Whitlockite-Type Structure as a Matrix for Optical Materials: Synthesis and Characterization of Novel TM-SM Co-Doped Phosphate Ca9Gd(PO4)7, a Single-Phase White Light Phosphors
Abstract
:1. Introduction
2. Materials and Methods
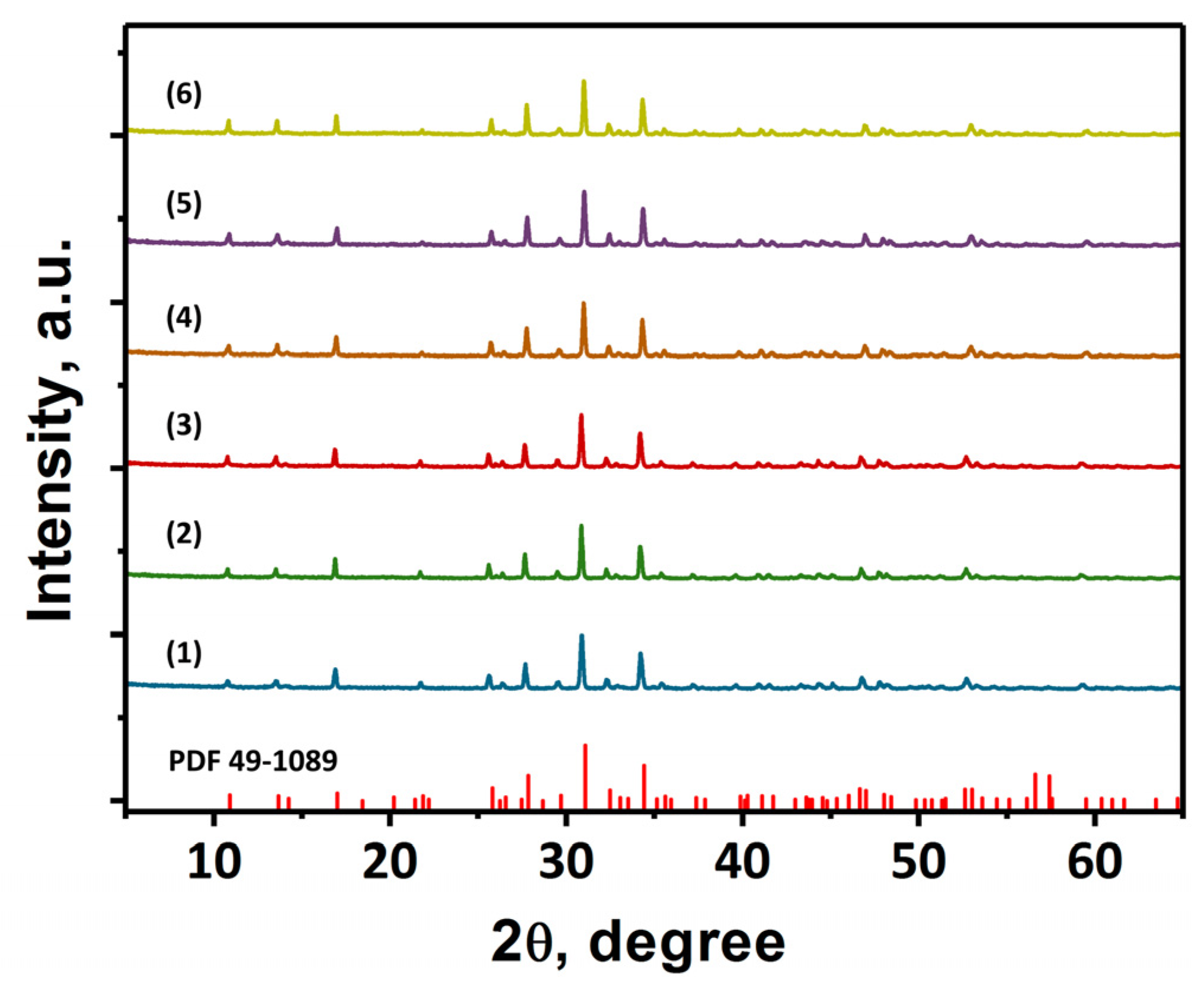
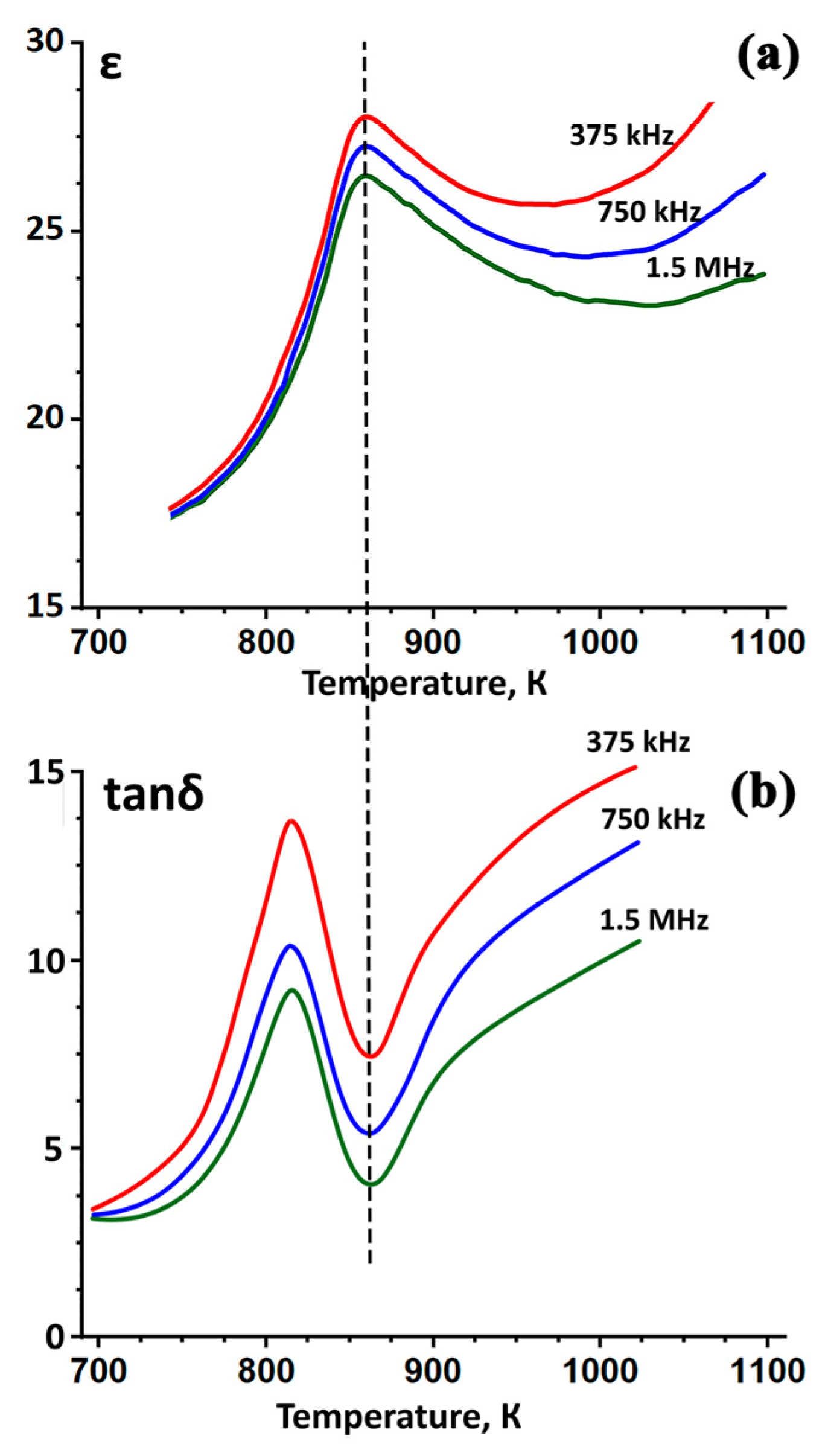
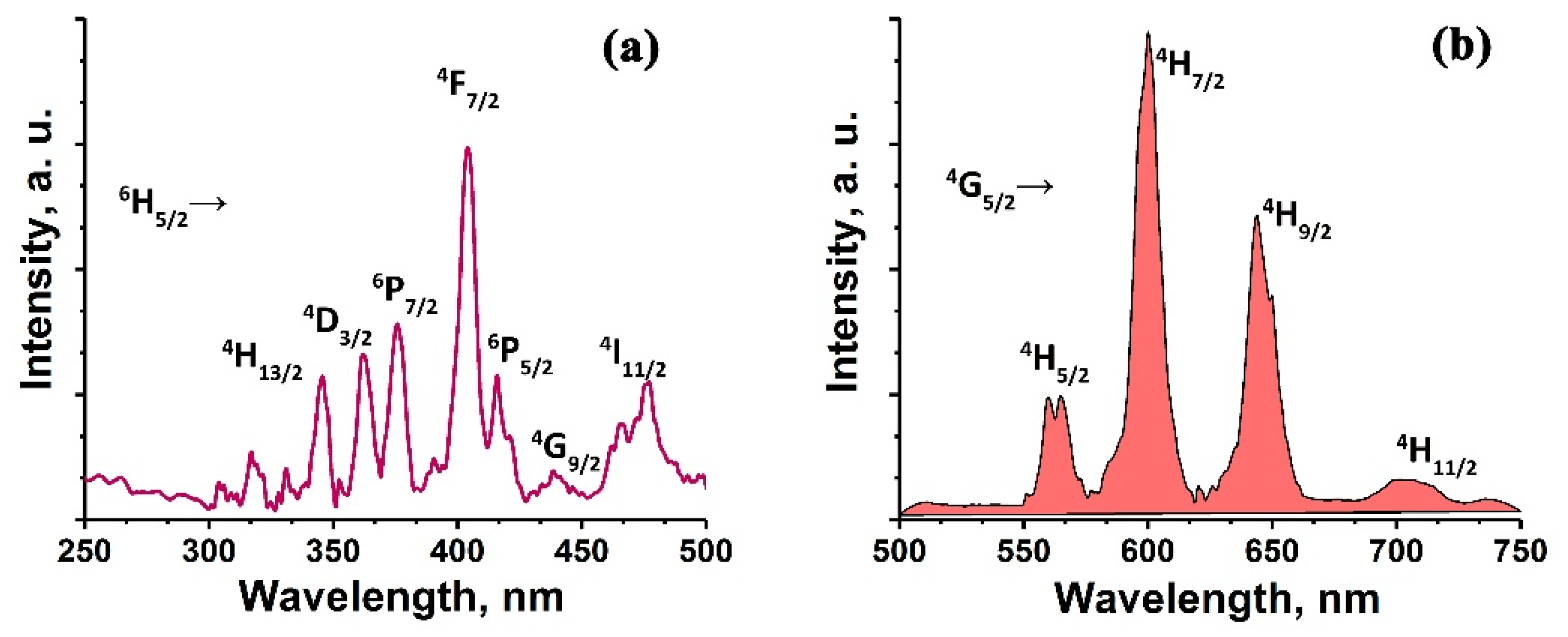
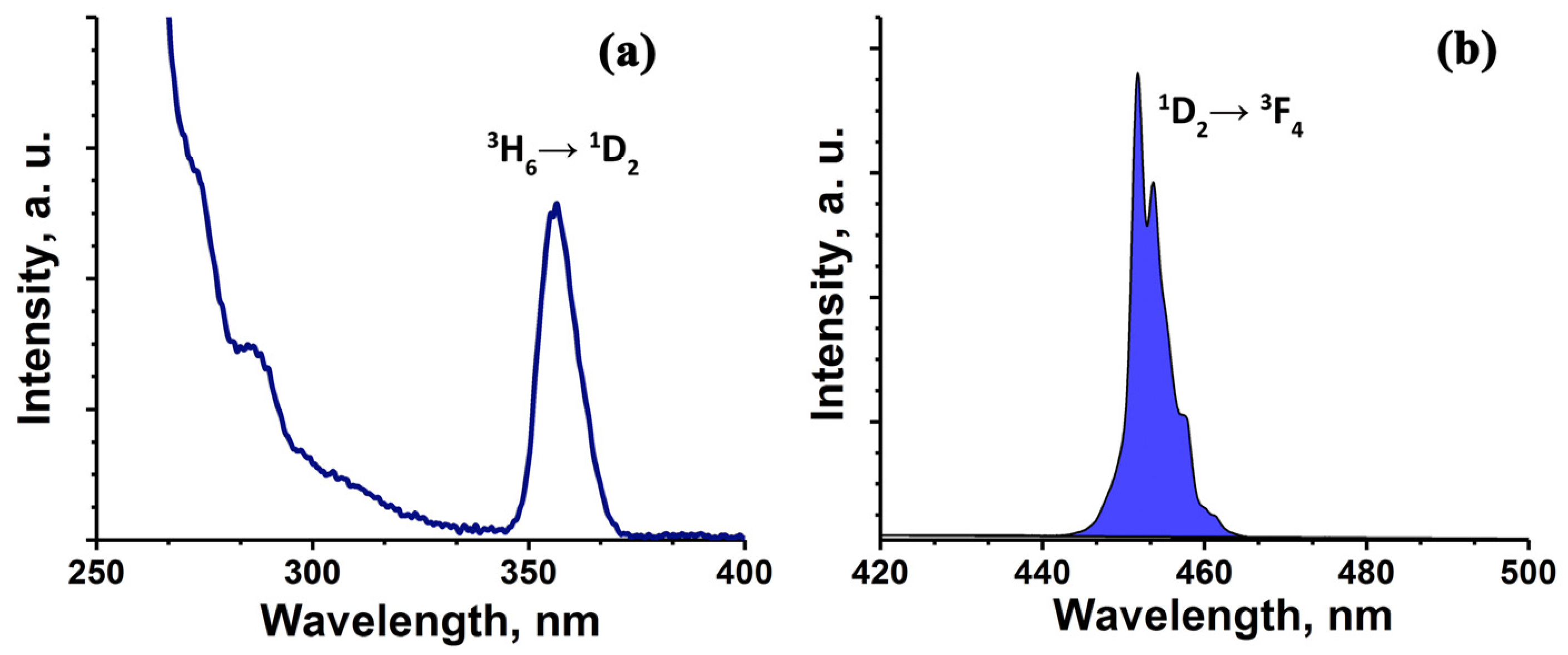
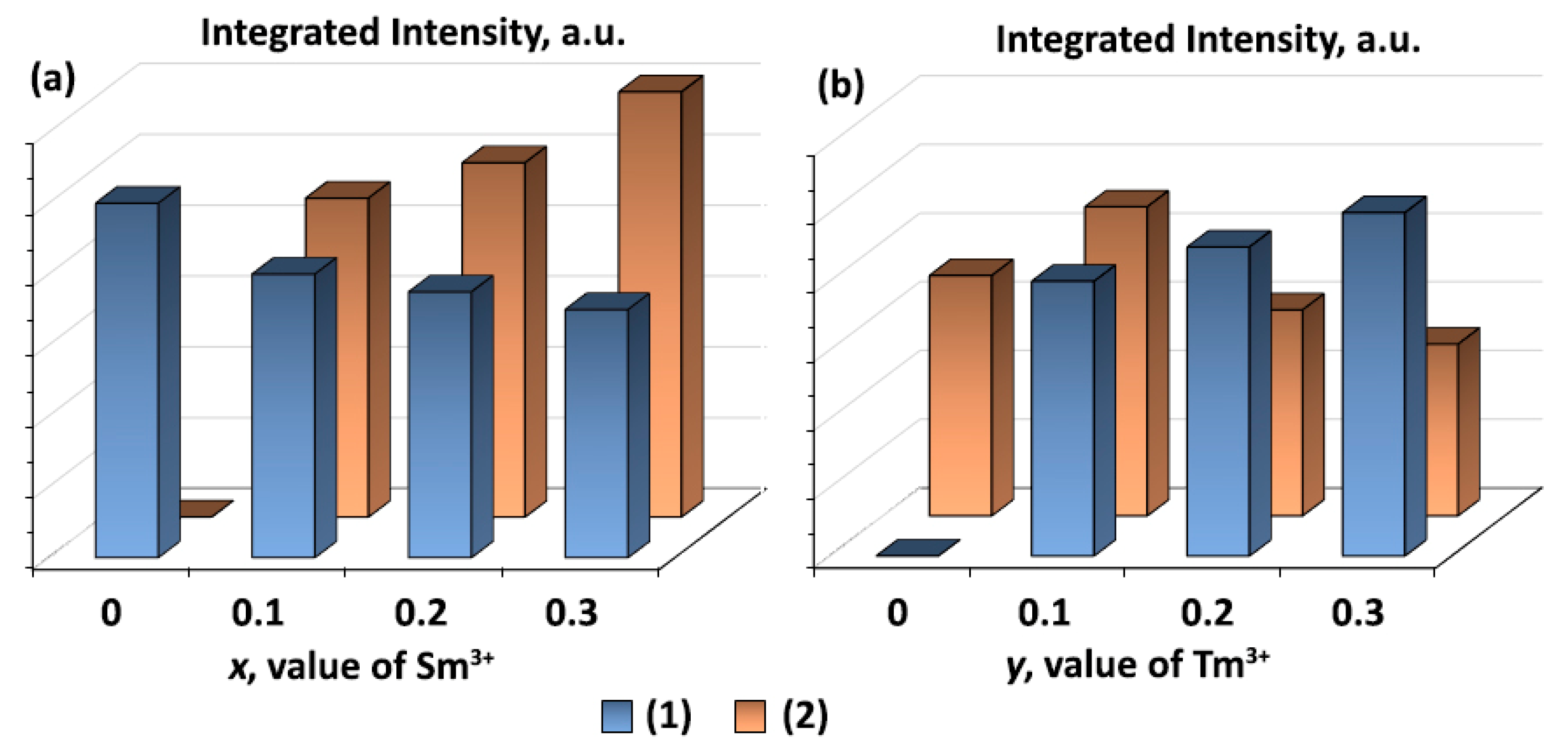
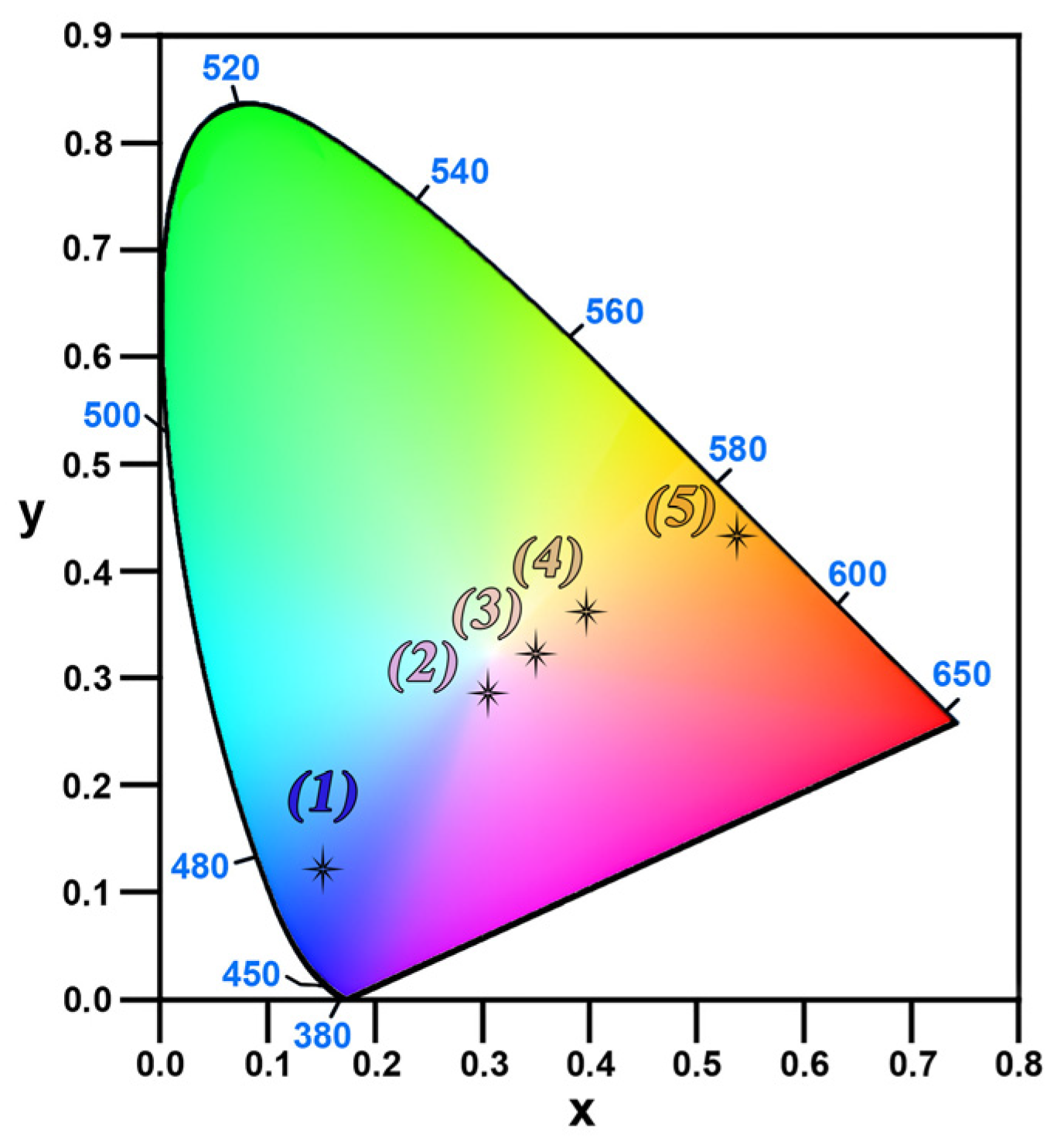
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Teterskii, A.V.; Stefanovich, S.Y.; Lazoryak, B.I.; Rusakov, D.A. Whitlockite solid solutions Ca9−xMxR(PO4)7 (x = 1, 1.5; M = Mg, Zn, Cd; R = Ln, Y) with antiferroelectric properties. Russ. J. Inorg. Chem. 2007, 52, 308–314. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Aksenov, S.M.; Nikiforov, I.V.; Stefanovich, S.Y.; Lazoryak, B.I. Symmetry inhomogeneity of Ca9–xZnxEu(PO4)7 phosphor determined by second-harmonic generation and dielectric and photoluminescence spectroscopy. Cryst. Growth Des. 2020, 20, 6461–6468. [Google Scholar] [CrossRef]
