Structural Variations across Wolframite Solid Solutions, (Fe,Mn)WO4
Abstract
:1. Introduction
2. Experimental Methods
2.1. Electron-Probe Microanalysis (EPMA)
2.2. Synchrotron High-Resolution Powder X-ray Diffraction (HRPXRD)
2.3. Rietveld Structural Refinement
3. Results and Discussion
3.1. Chemical Composition of Natural Wolframite Samples
3.2. High-Resolution Powder X-ray Diffraction (HRPXRD) Traces for Natural Wolframite Samples
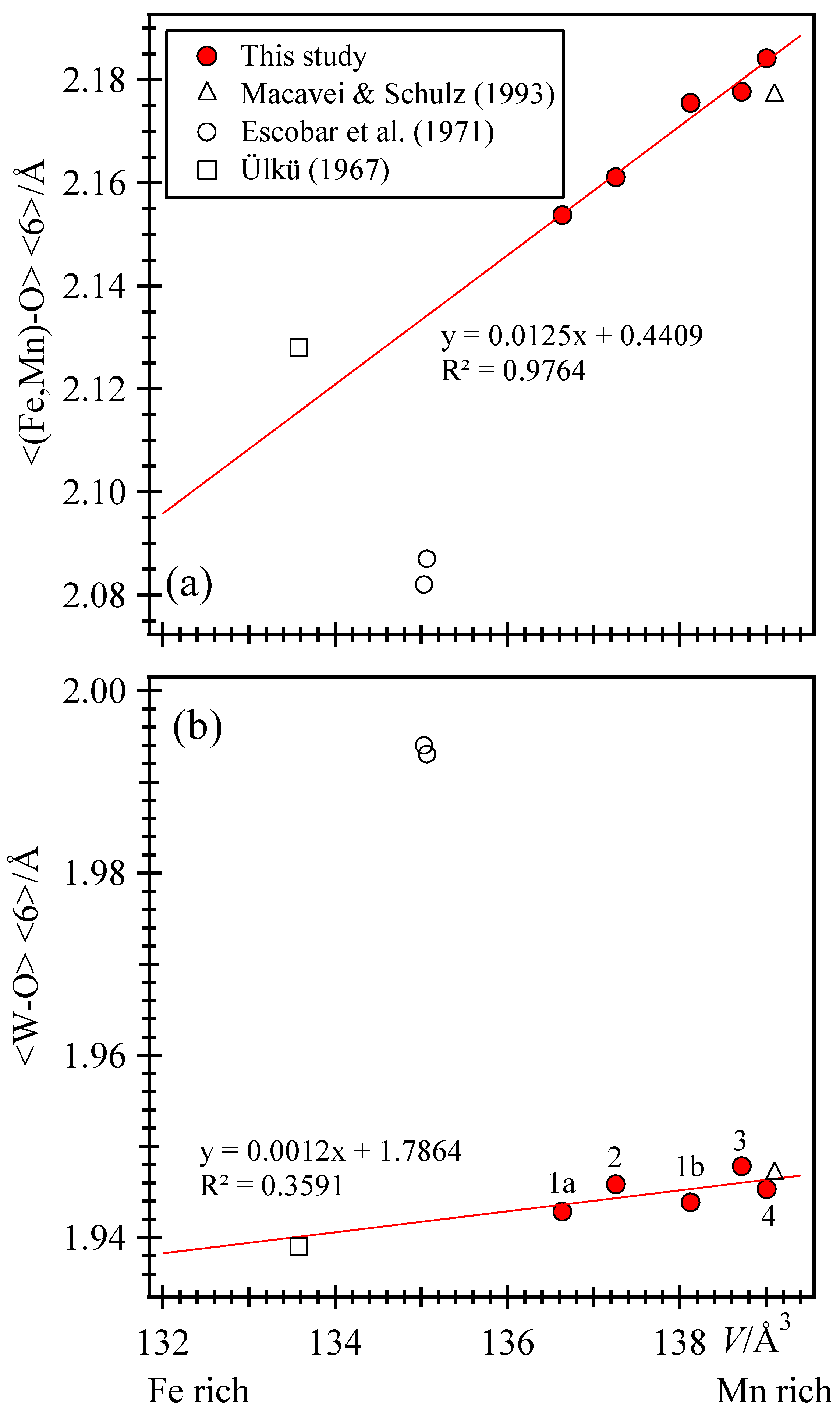
3.3. Variations of the Unit-Cell Parameters across Wolframite, (Fe,Mn)WO4, Solid Solutions
3.4. Variations of Structural Parameters across Wolframite, (Fe,Mn)WO4, Solid Solutions
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vegard, L. Results of crystal analysis. In Space Lattices and Atomic Dimensions; I Kommission hos J. Dybwad: Oslo, Norway, 1925. [Google Scholar]
- Cid-Dresdner, H.; Escobar, C. The crystal structure of ferberite, FeWO4. Z. Krist. 1968, 127, 61–72. [Google Scholar] [CrossRef]
- Escobar, C.; Cid-Dresdner, H.; Kittl, P.; Dümler, I. The relation between “light wolframite” and common wolframite. Am. Mineral. 1971, 56, 489–498. [Google Scholar]
- Ülkü, D. Untersuchungen zur Kristallstruktur und magnetischen Struktur des Ferberits FeWO4. Z. Krist. 1967, 124, 192–219. [Google Scholar] [CrossRef]
- Macavei, J.; Schulz, H. The crystal structure of wolframite type tungstates at high pressure. Z. Krist. 1993, 207, 193–208. [Google Scholar]
- Errandonea, D.; Ruiz-Fuertes, J. A brief review of the effects of pressure on wolframite-type oxides. Crystals 2018, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Lee, P.L.; Shu, D.; Ramanathan, M.; Preissner, C.; Wang, J.; Beno, M.A.; Von Dreele, R.B.; Ribaud, L.; Kurtz, C.; Antao, S.M.; et al. A twelve-analyzer detector system for high-resolution powder diffraction. J. Synchrotron Radiat. 2008, 15, 427–432. [Google Scholar] [CrossRef]
