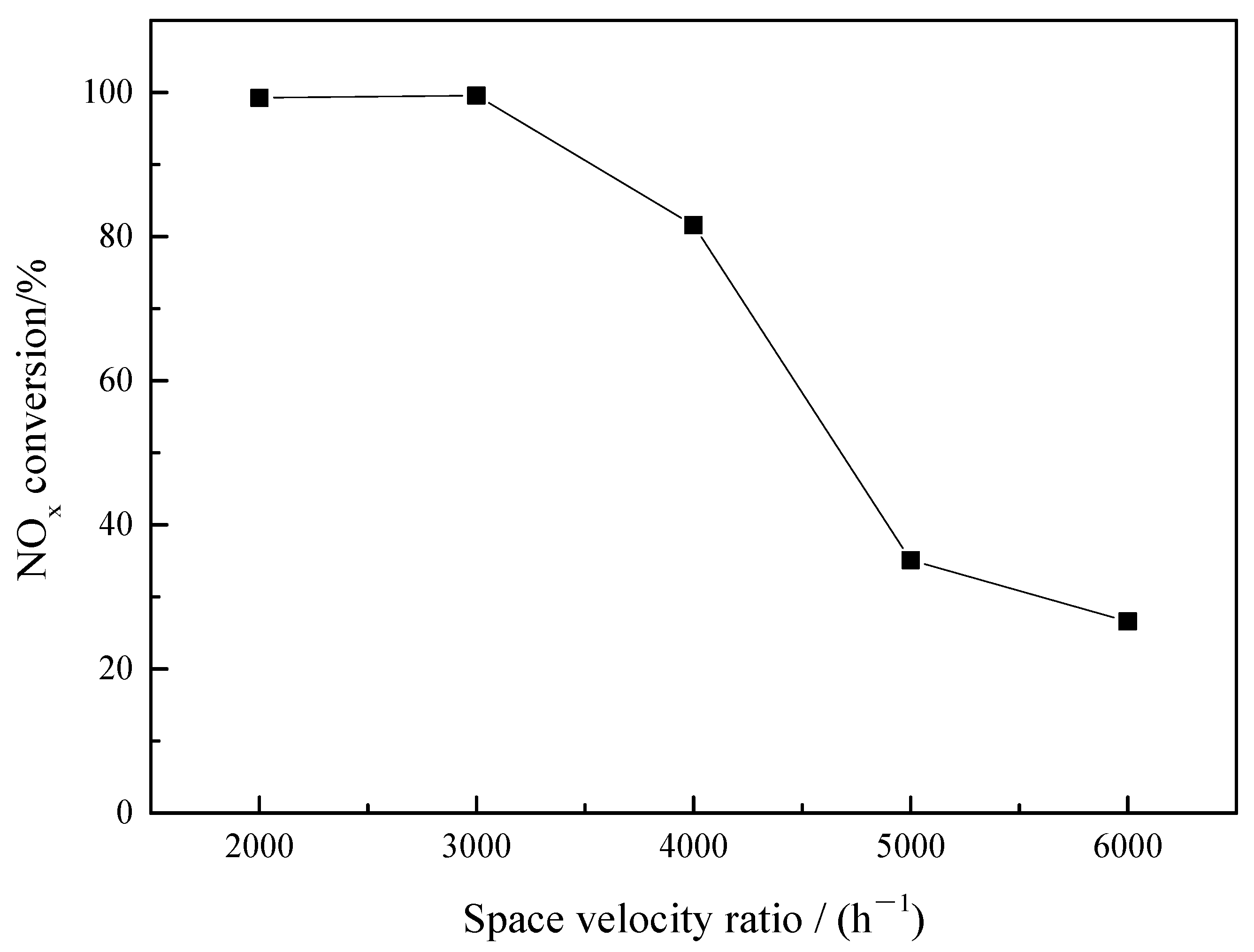
3.1.2. Effect of Space Velocity on Catalytic Reduction of NO in Sintered Ore
3.2. Double-Layer Ignition Sintering
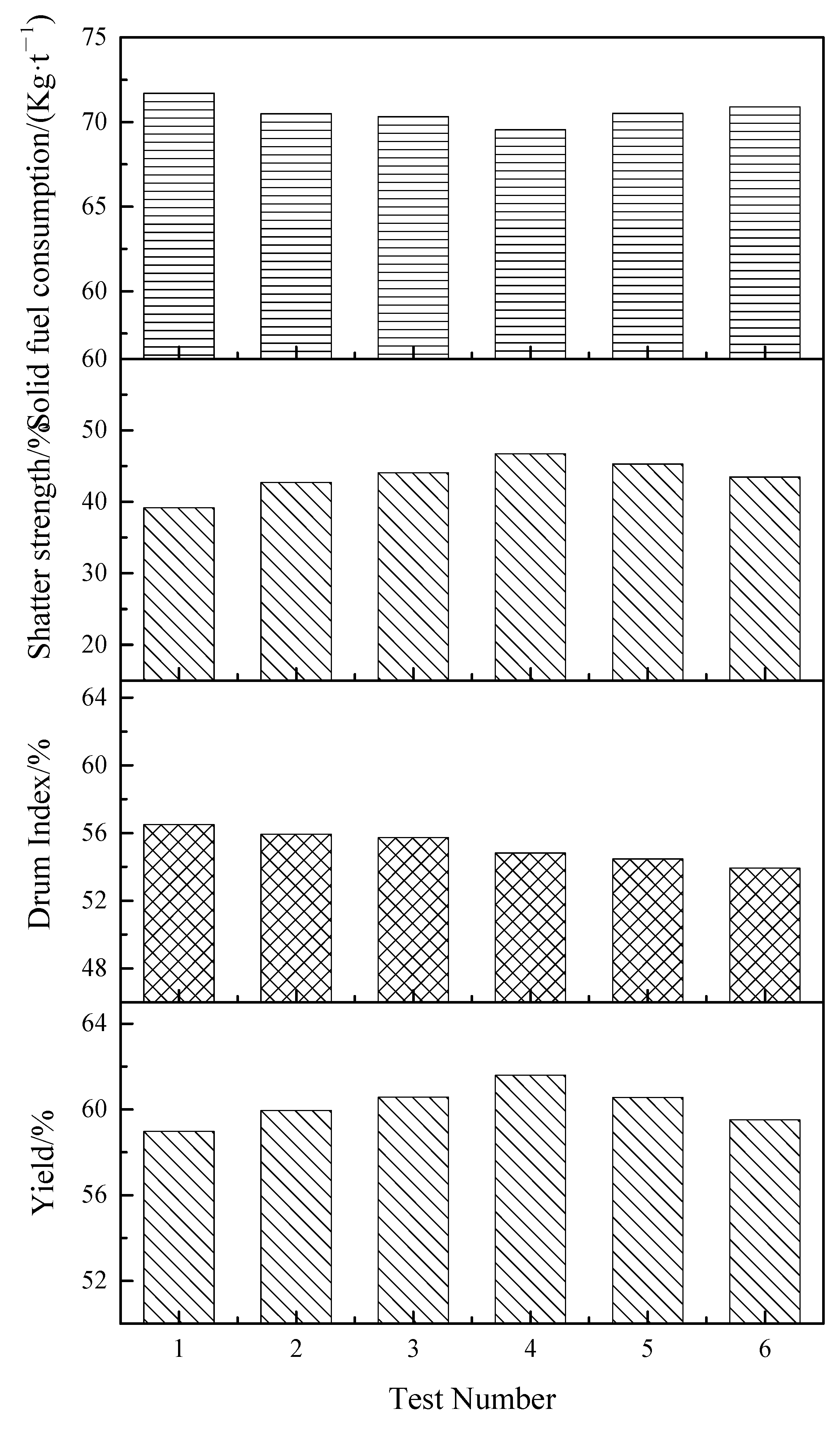
3.2.1. Sintering Performance
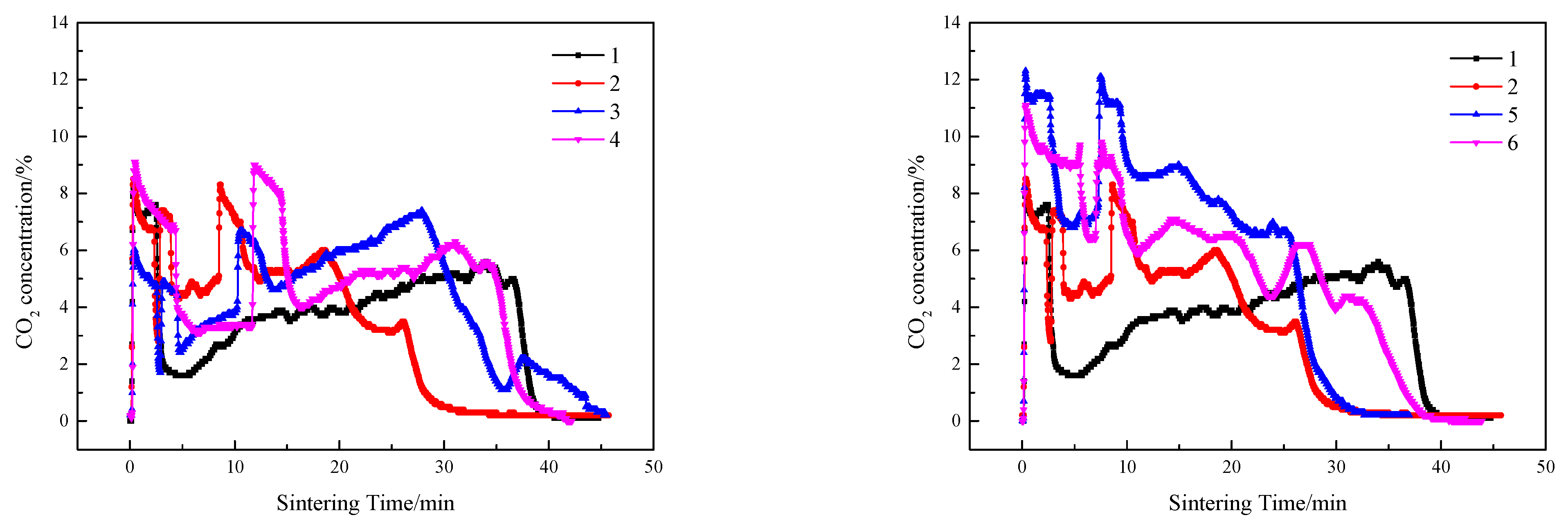
3.2.2. Sintering Flue Gas
3.2.3. Mechanism of NOx Degradation in Double-Layer Ignition Sintering
4. Conclusions
- (1)
- The sintered ores are beneficial to promote the reaction process of CO reducing NO. The reduction of NO by CO can only occur at high temperature above 600 °C, and it is difficult to proceed at low temperature. When sinter is used as catalyst, the conversion rate of NO reduced by sintered ore increased significantly, reaching 99.58% at 500 °C.
- (2)
- Compared with single-layer sintering, the sinter yield of double-layer ignition sintering is increased, solid fuel consumption is slightly reduced, falling strength is slightly increased, and drum strength is slightly decreased. Under the conditions of layer height proportion of 320/400 mm (lower/upper) and ignition time interval of 10 min, the yield, drum strength, shatter strength, and solid fuel consumption were reached 61.60%, 54.82%, 46.75%, and 69.55%, respectively.