- Nikiforov, I.V.; Deyneko, D.V.; Duskaev, I.F.; Lazoryak, B.I. Green luminophors in the family of phosphates with whitlockite structure. J. Struct. Chem. 2021, 62, 1621–1630. [Google Scholar] [CrossRef]
- Lazoryak, B.I.; Zhukovskaya, E.S.; Baryshnikova, O.V.; Belik, A.A.; Leonidova, O.N.; Deyneko, D.V.; Savon, A.E.; Dorbakov, N.G.; Morozov, V.A. Luminescence, structure and antiferroelectric-type phase transition in Ca8ZnEu(PO4)7. Mater. Res. Bull. 2018, 104, 20–26. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Morozov, V.A.; Hadermann, J.; Savon, A.E.; Spassky, D.A.; Stefanovich, S.Y.; Belik, A.A.; Lazoryak, B.I. A novel red Ca8.5Pb0.5Eu(PO4)7 phosphor for light emitting diodes application. J. Alloy. Compd. 2015, 647, 965–972. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.; Lazoryak, B.I.; Spassky, D.A.; Leonidov, I.I.; Stefanovich, S.Y.; Petrova, D.A.; Aksenov, S.M.; Burns, P.C. Ca8MgSm1−x(PO4)7: xEu3+, promising red phosphors for WLED application. J. Alloy. Compd. 