- Wang, J.; Toby, B.H.; Lee, P.L.; Ribaud, L.; Antao, S.M.; Kurtz, C.; Ramanathan, M.; Von Dreele, R.B.; Beno, M.A. A dedicated powder diffraction beamline at the advanced photon source: Commissioning and early operational results. Rev. Sci. Instrum. 2008, 79, 85105. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Wang, J.; Lee, P.L.; Toby, B.H. State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite. Can. Mineral. 2008, 46, 1501–1509. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal-structure analysis of four mineral samples of anhydrite, CaSO4, using synchrotron high-resolution powder X-ray diffraction data. Powder Diffr. 2011, 26, 326–330. [Google Scholar] [CrossRef]
- Parise, J.B.; Antao, S.M.; Michel, F.M.; Martin, C.D.; Chupas, P.J.; Shastri, S.; Lee, P.L. Quantitative high-pressure pair distribution function analysis. J. Synchrotron Radiat. 2005, 12, 554–559. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Parise, J.B. Haüyne: Phase transition and high-temperature structures obtained from synchrotron radiation and Rietveld refinements. Mineral. Mag. 2004, 68, 499–513. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Hersi, A.A. Single-crystal XRD, TEM, and thermal studies of the satellite reflections in nepheline. Can. Mineral. 2003, 41, 759–783. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Mulder, W.H.; Lee, P.L. The R-3c→R-3m transition in nitratine, NaNO3, and implications for calcite, CaCO3. Phys. Chem. Miner. 2008, 35, 545–557. [Google Scholar] [CrossRef]
- Zaman, M.; Schubert, M.; Antao, S. Elevated radionuclide concentrations in heavy mineral-rich beach sands in the Cox’s Bazar region, Bangladesh and related possible radiological effects. Isot. Environ. Health Stud. 2012, 48, 512–525. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report, LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Cagliotti, G.; Paoletti, A.; Ricci, F.P. Choice of collimators for a crystal spectrometer for neutron diffraction. Nucl. Instrum. 