- (3)
- In contrast to the single-layer sintering, NOx concentration under the 16% baseline oxygen content (c(NOx)) in the flue gas of double-layer ignition sintering is reduced. The CO2 concentration in flue gas of double-layer ignition sintering was higher, and the O2 concentration was lower. The oxidizing atmosphere in the upper sintering flue gas is weakened, and the high-temperature hot sintered ore in the lower layer provides favorable conditions for catalytic reduction of NOx.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rocha, L.; Kim, H.; Lee, C.; Jung, S.M. Mechanism of NOx Formation from Nitrogen in the Combustion of the Coals Used in Sintering Process. Metall. Mater. Trans. B 2020, 51, 2068–2078. [Google Scholar] [CrossRef]
- Chen, Y.G.; Guo, Z.C.; Wang, Z. Influence of CeO2 on NOx emission during iron ore sintering. Fuel Processing Technol. 2009, 90, 933–938. [Google Scholar] [CrossRef]
- Lu, P.; Hao, J.T.; Yu, W.; Zhu, X.M.; Dai, X. Effects of water vapor and Na/K additives on NO reduction through advanced biomass reburning. Fuel 2015, 170, 60–66. [Google Scholar] [CrossRef]
- Fan, X.H.; Yu, Z.Y.; Gan, M.K.H.; Chen, X.L.; Chen, Q.; Liu, S.; Huang, Y.S. Elimination Behaviors of NOx in the Sintering Process with Flue Gas Recirculation. ISIJ Int. 2015, 55, 2074–2081. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.J.; Wei, Y.Q.; Yang, L.Q. Research of emission reduction of nitrogen oxide in Chinese iron and steel enterprises. Environ. Eng. 2012, 5, 118–123. [Google Scholar]
- Zhu, T.Y. Sintering Flue Gas Purification Technology; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Ni, W.J.; Li, H.F.; Zhang, Y.Y.; Zou, Z.S. Effects of Fuel Type and Operation Parameters on Combustion and NOx Emission of the Iron Ore Sintering Process. Energies 2019, 12, 213. [Google Scholar] [CrossRef] [Green Version]
- Speth, K.; Murer, M.; Spliethoff, H. Experimental Investigation of Nitrogen Species Distribution in Wood Combustion and Their Influence on NOx Reduction by Combining Air Staging and Ammonia Injection. Energy Fuels 2016, 30, 5816–5824. [Google Scholar] [CrossRef]
- Xu, M.X.; Li, S.Y.; Wu, Y.H.; Jia, L.S.; Lu, Q.G. Effects of CO2 on the fuel nitrogen conversion during coal rapid pyrolysis. Fuel 2016, 184, 430–439. [Google Scholar] [CrossRef]
- Gan, M.; Fan, X.H.; Lv, W.; Chen, X.L.; Yu, Z.Y.; Zhou, Y. Fuel pre-granulation for reducing NOx emissions from the iron ore sintering process. Powder Technol. 2016, 301, 478–485. [Google Scholar] [CrossRef]
- Wo, C.N. NOx Formation of Pulverized Coal under Pressure Oxygen-Enriched Combustion. Master’s Thesis, Zhejiang University, Hangzhou, China, 2020. [Google Scholar]
- Gan, M.; Fan, X.; Ji, Z.; Jiang, T.; Chen, X.; Yu, Z.; Li, G.; Yin, L. Application of biomass fuel in iron ore sintering: Influencing mechanism and emission reduction. Ironmak. Steelmak. Processes Prod. Appl. 2014, 42, 27–33. [Google Scholar] [CrossRef]
- Mo, C.L.; Teo, C.S.; Hamilton, I.; Morrison, J. Admixing Hydrocarbons in Raw Mix to Reduce NOx Emission in Iron Ore Sintering Process. ISIJ Int. 1997, 37, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.G.; Guo, Z.C.; Wang, Z. Application of Modified Coke to NOx Reduction with Recycling Flue Gas during Iron Ore Sintering Process. ISIJ Int. 2008, 11, 1517–1523. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Wang, J.Y.; Wei, S.S.; Guo, Z.G.; Yang, J.; Wang, Q.W. Optimization of gaseous fuel injection for saving energy consumption and improving imbalance of heat distribution in iron ore sintering. Appl. Energy 2017, 207, 230–242. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Fan, X.H.; Gan, M.; Chen, X.L.; Lv, W. NOx Reduction in the Iron Ore Sintering Process with Flue Gas Recirculation. JOM 2017, 69, 1570–1574. [Google Scholar] [CrossRef]