2019, 776, 897–903. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Spassky, D.A.; Morozov, V.A.; Aksenov, S.M.; Kubrin, S.P.; Molokeev, M.S.; Lazoryak, B.I. Role of the Eu3+ distribution on the properties of β-Ca3(PO4)2 phosphors: Structural, luminescent, and 151Eu Mössbauer spectroscopy study of Ca9.5−1.5xMgEux(PO4)7. Inorg. Chem. 2021, 60, 3961–3971. [Google Scholar] [CrossRef]
- Xie, F.; Xu, D.; Wu, Z.; Molokeev, M.S.; Milićević, B.; Li, H.; Shi, J. Improving thermal stability of novel single-component white-light emitting phosphor Ca8MgLu(PO4)7:Tm3+, Dy3+ by back-energy-transfer. J. Lumin. 2020, 227, 117516. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Guo, L.; Zhang, F.; Wen, Y.; Liu, B.; Huang, Y. Vacuum ultraviolet and near-infrared excited luminescence properties of Ca3(PO4)2:RE3+, Na+ (RE = Tb, Yb, Er, Tm, and Ho). J. Solid State Chem. 2011, 184, 2178–2183. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Wu, Y.-N.; Shen, X.-H.; Ren, Y.-J.; Zhang, W.-G.; Wang, D.-J. Enhanced novel orange red emission in Ca3(PO4)2: Sm3+ by charge compensation. Opt. Laser Technol. 2014, 62, 63–68. [Google Scholar] [CrossRef]