1958, 3, 223–228. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye-Scherrer synchrotron X-ray data from alumina. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M. Is near-endmember birefringent grossular non-cubic? New evidence from synchrotron diffraction. Can. Mineral. 2013, 51, 771–784. [Google Scholar] [CrossRef]
- Antao, S.M. The mystery of birefringent garnet: Is the symmetry lower than cubic? Powder Diffr. 2013, 28, 281–288. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. A two-phase intergrowth of genthelvite from Mont Saint-Hilaire, Quebec. Can. Mineral. 2010, 48, 1217–1223. [Google Scholar] [CrossRef]
- Antao, S.M.; Klincker, A.M. Crystal structure of a birefringent andradite-grossular from Crowsnest Pass, Alberta, Canada. Powder Diffr. 2014, 29, 20–27. [Google Scholar] [CrossRef]
- Antao, S.M.; Mohib, S.; Zaman, M.; Marr, R.A. Ti-rich andradites: Chemistry, structure, multi-phases, optical anisotropy, and oscillatory zoning. Can. Mineral. 2015, 53, 133–158. [Google Scholar] [CrossRef]
- Antao, S.M. Crystal structure of morimotoite from Ice River, Canada. Powder Diffr. 2014, 29, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M.; Round, S.A. Crystal chemistry of birefringent spessartine. Powder Diffr. 2014, 29, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Antao, S.M.; Hassan, I.; Parise, J.B. The structure of danalite at high temperature obtained from synchrotron radiation and Rietveld refinements. Can. Mineral. 2003, 41, 1413–1422. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Parise, J.B. Cation ordering in magnesioferrite, MgFe2O4, to 982 °C using in situ synchrotron X-ray powder diffraction. Am. Mineral. 2005, 90, 219–228. [Google Scholar] [CrossRef]
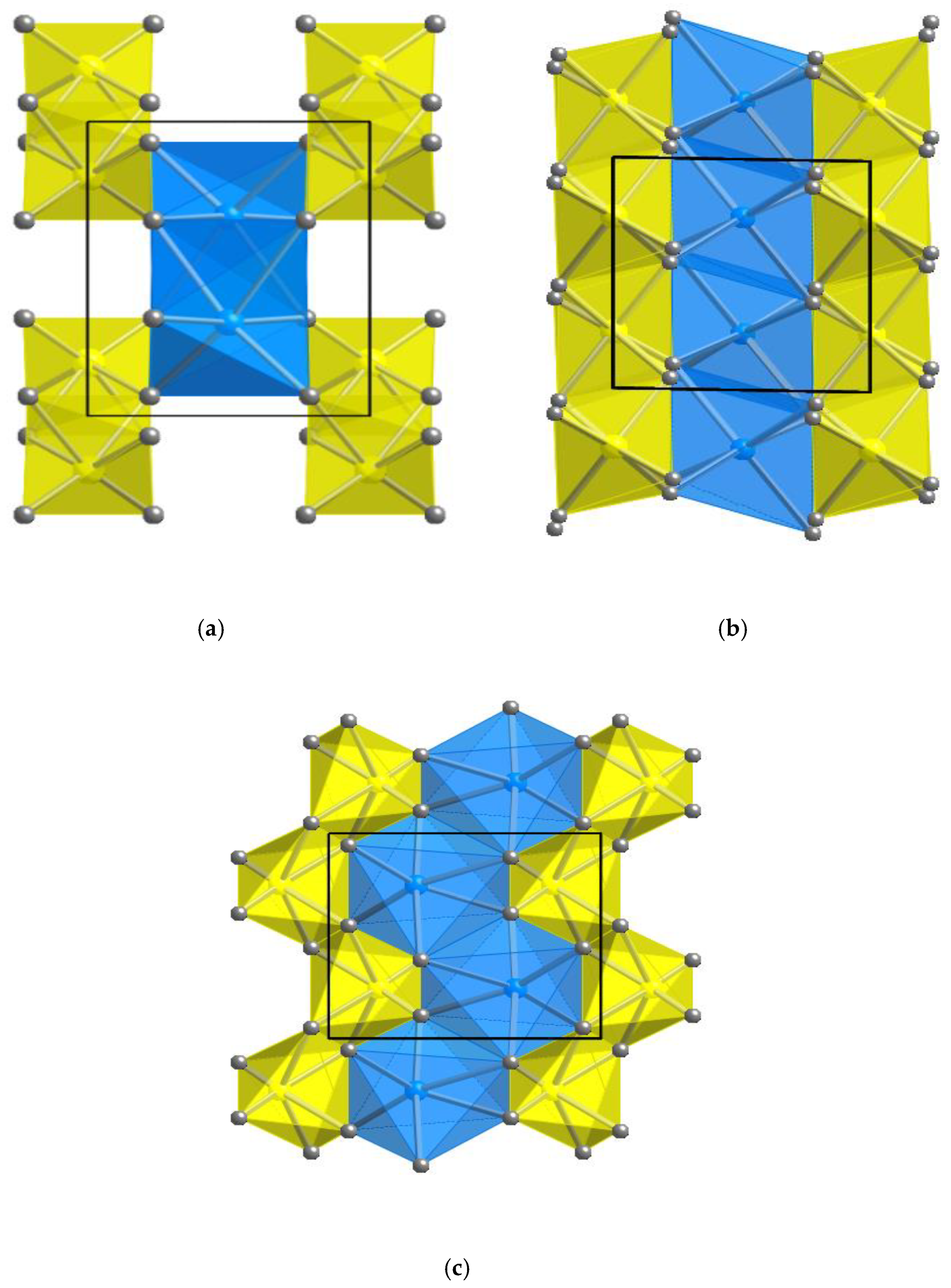




| Oxides | Radii (Å) * | 1a | 1b | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| wt. % | ||||||