- Locci, C.; Vervisch, L.; Farcy, B.; Domingo, P.; Perret, N. Selective Non-Catalytic Reduction (SNCR) of Nitrogen Oxide Emissions: A Perspective from Numerical Modeling. Flow Turbul. Combust. 2018, 100, 301–340. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Baeyens, J.; Seville, J. NOx formation and selective non-catalytic reduction (SNCR) in a fluidized bed combustor of biomass. Biomass Bioenergy 2010, 34, 1393–1409. [Google Scholar] [CrossRef]
- Procházka, L.; Mec, P. Possibility of using fly ash after denitrification by SNCR as admixture in alkali-activated materials. Mater. Today Proc. 2020, 37, 42–47. [Google Scholar] [CrossRef]
- Chen, X.P.; Liu, Q.; Wu, Q.; Luo, Z.K.; Zhao, W.T.; Chen, J.J.; Li, J.H. A hollow structure WO3@CeO2 catalyst for NH3-SCR of NOx. Catal. Commun. 2021, 149, 106252. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Zhao, S.; Wang, Y.L.; Wenren, Y.S.; Zhang, X.M. Plasma-enhanced low temperature NH3-SCR of NOx over a Cu-Mn/SAPO-34 catalyst under oxygen-rich conditions. Appl. Catal. B Environ. 2021, 286, 119886. [Google Scholar] [CrossRef]
- Xu, G.Y.; Guo, X.L.; Cheng, X.X.; Yu, J.; Fang, B.Z. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale 2021, 13, 7052–7080. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.; Moreno-Pirajan, J.C. Adsorption microcalorimetry Characterisation of activated carbons and their application in the study of NOx retention. J. Therm. Anal. Calorim. 2015, 121, 245–255. [Google Scholar]
- Song, Z.J.; Wang, B.; Yang, W.; Chen, T.; Sun, L. Research on NO and SO2 removal using TiO2 supported iron catalyst with vaporized H2O2 in a catalytic oxidation combined with absorption process. Environ. Sci. Pollut. R 2020, 27, 18329–18344. [Google Scholar] [CrossRef] [PubMed]
- Sahin, Z.; Tuti, M.; Durgun, O. Experimental investigation of the effects of water adding to the intake air on the engine performance and exhaust emissions in a DI automotive diesel engine. Fuel 2014, 115, 884–895. [Google Scholar] [CrossRef]
- Xiao-Hui, J.I. Influence of O2 Content in Circulating Flue Gas on Iron Ore Sintering. J. Iron Steel Res. Int. 2013, 20, 1–6. [Google Scholar]
- Menad, N.; Tayibi, H.; Carcedo, F.G.; Hernandez, A. Minimization methods for emissions generated from sinter strands: A review. J. Clean. Prod. 2006, 14, 740–747. [Google Scholar] [CrossRef]
- Fleischander, A.; Aichinger, C.; Zwittag, E. New developments for achieving environmentally friendly sinter production-Eposint and MEROS. China Metall. 2008, 18, 41–46. [Google Scholar]
- Sidorkin, V.T.; Tugov, A.N.; Moshnikov, A.N.; Vereshchetin, V.A.; Bersenev, K.G. Effect of Flue Gas Recirculation on the Technical and Environmental Performance of a Boiler. Power Technol. Eng. 2016, 49, 354–358. [Google Scholar] [CrossRef]
- Vanderheyden, B.; Borlee, J.; Brouhon, M. Impact of Waste Gas Recycling on Sintering Performances and Emissions; U.S. Department of Energy, Office of Scientific and Technical Information: Pittsburgh, PA, USA, 1996; Volume 55, p. 807.