- Xie, M.; Liang, H.; Huang, Y.; Gao, Z.; Tao, Y. Host absorption sensitizing and energy transfer to Eu3+ by Gd3+ in Ba6Gd2−xNa2Eux(PO4)6F2. J. Solid State Chem. 2013, 201, 18–23. [Google Scholar] [CrossRef]
- Guo, N.; Liang, Q.; Li, S.; Ouyang, R.; Lü, W. Triple energy transfer and color tuning in Tb3+ and Eu3+-coactivated apatite-type gadolinium-containing phosphors. Opt. Mater. 2017, 73, 570–576. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Shekhovtsov, A.; Kosmyna, M.; Loiko, P.; Vilejshikova, E.; Minikayev, R.; Romanowski, P.; Wierzchowski, W.; Wieteska, K.; Paulmann, C.; et al. Structure and thermal expansion of Ca9Gd(VO4)7: A combined powder-diffraction and dilatometric study of a Czochralski-grown crystal. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 411, 100–111. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Noh, H.M.; Jeong, J.H.; Jang, K.; Shin, D.S. Chemical bond parameters, charge transfer band, tunable white light of Tm3+ and Sm3+ coactivated Ca9Gd(VO4)7. J. Alloy. Compd. 2015, 618, 649–655. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, F.; Li, G.Q.; Zhang, J.; Zhang, W.F. Red-emitting phosphor series: Ca9Y(PO4)7(1−x)(VO4)7x: Eu3+ (x = 0−1) with improved luminescence thermal stability by anionic polyhedron substitution. J. Mater. Sci. Mater. Electron. 2019, 30, 8838–8846. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.V.; Spassky, D.A.; Berdonosov, P.S.; Dzhevakov, P.B.; Lazoryak, B.I. Sr8MSm1-xEux(PO4)7 phosphors derived by different synthesis routes: Solid state, sol-gel and hydrothermal, the comparison of properties. J. Alloy. Compd. 2021, 887, 161340. [Google Scholar] [CrossRef]
- Dikhtyar, Y.Y.; Deyneko, D.V.; Boldyrev, K.N.; Borovikova, E.Y.; Lipatiev, A.S.; Stefanovich, S.Y.; Lazoryak, B.I. Luminescent properties of Er3+ in centrosymmetric and acentric phosphates Ca8MEr(PO4)7 (M = Ca, Mg, Zn) and Ca9−xZnxLa(PO4)7:Er3+. Mater. Res. Bull. 2021, 138, 111244. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.; Spassky, D.; Dikhtyar, Y.Y.; Aksenov, S.M.; Stefanovich, S.Y.; Lazoryak, B.I. Luminescence of Eu3+ as a probe for the determination of the local site symmetry in β-Ca3(PO4)2-related structures. CrystEngComm 2019, 21, 5235–5242. [Google Scholar] [CrossRef]
- Bessière, A.; Benhamou, R.A.; Wallez, G.; Lecointre, A.; Viana, B. Site occupancy and mechanisms of thermally stimulated luminescence in Ca9Ln(PO4)7 (Ln=lanthanide). Acta Mater. 2012, 60, 6641–6649. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic study of inter atomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 1976, 32, 751–767. [Google Scholar]
- Zhang, Z.-J.; Yang, W. Luminescence characteristic of RE (RE = Pr, Sm, Eu, Tb, Dy) and energy levels of lanthanide ions in Gd5Si3O12N. Solid State Sci. 2017, 72, 64–70. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wen, Y.; Zhang, F.; Liu, B. Luminescence properties of Ca10K(PO4)7:RE3+ (RE=Ce, Tb, Dy, Tm and Sm) under vacuum ultraviolet excitation. J. Alloy. Compd. 2011, 509, 4649–4652. [Google Scholar] [CrossRef]