| MnO | 15.14 | 20.07 | 17.66(29) | 21.89(46) | 23.41(12) | |
| FeO | 8.71 | 3.42 | 5.89(26) | 1.51(56) | 0.07(2) | |
| ZnO | 0.02 | 0.04 | 0.02(3) | 0.01(1) | 0.04(2) | |
| MgO | 0.02 | 0.00 | 0.00(2) | 0.00(1) | 0.00(1) | |
| NiO | 0.02 | 0.04 | 0.00(2) | 0.03(2) | 0.02(2) | |
| CaO | 0.00 | 0.00 | 0.00(1) | 0.00(0) | 0.00(0) | |
| PbO | 0.00 | 0.00 | 0.00(0) | 0.00(0) | 0.00(0) | |
| WO3 | 76.11 | 76.00 | 76.47 | 76.29(16) | 76.05(39) | |
| MoO3 | 0.00 | 0.00 | 0.00(0) | 0.00(0) | 0.00(0) | |
| SO3 | 0.06 | 0.21 | 0.26(8) | 0.05(9) | 0.08(11) | |
| TiO2 | 0.02 | 0.00 | 0.00(5) | 0.00(0) | 0.01(1) | |
| V2O3 | 0.00 | 0.00 | 0.00(0) | 0.00(0) | 0.00(0) | |
| Cr2O3 | 0.01 | 0.00 | 0.00(1) | 0.01(1) | 0.00(1) | |
| Total | 100.11 | 99.78 | 100.30 | 99.79 | 99.68 | |
| Cations | - | apfu | ||||
| Mn2+ | 0.83 | 0.645 | 0.856 | 0.748(12) | 0.935(19) | 1.000(5) |
| Fe2+ | 0.78 | 0.367 | 0.144 | 0.247(11) | 0.064(23) | 0.003(1) |
| Zn2+ | 0.74 | 0.001 | 0.001 | 0.001(1) | 0.000(0) | 0.002(1) |
| Mg2+ | 0.72 | 0.001 | 0.000 | 0.000(1) | 0.000(1) | 0.000(1) |
| Ni2+ | 0.69 | 0.001 | 0.001 | 0.000(1) | 0.001(1) | 0.001(1) |
| Ca2+ | 1.00 | 0.000 | 0.000 | 0.000(0) | 0.000(0) | 0.000(0) |
| Pb2+ | 1.19 | 0.000 | 0.000 | 0.000(0) | 0.000(0) | 0.000(0) |
| ΣA | 1.015 | 1.002 | 0.996 | 1.000 | 1.006 | |
| W6+ | 0.60 | 0.992 | 0.991 | 0.992(2) | 0.998(3) | 0.994(3) |
| Mo6+ | 0.59 | 0.000 | 0.000 | 0.000(0) | 0.000(0) | 0.000(0) |
| S6+ | 0.29 | 0.002 | 0.008 | 0.010(3) | 0.002(3) | 0.003(4) |
| Ti4+ | 0.61 | 0.001 | 0.000 | 0.000(2) | 0.000(0) | 0.000(0) |
| V3+ | 0.64 | 0.000 | 0.000 | 0.000(0) | 0.000(0) | 0.000(0) |
| Cr3+ | 0.62 | 0.000 | 0.000 | 0.000(0) | 0.000(0) | 0.000(0) |
| ΣB | 0.995 | 0.999 | 1.002 | 1.000 | 0.997 | |
| Σ(A + B) | 2.010 | 2.001 | 1.998 | 2.000 | 2.003 | |
| % Hü | 63.74 | 85.60 | 75.18 | 93.59 | 99.70 |