- Schmid, H.; Zwittag, E.; Reidetschlager, J.; Kainz, K.; Guan, Y. Eposint—A New Waste—Gas Recycling System for Sinter Plants. World Iron Steel 2007, 3, 6–9. [Google Scholar]
- Pourhoseini, S.H.; Taghvaei, I.; Moghiman, M.; Baghban, M. Tangential Flue Gas Recirculation (TFGR) technique for enhancement of radiation characteristics and reduction of NOx emission in natural gas burners. J. Nat. Gas Sci. Eng. 2021, 94, 104130. [Google Scholar] [CrossRef]
- Pan, J. Research on Basic Theory and Technology of Reduction Emission of Sintering Flue Gas in Iron Ore. Ph.D. Thesis, Central South University, Changsha, China, 2007. [Google Scholar]
- Zhong, Q.; Liu, H.B.; Xu, L.P.; Zhang, X.; Rao, M.J.; Peng, Z.W.; Li, G.H.; Jiang, T. An efficient method for iron ore sintering with high-bed layer: Double-layer sintering. J. Iron Steel Res. Int. 2021, 28, 1366–1374. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Fan, X.H.; Gan, M.; Chen, X.L. Effect of Ca-Fe oxides additives on NOx reduction in iron ore sintering. J. Iron Steel Res. Int. 2017, 24, 18–23. [Google Scholar] [CrossRef]
- Yunas, J.; Sulaiman, N.H.; Ghazali, M.J. Comparative Study of the Calcium Ferrite Nanoparticles (CaFe2O4-NPs) Synthesis Process. In Proceedings of the 2018 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 15–17 August 2018; pp. 101–103. [Google Scholar]
- Wang, X.; Liu, Y.; Han, H.; Zhao, Y.; Ma, W.; Sun, H. Polyaniline coated Fe3O4 hollow nanospheres as anode materials for lithium ion batteries. Sustain. Energy Fuels 2017, 1, 915–922. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Zhou, R.X. Ce doping effect on performance of the Fe/b catalyst for NOx reduction by NH3. UEL Processing Technol. 2015, 133, 220–226. [Google Scholar] [CrossRef]
- Frank, B.; Renken, A. Kinetics and deactivation of the NO reduction by CO on Pt-supported catalysts. Chem. Eng. Technol. 1999, 22, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Gan, M.; Fan, X.H.; Yu, Z.Y.; Chen, X.L.; Ji, Z.Y.; Lv, W.; Liu, S.; Huang, Y.-S. A laboratory-based investigation into the catalytic reduction of NOx in iron ore sintering with flue gas recirculation. Ironmak. Steelmak. 2016, 43, 441–449. [Google Scholar] [CrossRef]
- Ge, X.; Chen, J.Q.; Zhang, H.L. Preparation, characterization and catalytic activity of the spinel ferrites. Chin. J. Inorg. Chem. 1999, 15, 730–731. [Google Scholar]
- Fan, X.H.; Yu, Z.Y.; Gan, M.; Chen, X.-L.; Ji, Z.Y. Combustion behavior and influence mechanism of CO on iron ore sintering with flue gas recirculation. J. Cent. South Univ. 2014, 21, 2391–2396. [Google Scholar] [CrossRef]
- Wu, S.L.; Chen, D.F.; Zhao, C.X.; Zhang, L.H.; Xue, F. Influence of Bed Depth on Sinter Flue Gas Composition and Emission. In Proceedings of the 5th International Congress on the Science and Technology of Ironmaking, Shanghai, China, 20–22 October 2009; pp. 519–523. [Google Scholar]
- Liu, F.Q.; Wang, S.L.; Gu, A.J. Analysis of influence factors of sinter strength and grain size composition. Ironmak. Technol. Newsl. 2009, 4, 27–29. [Google Scholar]
- Jiang, D.J. Influence of fuel structure on sintering character and comprehensive effect. Iron Steel 2020, 55, 31–41. [Google Scholar]
- Xu, B.; Zhuang, J.M.; Bai, G.H.; Liang, J.S. On Improving the Particle Size of Sinter. Sinter. Pelletizing 2001, 26, 19–21. [Google Scholar]