- Gruber, J.B.; Conway, J.G. Electronic energy levels and crystal quantum states of Tm (IV). J. Chem. Phys. 1960, 32, 1178. [Google Scholar] [CrossRef]
- Baklanova, Y.V.; Lipina, O.A.; Surat, L.L.; Chufarov, A.Y.; Tyutyunnik, A.P.; Zubkov, V.G. Luminescence Properties of Sr2La8−xTmx(GeO4)6O2 Apatites (x = 0.1–1.0) in the Visible and Short-Wave IR Spectral Ranges. Phys. Solid State 2020, 62, 1407–1414. [Google Scholar] [CrossRef]
- Ferhi, M.; Horchani-Naifer, K.; Férid, M. Spectroscopic properties of Eu3+-doped KLa(PO3)4 and LiLa(PO3)4 powders. Opt. Mater. 2011, 34, 12–18. [Google Scholar] [CrossRef]



| Dopantion | Radius, Å/CN | Dr, % | |||
|---|---|---|---|---|---|
| Gd3+ | Ca2+ | ||||
| 1.05 Å/8 | 0.94 Å/6 | 1.12 Å/8 | 1.00 Å/6 | ||
| Tm3+ | 0.88/6 | − | 6.8 | − | 13.6 |
| 0.99/8 | 6.1 | − | 13.1 | − | |
| Sm3+ | 0.96/6 | − | 1.1 | − | 5.3 |