| No. | Localities | a/Å | b/Å | c/Å | β/° | V/Å3 | * R(F2) | χ2 | Nobs | Npts | Var. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | Oregon Mine, Boulder, Colorado (VC758) | 4.78219(4) | 5.73389(4) | 4.98336(3) | 90.5631(6) | 136.640(2) | 0.0208 | 1.699 | 2134 | 43,067 | 43 |
| 1b | VC758 | 4.81212(3) | 5.74991(4) | 4.99266(3) | 90.9304(6) | 138.125(2) | |||||
| 2 | Tae Wha mine, Korea (VC315) | 4.79440(2) | 5.74073(2) | 4.98734(2) | 90.7109(3) | 137.258(1) | 0.0178 | 1.623 | 1065 | 43,067 | 28 |
| 3 | Silverton, Colorado (VC692) | 4.82435(2) | 5.75627(2) | 4.99611(1) | 91.0773(2) | 138.7188(8) | 0.0271 | 3.664 | 983 | 41,444 | 28 |
| 4 | Pasto Bueno, Peru (UC09059) | 4.82991(2) | 5.75974(2) | 4.99795(2) | 91.1475(2) | 139.0101(9) | 0.0238 | 2.791 | 1032 | 43,067 | 28 |
| Atom | -Site | 1a | 1b | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| Mn/Fe | x | ½ | ½ | ½ | ½ | ½ |
| y | 0.3202(2) | 0.3149(3) | 0.3181(1) | 0.3139(1) | 0.3131(1) | |
| 2f | z | ¾ | ¾ | ¾ | ¾ | ¾ |
| U | 0.29(1) | 0.29(1) | 0.31(1) | 0.54(2) | 0.31(1) | |
| sof | 0.65 Mn 0.35 Fe | 0.86 Mn 0.24 Fe | 0.75 Mn 0.25 Fe | 0.94 Mn 0.06 Fe | 1 Mn 0 Fe | |
| W | x | 0 | 0 | 0 | 0 | 0 |
| y | 0.17974(6) | 0.18025(8) | 0.17999(3) | 0.17988(4) | 0.17975(4) | |
| 2e | z | ¼ | ¼ | ¼ | ¼ | ¼ |
| U | 0.109(4) | 0.109(4) | 0.109(4) | 0.323(4) | 0.273(4) | |
| O1 | x | 0.2149(6) | 0.2084(7) | 0.2139(3) | 0.2137(4) | 0.2136(4) |
| y | −0.0980(5) | −0.1063(6) | −0.1032(3) | −0.0992(3) | −0.1035(3) | |
| 4g | z | 0.4433(7) | 0.4351(8) | 0.4396(4) | 0.4392(4) | 0.4458(4) |
| U | 0.07(3) | 0.07(3) | 0.05(3) | 0.47(3) | 0.25(5) | |
| O2 | x | 0.2628(6) | 0.2479(7) | 0.2544(3) | 0.2539(3) | 0.2523(3) |
| y | 0.3655(5) | 0.3766(6) | 0.3702(3) | 0.3730(3) | 0.3680(3) | |
| 4g | z | 0.3972(7) | 0.3880(8) | 0.3945(4) | 0.3893(4) | 0.3952(4) |
| Bond | 1a | 1b | 2 | 3 | 4 |
|---|---|---|---|---|---|
| (Fe,Mn)O6 octahedron | |||||
| Fe–O1 × 2 | 2.106(3) | 2.074(3) | 2.081(2) | 2.093(2) | 2.094(2) |
| Fe–O2 × 2 | 2.099(4) | 2.189(4) | 2.137(2) | 2.166(2) | 2.143(2) |