- Qian, F.; Chyang, C.S.; Chiou, J.B.; Tso, J. Effect of Flue Gas Recirculation (FGR) on NOx Emission in a Pilot-Scale Vortexing Fluidized-Bed Combustor. Energy Fuels 2011, 25, 5639–5646. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, M.X.; Liu, Z.H.; Cheng, M.; Chen, J.Z. Modeling NOx emission of coke combustion in iron ore sintering process and its experimental validation. Fuel 2016, 179, 322–331. [Google Scholar] [CrossRef]
- Xu, C.B.; Wu, S.L.; Cang, D.Q.; GeXi, R.F. Catalytical performance of several metallic oxides on elimination of NO in NO-CO-CO2-N2 system. Chin. Environ. Sci. 1998, 18, 1–6. [Google Scholar]
- Okazakf, K.; Ando, T. NOx reduction mechanism in coal combustion with recycled CO2. Energy 1997, 22, 207–215. [Google Scholar] [CrossRef]







| TFe | FeO | CaO | MgO | Al2O3 | SiO2 |
|---|---|---|---|---|---|
| 56.89 | 7.32 | 10.87 | 1.9 | 1.91 | 5.48 |
| Raw Materials | TFe | FeO | SiO2 | CaO | Al2O3 | MgO | MnO2 | V2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Iron ore fines | 61.47 | 2.88 | 5.4 | 0.16 | 1.52 | 0.08 | 0.31 | 0.008 | 5.14 |
| Return fines | 54.66 | 8.89 | 5.73 | 10.79 | 1.6 | 1.62 | 0.081 | 0.012 | 1.21 |
| Calcined lime | 0.49 | 0 | 8.24 | 67.08 | 1.98 | 3.23 | 0.005 | 0.001 | 17.07 |
| Limestone | 0.13 | 0 | 4.81 | 50.44 | 0.58 | 1.61 | 0.005 | 0.001 | 40.74 |
| Dolomite | 0.12 | 0 | 3.21 | 31.21 | 0.09 | 20.43 | 0.005 | 0.01 | 44.09 |
| Coal | 1.98 | 0 | 4.42 | 2.84 | 1.80 | 0.41 | 0.07 | 0.001 | 85.36 |
| Mad | Aad | Vad | FCad |
|---|---|---|---|
| 1.45 | 15.06 | 3.63 | 79.86 |
| Test Number | Lower Layer Height/mm | Upper Layer Height/mm | Ignition Time Interval/min |
|---|---|---|---|
| 1 | 720 | 0 | - |
| 2 | 320 | 400 | 5 |
| 3 | 320 | 400 | 7.5 |
| 4 | 320 | 400 | 10 |
| 5 | 360 | 360 | 5 |
| 6 | 400 | 320 | 5 |
| Raw Materials | Iron Ore Fines | Return Fines | Coal | Calcined Lime | Limestone | Dolomite | Total Mass |
|---|---|---|---|---|---|---|---|
| Mass fraction | 56.0 | 25.0 | 4.0 | 3.0 | 5.5 | 6.5 | 100.0 |
| Scheme | Equation | Unit | Symbols |
|---|---|---|---|
| Yield | η = (m0 − m1/m0) × 100% | % | m0/kg—mass of sintered ore without hearth |
| m1/kg—mass of return fines (<5 mm) after the shatter test | |||
| Shatter strength | F = m1/m0 × 100% | % | m0/kg—mass of sintered ore |
| m1/kg—mass of sintered ore (>10 mm) after the shatter test | |||
| Drum index | T = m1/m0 × 100% | % | m0/kg—mass of sample |
| m1/kg—mass of >6.3 mm sample after drum test |
| Size Distribution of Sintered Ore | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| >40 mm/% | 2.73 | 2.38 | 2.61 | 3.09 | 2.44 | 1.12 |
| 40~25 mm/% | 7.47 | 9.96 | 9.91 | 10.57 | 12.67 | 11.59 |
| 25~16 mm/% | 12.66 | 14.72 | 13.59 | 16.18 | 13.27 | 14.58 |
| 16~10 mm/% | 16.30 | 16.09 | 17.96 | 16.92 | 16.03 | 16.17 |
| 10~5 mm/% | 21.68 | 18.65 | 18.33 | 16.64 | 17.87 | 17.94 |
| <5 mm/% | 39.16 | 38.21 | 37.60 | 36.61 | 37.72 | 38.60 |
| Total/% | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Wan, J.; Chen, T.; Zhou, X.; Luo, Y.; Liu, J.; Hu, M.; Wang, Z. Study on Double-Layer Ignition Sintering Process Based on Autocatalytic Denitrification of Sintering Layer. Minerals 2022, 12, 33. https://doi.org/10.3390/min12010033
Shi B, Wan J, Chen T, Zhou X, Luo Y, Liu J, Hu M, Wang Z. Study on Double-Layer Ignition Sintering Process Based on Autocatalytic Denitrification of Sintering Layer. Minerals. 2022; 12(1):33. https://doi.org/10.3390/min12010033
Chicago/Turabian StyleShi, Benjing, Junying Wan, Tiejun Chen, Xianlin Zhou, Yanhong Luo, Jiawen Liu, Mengjie Hu, and Zhaocai Wang. 2022. "Study on Double-Layer Ignition Sintering Process Based on Autocatalytic Denitrification of Sintering Layer" Minerals 12, no. 1: 33. https://doi.org/10.3390/min12010033