| 1.08/8 | 2.7 | − | 3.7 | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforov, I.V.; Deyneko, D.V.; Spassky, D.A.; Lazoryak, B.I.; Aksenov, S.M. Whitlockite-Type Structure as a Matrix for Optical Materials: Synthesis and Characterization of Novel TM-SM Co-Doped Phosphate Ca9Gd(PO4)7, a Single-Phase White Light Phosphors. Minerals 2022, 12, 76. https://doi.org/10.3390/min12010076
Nikiforov IV, Deyneko DV, Spassky DA, Lazoryak BI, Aksenov SM. Whitlockite-Type Structure as a Matrix for Optical Materials: Synthesis and Characterization of Novel TM-SM Co-Doped Phosphate Ca9Gd(PO4)7, a Single-Phase White Light Phosphors. Minerals. 2022; 12(1):76. https://doi.org/10.3390/min12010076
Chicago/Turabian StyleNikiforov, Ivan V., Dina V. Deyneko, Dmitry A. Spassky, Bogdan I. Lazoryak, and Sergey M. Aksenov. 2022. "Whitlockite-Type Structure as a Matrix for Optical Materials: Synthesis and Characterization of Novel TM-SM Co-Doped Phosphate Ca9Gd(PO4)7, a Single-Phase White Light Phosphors" Minerals 12, no. 1: 76. https://doi.org/10.3390/min12010076