| Fe–O2 × 2 | 2.256(3) | 2.263(3) | 2.265(2) | 2.274(2) | 2.316(2) |
| <(Fe,Mn)–O> <6> | 2.154 | 2.176 | 2.161 | 2.178 | 2.184 |
| Average edge length | 3.0239 | 3.0461 | 3.0306 | 3.0508 | 3.0590 |
| Average angle | 104.570 | 104.048 | 104.378 | 104.125 | 104.058 |
| Polyhedral volume | 12.9526 | 13.2503 | 13.0622 | 13.3194 | 13.4182 |
| Octahedral angle variance | 65.4243 | 83.6260 | 70.0429 | 78.6334 | 82.4303 |
| Mean octahedral quadratic elongation | 1.0199 | 1.0252 | 1.0212 | 1.0236 | 1.0255 |
| WO6 octahedron | |||||
| W–O1 × 2 | 2.121(3) | 2.132(4) | 2.136(2) | 2.122(2) | 2.155(2) |
| W–O1 × 2 | 1.909(3) | 1.926(4) | 1.919(2) | 1.936(2) | 1.906(2) |
| W–O2 × 2 | 1.798(3) | 1.773(4) | 1.782(2) | 1.786(2) | 1.776(2) |
| <W–O> <6> | 1.943 | 1.944 | 1.946 | 1.948 | 1.945 |
| Average edge length | 2.7145 | 2.7215 | 2.7222 | 2.7258 | 2.7218 |
| Average angle | 103.519 | 104.010 | 103.821 | 103.840 | 103.808 |
| Polyhedral volume | 9.2754 | 9.3836 | 9.3925 | 9.4119 | 9.3755 |
| Octahedral angle variance | 118.6792 | 97.5426 | 102.6663 | 104.9690 | 103.9077 |
| Mean octahedral quadratic elongation | 1.0407 | 1.0347 | 1.0363 | 1.0362 | 1.0377 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umbsaar, D.A.; Antao, S.M. Structural Variations across Wolframite Solid Solutions, (Fe,Mn)WO4. Minerals 2022, 12, 42. https://doi.org/10.3390/min12010042
Umbsaar DA, Antao SM. Structural Variations across Wolframite Solid Solutions, (Fe,Mn)WO4. Minerals. 2022; 12(1):42. https://doi.org/10.3390/min12010042
Chicago/Turabian StyleUmbsaar, Darren A., and Sytle M. Antao. 2022. "Structural Variations across Wolframite Solid Solutions, (Fe,Mn)WO4" Minerals 12, no. 1: 42. https://doi.org/10.3390/min12010042
APA StyleUmbsaar, D. A., & Antao, S. M. (2022). Structural Variations across Wolframite Solid Solutions, (Fe,Mn)WO4. Minerals, 12(1), 42. https://doi.org/10.3390/min12010042






